Abstract
Previous studies have identified a population of neurons in the rat brain that discharge as a function of the animal's directional heading in the horizontal plane, independent of their location and on-going behaviour. Most studies on head direction (HD) cells have explored how they respond in two-dimensional environments within the horizontal plane. Many animals, however, live and locomote in a three-dimensional world. This paper reviews how HD cells respond when the animal locomotes on a vertical surface or inverted on a ceiling. We found that HD cells fire in a normal, direction-dependent manner when the rat is in the vertical plane, but not when the animal is inverted. Recent behavioural studies reported that rats are capable of accurately performing a navigational task when inverted, but only when the task was simple and started from not more than one or two entry points. Probe trials found that they did not have a flexible, map-like representation of space when inverted. The loss of the directional signal when the animal is in an inverted orientation may account for the absence of the map-like representation. Taken together, these findings indicate that a normal otolith signal contributes an important role to HD cell discharge.
 Jeffrey S. Taube earned his PhD from the University of Washington in 1986 under the supervision of Dr Phillip Schwartzkroin. After two periods of post-doctorate research with Dr James Ranck at SUNY Brooklyn and Dr Carl Cotman at UC Irvine, he joined the faculty at Dartmouth College in 1990. His research interests have centred around two main themes: understanding (1) the biological basis of spatial orientation and navigation and (2) the neurobiological mechanisms underlying learning and memory. To pursue these questions he uses electrophysiological recording techniques to record from single neurons in behaving rats. His work has focused on a population of cells in the rat hippocampal formation that discharge as a function of the animal's head direction in the horizontal plane, independent of its behaviour and location in the environment. His current research is designed to determine how the head direction signal is derived and processed from known sensory inputs. A second aim of his research is to determine the functional significance of the head direction cell signal to the organism; that is, how does an animal use these cells for orientation and navigation?
Jeffrey S. Taube earned his PhD from the University of Washington in 1986 under the supervision of Dr Phillip Schwartzkroin. After two periods of post-doctorate research with Dr James Ranck at SUNY Brooklyn and Dr Carl Cotman at UC Irvine, he joined the faculty at Dartmouth College in 1990. His research interests have centred around two main themes: understanding (1) the biological basis of spatial orientation and navigation and (2) the neurobiological mechanisms underlying learning and memory. To pursue these questions he uses electrophysiological recording techniques to record from single neurons in behaving rats. His work has focused on a population of cells in the rat hippocampal formation that discharge as a function of the animal's head direction in the horizontal plane, independent of its behaviour and location in the environment. His current research is designed to determine how the head direction signal is derived and processed from known sensory inputs. A second aim of his research is to determine the functional significance of the head direction cell signal to the organism; that is, how does an animal use these cells for orientation and navigation?
Accurate navigation initially involves perception of one's spatial orientation in the environment, and includes awareness about location and directional heading. The neural mechanisms that underlie locational and directional heading perceptions have received considerable attention – particularly in a rodent model. Place cells in the hippocampus, which fire in an allocentric location-specific manner, are ideally suited for providing information about the organism's perceived spatial location (O’Keefe, 1976; Best et al. 2001). In contrast, head direction (HD) cells, which fire as a function of the organism's directional heading in the horizontal (yaw) plane, provide information about the organism's perceived directional heading. HD cells have been identified in a number of different brain areas, but are particularly prevalent throughout the Papez circuit within the limbic system (Taube et al. 1990a,b; Taube, 2007). The preferred firing direction of HD cells are controlled by visual landmarks, although visual cues are not necessary for the presence of direction-specific firing. Interfering with vestibular inputs disrupts the HD signal and researchers have identified the pathways originating in the vestibular nuclei that project rostrally and contribute to the generation of the HD signal (Stackman & Taube, 1997; Sharp et al. 2001; Bassett et al. 2007). Motor and proprioceptive information has also been shown to influence the HD signal, particularly the cell's preferred firing direction when an animal is locomoting into a novel environment that is devoid of familiar landmark cues and must rely on path integration to maintain its sense of directional heading (Stackman et al. 2003). Further, some studies have found a reduction in the peak firing rate of a HD cell in response to passive rotation through the cell's preferred direction (Taube et al. 1990b; Knierim et al. 1995; Taube, 1995; Zugaro et al. 2001), but others have not observed this occurrence (Shinder & Taube, 2009). Nonetheless, it is important to note that HD cell discharge continues even in the absence of movement – such as when the animal is still (see Taube, 2007, for a review of this topic).
The vast majority of studies on these spatial cells have explored their responses when the animal locomotes along a two-dimensional surface in the horizontal plane. However, many animals inhabit and move about in a more three-dimensional world – travelling between different horizontal planes, climbing in a vertical plane, or locomoting in an inverted, upside-down position. Squirrels and marine mammals are but a few of the well-known species that routinely locomote in a three-dimensional environment. In addition to understanding the 3D properties of HD cells, studying how HD cells respond in different planes or when the animal is inverted may provide insights into the contributions the otolith organs serve in generating or modulating the directional signal. This review parallels my presentation at the symposium on Neural processes of orientation and navigation and describes how HD cells respond when the animal locomotes in a vertical plane or upside-down across a ceiling. How HD cells respond in these three planes when the animal is in zero gravity (0-g) is also reviewed. All HD cells discussed below were recorded in the anterior dorsal thalamus (ADN) of female Long–Evans rats that were between 4 and 8 months old. All procedures involving animals were performed in compliance with institutional standards as set forth by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
HD cell responses in the vertical plane
Our first set of experiments determined how HD cells respond as a rat locomotes from a horizontal plane into a vertical plane – one which is 90 deg orthogonal to the floor of the recording chamber (Stackman et al. 2000). These experiments also explored whether HD cell activity was affected when the rat was in a second horizontal plane that was significantly separated from, but still in sight of, the first horizontal plane. HD cell activity was recorded in a tall cylinder that contained a wide rim (annulus) around the top with four equally spaced food wells. A vertical wire mesh ‘ladder’ attached to the inside cylinder wall allowed the rat to access the annulus. HD cells were monitored as rats climbed up and down the wire mesh to retrieve food pellets on the floor and annulus. The wire mesh was positioned at 0, 90, 180 and 270 deg relative to the cell's preferred direction. HD cell discharge properties were similar when the rat locomoted in either horizontal plane (floor or annulus). When the wire mesh position corresponded with the cell's preferred direction (0 deg position), HD cells continued to fire at peak rates as the rat climbed up the wire mesh, but not when climbing down. With the mesh at the 180 deg position (opposite to the cell's preferred firing direction), cell firing continued when the rat ran down the mesh, but not when it ran up. There was an absence of firing when the rat ran up or down the ladder in the 90 and 270 deg positions. These studies indicate that cell discharge continues on a vertical surface if the rat approaches and locomotes into this plane while facing the cell's preferred direction. This finding is consistent with the hypothesis that HD cells may define the horizontal reference frame as the plane of locomotion of the animal. Preliminary experiments that used a spiral track positioned vertically that enabled the rat to sample all 360 deg in the vertical plane supported this view (Taube, 2005).
HD cell responses in 3-D in 0-g parabolic flight
Astronauts working in 0-g often experience visual reorientation illusions (VRIs) that sometimes trigger bouts of space sickness and often lead to reaching errors. On earth, subjects are often disoriented when they are upside-down. Our goal was to better understand orientation and navigation in 1-g and 0-g by determining how visual, gravitational and other cues anchor the three-dimensional response characteristics of HD cells. Specifically, we were interested in determining whether HD cells continue to respond in 0-g parabolic flight? If so, do the three-dimensional responses become more labile in 0-g, since the gravitational ‘down’ reference is absent? Finally, we also sought to determine whether the azimuthal response plane was aligned with the animal's plane of locomotion or with the visual reference frame.
Unrestrained rats locomoted in a clear Plexiglass rectangular cage, which had wire mesh covering the floor, ceiling and one wall (Taube et al. 2004). The cage was visually up–down symmetrical and the surrounding environmental cues were arranged so that up–down visual cues were ambiguous. We recorded from seven ADN HD cells from six rats with generally consistent responses observed across all cells. Each HD cell was monitored across about 40 episodes of 0-g. All cells maintained their direction-specific discharge when the rat was on the cage floor during the 0-g and 1.8-g pull-out periods. In contrast, direction-specific firing was usually disrupted when the rats were placed on the ceiling or wall and there was no single direction at which the cells fired. There also appeared to be an increase in background firing. While the rat was on the ceiling, three cells showed occasional bursts of firing when the rat's head was oriented in directions that were flipped relative to the long axis of symmetry of the chamber when compared to the cell's preferred firing direction on the floor. These responses suggest that during these particular parabolas the rats maintained a normal allocentric frame of reference in 0-g and 1-g when on the floor, but when placed on the ceiling or wall in 0-g, the rats appeared to be disoriented (as judged by the loss of directional specificity in HD cell firing). The occasional reversal of the HD cell's preferred direction across the cage axis of symmetry suggested that the rats may have experienced a VRI.
HD cell responses when upside-down on the ceiling
To determine how HD cells respond under normal gravity when an animal locomotes in an inverted position across a ceiling, food-restricted rats were trained to run around a 1 foot (∼30.5 cm) wide square-ring track (∼122 cm ×∼122 cm) that was oriented vertically (Fig. 1A) (Calton & Taube, 2005). Each surface contained a wire mesh to allow the rats to grasp and climb on. The floor surface was divided into two compartments separated by a partition. When in one floor compartment, the only way the rat could reach the other floor compartment was to climb up the wall, traverse the ceiling, and then climb down the other wall. Food was available in only one floor compartment and the rat started in the opposite floor compartment. The amount of food reward was limited so that once the rat reached the goal, it had to run back to the original compartment to receive additional food. Thus, the rat learned to shuttle back and forth between the two floor compartments by traversing the walls and ceiling. The apparatus was centred in a white-painted square room that had a large black curtain hanging from one wall. This curtain provided the major salient orienting cue for the rat. The apparatus could be rotated in the azimuthal plane so that we could examine HD cell responses either when the preferred direction was aligned with the plane of the apparatus or when the preferred direction was orthogonal to it. In addition to the ceiling mounted video camera, we had three additional cameras mounted to view the ceiling and the two vertical walls. Results showed that all HD cells showed robust direction-specific firing while the animal locomoted upright on the floor and walls, but surprisingly most cells lost direction-specific firing when locomoting upside-down on the ceiling (Fig. 1B). The remaining few cells showed mild directional responses when the rat was inverted on the ceiling, but these responses were markedly attenuated compared to the directional responses on the floor and walls. The cells that maintained some semblance of directional firing on the ceiling discharged in the same preferred direction with respect to the room as they discharged when the rat was on the floor. For all HD cell responses, firing on the walls was dependent on the direction from which the rat approached the wall and were similar to the findings described above for vertical wall climbing. Thus, if the rat approached the vertical surface from the cell's preferred direction, then cell firing continued as the rat locomoted either up or down a particular wall. Conversely, if the rat approached the vertical surface from a head orientation that was not facing the cell's preferred direction, then the cell didn't fire as the rat traversed the wall up or down. Consistent with these findings are preliminary studies showing that HD cells lose their direction-specific firing when the rat is passively pitched into an inverted orientation (Shinder & Taube, 2010).
Figure 1. HD cell responses on the floor, wall, and ceiling in a 3D task.
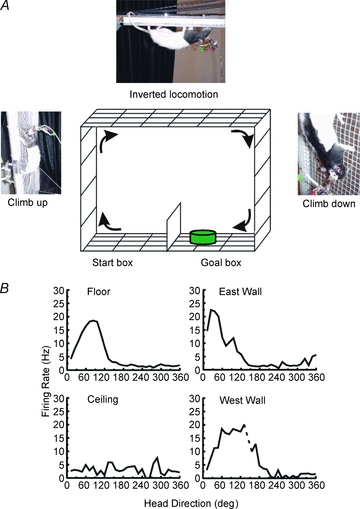
A, schematic drawing of the ring shuttle box task. The animal started in the floor compartment on the left and had to climb the West wall, traverse the ceiling, climb down the East wall, and end in the Goal box compartment in order to receive a reward. After about 10 s in the Goal compartment, the food cup was moved to the other side of the floor compartment and the rat had to shuttle back via the walls and ceiling to gain access to the food again. B, firing rate vs. HD tuning curves for when the rat was on the four surfaces. Results showed that directional firing was maintained on both walls, but was disrupted when the rat was on the ceiling.
Why was directional tuning lost during inversion?
There are several possible explanations that might account for the loss of directional tuning during inversion. One important factor to consider is whether the nature of the rat's locomotion when inverted played a role in the diminishment of the HD signal response. Certainly, the rats walked slower and used a different pattern of movement when inverted compared to their normal gait in the horizontal plane. Although one might argue that this different patterned movement led to the altered directional activity, the rats movement patterns and gait were also different when they locomoted along the vertical walls, and yet directional firing remained intact under these conditions. Thus, while we cannot rule out the possibility that an abnormal movement pattern when inverted may have contributed to the reduction in the HD signal, we feel this possibility is unlikely. Another possible factor contributing to the loss of direction firing is stress. However, the rats were trained on the square ring apparatus task for 2–3 months prior to recording cells, and they readily performed the task when the recordings were conducted. This situation makes it less likely that stress played a large role in the loss of directional activity. Whether further training beyond 3 months would have led to more direction-specific firing is possible, but it is hard to imagine how this might occur, given that the rats were well-trained and very familiar with the task. Furthermore, as mentioned above, there were a few cells that retained some semblance of directional tuning when inverted, and these cells were sometimes recorded several days prior to other cells that contained no directional tuning during inverted locomotion. If training experience was a strong factor in the loss of directional tuning, one would predict that cells recorded in the later stages of recordings would show directional tuning – this was not the case.
Another possibility to consider for the loss of directional activity is that if the animals define their plane of locomotion as the horizontal reference plane and then shift this plane with them as they locomote into the vertical plane, and then onto the ceiling when going inverted, the cells would fire in the opposite direction when on the ceiling compared to their response on the ground. This situation would obviously cause a conflict with the surrounding visual landmarks and possibly lead to confusion about the animal's true orientation. To avoid this circumstance the directional system might have evolved in a way as to ‘shut-down’ when the animal is inverted. By suspending operation of the system, the animal would avoid any potential conflict in their perceived orientation.
In the findings described above, HD cell responses in the vertical plane were similar to those in the horizontal plane, and can be accounted for by treating the vertical plane as an extension of the floor, where the animal shifts its allocentric reference frame to realign with the current plane of locomotion. This process, however, cannot explain the disruption observed in responses during inverted locomotion, and again raises the issue of why an inverted orientation should be so disruptive. There are two further possibilities that were not discussed above and are not mutually exclusive. First, the task we used was not sufficiently demanding to ‘force’ the animals to maintain their perceived spatial orientation while upside-down. The ring apparatus task is not particularly spatially demanding, as the rat only has to move straight forward continuously on the mesh in order to reach the reward compartment. Although this explanation is plausible, directional activity is not reduced in the traditional food foraging task in the cylinder, a task that does not require much navigation. In addition, this explanation also has difficulty accounting for why an attractor network, which is thought to underlie the HD signal (Skaggs et al. 1995; Redish et al. 1996; Zhang, 1996), would shut down when the animal is inverted. Most attractor networks are modelled in a way that they are continuously operative and do not shut down periodically even when the animal is not engaging that information. Nonetheless, because attractor network models remain just that – unproven models of how the HD signal is generated – it remains possible that the signal failed because the animal was not sufficiently engaged in a demanding navigational task. In sum, although the low spatial demand explanation does not seem to sustain much support, it cannot be excluded. Second, the signals arising from the otoliths during inversion are structured as to be completely unfamiliar to the animal. In this scenario, the rats’ common everyday experiences are such that they do not develop sufficient experiences during post-natal life to encode the types of signals that originate from the otoliths during inverted locomotion. As a result, in the absence of this experience, the brain may find the novel otolith signals to be sufficiently ‘foreign’ that it cannot process them correctly, and consequently the directional signal becomes disrupted. In essence, the neural system that derives perceived directional heading evolved to process otolith information with certain characteristics – and when it receives altered and unfamiliar information it cannot handle, the system ‘breaks down’. This situation may account for the disrupted HD cell signal with inverted locomotion despite extensive training of the animals on the task. Similar disorienting effects frequently occur in humans when encountering an unfamiliar otolith signal when in a supine position (Howard & Hu, 2001; Klier et al. 2005).
Behavioural performance during inverted locomotion
To address whether the loss of direction-specific firing while inverted was due to low spatial demand, we designed a more navigationally challenging task that required the rats to navigate accurately from an inverted position (Valerio et al. 2010). The task was modelled after the Morris water maze (Morris, 1981) and the Barnes hole board task (Barnes, 1979) and is referred to as the ‘Inverted Hole Board Escape Task’ (IHBET). A 1.2 m diameter wire mesh was suspended from the ceiling (Fig. 2A). Four holes spaced uniformly in the four quadrants were cut into the mesh. One of these holes allowed access to the top surface of the platform and enabled the rat to climb through and into an upright position. The other three ‘false’ holes were blocked with a piece of wood and prevented the rat from climbing through the hole. Clinging to the mesh or locomoting in an inverted position is taxing for the rats and they prefer to avoid such positions. Thus, in this task the rat is motivated to find the open hole in order to avoid being continually in an inverted position. The room contained several prominent visual landmarks that the rats could use for spatial reference points. The rats were started from entry points around the perimeter of the platform.
Figure 2. Behavioral performance in an upside-down spatial task.
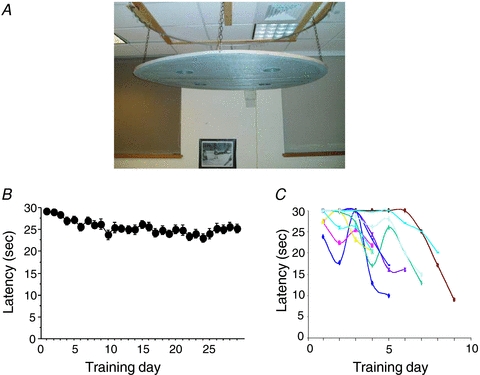
A, suspended platform apparatus for the inverted hole board escape task. Rats were released on the perimeter and had to move inverted to the correct hole in order to return to an upright posture. Three of the four holes were blocked with a piece of wood. B, escape latencies averaged across each day for 29 days in the condition where the rat was randomly released from one of four entry points. The plot shows that little improvement was observed over the course of 29 days. Error bars represent the s.e.m. C, escape latencies from individual rats when released from the same single entry point across days. All rats were able to learn the correct hole location and reached criterion performance within 9 days.
In Experiment 1, rats were trained to navigate to the escape hole by locomoting from either one- or four-start points. Interestingly, no animals from the four-start point group reached criterion, even after 29 days of training (Fig. 2B). Animals in the one-start point group reached criterion after about six training sessions (Fig. 2C). Rats could also learn the location of the hole when released from two nearby start points that were close to the hole. In Experiment 2, probe tests revealed that animals navigating from either one- or two-start points used distal visual landmarks for accurate orientation. However, subsequent probe tests revealed that their performance was markedly attenuated when navigating to the escape hole from a novel start point at the periphery or even at the maze centre. This absence of flexibility while navigating upside-down was confirmed in Experiment 3 where we demonstrated that the rats did not learn to reach a place, but instead learned separate trajectories to the target hole. These results suggested that inverted navigation primarily involved a simple directional strategy based on visual landmarks, rather than a flexible cognitive map-based strategy. Taken together, one might speculate that the loss of the directional signal when inverted prevents the animal from using a cognitive map-based strategy.
HD cell responses in otoconia-deficient mice
As mentioned above, the HD signal is dependent on an intact vestibular system, which includes the semicircular canals and otolith organs. While previous studies indicate that the semicircular canals provide a necessary component of the HD signal, since occlusion of the canals disrupts the directional signal (Muir et al. 2009), the involvement of otolithic information has remained less clear. Thus, to determine the importance of the otolith signal to HD cell firing, we recorded cell activity in the ADN of otoconia-deficient tilted mice during locomotion within a cylinder and compared their activity to normal C57BL/6J mice (Yoder & Taube, 2009). HD cells were identified in tilted mice, but directional firing properties were not as robust as those of C57BL/6J mice. Most HD cells in tilted mice were controlled by landmark rotation, but showed substantial signal degradation across trials. These results support current models that suggest otolithic information is involved in the perception of directional heading (Angelaki & Cullen, 2008).
Conclusions
In summary, HD cells maintain their direction-specific firing when the rat is in the vertical plane, but usually lose their directional tuning when the animal is inverted. The absence of a strong directional signal when inverted may account for the poor performance observed on the inverted spatial task. Even though the rats could learn a simpler version of the inverted task (using only 1–2 entry points), they did not have a more flexible, cognitive map-like representation of their environment that they could rely on to find the correct hole from a novel start point. Instead, they relied on a directional strategy where they moved toward a constellation of visual cues. Although further research is necessary to understand why directional tuning was lost despite the presence of salient visual cues, recordings from otolith-deficient mice showed that HD cells were still present in these animals. This finding suggests that it may be the resulting unfamiliar otolith signal that contributes to the loss of the HD signal and their subsequent poor performance when they are in an inverted orientation.
Acknowledgments
This research was supported through grants from the National Institutes of Health NS053907, DC009318. The author thanks the following people for their important contributions to the experiments described in this report: Joel Brown, Jeremy Chan, Benjamin Clark, Jeffrey Calton, Russell Frohardt, Carlton Frost, Mark Harris, Stanley Kim, Gary Muir, Charles Oman, Jennifer Rilling, Michael Shinder, Robert Stackman, Matthew Tullman, Stephane Valerio, Sarah Wang and Ryan Yoder.
Glossary
Abbreviations
- ADN
anterior dorsal thalamus
- HD
head direction
- VRI
visual reorientation illusion
References
- Angelaki DE, Cullen KE. Vestibular system: the many facets of a multimodal sense. Ann Rev Neurosci. 2008;31:125–150. doi: 10.1146/annurev.neuro.31.060407.125555. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Bassett JP, Tullman ML, Taube JS. Lesions of the tegmento-mammillary circuit in the head direction system disrupts the head direction signal in the anterior thalamus. J Neurosci. 2007;27:7564–7577. doi: 10.1523/JNEUROSCI.0268-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best PJ, White AM, Minai A. Spatial processing in the brain: the activity of hippocampal place cells. Ann Rev Neurosci. 2001;24:459–486. doi: 10.1146/annurev.neuro.24.1.459. [DOI] [PubMed] [Google Scholar]
- Calton JL, Taube JS. Degradation of head direction cell activity during inverted locomotion. J Neurosci. 2005;25:2420–2428. doi: 10.1523/JNEUROSCI.3511-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard IP, Hu G. Visually induced reorientation illusions. Perception. 2001;30:583–600. doi: 10.1068/p3106. [DOI] [PubMed] [Google Scholar]
- Klier EM, Angelaki DE, Hess BJ. Roles of gravitational cues and efference copy signals in the rotational updating of memory saccades. J Neurophysiol. 2005;94:468–478. doi: 10.1152/jn.00700.2004. [DOI] [PubMed] [Google Scholar]
- Knierim JJ, Kudrimoti HS, McNaughton BL. Place cells, head direction cells, and the learning of landmark stability. J Neurosci. 1995;15:1648–1659. doi: 10.1523/JNEUROSCI.15-03-01648.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RGM. Spatial localisation does not require local cues. Learning Motivation. 1981;12:239–260. [Google Scholar]
- Muir GM, Brown JE, Carey JP, Hirvonen TP, Della Santina CC, Minor LB, Taube JS. Semicircular canal occlusions disrupt head direction cell activity in the anterordorsal thalamus of chinchillas. J Neurosci. 2009;29:14521–14533. doi: 10.1523/JNEUROSCI.3450-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J. Place units in the hippocampus of the freely moving rat. Exp Neurol. 1976;51:78–109. doi: 10.1016/0014-4886(76)90055-8. [DOI] [PubMed] [Google Scholar]
- Redish AD, Elga AN, Touretzky DS. A coupled attractor model of the rodent head direction system. Network: Computation in Neural Systems. 1996;7:671–685. [Google Scholar]
- Sharp PE, Blair HT, Cho J. The anatomical and computational basis of the rat head-direction cell signal. Trends Neurosci. 2001;24:289–294. doi: 10.1016/s0166-2236(00)01797-5. [DOI] [PubMed] [Google Scholar]
- Shinder ME, Taube JS. Washington, DC: Society for Neuroscience; 2009. Active and passive movement are encoded equally by head direction cells of the anterodorsal thalamus. Program No. 193.3. 2009 Abstract Viewer/Itinerary Planner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinder ME, Taube JS. Washington, DC: Society for Neuroscience; 2010. Responses of head direction cells in the anterodorsal thalamus during inversion. Program No. 895.1. 2010 Abstract Viewer/Itinerary Planner. [Google Scholar]
- Skaggs WE, Knierim JJ, Kudrimoti HS, McNaughton BL. A model of the neural basis of the rat's sense of direction. In: Tesauro G, Touretzky DS, Leen TK, editors. Advances in Neural Information Processing Systems. Vol. 7. Cambridge, MA: MIT Press; 1995. pp. 173–180. [PubMed] [Google Scholar]
- Stackman RW, Golob EJ, Bassett JP, Taube JS. Passive transport disrupts directional path integration by rat head direction cells. J Neurophysiol. 2003;90:2862–2874. doi: 10.1152/jn.00346.2003. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Taube JS. Firing properties of head direction cells in rat anterior thalamic neurons: Dependence upon vestibular input. J Neurosci. 1997;17:4349–4358. doi: 10.1523/JNEUROSCI.17-11-04349.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman RW, Tullman ML, Taube JS. Maintenance of rat head direction cell firing during locomotion in the vertical plane. J Neurophysiol. 2000;83:393–405. doi: 10.1152/jn.2000.83.1.393. [DOI] [PubMed] [Google Scholar]
- Taube JS. Head direction cells recorded in the anterior thalamic nuclei of freely moving rats. J Neurosci. 1995;15:70–86. doi: 10.1523/JNEUROSCI.15-01-00070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS. Head direction cell activity: landmark control and responses in three dimensions. In: Wiener SI, Taube JS, editors. Head Direction Cells and the Neural Mechanisms of Spatial Orientation. Cambridge, MA: MIT Press; 2005. pp. 45–67. [Google Scholar]
- Taube JS. The head direction signal: origins and sensory-motor integration. Ann Rev Neurosci. 2007;30:181–207. doi: 10.1146/annurev.neuro.29.051605.112854. [DOI] [PubMed] [Google Scholar]
- Taube JS, Muller RU, Ranck JB., Jr Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci. 1990a;10:420–435. doi: 10.1523/JNEUROSCI.10-02-00420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS, Muller RU, Ranck JB., Jr Head-direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulations. J Neurosci. 1990b;10:436–447. doi: 10.1523/JNEUROSCI.10-02-00436.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS, Stackman RW, Calton JL, Oman CM. Rat head direction cell responses in 0-G parabolic flight. J Neurophysiol. 2004;92:2887–2997. doi: 10.1152/jn.00887.2003. [DOI] [PubMed] [Google Scholar]
- Valerio S, Clark BJ, Harris MJ, Frost CP, Chan JHM, Taube JS. Directional learning, but no spatial mapping by rats performing a navigational task in an inverted orientation. Neurobiol Learn Mem. 2010;93:495–505. doi: 10.1016/j.nlm.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder RM, Taube JS. Head direction cell activity in mice: robust directional signal depends on intact otoliths. J Neurosci. 2009;29:1061–1076. doi: 10.1523/JNEUROSCI.1679-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K. Representation of spatial orientation by the intrinsic dynamics of the head direction cell ensemble. J Neurosci. 1996;16:2112–2126. doi: 10.1523/JNEUROSCI.16-06-02112.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zugaro MB, Tabuchi E, Fouquier C, Berthoz A, Wiener SI. Active locomotion increases peak firing rates of anterodorsal thalamic head direction cells. J Neurophysiol. 2001;86:692–702. doi: 10.1152/jn.2001.86.2.692. [DOI] [PubMed] [Google Scholar]


