Abstract
Colonic chloride secretion is induced by chemical stimuli via the enteric nervous reflex. We have previously demonstrated that propionate stimulates chloride secretion via sensory and cholinergic systems of the mucosa in rat distal colon. In this study, we demonstrate non-neuronal release of ACh in the secretory response to propionate using an Ussing chamber. Mucosa preparations from the colon, not including the myenteric and submucosal plexuses, were used. Luminal addition of propionate and serosal addition of ACh caused biphasic changes in short-circuit current (Isc). TTX (1 μm) had no effects, while atropine (10 μm) significantly inhibited the Isc response to propionate and abolished that to ACh. In response to luminal propionate stimulation, ACh was released into the serosal fluid. A linear relationship was observed between the maximal increase in Isc and the amounts of ACh released 5 min after propionate stimulation. This ACh release induced by propionate was not affected by atropine and bumetanide, although both drugs significantly reduced the Isc responses to propionate. Luminal addition of 3-chloropropionate, an inactive analogue of propionate, abolished both ACh release and Isc response produced by propionate. RT-PCR analysis indicated that isolated crypt cells from the distal colon expressed an enzyme of ACh synthesis (ChAT) and transporters of organic cation (OCTs), but not neuronal CHT1 and VAChT. The isolated crypt cells contained comparable amounts of ACh to the residual muscle tissues including nerve plexuses. In conclusion, the non-neuronal release of ACh from colonocytes coupled with propionate stimulation plays a key role in chloride secretion, via the paracrine action of ACh on muscarinic receptors of colonocytes.
Non-technical summary
ACh is the best characterized neurotransmitter that is synthesized in cholinergic neurons in the brain and gut wall. In the gut, acetylcholine is released from the nerve endings in response to luminal stimuli and regulates the movement of gut contents via stimulating muscle contraction and epithelial ion secretion. We show that acetylcholine is synthesized in colonic epithelial cells and released on the serosal side by luminal chemical stimulation of the short chain fatty acid propionate and causes chloride secretion. These results suggest that non-neuronal release of acetylcholine in response to luminal stimuli plays a role in colonic chloride secretion.
Introduction
Chemical stimulation of the intestinal mucosa can elicit secretory and contractile responses via sensory input to receptor cells and the enteric nervous reflex (Hubel, 1985). Nutrients sensing regulates digestion, absorption and motility, while noxious stimuli evoke defence functions that flush out harmful microbes and toxins (Furness et al. 2004).
Short-chain fatty acids (SCFAs), which are major products of microbial fermentation in the large intestine, act not only as energy sources (Livesey & Elia, 1995) but also as chemical stimulators for mesenteric vasodilatation (Knock et al. 2002), colonic contraction (Yajima, 1985; Mitsui et al. 2005) and peristalsis (Grider & Piland, 2007), mucin secretion (Sakata & Setoyama, 1995), and chloride secretion (Yajima, 1988). The secretory response to SCFAs, in combination with the contractile response, seems to act as a lubricant for the movement of luminal content in the colon.
We previously demonstrated that luminal addition of propionate or other SCFAs transiently stimulated chloride secretion and resulted in an increase in short-circuit current (Isc) and conductance in rat distal colon in vitro (Yajima, 1988). Hubel and Russ confirmed the Isc responses to luminal propionate in rat distal colon and further showed that the propionate responses were not affected by tachyphylaxis to various transmitters localized in the intestinal wall, calcitonin gene-related peptide, 5-HT, histamine, neurotensin and substance-P (Hubel & Russ, 1993), whereas atropine (10 μm) and local anaesthetics (50–100 μm) reduced the propionate responses by 81–90% and 76–82%, respectively (Yajima, 1988; Hubel & Russ, 1993). Furthermore, superficial mucosal damage with hypertonic sodium sulphate (2 m) or xylose (4.5 m) reduced the propionate response by 90% and 86%, respectively (Hubel & Russ, 1993). Taken together, it could be speculated that ACh may be released from cholinergic secretomotor neurons or colonic epithelial cells; however, the exact site of ACh release was not fully revealed.
ACh is the best characterized neurotransmitter that is synthesized in cholinergic neurons and released via vesicular machinery in response to physiological and pharmacological stimulation. ACh acts through nicotinic and muscarinic receptors in nerves and peripheral tissues. The digestive tract is richly innervated by cholinergic neurons (Harrington et al. 2010a). Epithelial cells and muscles express many subtypes of muscarinic receptors that are involved in the reflex motor and secretory responses to mechanical and chemical signals from the luminal side of the digestive tract (Raybould et al. 2004).
Besides neuronal ACh, the synthesis and storage of ACh in a broad variety of non-neuronal cells, particularly in surface epithelia of the airway, bladder, placenta and skin of rodents and humans has been recently discovered (Wessler & Kirkpatrick, 2008). The presence of non-neuronal ACh is also reported in the epithelial cells of the small and large intestines of rats and humans (Klapproth et al. 1997). Non-neuronal ACh is physiologically expected to act as a local cellular signalling or trophic molecule (Klapproth et al. 1997). However, the physiological significance of the synthesis and release of non-neuronal ACh in the intestine are still unclear, although there are a few reports regarding changes in the epithelial expression of ChAT in inflammatory bowel diseases, including ulcerative colitis (Gareau et al. 2007; Jonsson et al. 2007).
In the present study, we examined the ACh release from the mucosa preparation of the colon, not including the myenteric and submucosal plexuses, in response to the luminal addition of propionate, and non-neuronal ACh storage in colonic crypt cells of rat. We have demonstrated for the first time that luminal propionate stimulation released non-neuronal ACh from colonic epithelial cells on the serosal side, and then induced chloride secretion, probably via paracrine action on muscarinic receptors, in colonocytes in rat.
Methods
Animals
This study was approved by the Hokkaido University Animal Committee, and all animals were maintained in accordance with the Hokkaido University guidelines for the care and use of laboratory animals. Male Sprague–Dawley rats (250–300 g) were used. They were fed a pelleted diet (Type MF, Oriental Yeast Co., Tokyo, Japan) ad libitum with free access to water, but were starved overnight before use.
Tissue preparation
Rats were asphyxiated with CO2 gas and exsanguinated. The colonic segments were removed and opened along the mesenteric border, and the luminal contents were washed out by warm Krebs bicarbonate saline solution. For mucosa preparation, which histologically consisted of the mucosa and the muscularis mucosae (data not shown), the submucosa together with the tunica muscularis were removed with fine forceps under a stereomicroscope.
Large and small epithelial sheets were made from the mucosa preparation. To measure the ACh release and electrical activity, two large sheets were prepared from each segment of the proximal or distal colon and were mounted in an Ussing chamber with a window size of 0.98 cm2 and a volume of 5 ml. Four small sheets were prepared from the distal colon for the measurement of electrical activity and were mounted in an Ussing chamber with a window size of 0.20 cm2 and a volume of 5 ml. To prepare four small sheets, the mucosa preparation of the distal colon was incised, first longitudinally and then transversely. Of the two small sheets from each transverse segment, one was treated with inhibitors of propionate response and the other, treated only with the solvent for the drug, served as control.
Short-circuit current measurement
The Ussing chambers were bathed on each side of the mucosa with a volume of 5 ml of bathing solution containing (in mm): NaCl, 119; CaCl2, 1.25; MgCl2 1; K2HPO4, 2.2; KH2PO4, 0.2; NaHCO3, 21 and glucose, 10. The solution was bubbled with a gas mixture of 95% O2 and 5% CO2 (pH 7.4, 37°C). The tissues were short-circuited by a voltage clamp (Nihon Koden, Tokyo, Japan) at zero potential automatically with compensation for solution resistance. Isc was continuously recorded and tissue conductance measured every minute. The Isc was referred to as positive when current flowed from the mucosal to the serosal side. The current was recorded by the Power Lab system (ADInstruments, Bella Vista, Australia).
Propionate and ACh stimulation
The tissues were left for about 40 min to stabilize Isc before the effects of propionate and drugs were studied. Sodium propionate (100 mm) and acetylcholine chloride (10 mm), 25 or 50 μl, were added to the mucosal and serosal sides in the Ussing chamber, respectively. Appropriate concentrations of other drugs in a volume of 5–10 μl were added to the mucosal or serosal side 10 min prior to propionate or ACh additions.
ACh release
After Isc of the mucosa preparation reached a stable baseline, ACh release experiments were initiated adding TTX (1 μm) to the serosal side and eserine (0.1 mm) to both sides. Ten minutes later, propionate (1 mm) was added to the luminal side. A volume of 200 μl from both luminal and serosal fluids was taken at 5 min intervals to measure ACh concentration. After sampling, 200 μl of bathing solution was added to both fluids. The samples were stored at −80°C until the ACh was analysed.
Crypt isolation
The proximal and distal colons were removed according to the previously described anatomical divisions (Yajima, 1985). The colon was opened longitudinally and rinsed in warm phosphate-buffered saline (PBS). Each colonic segment was incubated in a 10 ml of Hanks’ balanced solution (Sigma-Aldrich Corp., St Louis, MO, USA) containing 5 mm dithiothreitol, 0.1% BSA, 1 mm glutamine and 30 mm EDTA (pH 7.4) for 15 min with shaking at 37°C. After incubation, the colons were gently scraped with a rubber policeman to isolate the crypts, which were centrifuged at 100 g for 3 min at 4°C. The crypt pellets were washed twice with cold PBS. The residual muscle tissues were vigorously scraped with a rubber policeman and washed with cold PBS. The crypt pellets and residual muscle tissues were weighed and stored at −80°C until analysis by RT-PCR and measurement of ACh.
RNA extraction and cDNA synthesis
Total RNA was extracted from the crypt pellets and residual muscle tissues with a QuickGene RNA tissue kit S II (Fujifilm Corp., Tokyo, Japan) and a RNase-free DNase set (Takara, Shiga, Japan) according to the manufacturer's instructions. cDNA was then synthesized from 150 ng of the total RNA with a ReverTra Ace qPCR RT kit (Toyobo, Osaka, Japan) according to the manufacturer's instructions.
RT-PCR analysis
RT-PCR was performed using a LightCycler 480 (Roche Applied Science, Tokyo, Japan). The expression of six genes (ChAT, CHT1, OCT1, OCT2, OCT3 and VAChT) was assessed in all the samples. The expression of β-actin (a housekeeping gene) was also assessed in this study. Specific primers and probe sets for each gene were designed according to the GenBank accession number with an online software provided by Roche Applied Science (https://www.roche-applied-science.com/). The sequences of the primers and probes used in this study are listed in Table 1. PCR for all the genes was performed under the following conditions: an initial denaturation at 95°C for 5 min, followed by 50 cycles of denaturation at 95°C for 10 s and combined annealing–extension at 60°C for 30 s. All reactions were performed using a LightCycler 480 Probes Master and Universal ProbeLibrary Set (Roche) according to the manufacturer's instructions.
Table 1.
The sequences of primers and probes used in real-time PCR analysis
| Gene name | Accession no. | Primer sequence (5′–3′) | Probe no. | |
|---|---|---|---|---|
| β-Actin | NM_031144 | Forward | cccgcgagtacaaccttct | 17 |
| Reverse | cgtcatccatggcgaact | |||
| ChAT | NM_001170593 | Forward | gcctcatctctggtgtgctt | 120 |
| Reverse | cagtcagtgggaagggagtg | |||
| CHT1 | NM_053521 | Forward | cattggtttgttggttggtg | 83 |
| Reverse | gctgtcccgttgatgtaacc | |||
| OCT1 | NM_012697 | Forward | gctagctgtgtccctgccta | 4 |
| Reverse | gggattctgggacaaacca | |||
| OCT2 | NM_031584 | Forward | ctgtgactctgcccaacttct | 15 |
| Reverse | ggagatcagccatcttggag | |||
| OCT3 | NM_019230 | Forward | gactggcgctatgtggagac | 22 |
| Reverse | gccgcagacaaggtcaaa | |||
| VAChT | NM_031663 | Forward | tgcaagagcactgtccaact | 10 |
| Reverse | cgaggggctagggtattcat | |||
Listed probe numbers indicate the product number of the Universal ProbeLibrary Set and Human and Extension Set sold by Roche Applied Science.
The relative expression level of each mRNA was calculated by the comparative Ct method (Nishimura et al. 2003), wherein the Ct value of the target mRNA was subtracted from the Ct value of the β-actin mRNA. Data are presented as fold-differences to the expression level of the brain.
ACh measurement
The concentrations of ACh in the fluids obtained from the ACh release experiments, isolated crypts cells and residual muscle tissues were measured by high-performance liquid chromatography (HPLC) (Elite LaChrom, Hitachi Co. Ltd, Tokyo, Japan) combined with a post column enzyme reactor (AC-ENZYMEPAK, Eicom Co. Kyoto, Japan) and an electrochemical detector (ECD-700, Eicom Co.). The mobile phase, applied at flow rate of 0.15 ml min−1, comprised 50 mm KHCO3 containing 300 mg l−1 sodium 1-decanesulfonate and 50 mg l−1 Na2-EDTA. After separation by a C18 polymer gel column (AC-GEL, 2 × 150 mm, Eicom Co.), ACh was converted to hydrogen peroxide by the post-column enzyme reactor with immobilized acetylcholine esterase and choline oxidase at 30°C. The hydrogen peroxide was detected with the electrochemical detector which was equipped with a platinum electrode (+450 mV set potential). The ACh concentration was estimated by the calibration curves of ACh standard using chromatography data software (D-2000 Elite, Hitachi Co. Ltd, Tokyo, Japan). Isopropyl homocholine was used as the internal standard.
The crypt pellets and residual muscle tissues were homogenized in 500 μl of PBS containing eserine (0.1 mm) by Micro Smash (MS-100, Tomy Medical Ltd, Tokyo, Japan) at 3800 rpm for 3 min at room temperature. The lysates were centrifuged at 15 000 g for 10 min at 4°C and the supernatants were used for ACh measurement after appropriate dilution. Samples were loaded onto an autosampler (D-2000 Elite, Hitachi Co Ltd) at 10°C, and a sample of 10 μl was injected into the HPLC system.
Drugs
Sodium propionate and 3-chloropropionic acid were obtained from Kanto Kagaku Co. Ltd (Tokyo, Japan). The free acid was neutralized with sodium hydroxide. Acetylcholine chloride, amiloride hydrochloride, atropine sulphate, bumetanide, dithiothreitol, EDTA, eserine hemisulphate, glutamine, tetrodotoxin and veratridine were obtained from Sigma-Aldrich Corp. Veratridine was dissolved in DMSO.
Statistics
Values are mean ±s.e.m. The significance of the difference between data of the control and experimental groups was determined by Student's t test or one-way ANOVA followed by Dunnett's test. Linear regression was performed to determine the significance of differences in the relationship between propionate-induced Isc and ACh release. Differences were considered significant when P values were less than 0.05.
Results
Electrical activity of mucosa preparation
The basal Isc of the mucosa preparation after stabilization was 48.2 ± 3.5 μA cm−2 (n= 16). Neuronal stimulations by bipolar rectangular electrical pulses (5 mA, 10 Hz) and serosal addition of veratridine (10 μm) caused an increase in Isc of 97.5 ± 13.5 μA cm−2 (n= 4) and 149.4 ± 4.0 μA cm−2 (n= 5), respectively. These neuronal responses were abolished by serosal addition of TTX (1 μm), but were not affected by serosal addition of atropine (10 μm).
Effects of TTX and atropine on Isc responses to propionate and ACh
The serosal addition of TTX (1 μm) decreased the basal Isc by 28.4 ± 4.8 μA cm−2 (n= 8), but the serosal addition of atropine (10 μm) had no effect on the basal Isc. The residual Isc after TTX treatment was almost abolished by the mucosal addition of amiloride (0.1 mm).
The luminal addition of propionate transiently increased Isc not only across the mucosa–submucosa preparation but also the mucosa preparation as previously reported (Yajima, 1988). In this study, we confirmed that the mucosa preparation responded to luminal addition of propionate (0.5 mm) as well as serosal addition of ACh (0.05 mm), both of which caused a biphasic increase in Isc. At first, a short downward spike followed by a higher transient increase in Isc of similar duration was observed (Fig. 1).
Figure 1. A typical trace of short-circuit current (Isc) measurement in the mucosa preparation.
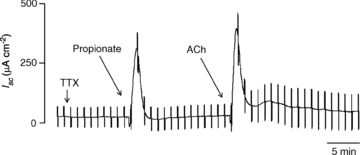
After the basal Isc was stabilized, TTX (1 μm) was added to the serosal fluid; 10 min later, propionate (0.5 mm) was added to the mucosal fluid. ACh (0.05 mm) was added to the serosal fluid after Isc returned to the basal level.
The Isc response to luminal propionate in the mucosa preparation was not significantly influenced by the serosal addition of TTX (1 μm) (Fig. 2). On the other hand, the Isc response to propionate was significantly inhibited by the serosal addition of atropine (10 μm) (Fig. 2). The serosal addition of TTX had no effect on ACh-induced Isc responses, while the serosal addition of atropine abolished the Isc responses to ACh (Fig. 2).
Figure 2. Effects of tetrodotoxin (TTX, 1 μm) and atropine (10 μm) on basal Isc and luminal propionate-induced Isc in mucosa preparation.
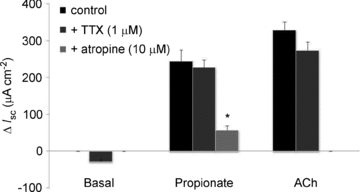
TTX (n= 8) and atropine (n= 8) were added to the serosal fluid 10 min before the luminal addition of propionate (0.5 mm), respectively. Values are means ±s.e.m. of the maximal increase in Isc and statistical significance was assessed with one-way ANOVA followed by Dunnett’ test. *P < 0.01 compared to the control.
Relationship between propionate- and ACh-induced Isc responses
Linear regression analysis of the maximal increases in Isc between propionate- and ACh-induced Isc responses showed a significant linear relationship (Fig. 3). This relationship, together with the results of the strong inhibition of Isc response to propionate with atropine, suggests that ACh release mediates the luminal propionate-induced Isc increase.
Figure 3. Relationship between propionate-induced Isc and ACh-induced Isc.
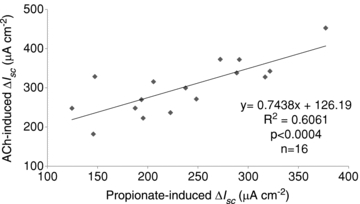
Linear regression analysis was performed based on the difference between the maximal increases of Isc induced by luminal propionate and that by serosal ACh in the same mucosa preparation.
ACh release induced by luminal propionate
To address the involvement of ACh in the luminal propionate-induced Isc increase, we directly measured ACh release from the mucosa preparation in response to luminal propionate in the presences of TTX (1 μm) and eserine (0.1 mm). The mucosal and serosal fluids were sampled every 5 min. Before the propionate stimulation, ACh release was not observed in either fluid. After the propionate stimulation, ACh was detected in the serosal fluid but not in the mucosal fluid (Fig. 4). The amount of ACh release induced by propionate was 803 ± 181 pmol (g tissue)−1 (n= 6) during the first 5 min period, 152 ± 61 pmol (g tissue)−1 (n= 6) during the second 5 min period, and no release during the third 5 min period.
Figure 4. ACh release induced by luminal propionate in the mucosa preparation.
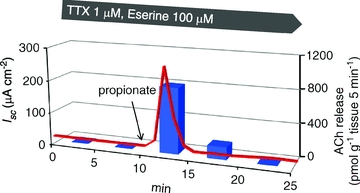
The concentration of ACh in the serosal fluid was measured every 5 min. Red line shows changes in Isc plotted by the mean value every minute (n= 6). Blue bars show ACh releases on the serosal fluid, which was plotted as the mean value every 5 min (n= 6). The amount of ACh release is shown in pmoles per gram wet weight of the tissue covered on the window of the Ussing chamber. Propionate (1 mm) was added to the mucosal fluid 10 min after the start.
There was a significant linear relationship between the values of maximal Isc increase and the amounts of ACh release after the propionate stimulation (Fig. 5).
Figure 5. Relationship between ACh release and Isc increase induced by luminal propionate.
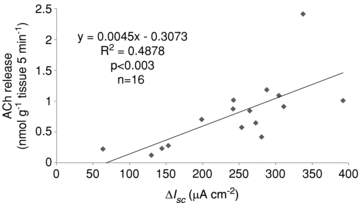
Linear regression analysis was performed based on the difference between the amount of ACh released for 5 min and the maximal increases in Isc induced by luminal propionate.
Effects of atropine, bumetanide and 3-Cl-propionate on the increase in Isc and ACh release induced by luminal propionate
Serosal additions of atropine (10 μm) and bumetanide (50 μm), a potent inhibitor of chloride secretion, in the presence of TTX significantly inhibited the Isc increase induced by luminal propionate, but both drugs had no effects on ACh release on the serosal side (Fig. 6).
Figure 6. Effects of atropine (10 μm), bumetanide (50 μm) and 3-Cl-propionate (1 mm) on Isc response and ACh release induced by luminal propionate in the mucosa preparation.
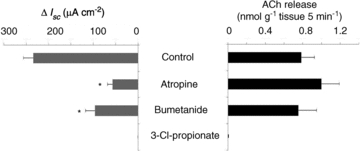
Atropine and bumetanide were added to the serosal fluid and 3-Cl-propionate was added to the mucosal fluid 10 min prior to luminal propionate (1 mm) stimulation. The concentration of ACh in the serosal fluid was measured at 5 min after luminal propionate stimulation. Values are means ±s.e.m. (n= 6–16). Statistical significances of the maximum increases in Isc and the ACh releases were assessed with one-way ANOVA followed by Dunnett's test. *P < 0.001 compared to the control.
We previously reported that 3-Cl-propionate, an inactive analogue of propionate, had no effect on potential difference (PD) across the everted rat colon, but competitively inhibited the luminal propionate-induced increase in lumen-negative PD (Yajima, 1989). In this study, luminal addition of 3-Cl-propionate had no effect on Isc response, but completely blocked the propionate-induced Isc increase as well as ACh release (Fig. 6). This result suggests that luminal propionate sensory input is received by a specific receptor site in the apical membrane of colonocytes and then causes ACh release.
Expression of genes related to ACh synthesis in isolated crypts
In light of the above data, it can be hypothesized that ACh release in response to luminal propionate in the mucosa preparation is of non-neuronal origin, and that epithelial cells are the most plausible candidates. To obtain colonic epithelium free of neuronal components, we isolated crypt cells from colonic sheets by means of Ca2+-free EDTA treatment (see details in Methods). The isolated crypt cells and residual muscle tissues were used for RT-PCR analysis of genes involved in ACh synthesis.
RT-PCR analysis showed that the crypt cells expressed higher mRNA levels of choline acetyltransferase (ChAT), an enzyme of ACh synthesis, compared to the residual muscle tissues (Fig. 7). On the other hand, mRNAs of the neuron-specific high affinity choline transporter (CHT1) and vesicular acetylcholine transporter (VAChT) were scarcely detected in the crypt cells. This indicates that the isolated crypt cells were not contaminated by the enteric nerve components. The increased mRNA expression of organic cation transporters, OCT1, 2 and 3, suggest that these transporters probably play a role in choline uptake in epithelial cells instead of CHT1.
Figure 7. Expression of genes related to ACh synthesis and storage.
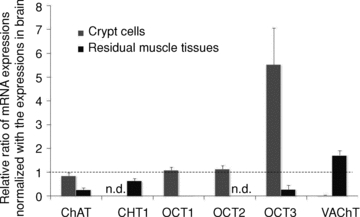
RT-PCR analysis indicates that ChAT and OCTs mRNAs were expressed in the isolated crypt cells, while neuronal marker mRNAs of CHT1 and VAChT were scarcely expressed. The dotted line indicates the level expressed in the brain. Values are means ±s.e.m. (n= 4).
ACh content in isolated crypt cells and residual muscle tissues
The ACh content in the isolated crypt cells of the distal colon was 11.9 ± 2.0 nmol (g wet wt)−1, which was not significantly different than that in the residual muscle tissues of proximal and distal colons (Fig. 8). On the other hand, the ACh content in the crypt cells of the proximal colon was significantly lower than that of the distal colon.
Figure 8. ACh content in the isolated crypt cells and the residual muscle tissues from the proximal and distal colon.
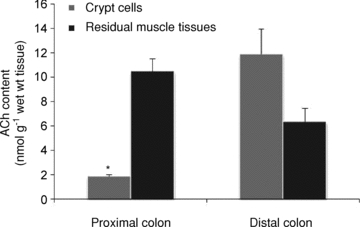
ACh concentrations in the tissues were measured after homogenizing with presence of eserine (0.1 mm) and centrifugation. The obtained supernatants were used in HPLC analysis. Values are means ±s.e.m. (n= 4). *P < 0.05 compared between crypt cells and residual muscle tissues by Student's t test.
Regional differences of propionate-induced ACh release and Isc in the colon
Based on the difference of ACh contents in the crypt cells of the proximal and distal colons, we measured ACh release and Isc response induced by luminal propionate stimulation in the proximal colon. As shown in Fig. 9, both ACh release and Isc response were significantly lower in the proximal colon than in the distal colon. These results suggest that the regional difference of the secretory responses to luminal propionate depends on the difference of ACh content in the colonic crypt cells.
Figure 9. Regional difference in ACh content, ACh release and Isc response between the proximal and distal colon.
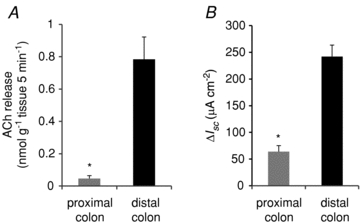
A, ACh release induced by luminal propionate (1 mm). B, Isc increase induced by luminal propionate (1 mm). Values are means ±s.e.m. (n= 4). *P < 0.05 compared between the proximal and distal colon by Student's t test.
Discussion
In the present study, we have demonstrated for the first time that the chemical stimulation by luminal propionate induced non-neuronal ACh release from the colonic epithelium on the serosal side, which induced an increase in the Isc, mainly due to bumetanide sensitive chloride secretion, in rat distal colon. This result was substantiated by the direct measurements of ACh release after the luminal propionate stimulation and of ACh content in the isolated colonic crypt cells. Furthermore, these findings were supported by the expression of genes associated with ACh synthesis in the isolated colonic crypt cells.
The mucosa preparation of rat distal colon consists of the epithelium and the muscularis mucosae, not including the myenteric and submucosal nerve plexuses. Bridges et al. (1986) reported that the mucosa preparation of rat distal colon contains the neuronal network called the mucosal nerve plexus, which influences colonic ion transport. It was shown that the Na+ channel activator, sea anemone toxin, and electrical field stimulation (EFS) stimulate the neurons of the mucosal plexus, and induce increases in Isc and conductance across the mucosa preparation, which are abolished by TTX (Bridges et al. 1986; Diener et al. 1989). Furthermore, it was suggested that the mucosa plexus is involved in a neuronally mediated cholinergic response according to the partial inhibition of the EFS-induced Isc increase by atropine (50 μm) (Diener et al. 1989). In this study, we confirmed that nerve stimulations with the Na+ channel activator veratridine and EFS induced increases in Isc in the mucosa preparation, which were completely abolished by TTX. It has been reported that veratridine causes ACh release from the submucosal neurons in rat colon (Gaginella et al. 1992). However, neither EFS nor veratridine could release ACh from the mucosal nerve plexus in the mucosa preparation since atropine had no effect on both induced Isc increase. On the other hand, the Isc response to luminal propionate in the mucosa preparation was not affected by serosal addition of TTX (1 μm) but was significantly inhibited by serosal addition of atropine (10 μm). This indicates that the Isc response to propionate is mediated by ACh that is released by means other than excitation of the mucosal neurons.
It is well known that muscarinic ACh receptors are expressed on intestinal epithelial cells (Hirota & McKay, 2006). In rat colonic epithelial cells, M1 and M3 muscarinic receptors are responsible for ACh receptor-mediated chloride secretion (O’Malley et al. 1995; Haberberger et al. 2006). In this study, the ACh-induced increases in Isc in the mucosa preparation were not affected by TTX but abolished by atropine, indicating a direct action of ACh on colonic epithelial muscarinic receptors. Hence, the TTX-resistant and atropine-sensitive Isc responses to propionate (Fig. 2) suggest ACh release from non-neuronal components in the colonic mucosa.
As expected, the luminal addition of propionate induced the largest ACh release on the serosal side in the mucosa preparation 5 min after the stimulation, indicating that the Isc increase observed during the same period must be elicited by the released ACh (Fig. 4). This speculation was supported by the observation of a highly significant linear relationship between the amount of ACh release and the maximal increase in Isc (Fig. 5). The evidences that atropine and bumetanide inhibited the Isc responses to luminal propionate but not the ACh release (Fig. 6) strongly suggests that the Isc increase induced by the luminal propionate is mediated by the ACh that is released into the serosal fluid. In this study, we adopted the HPLC technique combined with enzyme reactors and an electrochemical detector, a highly sensitive and specific method to measure ACh concentration in the experimental samples. In addition, sufficient concentrations of eserine to block acetylcholine esterase (AChE) activity in the mucosa preparation were added to both sides of the Ussing chambers since AChE is richly involved in the nerve fibres in rat colonic mucosa (Mestres et al. 1992).
It has been generally accepted that cells or tissues undergo a process of desensitization following sustained stimulation with a high concentration of receptor agonist. We have previously demonstrated that the secretory response to SCFAs is rapidly desensitized by the repeated stimulation of the same or different acids. However, cross-desensitization between ACh and propionate does not occur in the mucosa–submucosa preparation of rat colon (Yajima, 1988). In the present study, the ACh release induced by propionate did not apparently affect the secretory response to ACh, suggesting that the ACh release induced by luminal propionate is rapidly desensitized at the SCFA receptor and does not reach enough concentration to cause desensitization of the muscarinic receptor.
Even though the ACh release was TTX resistant, this does not exclude the possibility that the ACh release in response to the luminal propionate in the mucosa preparation was of neural origin as it has been shown that the mucosa is richly innervated by the nerve fibres of postganglionic cholinergic neurons in the submucosal plexus (Harrington et al. 2010a). Therefore, to evaluate the synthesis and storage of ACh in colonic epithelial cells, we prepared a colonic crypt free of mucosal nerve elements and measured the mRNA expressions of genes involved in ACh synthesis. The negligible expression of CHT1 and VAChT, specific markers of central and peripheral nerves (Harrington et al. 2010b), in the crypt cells compared to the residual muscle tissues containing enteric nerves (Fig. 7) shows that the isolated crypt cells were free of the contamination of neuronal elements. Higher mRNA expression of the ChAT gene in the crypt cells rather than in the residual muscle tissues indicates that the colonic epithelial cells have the ability to synthesize ACh. This was confirmed by direct measurement of the ACh contents in the crypt cells and the residual muscle tissues (Fig. 8).
Klapproth et al. (1997) previously reported that non-neuronal ACh content in the small and large intestines of rat was 1.3 ± 0.3 (n= 4), 1.4 ± 0.4 (n= 5) nmol (g wet wt)−1, respectively. They sampled the tissues above the muscularis mucosae by scraping the mucosal surface with a cotton-tipped applicator, indicating that their samples contained submucosal nerve components. Their value of ACh content in the large intestine is close to that obtained in the crypt cells from the proximal colon (1.8 ± 0.2 nmol (g wet wt)−1, n= 4) and less than that from the distal colon (11.9 ± 2.0 nmol (g wet wt)−1, n= 4). The ACh content in the crypt cells from the rat distal colon is approximately three orders of magnitude less than that in different regions of the rat brain (Vizi & Palkovits, 1978) and in the myenteric plexus in the guinea-pig distal colon (Giaroni et al. 1999).
Choline is a key substrate for ACh synthesis. In the neurons, the uptake of choline is mediated by the high-affinity choline transporter, CHT1 (Brandon et al. 2004). In the crypt cells, the expression of CHT1 was not detected by RT-PCR, but higher expression of mRNAs for the polyspecific organic cation transporters, OCT1, 2 and 3, was found in comparison with nerve-containing residual muscle tissues. Hence, the OCTs and not CHT1 may play a role in choline uptake for ACh synthesis in the colonic epithelial cells as choline is one of the endogenous substrates of OCTs (Koepsell et al. 2007).
The regional differences in the secretory responses to the luminal propionate in rat colon (Yajima, 1989) can be explained by the regional differences of the ACh content and release in the proximal and distal colon (Fig. 9). On the other hand, that there was no significant difference in ACh content in the nerve containing muscle tissues of the proximal and distal colon strongly supports the hypothesis that the secretory response to the luminal propionate depends on colonic epithelial ACh synthesis and release.
The ACh release from non-neuronal epithelial cells was reported for the first time in the human placenta (Wessler et al. 2001). By the superfusion of the villus isolated from the placenta, the ACh releases were observed at a rate of 0.13 nmol (g wet wt)−1 min−1. Interestingly, this value is comparable to that (0.16 nmol (g wet wt)−1 min−1) observed in the propionate-induced ACh release from rat colonic mucosa in this study. In the placenta villus, however, the ACh release spontaneously occurred without any stimulation, but not in the colonic mucosa. The stimulus-coupled release of non-neuronal ACh was recently reported in the human bladder strip with urothelium, in which the stretch tension enhanced the ACh release in the presence of TTX (Yoshida et al. 2008). To address the physiological role of non-neuronal ACh release from the epithelium, it is important to know whether ACh is released on the luminal or serosal side. However, the previous studies did not reveal the direction of ACh release from the epithelial cells of the placenta and bladder. In the present study, we clearly demonstrated that the ACh release coupled with the luminal propionate stimulation occurred on the serosal side, but not luminal side, in the rat colon using an Ussing chamber.
In general, a stimulus-coupled release of transmitters is observed in excitable cells such as neurons and endocrine cells. The release of ACh in response to luminal propionate stimulation (Fig. 4) appears to be similar to the stimulus-coupled phenomenon, probably via ACh-containing sensory cells on colonic mucosa. In our previous study, we have speculated that the sensory stimulation of secretory response to luminal SCFAs is received at a specific receptor site in the mucosal surface of the rat colon (Yajima, 1988). The prior addition of luminal 3-Cl-propionate completely blocked the Isc response and also abolished the ACh release in response to the luminal propionate (Fig. 6). These results indicate that epithelial cells storing ACh have a receptor site for propionate, although a specific epithelial cell type storing ACh in the colonic epithelial cells has not yet identified. The SCFA receptor, free fatty acid receptor 2 and 3 (FFA2 and FFA3), that was immunohistochemically demonstrated in the colon of rats and humans (Karaki et al. 2006; Tazoe et al. 2008) is a possible receptor in the colonic secretory response to luminal SCFAs. Further studies are required to identify the specific cell type on colonic mucosa that respond to luminal propionate, and to determine the mechanism of ACh release.
Acknowledgments
We thank Prof. A. Kuwahara for the critical reading of the article and Ms M. Harada for animal care and preparation of buffer and experimental equipment.
Glossary
Abbreviations
- ChAT
choline acetyltransferase
- CHT1
high affinity choline transporter
- OCT
organic cation transporter
- SCFA
short chain fatty acid
- VAChT
vesicular acetylcholine transporter
Author contributions
Conception and design of the experiment: T.Y. Collection, analysis and interpretation of data: T.Y., R.I., M.M. and M.Y. Drafting the article or revising it critically: T.Y. and M.Y. All authors approved the final version of this manuscript.
References
- Brandon EP, Mellott T, Pizzo DP, Coufal N, D’Amour KA, Gobeske K, Lortie M, Lopez-Coviella I, Berse B, Thal LJ, Gage FH, Blusztajn JK. Choline transporter 1 maintains cholinergic function in choline acetyltransferase haploinsufficiency. J Neurosci. 2004;24:5459–5466. doi: 10.1523/JNEUROSCI.1106-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RJ, Rack M, Rummel W, Schreiner J. Mucosal plexus and electrolyte transport across the rat colonic mucosa. J Physiol. 1986;376:531–542. doi: 10.1113/jphysiol.1986.sp016168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener M, Knobloch SF, Bridges RJ, Keilmann T, Rummel W. Cholinergic-mediated secretion in the rat colon: neuronal and epithelial muscarinic responses. Eur J Pharmacol. 1989;168:219–229. doi: 10.1016/0014-2999(89)90568-2. [DOI] [PubMed] [Google Scholar]
- Furness JB, Jones C, Nurgali K, Clerc N. Intrinsic primary afferent neurons and nerve circuits within the intestine. Prog Neurobiol. 2004;72:143–164. doi: 10.1016/j.pneurobio.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Gaginella TS, Grisham MB, Thomas DB, Walsh R, Moummi C. Oxidant-evoked release of acetylcholine from enteric neurons of the rat colon. J Pharmacol Exp Ther. 1992;263:1068–1073. [PubMed] [Google Scholar]
- Gareau MG, Jury J, Perdue MH. Neonatal maternal separation of rat pups results in abnormal cholinergic regulation of epithelial permeability. Am J Physiol Gastrointest Liver Physiol. 2007;293:G198–203. doi: 10.1152/ajpgi.00392.2006. [DOI] [PubMed] [Google Scholar]
- Giaroni C, Somaini L, Marino F, Cosentino M, Senaldi A, De Ponti F, Lecchini S, Frigo G. Modulation of enteric cholinergic neurons by hetero- and autoreceptors: cooperation among inhibitory inputs. Life Sci. 1999;65:813–821. doi: 10.1016/s0024-3205(99)00308-2. [DOI] [PubMed] [Google Scholar]
- Grider JR, Piland BE. The peristaltic reflex induced by short-chain fatty acids is mediated by sequential release of 5-HT and neuronal CGRP but not BDNF. Am J Physiol Gastrointest Liver Physiol. 2007;292:G429–437. doi: 10.1152/ajpgi.00376.2006. [DOI] [PubMed] [Google Scholar]
- Haberberger R, Schultheiss G, Diener M. Epithelial muscarinic M1 receptors contribute to carbachol-induced ion secretion in mouse colon. Eur J Pharmacol. 2006;530:229–233. doi: 10.1016/j.ejphar.2005.11.055. [DOI] [PubMed] [Google Scholar]
- Harrington AM, Hutson JM, Southwell BR. Cholinergic neurotransmission and muscarinic receptors in the enteric nervous system. Prog Histochem Cytochem. 2010a;44:173–202. doi: 10.1016/j.proghi.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Harrington AM, Lee M, Ong SY, Yong E, Farmer P, Peck CJ, Chow CW, Hutson JM, Southwell BR. Immunoreactivity for high-affinity choline transporter colocalises with VAChT in human enteric nervous system. Cell Tissue Res. 2010b;341:33–48. doi: 10.1007/s00441-010-0981-9. [DOI] [PubMed] [Google Scholar]
- Hirota CL, McKay DM. Cholinergic regulation of epithelial ion transport in the mammalian intestine. Br J Pharmacol. 2006;149:463–479. doi: 10.1038/sj.bjp.0706889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel KA. Intestinal nerves and ion transport: stimuli, reflexes, and responses. Am J Physiol Gastrointest Liver Physiol. 1985;248:G261–271. doi: 10.1152/ajpgi.1985.248.3.G261. [DOI] [PubMed] [Google Scholar]
- Hubel KA, Russ L. Mechanisms of the secretory response to luminal propionate in rat descending colon in vitro. J Auton Nerv Syst. 1993;43:219–229. doi: 10.1016/0165-1838(93)90328-r. [DOI] [PubMed] [Google Scholar]
- Jonsson M, Norrgard O, Forsgren S. Presence of a marked nonneuronal cholinergic system in human colon: study of normal colon and colon in ulcerative colitis. Inflamm Bowel Dis. 2007;13:1347–1356. doi: 10.1002/ibd.20224. [DOI] [PubMed] [Google Scholar]
- Karaki S, Mitsui R, Hayashi H, Kato I, Sugiya H, Iwanaga T, Furness JB, Kuwahara A. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 2006;324:353–360. doi: 10.1007/s00441-005-0140-x. [DOI] [PubMed] [Google Scholar]
- Klapproth H, Reinheimer T, Metzen J, Munch M, Bittinger F, Kirkpatrick CJ, Hohle KD, Schemann M, Racke K, Wessler I. Non-neuronal acetylcholine, a signalling molecule synthezised by surface cells of rat and man. Naunyn Schmiedebergs Arch Pharmacol. 1997;355:515–523. doi: 10.1007/pl00004977. [DOI] [PubMed] [Google Scholar]
- Knock G, Psaroudakis D, Abbot S, Aaronson PI. Propionate-induced relaxation in rat mesenteric arteries: a role for endothelium-derived hyperpolarising factor. J Physiol. 2002;538:879–890. doi: 10.1113/jphysiol.2001.013105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res. 2007;24:1227–1251. doi: 10.1007/s11095-007-9254-z. [DOI] [PubMed] [Google Scholar]
- Livesey G, Elia M. Short-chain fatty acids as an energy source in the colon: metabolism and clinical implications. In: Cummings JH, Rombeau JL, Sakata T, editors. Physiological and Clinical Aspects of Short-Chain Fatty Acids. Cambridge: Cambridge University Press; 1995. pp. 427–481. [Google Scholar]
- Mestres P, Diener M, Rummel W. Histo- and immunocytochemical characterization of the neurons of the mucosal plexus in the rat colon. Acta Anat (Basel) 1992;143:268–274. doi: 10.1159/000147261. [DOI] [PubMed] [Google Scholar]
- Mitsui R, Ono S, Karaki S, Kuwahara A. Neural and non-neural mediation of propionate-induced contractile responses in the rat distal colon. Neurogastroenterol Motil. 2005;17:585–594. doi: 10.1111/j.1365-2982.2005.00669.x. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Ueda N, Naito S. Effects of dimethyl sulfoxide on the gene induction of cytochrome P450 isoforms, UGT-dependent glucuronosyl transferase isoforms, and ABCB1 in primary culture of human hepatocytes. Biol Pharm Bull. 2003;26:1052–1056. doi: 10.1248/bpb.26.1052. [DOI] [PubMed] [Google Scholar]
- O’Malley KE, Farrell CB, O’Boyle KM, Baird AW. Cholinergic activation of Cl– secretion in rat colonic epithelia. Eur J Pharmacol. 1995;275:83–89. doi: 10.1016/0014-2999(94)00758-y. [DOI] [PubMed] [Google Scholar]
- Raybould HE, Cooke HJ, Christofi FL. Sensory mechanisms: transmitters, modulators and reflexes. Neurogastroenterol Motil. 2004;16(Suppl 1):60–63. doi: 10.1111/j.1743-3150.2004.00477.x. [DOI] [PubMed] [Google Scholar]
- Sakata T, Setoyama H. Local stimulatory effect of short-chain fatty acids on the mucus release from the hindgut mucosa of rats (Rattus norvegicus) Comp Biochem Physiol A Physiol. 1995;111:429–432. doi: 10.1016/0300-9629(95)00033-4. [DOI] [PubMed] [Google Scholar]
- Tazoe H, Otomo Y, Kaji I, Tanaka R, Karaki SI, Kuwahara A. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J Physiol Pharmacol. 2008;59(Suppl 2):251–262. [PubMed] [Google Scholar]
- Vizi S, Palkovits M. Acetylcholine content in different regions of the rat brain. Brain Res Bull. 1978;3:93–96. doi: 10.1016/0361-9230(78)90032-1. [DOI] [PubMed] [Google Scholar]
- Wessler I, Kirkpatrick CJ. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br J Pharmacol. 2008;154:1558–1571. doi: 10.1038/bjp.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessler I, Roth E, Schwarze S, Weikel W, Bittinger F, Kirkpatrick CJ, Kilbinger H. Release of non-neuronal acetylcholine from the human placenta: difference to neuronal acetylcholine. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:205–212. doi: 10.1007/s002100100445. [DOI] [PubMed] [Google Scholar]
- Yajima T. Contractile effect of short-chain fatty acids on the isolated colon of the rat. J Physiol. 1985;368:667–678. doi: 10.1113/jphysiol.1985.sp015882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima T. Luminal propionate-induced secretory response in the rat distal colon in vitro. J Physiol. 1988;403:559–575. doi: 10.1113/jphysiol.1988.sp017264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima T. Chemical specificity of short-chain fatty acid-induced electrogenic secretory response in the rat colonic mucosa. Comp Biochem Physiol A Comp Physiol. 1989;93:851–856. doi: 10.1016/0300-9629(89)90511-2. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Masunaga K, Satoji Y, Maeda Y, Nagata T, Inadome A. Basic and clinical aspects of non-neuronal acetylcholine: expression of non-neuronal acetylcholine in urothelium and its clinical significance. J Pharmacol Sci. 2008;106:193–198. doi: 10.1254/jphs.fm0070115. [DOI] [PubMed] [Google Scholar]


