Abstract
Women are more susceptible to orthostatic intolerance. Peripheral α-adrenergic responsiveness is important in orthostasis and is lower in women compared to men, and is modulated by female sex hormones. We tested the hypothesis that oestradiol attenuates peripheral cutaneous adrenergic responses in women with low orthostatic tolerance (LT), whereas progesterone enhances adrenergic responses in women with high orthostatic tolerance (HT). After completing a maximal lower body negative pressure test to determine level of orthostatic tolerance (cumulative stress index, CSI), women self administered a gonadotropin releasing hormone (GnRH) antagonist for 16 days to suppress endogenous sex hormone production. Oestradiol (E2, 0.2 mg day−1, patch; days 4–16), and progesterone (P4, 200 mg day−1, oral; days 12–16) were administered. Skin blood flow responses to graded intradermal microdialysis infusions of noradrenaline (NA) were measured during GnRH antagonist, E2, and E2+P4, in eight HT (s.e.m. = 22 ± 1 years, CSI −871 ± 86 mmHg min) and eight LT (21 ± 1 years, CSI −397 ± 65 mmHg min) women. In separate probes, NA was infused alone, and co-infused with the nitric oxide synthase inhibitor NG-monomethyl-l-arginine (l-NMMA, 10 mm), the non-selective cyclooxygenase inhibitor ketorolac tromethamine (Keto, 10 mm), and combined l-NMMA + Keto (10 mm each). Progesterone administration enhanced adrenergic responses in HT women (logEC50 GnRH −4.02 ± 0.39, E2+P4−5.18 ± 0.31, P < 0.05); this response was reversed with Keto (E2+P4 logEC50 NA+Keto −3.82 ± 0.35, P < 0.05). In contrast, no change in adrenergic responsiveness occurred in LT women during any hormone condition. These data indicate differential regulation of cutaneous adrenergic responses by progesterone via the cyclooxygenase pathway in women with high and low orthostatic tolerance.
Non-technical summary
Women experience orthostatic intolerance (the inability to maintain blood pressure during postural changes) more frequently than men. This difference between men and women may in part be related to how oestradiol and progesterone influence dilatation and constriction of blood vessels. We show that progesterone enhances vasoconstriction in women who have a higher tolerance to orthostatic stress, but not in women with low tolerance. The increase in vasoconstriction with progesterone administration in women with high tolerance appears to be mediated by prostaglandins (hormone-like substances that assist in maintaining bodily functions). These results show that progesterone alters blood vessel constriction and can help us understand blood pressure regulation in women.
Introduction
Assumption of upright posture produces a sudden downward shift of central blood volume, with pooling of as much as 700 ml of blood in the lower abdomen and lower limbs and away from the central and cerebral areas (Rowell, 1993). If not for mechanisms evolved to maintain blood pressure, this loss of central blood volume would result in syncope.
During orthostatic challenges, both initial and prolonged mechanisms contribute to maintain blood pressure. The initial, neural reflex response includes both central and peripheral cardiovascular adjustments, which include an increase in sympathetic outflow, heart rate and peripheral vasoconstriction. Peripheral vasoconstriction in muscle and skin accounts for up to 40% of the change in total vascular resistance, with vasoconstriction of the splanchnic region and kidneys contributing the balance (Rowell et al. 1972; Rowell, 1993). Thus, reduced peripheral vasoconstrictive responses to adrenergic stimuli are likely to contribute to orthostatic intolerance. The degree to which an individual can vasoconstrict in response to changes in posture may determine his or her orthostatic tolerance (Fu et al. 2004b) and may partially explain why women are more susceptible to orthostatic intolerance compared to men (White et al. 1996; Convertino, 1998). For example, women have a lower vasoconstricting response to α-adrenergic stimuli, as measured by finger blood flow changes during brachial artery infusion of phenylephrine (Freedman et al. 1987). An additional study measuring forearm blood flow using plethysmography demonstrated that women have greater β2-adrenergically mediated vasodilatation compared to men (Kneale et al. 2000). In women presumed to have normal orthostatic tolerance, vasoconstriction is enhanced during the luteal phase of the menstrual cycle (when both oestrogens and progesterone levels are elevated) compared to the early follicular phase (when oestrogens and progesterone are at their lowest) (Freedman & Girgis, 2000). Thus, it is likely that the oestradiol and progesterone modulation of vasodilatory and vasoconstrictor responses play a role in orthostatic tolerance.
Adrenergic responsiveness of the cutaneous circulation was used in the present study as a model to investigate mechanisms controlling peripheral adrenergic control of blood pressure. Under resting conditions, the cutaneous circulation is under adrenergic control (Hodges & Johnson, 2009), and cutaneous blood flow plays a major role in the peripheral vasoconstrictor response to orthostatic challenge during normothermic conditions, especially at high levels of orthostatic stress (Beiser et al. 1970; Rowell et al. 1973; Tripathi & Nadel, 1986). Furthermore, the cutaneous circulation can be used as a generalized model of microvascular function (Holowatz et al. 2008) because responses in the cutaneous microvasculature parallel those in other parts of the body (Abularrage et al. 2005; Holowatz et al. 2008) and can predict chronic disease associated with a number of syndromes (Stewart et al. 2004, 2007). Finally, the skin is a readily accessible vascular bed (Wilson et al. 2003) and so allows for the exploration of important mechanisms relating to nitric oxide (NO) and prostaglandin inhibition (via intradermal microdialysis) without exposing the whole body to these drugs.
To examine the effects of female reproductive hormones on adrenergic responsiveness in relation to orthostatic tolerance, we suppressed endogenous production of oestrogen and progesterone using the gonadotropin-releasing hormone (GnRH) antagonist, ganirelix acetate, and then administered 17β-oestradiol alone, followed by combined oestradiol and progesterone. Specifically, the purpose of this investigation was to determine the effects of oestradiol (E2) and progesterone (P4) on cutaneous vasoconstrictor responses in women with high (HT) and low (LT) orthostatic tolerance. We hypothesized that (1) oestradiol attenuates cutaneous adrenergic responsiveness more in LT compared to HT women; and (2) progesterone enhances cutaneous adrenergic responsiveness more in HT compared to LT women. Our overall hypothesis was that women with low orthostatic tolerance are more sensitive to the vasodilatory effects of oestradiol, but less sensitive to progesterone induced vasoconstriction compared to women with high orthostatic tolerance.
Methods
Eighteen healthy young women were recruited for the study. All women were normotensive and non-smoking and had a body mass index (BMI) <30 kg m−2. Women were excluded if they had a history of blood clots, high blood pressure, stroke, epilepsy, diabetes, or cancer. Pregnant women or those who had irregular menstrual cycles were excluded. Subjects refrained from caffeine (12 h), alcohol (12 h), and exercise (24 h) prior to testing. Women were instructed to drink 7 ml kg−1 of water the night before the study, but restricted their fluid intake the morning of the study to avoid the need to change postures during the protocol to void. All subjects gave written informed consent to participate in the study, which conformed to the Declaration of Helsinki and had prior approval by the Human Investigation Committee of Yale University School of Medicine.
Experimental design
Determination of orthostatic tolerance
Each woman completed a lower body negative pressure (LBNP) test to determine her level of orthostatic tolerance. Experiments were conducted in a temperature controlled room (27°C, <30% rh). All subjects lay in the supine position with their legs inside the LBNP box, which was sealed at the level of the iliac crest. An intravenous catheter was placed in the left arm for blood sampling. Subjects were instrumented for measurements of heart rate (single lead ECG), beat-to beat blood pressure (BP, Finometer, Finapres Medical Systems, Amsterdam, The Netherlands), and respiration (Pneumotrace II model 1132, UFI, Morro Bay, CA, USA). An automated upper arm blood pressure cuff was also used for standard brachial blood pressure measurements (Colin Medical Instruments, Komaki, Japan) during the LBNP test as a backup for subject safety, but the Finometer data were used in all analyses. After a 30 min supine rest period, a blood sample was taken and 5 min of baseline measurements commenced. The LBNP test started with applying negative pressure at −15 mmHg for 3 min, followed by −20 mmHg for 3 min. Each subsequent stage decreased in pressure by −10 mmHg (−30, −40, −50 mmHg, etc.) in 3 min intervals until presyncope. Test termination was determined using any one of the following criteria: a decrease in systolic BP < 80 mmHg; a decrease in systolic BP to <90 associated with symptoms of lightheadedness, nausea, sweating or diaphoresis; or progressive symptoms of presyncope accompanied by a request from the subject to terminate the test. A second blood sample was taken at test termination. Blood samples were analysed for haemoglobin, haematocrit, noradrenaline (NA), adrenaline (Adr), and plasma renin activity (PRA). A cumulative stress index (CSI) was calculated for each woman by summing the product of the negative pressure (mmHg) and the time (minutes) spent at that stage (Fu et al. 2004b, 2005). A more negative CSI indicated a higher negative pressure attained prior to presyncopal symptoms and thus higher orthostatic tolerance.
Experimental protocol
All women completed three experimental conditions: (1) gonadotropin-releasing hormone (GnRH) antagonist, (2) GnRH antagonist + oestradiol, and (3) GnRH antagonist + oestradiol + progesterone. Each experimental visit was separated by 1 week. During each visit, we measured the cutaneous vascular responses to graded noradrenaline infusions via intradermal microdialysis (see below). Red blood cell flux, an index of skin blood flow (SkBF), was measured over each site of microdialysis infusion using laser Doppler flowmetry (LDF, Perimed, Järfälla, Sweden). Experiments were conducted in a temperature controlled room (27°C).
Hormonal intervention
GnRH antagonist (ganirelex acetate)
Ganirelix acetate is a synthetic decapeptide with high antagonistic activity against naturally occurring gonadotropin-releasing hormone (GnRH). Ganirelix acetate is derived from native GnRH with substitutions at positions 1, 2, 3, 6, 8 and 10. When ganirelix acetate is given in therapeutic doses it acts by competitively blocking the GnRH receptors on the pituitary gonadotroph and subsequent transductions pathway. It induces a rapid, reversible suppression of gonadotropin secretion (Oberye et al. 1999a,b;). In young women with regular menstrual cycles, continued administration of ganirelix acetate leads to suppression of oestrogens and progesterone to postmenopausal levels. These decreases occur after 36–48 h of administration, and the suppression of the hypothalamic–pituitary–ovarian axis is reversed upon cessation of drug therapy (Oberye et al. 1999a,b;).
Women self-administered (subcutaneous injection) a GnRH antagonist (Ganirelix acetate, 250 μg in 0.5 ml normal saline, Organon, Roseland, NJ, USA) daily to suppress endogenous reproductive hormone production for 16 days. Women began using the GnRH antagonist on days 25–28 of their menstrual cycle, and continued daily for 16 days. Oestradiol (E2, 0.2 mg day−1 patch, Vivelle, CIBA Pharmaceuticals, Summit, NJ, USA) was administered on days 4–16, and progesterone (P4, 200 mg day−1 Prometrium, oral, Solvay Pharmaceuticals, Marietta, GA, USA) was added on days 13–16. Women using hormonal oral contraceptive pills (n= 5) stopped taking their pills and began taking the injections on what would have been the final day of the pill cycle; they were not tested until a full 3 days after stopping their contraceptive pills.
Skin blood flow studies
Subjects arrived at the laboratory at approximately 07.00 h, after refraining from caffeine and alcohol for at least 12 h. After giving a urine sample, they were weighed. Subjects were then seated in a semi-recumbent position for instrumentation (see below), and remained in that position throughout the study.
Microdialysis probe placement
Under sterile conditions, four 27-gauge needles were inserted on the dorsal aspect of the forearm (intradermal). The entrance and exit sites were 2 cm apart and the placement of each needle was separated by at least 2 cm. Microdialysis probes were threaded through the lumen of the needle. The needle was removed, leaving the hollow fibre portion of the microdialysis probe in place under the skin. All four microdialysis probes were infused with 0.9% saline (2 μl min−1; microinfusion pump, Harvard Apparatus, Holliston, MA, USA) for 120 min after placement to allow for recovery. Following probe placement, an i.v. catheter was inserted for blood sampling. The first blood sample was taken a minimum of 60 min after catheter placement. A total of three venous blood samples were taken (beginning, middle and end of protocol) for the analysis of haemoglobin and haematocrit. Blood was also drawn for the measurement of plasma oestradiol (p[E2]) and progesterone (p[P4]) concentration, and plasma concentrations of NA, Adr and PRA at the beginning and end of each SkBF experimental visit.
Cutaneous vascular responsiveness
Skin blood flow was measured by LDF probes over the four separate sites corresponding to the microdialysis probes throughout the experimental protocol, and beat-by-beat BP (Finometer) was measured at the finger on the contra lateral hand. After the 120 min recovery period from probe placement, baseline SkBF measurements were made for 10 min. Each microdialysis probe was then infused with one of the following: (1) 0.9% saline; (2) the nitric oxide synthase (NOS) inhibitor NG-monomethyl-l-arginine (l-NMMA, 10 mm); (3) the non-selective cyclooxygenase inhibitor ketorolac tromethamine (Keto, 10 mm); (4) combined l-NMMA + ketorolac (10 mm each). All probes were infused at a rate of 5 μl min−1 for 45 min. Skin temperature was maintained at 34°C through heating units within the laser Doppler probes during microdialysis infusions. After infusing the pharmacological blocking agents, increasing doses of noradrenaline (NA) were continuously infused at a rate of 5 μl min−1 for 15 min at the following doses: 1 × 10−8, 1 × 10−6, 1 × 10−5, 1 × 10−4, 1 × 10−3m. Each dose of NA was co-infused with one of the respective pharmacological agents listed above. Therefore, all four microdialysis probes were progressively infused with noradrenaline, with each individual microdialysis probe receiving one of the three pharmacological agents, in addition to one probe receiving NA alone. A 5 min saline wash-out was used between each dose of noradrenaline. All microdialysis syringes were prepared the morning of the study by the Investigational Drug Services at Yale New Haven Hospital.
Blood analysis
An aliquot was transferred into a tube without anticoagulant for the determination of p[E2], and p[P4]. The samples were centrifuged, frozen immediately and stored at −80°C until analysis. Catecholamines were analysed using high performance liquid chromatography (HPLC) with electrochemical detection (Colorchem Detector, ESA Corp., Acton, MA, USA) with intra-assay and inter-assay coefficients of variation of 1% and 10%, respectively. Plasma concentrations of E2 and P4, and PRA were measured using competitive binding radioimmunoassay methods. Intra-assay coefficient of variation for the mid-range standard for p[E2] (s.e.m. = 180 ± 13 pg ml−1) was 2.4% (Siemens Healthcare Diagnostics, Los Angeles, CA, USA), and for p[P4] (3.5 ± 0.2 ng ml−1) was 1.8% (Siemens Healthcare Diagnostics). Intra- and inter-assay coefficients of variation for PRA (standards range 3.6–6.6 ng angiotensin I ml−1 h−1) were 2.3% and 2.7% (Diasorin, Stillwater, MN, USA). Intra- and inter-assay coefficients of variation for the mid-range standards for aldosterone (175 ± 18 pg ml−1) were 1.7% and 1.9% (Siemens Healthcare Diagnostics), and for angiotensin II (14.5 ± 4.3 pmol l−1) were 2.0% and 3.2%, respectively (IBL America, Minneapolis, MN, USA).
Data analysis
The women were divided into low and normal/high orthostatic tolerance groups; low orthostatic tolerance was defined a priori as CSI ≤−600 mmHg min based on previous data reported in the literature (Sather et al. 1986; Fu et al. 2004a, 2005). Laser Doppler flowmetry data were recorded at 1000 Hz using LabChart 7 (ADInstruments, Bella Vista, NSW, Australia). The final 2 min of SkBF and mean arterial blood pressure (MAP) at each NA dose were used for analysis, and all SkBF responses were closely inspected to ensure a plateau had been reached at each level. Cutaneous vascular conductance (CVC) was calculated as mean SkBF/MAP, and expressed as a percentage of baseline. Noradrenaline doses were transformed to logarithmic concentrations, and CVC normalized so that baseline CVC = 100% (pre-NA infusion), and percentage baseline CVC at the highest NA concentration = 0. We did not observe a significant vasoconstriction at the first concentration (10−8) of NA. We used a sigmoidal dose–response curve with variable slope, equivalent to a four-parameter logistic equation (Dodson & Rhoden, 2001; Wilson et al. 2002), with constraints set for the bottom (zero) and top (100) parameters (Prism v4, GraphPad Software Inc., La Jolla, CA, USA) to best fit parameters of the model. The log EC50 (dose where 50% of the drug has maximal effects) and Hill slope (to define the sensitivity to an adrenergic stimulus) of the dose–response curves were determined by non-linear regression curve fitting of mean dose–response data fitted to the equation Y=Ymin+ (Ymax−Ymin)/(1 +[EC50/X]n), where Ymin and Ymax are the minimal and maximal responses, respectively, X is the NA concentration, and n is the Hill slope (Prism). We analysed the differences within each tolerance group across hormone conditions using Friedman's test for repeated measures comparisons (Prism). Differences in baseline characteristics between the two groups were determined by independent t tests. Differences were considered statistically significant when P < 0.05. All data are presented as means ±s.e.m.
Sample size calculation
Sample size calculations were based on our primary outcome variable of interest: the log EC50 and slope of our SkBF-NA dose–response curves. The desired statistical test was two-sided, and we assumed an α level equal to 0.01 to account for multiple comparisons (Hintze, 2001). Holowatz et al. (2003) report a percentage change in CVC using l-NAME during microdialysis with laser Doppler techniques of 23 ± 0.8%. Given eight women per group and α= 0.01, this effect size would allow us >80% power to differentiate these changes from chance (Hintze, 2001).
Results
Two women experienced symptoms during the GnRH antagonist intervention (one woman had mild vasomotor symptoms, and another experienced breast tenderness), but symptoms did not cause any woman to leave the study. One woman (high tolerance, HT) discontinued the study due to scheduling conflicts, and insufficient data were collected in a second woman (low tolerance, LT) because of technical difficulties during the infusion studies. Therefore data are reported on 16 women (eight HT, eight LT). The high and low tolerance women were similar with respect to age, height, mass and BMI (Table 1). All women included in the analysis reached maximal tolerance as defined by criteria previously described, and their haemodynamic and hormonal responses to the maximal LBNP test were similar in both high and low tolerance women (Table 2).
Table 1.
Subject characteristics and hormonal profile
| HT women | LT women | |||||
|---|---|---|---|---|---|---|
| Age (years) | 22 ± 1 | 21 ± 1 | ||||
| Height (cm) | 161 ± 2 | 159 ± 1 | ||||
| Mass (kg) | 61 ± 3 | 56 ± 1 | ||||
| BMI (kg m−2) | 24 ± 1 | 22 ± 1 | ||||
| CSI (mmHg min) | −871 ± 86 | [range −605 to −1257] | −397 ± 65 | [range −118 to −600] | ||
| GnRH | E2 | E2+P4 | GnRH | E2 | E2+P4 | |
| p[E2] (pg ml−1) | 38 ± 9 | 203 ± 45† | 179 ± 28† | 1 ± 9 | 239 ± 30† | 221 ± 33† |
| p[P4] (ng ml−1) | 0.9 ± 0.3 | 0.6 ± 0.1 | 20.6 ± 5.6† | 0.6 ± 0.1 | 0.6 ± 0.1 | 15.8 ± 5.3† |
Data are presented as means ±s.e.m. HT, high tolerance; LT, low tolerance; BMI, body mass index; CSI, cumulative stress index; GnRH, gonadotropin releasing hormone; E2, oestradiol; E2+P4, oestradiol combined with progesterone; p[E2], plasma oestradiol concentration; p[P4], plasma progesterone concentration.
P < 0.05 compared to GnRH antagonist.
Table 2.
Haemodynamic and hormonal responses to lower body negative pressure
| Lower body negative pressure max test | ||||
|---|---|---|---|---|
| HT women | LT women | |||
| Baseline | Max | Baseline | Max | |
| SBP (mmHg) | 113 ± 4 | 81 ± 6† | 114 ± 6 | 89 ± 7† |
| DBP (mmHg) | 56 ± 4 | 44 ± 4† | 53 ± 4 | 44 ± 5 |
| MAP (mmHg) | 72 ± 4 | 55 ± 4† | 68 ± 5 | 56 ± 5† |
| HR (bpm) | 68 ± 2 | 101 ± 6† | 66 ± 4 | 92 ± 5† |
| p[NA] (pg ml−1) | 201 ± 18 | 339 ± 20† | 173 ± 13 | 259 ± 19† |
| p[Adr] (pg ml−1) | 18 ± 3 | 61 ± 30 | 24 ± 7 | 63 ± 17 |
| PRA (ng Ang I ml−1 h−1) | 1.8 ± 0.1 | 4.1 ± 0.2† | 2.0 ± 0.3 | 3.4 ± 0.3† |
| Haematocrit (%) | 36.7 ± 0.8 | 38.9 ± 1.0† | 36.1 ± 1.1 | 37.4 ± 1.1† |
| Haemoglobin (mg dl−1) | 11.4 ± 0.4 | 12.1 ± 0.5† | 11.6 ± 0.5 | 11.9 ± 0.4 |
Data are presented as means ±s.e.m. HT, high tolerance; LT, low tolerance; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate; p[NA], plasma noradrenaline concentration; p[Adr], plasma adrenaline concentration; PRA, plasma renin activity; Ang I, angiotensin I.
P < 0.05 compared to baseline within group.
Both endogenous oestradiol and progesterone production were suppressed during GnRH antagonist administration (Table 1). As expected, p[E2] increased during E2 administration, while p[P4] remained suppressed, whereas both p[E2] and p[P4] increased during combined E2+P4 administration. The hormone levels were similar between groups under all conditions. Plasma renin activity and p[NA] were similar between HT and LT women and across hormone conditions (data not shown).
Skin blood flow responses
Women with high orthostatic tolerance
Oestradiol administration did not alter the cutaneous vasoconstricting response to NA compared to GnRH antagonist administration in women with high orthostatic tolerance (Table 3, Fig. 1A). However, the combined E2+P4 condition induced a leftward shift in the SkBF-NA dose–response curve compared to GnRH antagonist administration indicating enhanced constriction (Fig. 1A, P < 0.05). The progesterone-induced vasoconstriction during combined E2+P4 was reversed with COX inhibition (Table 3, Fig. 2C, P < 0.05). Although NOS inhibition had no consistent effect under any hormone condition, COX inhibition enhanced the vasoconstrictor response during GnRH antagonist administration compared to NA alone (Table 3, Fig. 2A, P < 0.05), and this effect was diminished by E2 administration (Table 3, Fig. 2B, P < 0.05). NOS inhibition and combined COX+NOS inhibition did not affect the SkBF-NA dose–response curve during GnRH antagonist, E2, or combined E2+P4 administration (Table 3). Baseline absolute CVC was not altered by hormone treatment or microdialysis infusion site (Table 4).
Table 3.
logEC50 of dose–response curves
| HT women | LT women | |||||
|---|---|---|---|---|---|---|
| GnRH | E2 | E2+P4 | GnRH | E2 | E2+P4 | |
| NA | −4.02 ± 0.39 | −4.43 ± 0.31 | −5.18 ± 0.31* | −4.41 ± 0.44 | −4.69 ± 0.26 | −4.94 ± 0.25 |
| NA+Keto | −5.60 ± 0.26† | −4.81 ± 0.26* | −3.82 ± 0.35*†‡ | −6.03 ± 0.35† | −4.50 ± 0.27* | −5.35 ± 0.15‡ |
| NA+l-NMMA | −4.09 ± 0.65 | −6.60 ± 0.56 | −5.09 ± 0.20 | −4.61 ± 0.68 | −5.57 ± 0.50 | −4.13 ± 0.39 |
| NA+Keto+l-NMMA | −4.24 ± 0.57 | −4.91 ± 0.38 | −4.84 ± 0.49 | −6.53 ± 0.56 | −4.99 ± 0.75 | −5.06 ± 0.39 |
Data are presented as means ±s.e.m. HT, high tolerance; LT, low tolerance; GnRH, gonadotropin releasing hormone; E2, oestradiol; E2+P4, oestradiol combined with progesterone; NA, noradrenaline; NA+Keto, noradrenaline combined with ketorolac tromethamine, a non-selective cyclooxygenase inhibitor; NA+l-NMMA, noradrenaline combined with NG-monomethyl-l-arginine, a nitric oxide synthase inhibitor; NA+Keto+l-NMMA, noradrenaline combined with ketorolac tromethamine and NG-monomethyl-l-arginine.
P < 0.05 compared to GnRH antagonist within group.
P < 0.05 compared to NA within group.
P < 0.05 compared to E2 within group.
Figure 1. Dose–response curves to cutaneous microdialysis infusions of noradrenaline (NA) during GnRH antagonist (GnRH), oestradiol (E2) and combined oestradiol and progesterone (E2+P4) in high tolerance (A) and low tolerance (B) women.
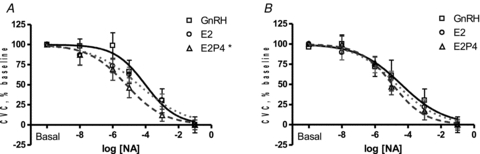
E2+P4 administration enhanced vasoconstriction compared to GnRH antagonist administration in high tolerance women (*P < 0.05) but not low tolerance women.
Figure 2. Effect of COX inhibition (Keto) on cutaneous vascular responses to NA in high tolerance women during GnRH antagonist (A), E2 (B) and E2+P4 (C).
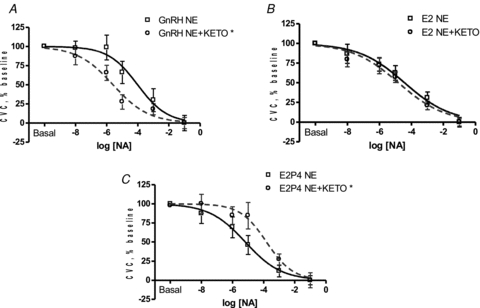
COX inhibition enhanced vasoconstriction during GnRH antagonist administration (*P < 0.05) but attenuated the response during E2+P4 administration (*P < 0.05).
Table 4.
Baseline cutaneous vascular conductance
| HT women | LT women | |||||
|---|---|---|---|---|---|---|
| GnRH | E2 | E2+P4 | GnRH | E2 | E2+P4 | |
| NA | 0.36 ± 0.12 | 0.33 ± 0.10 | 0.32 ± 0.05 | 0.31 ± 0.09 | 0.32 ± 0.07 | 0.32 ± 0.05 |
| NA+Keto | 0.24 ± 0.05 | 0.31 ± 0.10 | 0.33 ± 0.07 | 0.41 ± 0.16 | 0.30 ± 0.06 | 0.29 ± 0.11 |
| NA+l-NMMA | 0.17 ± 0.02 | 0.27 ± 0.05 | 0.23 ± 0.06 | 0.17 ± 0.03 | 0.18 ± 0.03 | 0.18 ± 0.03 |
| NA+Keto+l-NMMA | 0.25 ± 0.07 | 0.18 ± 0.03 | 0.23 ± 0.06 | 0.31 ± 0.06 | 0.23 ± 0.06 | 0.26 ± 0.05 |
Data are presented as means ±s.e.m. in arbitrary units. HT, high tolerance; LT, low tolerance; GnRH, gonadotropin releasing hormone; E2, oestradiol; E2+P4, oestradiol combined with progesterone; NA, noradrenaline; NA+Keto, noradrenaline combined with ketorolac tromethamine, a non-selective cyclooxygenase inhibitor; NA+l-NMMA, noradrenaline combined with NG-monomethyl-l-arginine, a nitric oxide synthase inhibitor; NA+Keto+l-NMMA, noradrenaline combined with ketorolac tromethamine and NG-monomethyl-l-arginine.
Women with low orthostatic tolerance
Neither E2 nor combined E2+P4 altered the vasoconstricting response to NA compared to GnRH antagonist alone in women with low orthostatic tolerance (Fig. 1B). Similar to women with high orthostatic tolerance, COX inhibition enhanced the vasoconstrictor response during GnRH antagonist administration compared to NA alone (Table 3, Fig. 3A, P < 0.05) and E2 administration diminished this effect (Table 3, Fig. 3B, P < 0.05). However, combined E2+P4 administration had no affect on the SkBF-NA dose–response curve during COX inhibition (Table 3, Fig. 3C). Finally, NOS inhibition and combined COX+NOS inhibition did not affect the SkBF-NA dose–response curve during GnRH antagonist, E2, or combined E2+P4 administration (Table 3). Baseline absolute CVC was not altered by hormone treatment or microdialysis infusion site (Table 4).
Figure 3. Effect of COX inhibition (Keto) on cutaneous vascular responses to NA in low tolerance women during GnRH antagonist (A), E2 (B) and E2+P4 administration (C).
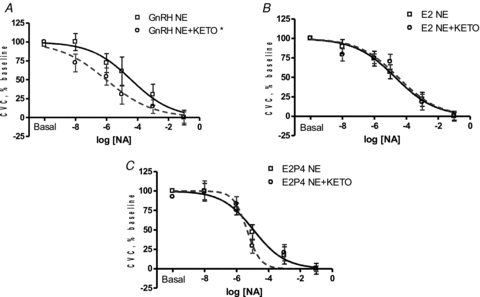
COX inhibition enhanced vasoconstriction during GnRH antagonist administration (*P < 0.05) but responses were similar during E2 and E2+P4 administration.
Discussion
This is the first study to examine the relationship among cutaneous adrenergic responsiveness, reproductive hormone exposure, and orthostatic tolerance in young healthy women. We utilized a controlled hormone intervention to determine the interaction between oestradiol and progesterone on cutaneous vasoconstrictor responsiveness and orthostatic tolerance in young, healthy, normotensive women. The main findings of the study are as follows: (1) combined oestradiol with progesterone exposure enhances cutaneous vasoconstriction in women with normal/high orthostatic tolerance; (2) the enhanced vasoconstriction seen with progesterone administration in women with high orthostatic tolerance is mediated by cyclooxygenase; and (3) there are no effects of either hormone (oestradiol alone or in combination with progesterone) on women with low tolerance. Thus, our data suggest differential regulation of cutaneous adrenergic responses between women with high and low tolerance during hormone exposure. Importantly, the effect on adrenergic responses in the high tolerance women appears to be mediated by cyclooxygenase supporting a role for prostaglandins in blood pressure regulation in young women.
Blood pressure is initially maintained during orthostatic challenge by baroreflex mediated increases in heart rate and peripheral vasoconstriction. Vasoconstriction in the forearm cutaneous circulation occurs during baroreceptor unloading under normothermic conditions (Rowell et al. 1973; Tripathi & Nadel, 1986), and as such is important to the maintenance of blood pressure during orthostasis. The cutaneous circulation is innervated by both sympathetic vasodilator and vasoconstrictor nerves, so poor peripheral regulation may be the result of dysfunction in one or both of these systems. Women are more susceptible to orthostatic intolerance, tend to have lower sympathetic outflow (Hart et al. 2009), and have attenuated peripheral adrenergic responsiveness (Freedman et al. 1987). Our data indicate that sex hormones modulate adrenergic control of the cutaneous circulation through a cyclooxygenase mechanism.
Women with high orthostatic tolerance
In women with high orthostatic tolerance, progesterone induced a leftward shift in the SkBF-NA dose–response curve, indicating enhanced cutaneous adrenergic responsiveness. These findings are consistent with earlier studies showing progesterone negates the vasodilatory effects of oestrogen (Zerr-Fouineau et al. 2009) and enhances α-adrenergic responsiveness (Freedman & Girgis, 2000). The enhanced cutaneous adrenergic response we observed with progesterone in the high tolerance women was reversed with non-selective COX inhibition, suggesting prostanoids are mediating the progesterone-induced vasoconstriction. To our knowledge, this is the first study demonstrating progesterone-induced cutaneous vasoconstriction is mediated through the COX pathway.
Cyclooxygenase breaks down arachidonic acid to produce both vasodilatory and vasoconstrictor prostanoids. Prostacyclin and its corresponding receptor (IP) primarily facilitate vasodilatation, whereas thromboxane and its receptor (TP) cause vasoconstriction. These prostanoids act on vascular smooth muscle cells, and are important in maintaining vascular homeostasis. We observed an enhanced cutaneous vasoconstrictor response with the non-selective COX inhibitor ketorolac during hormone suppression, suggesting inhibition of prostacyclin or IP receptors. Evidence in cell and animal models indicates that both oestrogen (Geary et al. 2000; Sobrino et al. 2010) and progesterone increase prostacyclin production (Hermenegildo et al. 2005), and progesterone may also decrease thromboxane production (Oviedo et al. 2011) and receptor density (Minshall et al. 2001). However our data suggest that progesterone may also either increase COX-derived constrictor products or alter prostanoid receptor expression, leading to enhanced cutaneous adrenergic responsiveness in young healthy women. Recent data suggest a shift toward vasoconstrictor prostanoids in the cutaneous microcirculation with ageing (Holowatz et al. 2009), such that the balance between TP and IP receptors is altered in a way that favours vasoconstriction (Tang & Vanhoutte, 2008). Based on our current findings, we speculate that female sex hormones may alter the ratio of TP and IP receptor expression, shifting the balance to a pro-constrictor state when both oestradiol and progesterone are elevated. Thus, in this healthy young group, the progesterone-induced increase in cutaneous vasoconstriction may be advantageous in maintaining peripheral vasoconstriction during orthostatic stress. Therefore, the progesterone associated increases in cutaneous adrenergic responsiveness in women with normal/high orthostatic tolerance together with plasma volume and extracellular fluid volume expansion (Stachenfeld & Taylor, 2005) may help to compensate for fluid shifts during orthostatic stress more rapidly, thereby maintaining blood pressure and improving tolerance. Finally, oestradiol administration alone or in combination with progesterone attenuated cutaneous adrenergic responses compared to GnRH antagonist during COX inhibition (Fig. 2, Table 3), suggesting that oestradiol administration can either override the effects of prostaglandin inhibition during adrenergic stimulation, or that oestradiol exposure reduces adrenergic responsiveness through another mechanism such as nitric oxide.
A surprising finding is that combined NOS and COX inhibition did not induce vasoconstriction, comparable to or greater than COX inhibition alone, which can only indicate an interaction between these two pathways. Crosstalk between the two systems has been documented (Mollace et al. 2005), and interestingly, NO inhibition may also result in an attenuated release in prostaglandin E2 (Salvemini et al. 1993). However, other studies have reported that NO inhibition may enhance the release of prostacyclin (Mollace et al. 2005) or endothelial-derived hyperpolarizing factor (Lenasi & Strucl, 2008), possibly serving as a redundant vasodilatory system. Furthermore, COX inhibition with ketorolac may alter NO bioavailability during thermoneutral baseline conditions (Holowatz et al. 2009). While the nature of this interaction between NO and COX is not apparent from our data, we suspect that either low NO bioavailability or altered prostanoid production through the reciprocal relationship between NO and COX is interfering with the adrenergic response.
Women with low orthostatic tolerance
In contrast to women with high tolerance, combined oestradiol and progesterone administration did not enhance cutaneous vasoconstrictor responses to adrenergic stimulation, indicating they are less sensitive to the vasoconstrictor effect of progesterone relative to women with normal/high tolerance. Similar to women with high orthostatic tolerance, COX inhibition enhanced vasoconstriction in women with low orthostatic tolerance during hormone suppression. This enhanced constriction was attenuated with oestradiol administration (similar to women with high tolerance), although this effect was not observed with combined oestradiol and progesterone administration. Thus, women with low orthostatic tolerance were insensitive to the vasoconstricting effects of progesterone and may lack COX-derived constrictor products (or sensitivity to them) in the periphery. We propose that an impaired or absent progesterone-mediated adrenergic vasoconstrictive response reduces resting peripheral vascular resistance, delaying cardiopulmonary and arterial baroreceptor responses, thereby reducing the speed and magnitude of changes in blood flow during shifts in posture. This delayed compensation to shifts in central volume leads to lower cardiac filling pressure and cardiac output, and increases the risk for a slow response to orthostatic challenges, and syncope. Thus, we propose that a key difference between the low and normal/high tolerance groups is the ability to produce COX-derived constrictor products via progesterone to support cutaneous adrenergic responsiveness during an orthostatic challenge. Based on the opposing actions of progesterone during COX inhibition in high tolerance women, we speculate that through the cyclooxygenase pathways, high tolerance women are able to regulate vasodilatory and vasoconstricting actions to balance blood pressure or fluid shifts whereas low tolerance women are either insensitive to the vasoconstrictor pathway or sensitive to vasodilatory prostanoids.
Similar SkBF responses in both groups
The vasodilatory effects of oestrogen are well established although the mechanisms are still widely debated (Orshal & Khalil, 2004). Oestrogen-induced vasodilatation primarily occurs through increasing NO bioavailability (Sudhir et al. 1996). Oestrogen exposure may attenuate peripheral vasoconstrictor responses, as demonstrated after 8 weeks of oestradiol supplementation in perimenopausal women (Sudhir et al. 1997). Nitric oxide attenuates cutaneous vasoconstrictor responsiveness to noradrenaline in men (Shibasaki et al. 2008). Thus, we hypothesized that oestradiol would attenuate NA-induced cutaneous vasoconstriction via NO in the women in our present study. However, we did not see an effect of oestradiol exposure on cutaneous adrenergic responses in either group (Fig. 1). Similarly, we did not see an impact of NOS inhibition (alone or in combination with COX inhibition) on cutaneous adrenergic responses. The reason for the lack of a response is not apparent from our data, but may be due to low NO bioavailability, and therefore any further attenuation of adrenergic effectiveness or sensitivity by inhibiting NOS plus COX would have been minimal. There are a number of other possible explanations; first, our GnRH antagonist suppressed oestradiol, which may have reduced the bioavailabity of NO, so any further changes induced by our NOS inhibitor would have been minimal. Ovariectomy impairs NO bioavailability and endothelial-dependent vasodilatation, which is not restored until after 3 months of oestrogen replacement (Virdis et al. 2000). Thus, our short period of oestradiol exposure (7 days) probably would not have been sufficient to restore NO production and induce vasodilatation (Sudhir et al. 1997). Second, the lack of an effect of NOS inhibition may also have been due to low baseline SkBF in the forearm, so vasoconstriction resulting from NA infusion through the microdialysis probes in the skin may have been difficult to detect. In order to avoid this problem, we used mild skin warming, but the heating remained below 34°C to avoid sympathetic stimulation (Hodges et al. 2009). Third, it is also possible that the impact of NO is small during cutaneous adrenergic stimulation, but would be seen under other conditions such as cholinergically induced cutaneous vasodilatation (Kellogg et al. 2005; Medow et al. 2008).
Limitations
One limitation in our study is that differences in the prostaglandin-mediated effects (constriction vs. dilatation) of progesterone may depend on the receptors present and the tissues studied (uterine, breast, vascular), and we only studied skin. In the current study we did not investigate the cellular mechanisms involved in these prostaglandin effects, and therefore cannot determine the downstream effects (i.e. inhibiting vasodilatation or inhibiting COX-derived constrictor products, or altering the balance between the two). Further studies using selective prostacyclin and thromboxane inhibitors are needed to elucidate these mechanisms. A second limitation may be that while the cutaneous circulation can be used as a generalized model of microvascular function (Holowatz et al. 2008), we cannot extrapolate our findings to other tissues or vascular beds, such as the muscle, kidneys or splanchnic region, key regions in the orthostatic response. Although studies in healthy subjects (Ray & Wilson, 2004; Okazaki et al. 2005; Medow et al. 2008) and patients with postural tachycardia syndrome (Stewart et al. 2003; Medow et al. 2005) report similarities in vascular responses between upper and lower extremities, direct measures of adrenergic responsiveness during difference hormone exposures would be of great value. Although changes in cutaneous blood flow are an integral part of the orthostatic response, vasoconstriction in the lower legs is important in buffering volume changes during orthostatic challenge, so future studies examining adrenergic responses during sex hormone exposure should include this vascular bed.
Summary
Our study is the first to examine interactions between female reproductive hormones, orthostatic tolerance, and cutaneous vascular responsiveness. Our findings support an important role for the prostaglandin system in the sex hormone effects on the cardiovascular system in women with normal to high orthostatic tolerance. Further, the women with lower orthostatic tolerance were insensitive to the progesterone mediated increases in adrenergically induced vasoconstriction in the skin, which may contribute to their lower orthostatic tolerance. Cyclooxygenase facilitates production of both vasodilatory and vasoconstrictive prostaglandins, so future studies may explore differences between the nature of prostanoid function in women with and without orthostatic intolerance
Perspectives
Orthostatic intolerance is a common cardiovascular dysfunction in young women, and is estimated to affect 500,000 Americans (Robertson, 1999). It is the second most common blood pressure regulation disorder, with hypertension being the first (Robertson, 1999), and its effects can be debilitating. Understanding the mechanisms contributing to orthostatic intolerance is important for advancing treatment options. For example, oestradiol has been the primary focus in understanding sex differences in cardiovascular disease, but our data suggest an important role for progesterone in supporting adrenergic responsiveness in young women. Previous studies indicate a greater ratio of constrictor prostanoids with ageing (Tang & Vanhoutte, 2008; Holowatz et al. 2009). Thus, coupled with our findings of progesterone-induced microvascular vasoconstriction mediated by prostaglandins, studies on adrenergic responsiveness during progesterone exposure in menopausal women are needed to determine if progesterone treatment is detrimental to older women, or any population at risk for hypertension.
Acknowledgments
We gratefully acknowledge Cheryl Leone, MA and Andy Grabarek, BS for technical assistance, Gary Mack, PhD for input on study design and analysis, Osama Abdelghany, PharmD, BCOP, for microdialysis drug preparations, and the subjects for their time. This research was supported by NIH Grant RO1 HL071159.
Glossary
Abbreviations
- Adr
adrenaline
- BP
blood pressure
- BMI
body mass index
- COX
cyclooxygenase
- CSI
cumulative stress index
- CVC
cutaneous vascular conductance
- E2
oestradiol
- GnRH
gonadotropin releasing hormone
- HT
high tolerance
- Keto
ketorolac tromethamine
- LDF
laser Doppler flowmetry
- LBNP
lower body negative pressure
- LT
low tolerance
- MAP
mean arterial pressure
- NA
noradrenaline
- NO
nitric oxide
- NOS
nitric oxide synthase
- l-NMMA
NG-monomethyl-l-arginine
- PRA
plasma renin activity
- P4
progesterone
- SkBF
skin blood flow
Author contributions
M.M.W. participated in the conceptualization underlying this work, data collection and analysis, and the writing of the manuscript. H.S.T. provided medical supervision of the subjects, participated in the conceptualization underlying this work and the writing of the manuscript. N.S.S. participated in the conceptualization underlying this work, data collection, supervision and data analysis, and the writing of the manuscript. All authors approved the final version of the manuscript. All experiments were performed at The John B. Pierce Laboratory.
References
- Abularrage CJ, Sidawy AN, Aidinian G, Singh N, Weiswasser JM, Arora S. Evaluation of the microcirculation in vascular disease. J Vasc Surg. 2005;42:574–581. doi: 10.1016/j.jvs.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Beiser GD, Zelis R, Epstein SE, Mason DT, Braunwald E. The role of skin and muscle resistance vessels in reflexes mediated by the baroreceptor system. J Clin Invest. 1970;49:225–231. doi: 10.1172/JCI106231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convertino VA. Gender differences in autonomic functions associated with blood pressure regulation. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1909–1920. doi: 10.1152/ajpregu.1998.275.6.R1909. [DOI] [PubMed] [Google Scholar]
- Dodson AM, Rhoden KJ. Bradykinin increases Na+-K+ pump activity in cultured guinea-pig tracheal smooth muscle cells. Br J Pharmacol. 2001;133:1339–1345. doi: 10.1038/sj.bjp.0704198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman RR, Girgis R. Effects of menstrual cycle and race on peripheral vascular α-adrenergic responsiveness. Hypertension. 2000;35:795–799. doi: 10.1161/01.hyp.35.3.795. [DOI] [PubMed] [Google Scholar]
- Freedman RR, Sabharwal SC, Desai N. Sex differences in peripheral vascular adrenergic receptors. Circ Res. 1987;61:581–585. doi: 10.1161/01.res.61.4.581. [DOI] [PubMed] [Google Scholar]
- Fu Q, Arbab-Zadeh A, Perhonen MA, Zhang R, Zuckerman JH, Levine BD. Hemodynamics of orthostatic intolerance: implications for gender differences. Am J Physiol Heart Circ Physiol. 2004a;286:H449–457. doi: 10.1152/ajpheart.00735.2002. [DOI] [PubMed] [Google Scholar]
- Fu Q, Witkowski S, Levine BD. Vasoconstrictor reserve and sympathetic neural control of orthostasis. Circulation. 2004b;110:2931–2937. doi: 10.1161/01.CIR.0000146384.91715.B5. [DOI] [PubMed] [Google Scholar]
- Fu Q, Witkowski S, Okazaki K, Levine BD. Effects of gender and hypovolemia on sympathetic neural responses to orthostatic stress. Am J Physiol Regul Integr Comp Physiol. 2005;289:R109–116. doi: 10.1152/ajpregu.00013.2005. [DOI] [PubMed] [Google Scholar]
- Geary GG, Krause DN, Duckles SP. Estrogen reduces mouse cerebral artery tone through endothelial NOS- and cyclooxygenase-dependent mechanisms. Am J Physiol Heart Circ Physiol. 2000;279:H511–519. doi: 10.1152/ajpheart.2000.279.2.H511. [DOI] [PubMed] [Google Scholar]
- Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance: implications for human blood pressure regulation. Hypertension. 2009;53:571–576. doi: 10.1161/HYPERTENSIONAHA.108.126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermenegildo C, Oviedo PJ, Garcia-Martinez MC, Garcia-Perez MA, Tarin JJ, Cano A. Progestogens stimulate prostacyclin production by human endothelial cells. Hum Reprod. 2005;20:1554–1561. doi: 10.1093/humrep/deh803. [DOI] [PubMed] [Google Scholar]
- Hintze J. NCSS and PASS Number Cruncher Statistical Systems. Kaysville, UT, USA: 2001. [Google Scholar]
- Hodges GJ, Johnson JM. Adrenergic control of the human cutaneous circulation. Appl Physiol Nutr Metab. 2009;34:829–839. doi: 10.1139/H09-076. [DOI] [PubMed] [Google Scholar]
- Hodges GJ, Kosiba WA, Zhao K, Johnson JM. The involvement of heating rate and vasoconstrictor nerves in the cutaneous vasodilator response to skin warming. Am J Physiol Heart Circ Physiol. 2009;296:H51–56. doi: 10.1152/ajpheart.00919.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowatz LA, Houghton BL, Wong BJ, Wilkins BW, Harding AW, Kenney WL, Minson CT. Nitric oxide and attenuated reflex cutaneous vasodilation in aged skin. Am J Physiol Heart Circ Physiol. 2003;284:H1662–1667. doi: 10.1152/ajpheart.00871.2002. [DOI] [PubMed] [Google Scholar]
- Holowatz LA, Jennings JD, Lang JA, Kenney WL. Ketorolac alters blood flow during normothermia but not during hyperthermia in middle-aged human skin. J Appl Physiol. 2009;107:1121–1127. doi: 10.1152/japplphysiol.00750.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol. 2008;105:370–372. doi: 10.1152/japplphysiol.00858.2007. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Zhao JL, Coey U, Green JV. Acetylcholine-induced vasodilation is mediated by nitric oxide and prostaglandins in human skin. J Appl Physiol. 2005;98:629–632. doi: 10.1152/japplphysiol.00728.2004. [DOI] [PubMed] [Google Scholar]
- Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol. 2000;36:1233–1238. doi: 10.1016/s0735-1097(00)00849-4. [DOI] [PubMed] [Google Scholar]
- Lenasi H, Strucl M. The effect of nitric oxide synthase and cyclooxygenase inhibition on cutaneous microvascular reactivity. Eur J Appl Physiol. 2008;103:719–726. doi: 10.1007/s00421-008-0769-8. [DOI] [PubMed] [Google Scholar]
- Medow MS, Glover JL, Stewart JM. Nitric oxide and prostaglandin inhibition during acetylcholine-mediated cutaneous vasodilation in humans. Microcirculation. 2008;15:569–579. doi: 10.1080/10739680802091526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medow MS, Minson CT, Stewart JM. Decreased microvascular nitric oxide-dependent vasodilation in postural tachycardia syndrome. Circulation. 2005;112:2611–2618. doi: 10.1161/CIRCULATIONAHA.104.526764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshall RD, Pavcnik D, Halushka PV, Hermsmeyer K. Progesterone regulation of vascular thromboxane A2 receptors in rhesus monkeys. Am J Physiol Heart Circ Physiol. 2001;281:H1498–1507. doi: 10.1152/ajpheart.2001.281.4.H1498. [DOI] [PubMed] [Google Scholar]
- Mollace V, Muscoli C, Masini E, Cuzzocrea S, Salvemini D. Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol Rev. 2005;57:217–252. doi: 10.1124/pr.57.2.1. [DOI] [PubMed] [Google Scholar]
- Oberye JJ, Mannaerts BM, Huisman JA, Timmer CJ. Pharmacokinetic and pharmacodynamic characteristics of ganirelix (Antagon/Orgalutran). Part II. Dose-proportionality and gonadotropin suppression after multiple doses of ganirelix in healthy female volunteers. Fertil Steril. 1999a;72:1006–1012. doi: 10.1016/s0015-0282(99)00414-8. [DOI] [PubMed] [Google Scholar]
- Oberye JJ, Mannaerts BM, Kleijn HJ, Timmer CJ. Pharmacokinetic and pharmacodynamic characteristics of ganirelix (Antagon/Orgalutran). Part I. Absolute bioavailability of 0.25 mg of ganirelix after a single subcutaneous injection in healthy female volunteers. Fertil Steril. 1999b;72:1001–1005. doi: 10.1016/s0015-0282(99)00413-6. [DOI] [PubMed] [Google Scholar]
- Okazaki K, Fu Q, Martini ER, Shook R, Conner C, Zhang R, Crandall CG, Levine BD. Vasoconstriction during venous congestion: effects of venoarteriolar response, myogenic reflexes, and hemodynamics of changing perfusion pressure. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1354–1359. doi: 10.1152/ajpregu.00804.2004. [DOI] [PubMed] [Google Scholar]
- Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;286:R233–249. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- Oviedo PJ, Sobrino A, Novella S, Rius C, Laguna-Fernandez A, Garcia-Perez MA, Tarin JJ, Cano A, Hermenegildo C. Progestogens reduce thromboxane production by cultured human endothelial cells. Climacteric. 2011 doi: 10.3109/13697131003602496. (in press) [DOI] [PubMed] [Google Scholar]
- Ray CA, Wilson TE. Comparison of skin sympathetic nerve responses to isometric arm and leg exercise. J Appl Physiol. 2004;97:160–164. doi: 10.1152/japplphysiol.00699.2003. [DOI] [PubMed] [Google Scholar]
- Robertson D. The epidemic of orthostatic tachycardia and orthostatic intolerance. Am J Med Sci. 1999;317:75–77. doi: 10.1097/00000441-199902000-00001. [DOI] [PubMed] [Google Scholar]
- Rowell L. Human Cardiovascular Control. New York: Oxford University Press; 1993. [Google Scholar]
- Rowell LB, Detry JM, Blackmon JR, Wyss C. Importance of the splanchnic vascular bed in human blood pressure regulation. J Appl Physiol. 1972;32:213–220. doi: 10.1152/jappl.1972.32.2.213. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Wyss CR, Brengelmann GL. Sustained human skin and muscle vasoconstriction with reduced baroreceptor activity. J Appl Physiol. 1973;34:639–643. doi: 10.1152/jappl.1973.34.5.639. [DOI] [PubMed] [Google Scholar]
- Salvemini D, Misko TP, Masferrer JL, Seibert K, Currie MG, Needleman P. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci U S A. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather TM, Goldwater DJ, Montgomery LD, Convertino VA. Cardiovascular dynamics associated with tolerance to lower body negative pressure. Aviat Space Environ Med. 1986;57:413–419. [PubMed] [Google Scholar]
- Shibasaki M, Low DA, Davis SL, Crandall CG. Nitric oxide inhibits cutaneous vasoconstriction to exogenous norepinephrine. J Appl Physiol. 2008;105:1504–1508. doi: 10.1152/japplphysiol.91017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrino A, Oviedo PJ, Novella S, Laguna-Fernandez A, Bueno C, Garcia-Perez MA, Tarin JJ, Cano A, Hermenegildo C. Estradiol selectively stimulates endothelial prostacyclin production through estrogen receptor-α. J Mol Endocrinol. 2010;44:237–246. doi: 10.1677/JME-09-0112. [DOI] [PubMed] [Google Scholar]
- Stachenfeld NS, Taylor HS. Progesterone increases plasma volume independent of estradiol. J Appl Physiol. 2005;98:1991–1997. doi: 10.1152/japplphysiol.00031.2005. [DOI] [PubMed] [Google Scholar]
- Stewart J, Kohen A, Brouder D, Rahim F, Adler S, Garrick R, Goligorsky MS. Noninvasive interrogation of microvasculature for signs of endothelial dysfunction in patients with chronic renal failure. Am J Physiol Heart Circ Physiol. 2004;287:H2687–2696. doi: 10.1152/ajpheart.00287.2004. [DOI] [PubMed] [Google Scholar]
- Stewart JM, Medow MS, Minson CT, Taneja I. Cutaneous neuronal nitric oxide is specifically decreased in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2007;293:H2161–2167. doi: 10.1152/ajpheart.00600.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JM, Medow MS, Montgomery LD. Local vascular responses affecting blood flow in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2003;285:H2749–2756. doi: 10.1152/ajpheart.00429.2003. [DOI] [PubMed] [Google Scholar]
- Sudhir K, Elser MD, Jennings GL, Komesaroff PA. Estrogen supplementation decreases norepinephrine-induced vasoconstriction and total body norepinephrine spillover in perimenopausal women. Hypertension. 1997;30:1538–1543. doi: 10.1161/01.hyp.30.6.1538. [DOI] [PubMed] [Google Scholar]
- Sudhir K, Jennings GL, Funder JW, Komesaroff PA. Estrogen enhances basal nitric oxide release in the forearm vasculature in perimenopausal women. Hypertension. 1996;28:330–334. doi: 10.1161/01.hyp.28.3.330. [DOI] [PubMed] [Google Scholar]
- Tang EH, Vanhoutte PM. Gene expression changes of prostanoid synthases in endothelial cells and prostanoid receptors in vascular smooth muscle cells caused by aging and hypertension. Physiol Genomics. 2008;32:409–418. doi: 10.1152/physiolgenomics.00136.2007. [DOI] [PubMed] [Google Scholar]
- Tripathi A, Nadel ER. Forearm skin and muscle vasoconstriction during lower body negative pressure. J Appl Physiol. 1986;60:1535–1541. doi: 10.1152/jappl.1986.60.5.1535. [DOI] [PubMed] [Google Scholar]
- Virdis A, Ghiadoni L, Pinto S, Lombardo M, Petraglia F, Gennazzani A, Buralli S, Taddei S, Salvetti A. Mechanisms responsible for endothelial dysfunction associated with acute estrogen deprivation in normotensive women. Circulation. 2000;101:2258–2263. doi: 10.1161/01.cir.101.19.2258. [DOI] [PubMed] [Google Scholar]
- White DD, Gotshall RW, Tucker A. Women have lower tolerance to lower body negative pressure than men. J Appl Physiol. 1996;80:1138–1143. doi: 10.1152/jappl.1996.80.4.1138. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Cui J, Crandall CG. Effect of whole-body and local heating on cutaneous vasoconstrictor responses in humans. Auton Neurosci. 2002;97:122–128. doi: 10.1016/s1566-0702(02)00046-2. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Shibasaki M, Cui J, Levine BD, Crandall CG. Effects of 14 days of head-down tilt bed rest on cutaneous vasoconstrictor responses in humans. J Appl Physiol. 2003;94:2113–2118. doi: 10.1152/japplphysiol.00067.2002. [DOI] [PubMed] [Google Scholar]
- Zerr-Fouineau M, Jourdain M, Boesch C, Hecker M, Bronner C, Schini-Kerth VB. Certain progestins prevent the enhancing effect of 17β-estradiol on NO-mediated inhibition of platelet aggregation by endothelial cells. Arterioscler Thromb Vasc Biol. 2009;29:586–593. doi: 10.1161/ATVBAHA.108.178004. [DOI] [PubMed] [Google Scholar]


