Abstract
Fetal growth depends on placental transfer of amino acids from maternal to fetal blood. The mechanisms of net amino acid efflux across the basal membrane (BM) of the placental syncytiotrophoblast to the fetus, although vital for amino acid transport, are poorly understood. We examined the hypothesis that facilitated diffusion by the amino acid transporters TAT1, LAT3 and LAT4 plays an important role in this process, with possible effects on fetal growth. Amino acid transfer was measured in isolated perfused human placental cotyledons (n= 5 per experiment) using techniques which distinguish between different transport processes. Placental TAT1, LAT3 and LAT4 proteins were measured, and mRNA expression levels (measured using real-time quantitative-PCR) were related to fetal and neonatal anthropometry and dual-energy X-ray absorptiometry measurements of neonatal lean mass in 102 Southampton Women's Survey (SWS) infants. Under conditions preventing transport by amino acid exchangers, all amino acids appearing in the fetal circulation were substrates of TAT1, LAT3 or LAT4. Western blots demonstrated the presence of TAT1, LAT3 and LAT4 in placental BM preparations. Placental TAT1 and LAT3 mRNA expression were positively associated with measures of fetal growth in SWS infants (P < 0.05). We provide evidence that the efflux transporters TAT1, LAT3 and LAT4 are present in the human placental BM, and may play an important role in the net efflux of amino acids to the fetus. Unlike other transporters they can increase fetal amino acid concentrations. Consistent with a role in placental amino acid transfer capacity and fetal growth TAT1 and LAT3 mRNA expression showed positive associations with infant size at birth.
Non-technical summary
Fetal growth depends on transfer of amino acids from the mother to the fetus via the placenta: the interface between the maternal and fetal circulations. We know how amino acids enter the placenta from the maternal blood, but it was not known how the amino acids exit the placenta to reach the fetus. Our work has now provided the first experimental evidence for a novel transport system which provides net amino acid transport to the fetus and influences fetal growth.
Introduction
The fetus is dependent on placental transport of amino acids for protein accretion, metabolic processes and biosynthetic pathways. If placental amino acid transfer is decreased, fetal growth becomes restricted (Cetin et al. 1988; Jansson et al. 2006). Both uptake by the placenta and transport from the placenta to the fetus are involved in this transfer. However, a key step, namely transport of amino acids from the placenta to the fetus, is poorly understood and cannot be explained by the transporters currently known to be present in the placenta.
The placental syncytiotrophoblast is the primary barrier between the maternal and fetal circulations. Amino acids must cross both the maternal-facing microvillous plasma membrane (MVM) and fetal-facing basal plasma membrane (BM) of the placental syncytiotrophoblast in order to reach the fetal circulation (Fig. 1). Placental amino acid transfer is an active process, as indicated by the fact that fetal plasma amino acid concentrations are higher than maternal plasma levels (Philipps et al. 1978).
Figure 1. Transport of amino acids across the placental syncytiotrophoblast.
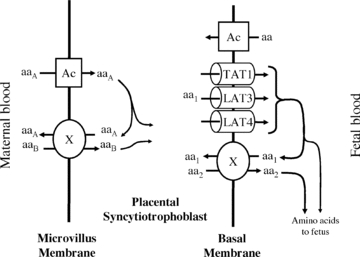
Amino acids are transported into the placenta across the microvillous membrane (MVM) of placental syncytiotrophoblast by active accumulative transporters (Ac) and exchangers (X). To mediate transport of all amino acids, those transported by accumulative transporters (aaA) must be taken up and exchanged back for those only transported by exchangers (aaB). Amino acids are transported out of the placenta across the basal membrane (BM) by facilitated transporters (TAT1, LAT3 and LAT4) and exchangers (X). The facilitated transporters TAT1, LAT3 and LAT4 transport specific amino acids (aa1) down their concentration gradient to the fetus. In order to transport other amino acids (aa2) to the fetus, aa1 must be exchanged for aa2 via exchangers (X). Thus the function of the exchangers is dependent on the activity of accumulative transporters on the MVM and facilitated transporters on the BM.
There are two classes of amino acid transporter known to be active in the human placenta: accumulative transporters and amino acid exchangers (Cleal et al. 2007; Lewis et al. 2007). Accumulative amino acid transporters mediate net uptake of specific amino acids into the syncytiotrophoblast. Amino acid exchange transporters swap one amino acid within the syncytiotrophoblast for one in the maternal or fetal circulation, thus altering the composition but not the overall quantity of amino acids transported (Fig. 1).
Amino acid exchange transport systems have been identified in the human placenta at the protein and RNA levels. To date, the amino acid exchangers LAT1 (SLC7A5), LAT2 (SLC7A8), y+LAT1 (SLC7A7), y+LAT2 (SLC7A6) and asc (SLC7A10) have been identified in human placental tissue at the mRNA level and ASC1 (SLC1A4) and ASC2 (SLC1A5) mRNA have been found in placental cell lines (Arriza et al. 1993; Kekuda et al. 1996). This does not reveal whether the exchanger protein is present, whether it is in syncytiotrophoblast and, if so, in which membrane the transporter is localized. Whilst syncytiotrophoblast membrane vesicle preparations show LAT, y+LAT and ASC activity on the BM, there is uncertainty about the identity of the LAT and y+LAT isoforms and whether b0,+ (SLC7A9) is on the BM (Furesz et al. 1995; Furesz & Smith, 1997; Ayuk et al. 2000). The present study therefore aimed to establish which exchangers function in the BM of intact human placental syncytiotrophoblast.
Accumulative amino acid transporters and exchangers can account for the net uptake of amino acids from the maternal circulation across the MVM into the placenta (Cleal & Lewis, 2008), but they cannot explain the efflux of amino acids from the placenta across the BM into the fetal circulation. We hypothesise that unidentified efflux transporters could mediate net transport of specific amino acids into the fetal circulation; their substrates could then swap for other amino acids via exchangers (Fig. 1). In support of this we have recently demonstrated that there is an unidentified efflux transporter for leucine on the BM (Cleal et al. 2007). The implications of this are important, as impairment in rate-limiting efflux transporter activity on the BM could restrict fetal growth. By analogy, activity of system A, which is the main accumulative transport system on the MVM, is decreased in placentas from growth restricted babies (Sibley et al. 1997; Jansson et al. 1998; Cetin, 2003).
We therefore sought to identify BM transporters which mediate efflux of amino acids from the placenta to the fetus and to develop a model for the functional interaction of efflux transporters and amino acid exchangers which could explain transport of all the essential amino acids to the fetus. Then, we related the expression of these to birth weight and neonatal lean mass. Our findings are important because reduced growth in utero not only has serious immediate consequences for the baby (Rosenberg, 2008), but is associated with increased risk of a range of chronic diseases in adult life (Gluckman et al. 2008).
Methods
Placentas
Placentas were collected from term pregnancies immediately after delivery, following written informed consent and with the approval of the South and West Hants Local Research Ethics Committee. The study conformed to the Declaration of Helsinki.
Perfusion experiments
Perfusion method
Placentas were perfused using the isolated perfused placental cotyledon methodology (Schneider et al. 1972), as previously described (Cleal et al. 2007). Catheters (Portex, Hythe, Kent, UK), 15 cm in length were inserted in the fetoplacental artery (polythene tubing: i.d. 1.0 mm, o.d. 1.6 mm) and fetoplacental vein (PVC tubing: i.d. 2 mm, o.d. 3 mm) of an intact cotyledon and sutured in place. On the maternal side five 10 cm lengths of polythene tubing (Portex; i.d. 0.58 mm, o.d. 0.96 mm) were inserted through the decidua and into the intervillous space. The fetal circulation and intervillous space were perfused with a modified Earle's bicarbonate buffer (EBB, 1.8 mm CaCl2, 0.4 mm MgSO4, 116.4 mm NaCl, 5.4 mm KCl, 26.2 mm NaHCO3, 0.9 mm NaH2PO4, 5.5 mm glucose) containing 35 g l−1 dextran (MW 64,000–74,000; Sigma-Aldrich Co., Poole, UK), 0.1% bovine serum albumin, and 5000 IU l−1 heparin equilibrated with 95% O2–5% CO2) using roller pumps (Watson Marlow, Falmouth, UK) at 6 and 14 ml min−1, respectively. Perfusion of the fetal circulation was established first, and, if fetal venous outflow was ≥95% of fetal arterial inflow, the maternal-side arterial perfusion with EBB was established 15 min later. Approximately 1 ml samples of fetal and maternal venous outflow were collected.
Paracellular diffusion
Creatinine has been shown to have free permeability through the human fetoplacental endothelium pores and was used as a marker of paracellular diffusion (Illsley et al. 1985). Creatinine was measured using an Infinity Creatinine assay kit (Thermo Electron, Noble Park, Victoria, Australia) in a microplate read at 490 nm. The intra- and interassay coefficients of variation were 1.9% and 3.8%, respectively.
Maternal tracer experiment
Following 40 min of initial perfusion, the maternal arterial perfusion was switched to EBB containing 50 μm of a specific 3H- or 14C-radiolabelled amino acid (l-lysine, l-threonine, l-glutamine, l-alanine, l-tyrosine, l-phenylalanine, l-isoleucine and l-valine) plus 20 μm unlabelled amino acid and 1.8 mm creatinine for a further 2 h 35 min (n= 5 placentas per amino acid). Sampling was continued every 5 min until 60 min as illustrated in Fig. 2.
Figure 2. Experimental protocol for the perfusion experiments (as described in Cleal et al. 2007).
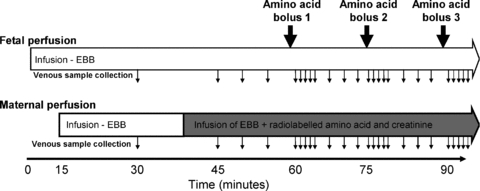
The fetal circulation was perfused from time 0 with Earl's bicarbonate buffer (EBB) and, if venous recovery was >95%, the maternal circulation from 15 min. At 40 min the maternal buffer was changed to one containing radiolabelled amino acids and creatinine as indicated by the shaded area. Boluses of unlabelled amino acids were injected into the fetal circulation, upstream of the pump, as indicated by the large arrows. Although only 3 boluses are shown in this diagram, up to 9 boluses of different amino acids were given in any one experiment. Samples were taken from the maternal and fetal venous circulation as indicated by the small arrows.
From 60 min, each of the following amino acids or system specific amino acid analogues were administered as a separate bolus (12.5 μmol in 1.5 ml EBB) to the fetal inflow line via an injection point prior to the pump over a 15 s period: l-glutamate, l-alanine, l-threonine, l-serine, l-glutamine, l-lysine, l-leucine, l-tryptophan, 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (BCH; a System L substrate). Given in this way, the bolus was diluted 7.7-fold as it went through the pump and the tubing prior to entering placental circulation. So a 12.5 μmol bolus in 1.5 ml gave a peak concentration of 1.0 mmol l−1. The boluses were given in random order at 15 min intervals followed by fetal and maternal venous sampling at +1, 2, 3, 4, 5, 8, 11 and 14 min (Fig. 2). Amino acid boluses were chosen to cover the range of amino acid exchangers in the placenta, and l-glutamate was included as a negative control as it is not exchanged by any exchanger expressed in the placenta. The total perfusion time for the placenta was 3 h 15 min; during this time the creatinine transfer and venous outflow volume did not change. There were no major shifts in placental perfusion pressure during the course of these experiments.
Approximately 1 ml samples of fetal and maternal venous outflow were collected. Appearance of labelled amino acids was measured in maternal and fetal venous samples (0.5 ml in 8 ml scintillation fluid) by liquid scintillation counting.
Protein measurement
Antibodies to transporters
Antibodies to a human TAT1 (SLC16A10) peptide (aa 316–326) were raised in rabbit, affinity purified (Cambridge Research Biochemicals, Billingham, UK) and used at a dilution of 1:500. Antibodies to a human LAT3 (SLC43A1) peptide (aa 338–350) and a human LAT4 (SLC43A2) peptide (aa 298–312) were raised in rabbit, affinity purified (Thermo Fisher Scientific, Rockford, IL, USA) and used at a dilution of 1:200 and 1:500 respectively.
Western blotting
The basal membrane (BM) of the placental syncytiotrophoblast was isolated as described previously (Glazier & Sibley, 2006). BM marker enrichment of dihydroalprenolol binding in the BM preparations compared to initial homogenates was 28.8 (7.9)-fold (mean and s.e.m.; n= 10). BM protein samples (15 μg, n= 5) were mixed with loading buffer (RunBlue LDS Sample Buffer with dithiothreitol at 10 mg ml−1; Expedion Ltd, Harston, Cambridgeshire, UK) and reduced at 70°C for 10 min. Samples were separated by electrophoresis in 4–12% SDS-PAGE gels and blotted on PVDF-membranes (Expedion). The membranes were blocked overnight at 4°C with 5% advanced blocking reagent (GE Healthcare, Little Chalfont, UK) in phosphate-buffered saline–0.1% Tween-20 (PBS-T, pH 7.4). Blots were incubated with primary antibody overnight at 4°C. After washing, blots were incubated for 2 h at room temperature with goat anti-rabbit secondary antibody conjugated to horseradish peroxidase (1:100,000; Abcam, UK). Immunoreactive signals were visualized using enhanced chemiluminescence (SuperSignal West Femto, Thermo Scientific, UK). The specificity of the TAT1 signal was verified using TAT1 antibody pre-incubated (1 h room temperature) with the antigen peptide (Cambridge Research Biochemicals); peptides were not available for LAT3 or LAT4. A negative control membrane probed only with secondary antibody was also used. Each membrane was reprobed for β-actin (1:10000) to confirm equal protein loading.
Southampton Women's Survey (SWS)
All data were collected following written informed consent and with the approval of the South and West Hants Local Research Ethics Committee. As previously described (Inskip et al. 2006) non-pregnant Southampton women aged 20–34 years were recruited to the SWS and those who became pregnant had ultrasound measurements of fetal head circumference growth velocity between 19 and 34 weeks gestation. The infant's gestational age at birth was calculated from the date of the mother's last menstrual period and ultrasonography. Trained research midwives recorded neonatal anthropometric measures (birth weight, head circumference, crown–heel length and crown–rump length). Within 2 weeks of the baby's birth, a subset of mothers were invited to attend for dual energy X-ray absorptiometry (DXA) measurement of the baby's lean and fat mass. This was measured using a Lunar DPX instrument with neonatal scan mode and specific paediatric software (paediatric small scan mode, v4.7c, GE Corp., Madison, WI, USA). The instrument underwent daily quality assessment, and was calibrated against a water phantom weekly. The baby was kept in position using rice bags placed over the bottom end of the towel. The manufacturer's short-term and long-term coefficients of variation (c.v.) for the DXA instrument were 0.8% and 1.4% respectively
Molecular biology
RNA extraction and cDNA synthesis
For RNA studies 102 SWS placentas were selected from 300 collected in total, based on availability of neonatal DXA data.
To ensure that the RNA extracted was representative of the placentas as a whole, five villous tissue samples were selected using a stratified random sampling method, and the snap frozen samples were pooled and powdered in a frozen tissue press. Total RNA was extracted from 30 mg powdered placental tissue using the RNeasy Fibrous Tissue Mini Kit (Qiagen, Crawley, West Sussex, UK) for RNA isolation according to the manufacturer's instructions. The integrity of total RNA was confirmed by visualization of ribosomal bands with ethidium bromide under ultra-violet illumination by agarose gel electrophoresis, in 1 × TAE (Tris-acetate-EDTA) buffer.
RNA (0.2 μg) was reverse transcribed with 0.5 μg random hexamer primer, 200 u MMLV (Moloney Murine Leukemia Virus) reverse transcriptase, 25 units recombinant RNasin ribonuclease inhibitor and 0.5 mm each of dATP, dCTP, dGTP and dTTP in a final reaction volume of 25 μl in 1× MMLV reaction buffer (Promega, UK). All 102 samples were produced in one batch to reduce variation.
Real-time quantitative PCR
The mRNA expression of TAT1, LAT3 and LAT4 was measured in SWS placentas (n= 102). Oligonucliotiode probes and primers were designed using the Roche ProbeFinder v. 2.45 for humans. Probes were supplied by Roche from the human universal probe library and primers were synthesised by Eurogentec (Seraing, Belgium). TAT1 forward 5′-ggtgtgaagaaggtttatctacagg-3′, reverse 5′-agggccccaaagatgcta-3′, probe no. 6; LAT3 forward 5′-gccctcatgattggctctta-3′, reverse 5′-ccggcatcgtagatcagc-3′, probe no. 29; LAT4 forward 5′-acaagtatggcccgaggaa-3′, reverse 5′-gcaatcagcaagcaggaaa-3′, probe no. 3. Control genes (topoisomerase DNA I (TOP1), ubiquitin C (UBC), phospholipase A2 (YWHAZ)) were selected using the geNormTM human Housekeeping Gene Selection Kit (Primer Design Ltd, Southampton, UK) (Cleal et al. 2009). Real-time PCR was performed using a Roche light-cycler 480. For Roche universal probe library probes, the cycle parameters were 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. For primer design Perfect Probes the cycle parameters were 95°C for 10 min, followed by 40 cycles of 95°C for 10 s and 60°C and 72°C for 15 s. Intra-assay c.v. values for each gene were 5–8%. Each of the 102 samples was run on the same plate in triplicate. Real-time quantitative PCR was performed twice for each gene. All mRNA levels were analysed relative to the geometric mean of the three control genes (Cleal et al. 2009).
Data analysis
Amino acid transport data were presented as means ±s.e.m. Loss of amino acids from the maternal circulation was derived as 100 – (maternal venous sample (counts per minute (c.p.m.)) as a percentage of maternal arterial sample (c.p.m.)). Gain of amino acids into the fetal circulation was derived as fetal venous sample (c.p.m.) as a percentage of maternal arterial sample (c.p.m.). Release of labelled amino acid into the fetal circulation (c.p.m.) following a fetal amino acid bolus was expressed as area under the response curve (AUC) calculated using the trapezium rule. AUC was determined over the 10 min period following the injection of the bolus with two measurements prior to this (e.g. −4 and −1 min) and following this (e.g. +11 and + 14 min) used to calculate the baseline. c.p.m. measurements of the maternal radiolabelled stock solution allowed this value to be converted to nmol. Data were compared to glutamate using ANOVA and Dunnet's post hoc test with SPSS (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant.
Variables that were not normally distributed were transformed to normality logarithmically. Pearson's correlation coefficients were used to analyse the mRNA data using Stata version 10.0 (StataCorp LP, College Station, TX, USA). Fetal variables were adjusted for sex. Birth weight, neonatal head circumference and crown heel length were adjusted for sex and gestational age. Neonatal DXA measurements were adjusted for sex, gestational age and age at DXA. As there was a question regarding sex differences in mRNA levels between male and female placentas all mRNA data were adjusted for the baby's sex (Cleal et al. 2010). To investigate whether there were sex differences in the relationships with gene expression, sex was included in all regression analysis.
Results
Perfusion experiments
Mean perfused cotyledon size was 29.5 ± 1.8 g; mean fetal venous outflow was 99.0 ± 0.3% of fetal arterial inflow. These values represent all placental cotyledons (n= 40) as no differences occurred between experimental sets. An example experiment showing the appearance of substrates in the fetal venous circulation following perfusion into the maternal artery is shown in Fig. 3.
Figure 3. A representative perfusion experiment showing l-[14C]isoleucine and creatinine in the fetoplacental venous perfusate.
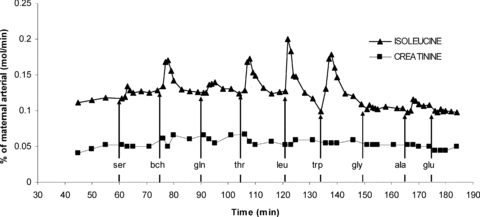
The concentration of substrate measured in the venous perfusate is expressed as a percentage of the substrate concentration (mol min−1) that was perfused into the maternal circulation. Perfusion of 50 μm l-[14C]isoleucine, 20 μm unlabelled l-isoleucine and 1.8 mm creatinine into the maternal arterial circulation was started at 40 min from the start of fetal perfusion. At 15 min intervals a 1.5 ml bolus containing 12.5 μmol of l-serine (ser), 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (bch), l-glutamine (gln), l-threonine (thr), l-leucine (leu), l-tryptophan (trp), glycine (gly), l-alanine (ala) or l-glutamate (glu) was injected into the fetal artery over 15 s. The difference between the isoleucine line and creatinine line represents the difference between carrier-mediated and non-mediated transport; the increase in the isoleucine line following a fetal bolus indicates exchange mediated transport.
Evidence of net amino acid efflux from placenta to fetus
At baseline (5 min interval before each amino acid bolus) all amino acids studied were taken up from the maternal circulation into the placenta at levels greater than via paracellular diffusion (as measured by disappearance of creatinine; P < 0.01, Fig. 4A). At baseline significantly more maternal l-alanine, l-tyrosine, l-phenylalanine, l-isoleucine and l-valine, but not l-lysine, l-threonine and l-glutamine, were transferred from the maternal to the fetal circulation as compared to creatinine transfer (P < 0.01, n= 5 placentas per maternal amino acid, Fig. 4B).
Figure 4. Evidence consistent with TAT1, LAT3 and LAT4 activity in the human placenta.
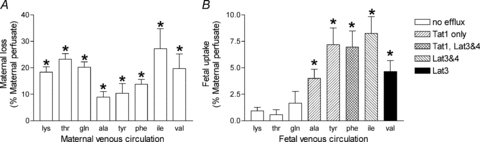
Amino acids were taken up by the placental syncytiotrophoblast (A) and specific amino acids were transported into the fetal circulation by non-exchange mechanisms (B, legend indicates putative transporters). Data (means +s.e.m.) as a percentage of substrate perfused into the maternal circulation. *P < 0.05, different from creatinine (a marker of paracellular diffusion), paired t test, n= 5 placentas per maternal amino acid.
Characterisation of amino acid exchange activity on the BM
In response to fetal 12.5 μmol boluses of specific amino acids, all labelled amino acids studied were released into the feto-placental circulation (Table 1, P < 0.01 compared to 12.5 μmol l-glutamate, n= 5 placentas per maternal amino acid). Maternal venous amino acid levels following fetal amino acid boluses were not significantly different from those following a l-glutamate bolus (data not shown).
Table 1.
Data indicating which fetal amino acids exchange for which maternal amino acids in the isolated perfused human placenta
| Fetal bolus | Maternal amino acid appearance in the fetal circulation (nmol) | |||||||
|---|---|---|---|---|---|---|---|---|
| Ala | Phe | Iso | Tyr | Gln | Lys | Thr | Val | |
| Glu | 0.1 ± 0.7 | −14 ± 3 | −8 ± 4 | −7 ± 6 | −7 ± 6 | 3 ± 3 | −7 ± 3 | 1 ± 2 |
| Ala | 174 ± 56* | 82 ± 45 | 68 ± 37 | 61 ± 21 | 88 ± 10* | — | 76 ± 17* | 40 ± 8* |
| Thr | 83 ± 13* | 108 ± 25 | 131 ± 39* | 72 ± 14 | 86 ± 13* | 11 ± 3 | 89 ± 13* | 78 ± 11* |
| Ser | 86 ± 25* | 32 ± 14 | 53 ± 15 | 57 ± 27 | 79 ± 4* | 25 ± 6 | 65 ± 9* | 41 ± 9* |
| Gln | 69 ± 15 | 74 ± 29 | 17 ± 6 | 39 ± 18 | 81 ± 17* | 66 ± 8* | 40 ± 6* | — |
| Lys | 7 ± 4 | 0.6 ± 10 | — | — | 10 ± 5 | 62 ± 10* | — | −5 ± 2 |
| Leu | 20 ± 3 | 277 ± 69* | 181 ± 32* | 172 ± 36* | 78.8 ± 16* | 81 ± 15 | 31 ± 7* | 101 ± 20* |
| Trp | — | 197 ± 30* | 164 ± 29* | 191 ± 24* | 12 ± 5 | −38 ± 4* | — | — |
| BCH | 5 ± 3 | 140 ± 50* | 115 ± 19* | 144 ± 25* | 14 ± 5 | 15 ± 7 | 6 ± 7 | — |
Mean ±s.e.m. difference from baseline. An l-glutamate (Glu) bolus was used as a control as it is not exchanged by any exchanger expressed in the placenta.
P < 0.05, significantly different to Glu.
n= 5 placentas per maternal amino acid. — indicates not done; l-alanine (Ala), l-threonine (Thr), l-serine (Ser), l-glutamine (Gln), l-lysine (Lys), l-leucine (Leu), l-tryptophan (Trp), 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (Bch), l-phenylalanine (Phe), l-isoleucine (Iso), l-tyrosine (Tyr), l-valine (Val).
TAT1, LAT3 and LAT4 protein expression
The presence of TAT1, LAT3 and LAT4 proteins in the basal (fetal facing) membrane of term human placenta was confirmed by Western blotting. TAT1 antibody demonstrated 48 and 133 kDa bands which were not present in the negative control and disappeared following pre-incubation with blocking peptide (Fig. 5A). LAT3 antibody demonstrated 28, 39 and 56 kDa bands, which were not present in the negative control (Fig. 5B). LAT4 antibody demonstrated 48, 50, 54, 71 and 80 kDa bands, which were not present in the negative control (Fig. 5C). Immunoblotting for β-actin on each blot indicated similar protein loading in each lane.
Figure 5. Immuno-localisation of TAT1, LAT3 and LAT4 protein to the basal membrane of human placenta.
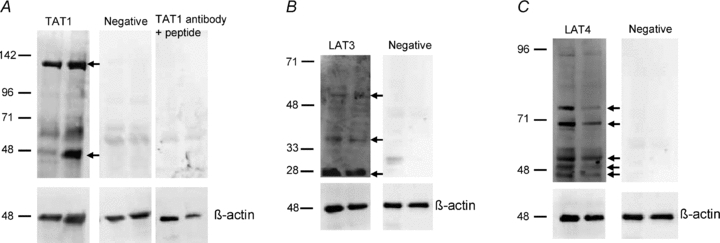
Western blot on placental basal membrane samples from term human placenta. Immunoblotting for β-actin on each blot indicated similar protein loading in each lane. A, TAT1 antibody demonstrated 48 and 133 kDa bands (arrows) which were not present in the negative control and disappeared following pre-incubation with blocking peptide. This matched the ∼50 kDa TAT1 protein and the higher molecular weight band thought to represent protein interaction suggested in previous studies in oocytes transfected with mTAT1 mRNA (Kim et al. 2002; Loubiere et al. 2010). B, LAT3 antibody demonstrated 28, 39 and 56 kDa bands (arrows), which were not present in the negative control. A similar pattern of bands has previously been shown in mouse tissue (Fukuhara et al. 2007). C, LAT4 antibody demonstrated bands at 48, 50 and 54 kDa (arrows) and two bands thought to be glycosylated forms (71 and 80 kDa); these were not present in the negative control. This is similar to the pattern seen in transfected HeLa cells (Bodoy et al. 2005).
TAT1, LAT3 and LAT4 mRNA expression
Characterisation of the subjects from the SWS cohort
The mean age (s.d.) of the 102 mothers at the birth of their child was 30.9 (3.9) years; 37.9% were primiparous. The median (inter-quartile range) gestational age was 39.6 (38.8–40.7) weeks. The mean (s.d.) placental/fetal weight ratio was 0.13 (0.02).
Cycle threshold values (means ±s.e.m.) for TAT1, LAT3 and LAT4 were 28.8 ± 0.1, 29.5 ± 0.04 and 22.0 ± 0.05, respectively. The mRNA levels (mean ±s.e.m.) for TAT1, LAT3 and LAT4 were 1.22 ± 0.05, 1.08 ± 0.03 and 1.38 ± 0.03, respectively, relative to the geometric mean of the housekeeping genes.
Relationships between TAT1, LAT3 and LAT4 mRNA levels in placentas at birth and fetal and neonatal factors
TAT1 mRNA levels in placentas positively correlated with change in fetal head circumference between 19 and 34 weeks gestation (Table 2). TAT1 mRNA levels positively correlated with birth weight (Fig. 6A and Table 2), neonatal lean mass (Fig. 6B and Table 2) and head circumference (Table 2). TAT1 mRNA was not related to neonatal fat mass or crown heel length (Table 2). LAT3 mRNA levels also positively correlated with neonatal head circumference (Table 2). LAT4 mRNA levels were not significantly related to the fetal or neonatal factors measured.
Table 2.
Relationship between placental TAT1, LAT3 and LAT4 mRNA levels at birth (adjusted for sex) and fetal and neonatal parameters (adjusted for sex and gestation length)
| TAT1 | LAT3 | LAT4 | |||||
|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | n | |
| 19–34 week head circumference z- score | 0.36 | 0.01* | 0.23 | 0.08 | −0.18 | 0.17 | 59 |
| Birth weight | 0.22 | 0.02* | 0.13 | 0.18 | −0.02 | 0.85 | 102 |
| Placental weight | 0.17 | 0.08 | 0.11 | 0.27 | −0.07 | 0.49 | 101 |
| Neonatal head circumference | 0.22 | 0.03* | 0.26 | 0.01* | 0.04 | 0.68 | 102 |
| Neonatal lean mass | 0.22 | 0.03* | 0.04 | 0.69 | −0.02 | 0.80 | 102 |
| Neonatal fat mass | 0.09 | 0.38 | 0.14 | 0.17 | −0.03 | 0.79 | 102 |
| Neonatal crown heel length | 0.11 | 0.29 | 0.05 | 0.60 | 0.01 | 0.93 | 102 |
P < 0.05, significant correlation; Pearson's correlation coefficient.
Figure 6. TAT1 mRNA expression is associated with fetal growth.
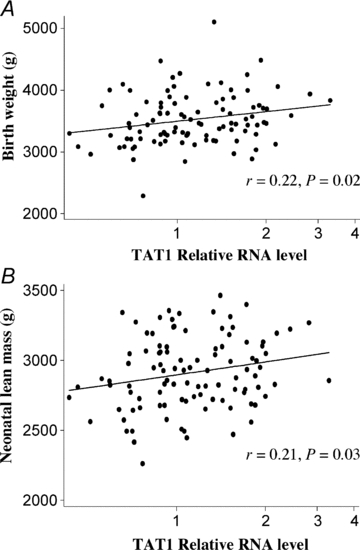
TAT1 relative mRNA expression in human placenta is positively correlated with birth weight (A) and neonatal lean mass (B) (n= 102, Pearson's correlation coefficient).
Discussion
This study suggests that the facilitated transporters TAT1, LAT3 and LAT4 play an important role in the efflux of amino acids across the BM of human placental syncytiotrophoblast to the fetus. Unlike other amino acid transporters, TAT1, LAT3 and LAT4 can mediate net transfer of amino acids to the fetus and as such may be key determinants of placental amino acid transfer capacity and fetal growth. Consistent with the hypothesis that these transporters may influence fetal growth, mRNA levels for TAT1 and LAT3 were positively correlated with fetal growth parameters.
Without amino acids in the fetal circulation, transport across the BM by exchange mechanisms cannot occur. However, in the isolated perfused placenta without amino acids in the fetal circulation, specific amino acids were transferred from the maternal to the fetal circulation by transporter-mediated routes. This demonstrates that non-exchange transport systems are present for l-alanine, l-tyrosine, l-phenylalanine, l-isoleucine and l-valine in the placental BM. Our previous data indicate that l-leucine, but not l-serine or glycine, is also transported across the placenta in this way (Cleal et al. 2007). The amino acids transported across the BM are substrates of the transporters TAT1, LAT3 or LAT4, which mediate facilitated diffusion (Ramadan et al. 2006; Bodoy et al. 2005). TAT1 alone transports l-tyrosine and l-alanine, whereas all three transporters transport l-phenylalanine and l-leucine. Both LAT3 and LAT4 transport l-isoleucine and LAT3 transports l-valine. These data are therefore consistent with the presence of these transporters in the human placenta.
Using Western blot analysis with custom-made antibodies we have generated this data suggesting that TAT1, LAT3 and LAT4 protein could be present on the fetal-facing BM of the human placental syncytiotrophoblast. TAT1 protein is predicted to be 55 kDa in size, and 48 and 133 kDa bands were detected, which is consistent with the ∼50 and ∼130 kDa band seen in oocytes transfected with mouse TAT1cDNA (Ramadan et al. 2007). It has been predicted that TAT1 binds with a glycoprotein such as CD147 to facilitate membrane localisation (Kim et al. 2002), consistent with the expression of CD147 in BM (Settle et al. 2004). LAT3 has two 56 kDa isoforms and a previous study in mouse liver indicates 28, 48 and 58 kDa bands (Fukuhara et al. 2007). In the present study we detected bands at approximately 28, 39 and 56 kDa which could represent LAT3 protein. Using the LAT4 antibody we detected three bands consistent with the 63, 53 and 57 kDa LAT4 isoforms and the approximately 60 kDa band shown in transfected HeLa cells (Bodoy et al. 2005). There were also two higher molecular weight bands consistent with glycosylated LAT4 protein, which has previously been shown in transfected HeLa cells (Bodoy et al. 2005).
These observations are consistent with in vivo stable isotope tracer studies in human pregnancy demonstrating rapid transfer of l-phenylalanine and l-leucine but only limited transfer of l-proline and glycine from mother to fetus (Paolini et al. 2001). In other epithelial tissues (gut and kidney) TAT1 protein and LAT4-like activity has been identified in the basal membrane, suggesting a role for these transporters more widely in transepithelial amino acid transfer (Kim et al. 2001; Bodoy et al. 2005).
Amino acid concentrations in placental tissues (built up by the activity of transporters on the MVM) are higher than those in either maternal or fetal blood (Philipps et al. 1978), indicating that net transport via TAT1, LAT3 and LAT4 would occur in the placental to fetal direction. While TAT1, LAT3 and LAT4 mediate efflux of amino acids, they have restricted substrate specificity and so could not supply the fetus with all the amino acids it requires. However, the amino acids transported by TAT1, LAT3 and LAT4 into fetal blood would be available for exchange for other amino acids within the placenta by amino acid exchangers. The establishment of a positive fetomaternal amino acid concentration gradient by 12–17 weeks of pregnancy (Jauniaux et al. 1999) suggests functional coupling of these facilitative transporters and amino acid exchangers occurs at the BM locus from early pregnancy onwards.
This study provides functional evidence for the activity of multiple amino acid exchangers based on measurements of amino acid exchange between the placenta and the fetal circulation. These exchangers, working together with the efflux transporters, can mediate the transfer of a much wider range of amino acids to the fetus than can either transporter type alone.
Fetal boluses of 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (BCH; a System L substrate) exchanged for maternal l-leucine (Cleal et al. 2007), l-isoleucine, l-phenylalanine and l-tyrosine, but not for l-alanine, l-glutamine, l-serine (Cleal et al. 2007) or l-threonine. This pattern is consistent with a predominant role for LAT1 rather than LAT2, which was thought to be the system L isoform on the BM on the basis of BM vesicle studies (Kudo & Boyd, 2001). The high levels of exchange of l-threonine (which is not exchanged by ASC1) for l-alanine and l-serine are consistent with the activity of ASC2 as there was no evidence for LAT2 activity; this does not exclude the expression of ASC1.
The evidence for y+LAT activity comes from exchange of l-lysine for itself (given that there is no evidence for b0,+). Additional evidence comes from the fact that y+LAT only mediates l-leucine influx (Pfeiffer et al. 1999; Broer et al. 2000). Our data demonstrate that a fetal l-leucine bolus stimulates l-lysine efflux but that a fetal l-lysine bolus does not stimulate l-leucine efflux, which is consistent with the activity of y+LAT. LAT1, y+LAT and ASC2 can therefore transport the amino acids l-serine, l-cystine, l-threonine, l-glutamine, l-histidine, l-lysine and l-arginine into the fetal circulation in exchange for the efflux transporter substrates.
The observed pattern of amino acid exchange was not consistent with activity of systems asc and b0,+ on the BM (Table 1). If system asc were active on the BM, we would expect exchange of glycine for itself and for l-serine and l-alanine, but this was not observed in either placental to fetal or fetal to placental direction (Cleal et al. 2007). If b0,+ were active on the BM we would expect to see exchange of l-lysine for l-alanine, l-serine and l-valine, and exchange of l-phenylalanine for l-lysine, l-serine, l-alanine and l-threonine, but this was not observed.
Working in combination, the efflux transporters and the exchangers can transport all the essential amino acids to the fetus. The only amino acids which cannot be transported by this efflux or exchange are the non-essential amino acids glycine, l-proline, l-aspartate l-asparagine and l-glutamate. However, l-proline is transported by ASC1 at low affinity and this may provide a route for l-proline transfer to the fetus (Zerangue & Kavanaugh, 1996). Stable isotope studies suggest that glycine and l-proline transfer across the human placenta is limited (Paolini et al. 2001) and umbilical venous–arterial difference measurements suggest that l-glutamate is actually taken up from the fetal circulation by the placenta (Cetin et al. 1988). Presumably other fetal requirements for these non-essential amino acids are met by synthesis de novo.
Impaired placental amino acid transporter activity (Glazier et al. 1997; Jansson et al. 1998) and altered fetal amino acid concentrations (Cetin et al. 1988) have been associated with reduced fetal growth. On the MVM, accumulative transporters, in particular systems A and y+, are important as they create the gradients within the placenta which drive uptake by exchangers and transport across the BM by facilitated diffusion and amino acid exchange. In particular System A transporter activity on the MVM is thought to influence fetal growth (Mahendran et al. 1993; Godfrey et al. 1998; Shibata et al. 2008). On the BM, TAT1, LAT3 and LAT4 may be rate-limiting for amino acid transfer and fetal growth as they are the only transporters which are known to mediate net efflux of amino acids to the fetus.
We found that both TAT1 and LAT3 mRNA expression in placentas are positively related to measures of fetal growth. TAT1 mRNA appears to be associated with fetal growth in terms of lean mass rather than adiposity or linear growth. As lean mass contains a high proportion of muscle, a protein rich tissue, its growth will require a substantial amino acid supply. However, we do not have any evidence as yet that the primary mechanism of regulation of TAT1, LAT3 and LAT4 activity is at the transcriptional level.
This study indicates that placental amino acid transport is a complex process which requires the interaction of different types of transporters. Because of this complexity it is difficult to determine intuitively how altering one component of the system will affect its function as a whole. Recent work has begun to model these processes mathematically to assist with understanding placental amino acid transport as a whole and the specific contributions of individual transport systems (Sengers et al. 2010).
In conclusion this study has shown the presence of three efflux transporters (TAT1, LAT3 and LAT4) active on the BM of human placenta which could mediate a quantitative increase in total fetal amino acids levels. The amino acid exchangers could then use these amino acids to alter qualitatively the composition of fetal amino acids, thus ensuring that the correct balance of amino acids is supplied to the fetus for growth to occur. This is a significant advance in our knowledge of how the placenta functions in order to provide the fetus with nutrients in utero. It is interesting to note also that mRNA expression of these transporters correlates with aspects of fetal growth in late gestation and with anthropometry at birth. Future studies aimed at understanding the regulation of these placental amino acid transporters may assist with identifying the causes and consequences of placental insufficiency and might lead to the development of therapeutic interventions to prevent or redress the effects of impaired fetal growth.
Acknowledgments
Southampton Women's Survey Study Group: D. J. P. Barker, H. M. Inskip, C. M. Law, V. Cox, P. Coakley, J. Hammond. This work was funded by the UK Medical Research Council, British Heart Foundation, Henry Smith's Charity, Gerald Kerkut Trust, Wessex Medical Trust, University of Southampton.
Glossary
Abbreviations
- MVM
maternal-facing microvillous plasma membrane
- BM
basal plasma membrane
Author contributions
All authors contributed to the writing of the manuscript, and approved the final version of the manuscript. Experiments were carried out at the Institute of Developmental Sciences, University of Southampton by J. C. and P. D., with statistical analysis carried out by G. N. and S. C. The overall conception, design and data interpretation for this study was carried out by J. C., R. L. J. G. and M. H. (SWS component N. H., S. R., C. C., and K. G.).
References
- Arriza JL, Kavanaugh MP, Fairman WA, Wu YN, Murdoch GH, North RA, Amara SG. Cloning and expression of a human neutral amino acid transporter with structural similarity to the glutamate transporter gene family. J Biol Chem. 1993;268:15329–15332. [PubMed] [Google Scholar]
- Ayuk PT, Sibley CP, Donnai P, D'Souza S, Glazier JD. Development and polarization of cationic amino acid transporters and regulators in the human placenta. Am J Physiol Cell Physiol. 2000;278:C1162–C1171. doi: 10.1152/ajpcell.2000.278.6.C1162. [DOI] [PubMed] [Google Scholar]
- Bodoy S, Martin L, Zorzano A, Palacin M, Estevez R, Bertran J. Identification of LAT4, a novel amino acid transporter with system L activity. J Biol Chem. 2005;280:12002–12011. doi: 10.1074/jbc.M408638200. [DOI] [PubMed] [Google Scholar]
- Broer A, Wagner CA, Lang F, Broer S. The heterodimeric amino acid transporter 4F2hc/y+LAT2 mediates arginine efflux in exchange with glutamine. Biochem J. 2000;349:787–795. doi: 10.1042/bj3490787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin I. Placental transport of amino acids in normal and growth-restricted pregnancies. Eur J Obstet Gynecol Reprod Biol. 2003;110(Suppl 1):S50–S54. doi: 10.1016/s0301-2115(03)00172-6. [DOI] [PubMed] [Google Scholar]
- Cetin I, Marconi AM, Bozzetti P, Sereni LP, Corbetta C, Pardi G, Battaglia FC. Umbilical amino acid concentrations in appropriate and small for gestational age infants: a biochemical difference present in utero. Am J Obstet Gynecol. 1988;158:120–126. doi: 10.1016/0002-9378(88)90792-2. [DOI] [PubMed] [Google Scholar]
- Cleal JK, Brownbill P, Godfrey KM, Jackson JM, Jackson AA, Sibley CP, Hanson MA, Lewis RM. Modification of fetal plasma amino acid composition by placental amino acid exchangers in vitro. J Physiol. 2007;582:871–882. doi: 10.1113/jphysiol.2007.130690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleal JK, Day P, Hanson MA, Lewis RM. Measurement of housekeeping genes in human placenta. Placenta. 2009;30:1002–1003. doi: 10.1016/j.placenta.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Cleal JK, Day PL, Hanson MA, Lewis RM. Sex differences in the mRNA levels of housekeeping genes in human placenta. Placenta. 2010;31:556–557. doi: 10.1016/j.placenta.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Cleal JK, Lewis RM. The mechanisms and regulation of placental amino acid transport to the human foetus. J Neuroendocrinol. 2008;20:419–426. doi: 10.1111/j.1365-2826.2008.01662.x. [DOI] [PubMed] [Google Scholar]
- Fukuhara D, Kanai Y, Chairoungdua A, Babu E, Bessho F, Kawano T, Akimoto Y, Endou H, Yan K. Protein characterization of Na+-independent system L amino acid transporter 3 in mice: a potential role in supply of branched-chain amino acids under nutrient starvation. Am J Pathol. 2007;170:888–898. doi: 10.2353/ajpath.2007.060428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furesz TC, Moe AJ, Smith CH. Lysine uptake by human placental microvillous membrane: comparison of system y+ with basal membrane. Am J Physiol Cell Physiol. 1995;268:C755–C761. doi: 10.1152/ajpcell.1995.268.3.C755. [DOI] [PubMed] [Google Scholar]
- Furesz TC, Smith CH. Identification of two leucine-sensitive lysine transport activities in human placental basal membrane. Placenta. 1997;18:649–655. doi: 10.1016/s0143-4004(97)90006-0. [DOI] [PubMed] [Google Scholar]
- Glazier JD, Cetin I, Perugino G, Ronzoni S, Grey AM, Mahendran D, Marconi AM, Pardi G, Sibley CP. Association between the activity of the system A amino acid transporter in the microvillous plasma membrane of the human placenta and severity of fetal compromise in intrauterine growth restriction. Pediatr Res. 1997;42:514–519. doi: 10.1203/00006450-199710000-00016. [DOI] [PubMed] [Google Scholar]
- Glazier JD, Sibley CP. In vitro methods for studying human placental amino acid transport: placental plasma membrane vesicles. Methods Mol Med. 2006;122:241–252. doi: 10.1385/1-59259-989-3:241. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey KM, Matthews N, Glazier J, Jackson A, Wilman C, Sibley CP. Neutral amino acid uptake by the microvillous plasma membrane of the human placenta is inversely related to fetal size at birth in normal pregnancy. J Clin Endocrinol Metab. 1998;83:3320–3326. doi: 10.1210/jcem.83.9.5132. [DOI] [PubMed] [Google Scholar]
- Illsley NP, Hall S, Penfold P, Stacey TE. Diffusional permeability of the human placenta. Contrib Gynecol Obstet. 1985;13:92–97. [PubMed] [Google Scholar]
- Inskip HM, Godfrey KM, Robinson SM, Law CM, Barker DJ, Cooper C. Cohort profile: The Southampton Women's Survey. Int J Epidemiol. 2006;35:42–48. doi: 10.1093/ije/dyi202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson N, Pettersson J, Haafiz A, Ericsson A, Palmberg I, Tranberg M, Ganapathy V, Powell TL, Jansson T. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J Physiol. 2006;576:935–946. doi: 10.1113/jphysiol.2006.116509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson T, Scholtbach V, Powell TL. Placental transport of leucine and lysine is reduced in intrauterine growth restriction. Pediatr Res. 1998;44:532–537. doi: 10.1203/00006450-199810000-00011. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Gulbis B, Gerloo E. Free amino acids in human fetal liver and fluids at 12–17 weeks of gestation. Hum Reprod. 1999;14:1638–1641. doi: 10.1093/humrep/14.6.1638. [DOI] [PubMed] [Google Scholar]
- Kekuda R, Prasad PD, Fei YJ, Torres-Zamorano V, Sinha S, Yang-Feng TL, Leibach FH, Ganapathy V. Cloning of the sodium-dependent, broad-scope, neutral amino acid transporter Bo from a human placental choriocarcinoma cell line. J Biol Chem. 1996;271:18657–18661. doi: 10.1074/jbc.271.31.18657. [DOI] [PubMed] [Google Scholar]
- Kim DK, Kanai Y, Chairoungdua A, Matsuo H, Cha SH, Endou H. Expression cloning of a Na+-independent aromatic amino acid transporter with structural similarity to H+/monocarboxylate transporters. J Biol Chem. 2001;276:17221–17228. doi: 10.1074/jbc.M009462200. [DOI] [PubMed] [Google Scholar]
- Kim DK, Kanai Y, Matsuo H, Kim JY, Chairoungdua A, Kobayashi Y, Enomoto A, Cha SH, Goya T, Endou H. The human T-type amino acid transporter-1: characterization, gene organization, and chromosomal location. Genomics. 2002;79:95–103. doi: 10.1006/geno.2001.6678. [DOI] [PubMed] [Google Scholar]
- Kudo Y, Boyd CA. Characterisation of l-tryptophan transporters in human placenta: a comparison of brush border and basal membrane vesicles. J Physiol. 2001;531:405–416. doi: 10.1111/j.1469-7793.2001.0405i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RM, Glazier J, Greenwood SL, Bennett EJ, Godfrey KM, Jackson AA, Sibley CP, Cameron IT, Hanson MA. l-Serine uptake by human placental microvillous membrane vesicles. Placenta. 2007;28:445–452. doi: 10.1016/j.placenta.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Loubiere LS, Vasilopoulou E, Bulmer JN, Taylor PM, Stieger B, Verrey F, McCabe CJ, Franklyn JA, Kilby MD, Chan SY. Expression of thyroid hormone transporters in the human placenta and changes associated with intrauterine growth restriction. Placenta. 2010;31:295–304. doi: 10.1016/j.placenta.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Mahendran D, Donnai P, Glazier JD, D'Souza SW, Boyd RD, Sibley CP. Amino acid (system A) transporter activity in microvillous membrane vesicles from the placentas of appropriate and small for gestational age babies. Pediatr Res. 1993;34:661–665. doi: 10.1203/00006450-199311000-00019. [DOI] [PubMed] [Google Scholar]
- Paolini CL, Marconi AM, Ronzoni S, Di Noio M, Fennessey PV, Pardi G, Battaglia FC. Placental transport of leucine, phenylalanine, glycine, and proline in intrauterine growth-restricted pregnancies. J Clin Endocrinol Metab. 2001;86:5427–5432. doi: 10.1210/jcem.86.11.8036. [DOI] [PubMed] [Google Scholar]
- Pfeiffer R, Rossier G, Spindler B, Meier C, Kuhn L, Verrey F. Amino acid transport of y+L-type by heterodimers of 4F2hc/CD98 and members of the glycoprotein-associated amino acid transporter family. EMBO J. 1999;18:49–57. doi: 10.1093/emboj/18.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipps AF, Holzman IR, Teng C, Battaglia FC. Tissue concentrations of free amino acids in term human placentas. Am J Obstet Gynecol. 1978;131:881–887. doi: 10.1016/s0002-9378(16)33136-2. [DOI] [PubMed] [Google Scholar]
- Ramadan T, Camargo SM, Herzog B, Bordin M, Pos KM, Verrey F. Recycling of aromatic amino acids via TAT1 allows efflux of neutral amino acids via LAT2–4F2hc exchanger. Pflugers Arch. 2007;454:507–516. doi: 10.1007/s00424-007-0209-3. [DOI] [PubMed] [Google Scholar]
- Ramadan T, Camargo SM, Summa V, Hunziker P, Chesnov S, Pos KM, Verrey F. Basolateral aromatic amino acid transporter TAT1 (Slc16a10) functions as an efflux pathway. J Cell Physiol. 2006;206:771–779. doi: 10.1002/jcp.20531. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. The IUGR newborn. Semin Perinatol. 2008;32:219–224. doi: 10.1053/j.semperi.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Schneider H, Panigel M, Dancis J. Transfer across the perfused human placenta of antipyrine, sodium and leucine. Am J Obstet Gynecol. 1972;114:822–828. doi: 10.1016/0002-9378(72)90909-x. [DOI] [PubMed] [Google Scholar]
- Sengers BG, Please CP, Lewis RM. Computational modelling of amino acid transfer interactions in the placenta. Exp Physiol. 2010;95:829–840. doi: 10.1113/expphysiol.2010.052902. [DOI] [PubMed] [Google Scholar]
- Settle P, Mynett K, Speake P, Champion E, Doughty IM, Sibley CP, D'Souza SW, Glazier J. Polarized lactate transporter activity and expression in the syncytiotrophoblast of the term human placenta. Placenta. 2004;25:496–504. doi: 10.1016/j.placenta.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Shibata E, Hubel CA, Powers RW, von Versen-Hoeynck F, Gammill H, Rajakumar A, Roberts JM. Placental system A amino acid transport is reduced in pregnancies with small for gestational age (SGA) infants but not in preeclampsia with SGA infants. Placenta. 2008;29:879–882. doi: 10.1016/j.placenta.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley C, Glazier J, D'Souza S. Placental transporter activity and expression in relation to fetal growth. Exp Physiol. 1997;82:389–402. doi: 10.1113/expphysiol.1997.sp004034. [DOI] [PubMed] [Google Scholar]
- Zerangue N, Kavanaugh MP. ASCT-1 is a neutral amino acid exchanger with chloride channel activity. J Biol Chem. 1996;271:27991–27994. doi: 10.1074/jbc.271.45.27991. [DOI] [PubMed] [Google Scholar]


