Abstract
Purpose
Clinical decision support (CDS) systems could be valuable tools in reducing aminoglycoside prescribing errors. We evaluated the impact of CDS on initial dosing, interval, and pharmacokinetic outcomes of amikacin and tobramycin therapy.
Methods
A complex CDS advisor to provide guidance on initial dosing and monitoring, using both traditional and extended interval dosing strategies, was integrated into computerized provider order entry (CPOE) and compared to a control group which featured close pharmacy monitoring of all aminoglycoside orders. A random sample of 118 patients from an academic, tertiary care medical center prescribed amikacin and tobramycin prior to advisor implementation was compared to 98 patients admitted following advisor implementation. Primary outcome was an initial dose within 10% of a dose calculated to be adherent to published dose guidelines. Secondary outcomes were a guideline-adherent interval, trough and peak concentrations in goal range, and incidence of nephrotoxicity.
Results
Of 216 patients studied, 97 were prescribed amikacin and 119 were prescribed tobramycin. The primary outcome of initial dosing consistent with guideline-based care increased from 40% in the pre-advisor arm to 80% in the post-advisor arm (p<0.001), with a number needed to treat of 3 patients to prevent one incorrect dose. Correct initial interval based on renal function also increased from 63% to 87% (p<0.001). The changes in initial dosing and interval resulted in an increase of trough concentrations in the goal range from 59% pre-advisor to 89% post-advisor implementation (p=0.0004). There was no significant difference in peak concentrations in goal range or incidence of nephrotoxicity (25% vs. 17%, p=0.2).
Conclusion
An advisor for aminoglycoside dosing and monitoring integrated into CPOE significantly improves initial dosing, selection of interval, and trough concentrations at goal compared to unassisted physician dosing.
Keywords: Aminoglycosides, Clinical Decision Support Systems, Pharmacokinetics, Amikacin, Tobramycin
Introduction
Clinical decision support (CDS) embedded into a Computerized Physician Order Entry (CPOE) system is an attractive method for providing around-the-clock dosing assistance to prescribers for medications with narrow therapeutic indices and serious toxicities, such as the aminoglycoside antibiotics. Medication dosing errors have been reported to be the most common type of preventable medication error.[1] One report from the Adverse Drug Event Prevention Study group found that 56% of the preventable medication errors occurred at the step of ordering.[2] Another study of hospitalized patients reported 60% of clinically significant preventable errors consisted of incorrect doses or frequencies.[1,3] CDS can potentially have a high impact by reducing prescribing errors for aminoglycosides, but as discussed in this paper, there are few clinical trials to validate this hypothesis.
Aminoglycosides exhibit concentration-dependent bactericidal effects, and optimal action exists when the peak concentration is approximately 10 times the minimum inhibitory concentration (MIC) of the organism.[4] Aminoglycoside goal concentrations vary by indication. While lower levels provide adequate treatment of urinary tract infections, high peak concentrations are necessary to obtain an adequate concentration of aminoglycoside at the alveolar site when treating pneumonia.[5] Achieving effective aminoglycoside serum concentrations early in the treatment course, ideally with the first dose, is important. Physicians must provide a dose that reaches the required peak levels, while allowing patients to have a period of low or no aminoglycoside level in the serum, given that this has been shown to minimize nephrotoxicity.[4,6–8] In some instances, institutions rely on a “pharmacy to dose” protocol beginning with the first or second dose and subsequent doses.[9] This can be time consuming or could delay receipt of the first dose of aminoglycoside. Providing standard or “default” doses based on drug indication using simple mechanisms within computerized physician order entry (CPOE) can lead to new ordering errors. [10–12] In a recent study, the majority (73%) of aminoglycoside orders based on a CPOE-based default dose prescribing system were incorrect, highlighting the need for individualized prescribing support within CPOE. [11]
The objective of this study was to evaluate the impact of an advanced clinical decision support system embedded within CPOE for aminoglycoside ordering, which we will refer to in the manuscript as the aminoglycoside advisor. The system and the evaluation is focused on initial dosing of the aminoglycosides amikacin and tobramycin compared to a historical control period where there was active pharmacy surveillance of aminoglycoside orders but no up front, patient-specific dosing assistance. Gentamicin orders were excluded from analysis because the aminoglycoside advisor was initially developed using iterative testing and implementation cycles to assist with gentamicin orders. We hypothesized that the advisor would increase the proportion of initial doses within 10% of a recommended dose calculated using published pharmacokinetic equations. [4, 7, 13–16] Secondary objectives included evaluating the effect the advisor had on initial dosing intervals, trough and peak concentrations in goal ranges, and nephrotoxicity.
Methods
Setting
Vanderbilt University Medical Center (VUMC) is a 593 bed tertiary care academic medical center with an active clinical pharmacy service. Medication orders are initiated by physicians through computerized physician order entry (CPOE), electronically transmitted to one of four pharmacies, then reviewed and dispensed by a pharmacist. Clinical staff and resident-level pharmacists round with medical and surgical teams Monday through Friday and routinely review medication lists and new drug orders within the previous 24 hours.
All aminoglycoside orders are reviewed by staff pharmacists before dispensing, and subsequently monitored daily by a therapeutic drug monitoring (TDM) team of clinical pharmacists. A pharmacy-to-dose protocol is not implemented currently, because of the volume of aminoglycoside orders overnight, when few clinical pharmacists are covering the hospital, and the concerns for delayed administration of antibiotic therapy. When the clinical pharmacist assigned to the TDM team detects the need for an aminoglycoside dose change or change to monitoring plan, the primary team is notified by phone. The aminoglycoside advisor was designed to complement the existing TDM services, by supporting the correct initial dosing of aminoglycoside therapy by physicians or non-physician prescribers. During the study, TDM services were delivered 7 days a week during both control and intervention periods by the same group of clinical pharmacists.
Design of Aminoglycoside Advisor
An aminoglycoside advisor was designed, piloted using gentamicin orders, and embedded into the CPOE system to provide proactive initial dosing support 24 hours a day. House staff were presented with the advisor and provided design and implementation feedback during weekly sessions led by the CPOE team during the piloting phase. The aminoglycoside advisor was designed to replace standard order procedures when a physician or other provider initiated an aminoglycoside drug order for any adult patient not receiving hemodialysis, but was still subject to pharmacist review. Secondly, it was designed to support both Extended Interval Dosing (EID) and Traditional Dosing (TD) and to assist prescribers in selecting one of these strategies. Prior to the advisor, aminoglycoside ordering via CPOE provided a pick list of doses or intervals, drug allergy inspection, and a reminder to order laboratory monitoring, but no other clinical decision support. When introduced, the advisor provided customized doses and intervals calculated via a pharmacokinetic model, drug level monitoring, and the opportunity to initiate Infectious Disease or Pharmacy consultations. The EID version (Figure I) appeared by default unless the patient was 65 years or older or had moderate to severe chronic kidney disease (calculated creatinine clearance ≤30ml/min), in which case, a similar screen (Figure II) was shown to assist with TD. For both EID and TD advisors, demographics and laboratory data (sex, age, weight, height, and serum creatinine) were automatically imported, using the most recent measurements from the electronic medical record (EMR). These values could be edited to incorporate information (such as an updated weight or serum creatinine measured outside of the institution) that was not currently available in electronic form. Internally, the advisor calculated an ideal body weight (IBW), a Dosing Body Weight (DBW), and a creatinine clearance using the Cockcroft-Gault equation.[17] The DBW, defined as adding 40% of the difference between the actual and IBW to the IBW, was used when the actual body weight was >120% of the IBW. Our advisor was based on validated pharmacokinetic equations that have been proven in clinical studies and recommended by expert opinion.[4,7,13–16] For traditional dosing, the advisor used the Sawchuck-Zaske method (Figure IIIa) of initial dosing based on IBW or DBW.[13] We designed the extended interval dosing (EID) advisor to follow the Hartford nomogram (Figure IIIb) which determines the dose using either actual or DBW.[4] As a second step when interacting with the advisor, a provider selected a dosing concentration (mg/kg) for EID or a desired target peak and trough concentration for TD. After pressing the “calculate dose” button, the advisor suggested a specific dose and interval, which could be altered by the provider, and offered the provider several monitoring options for drug serum levels and creatinine. A final option allowed prescribers to request Infectious Disease or Clinical Pharmacy consult services. As shown in Figures I and II, there is text to warn providers that the advice is not designed for dialysis, severe burns, and patients with rapidly changing renal function.
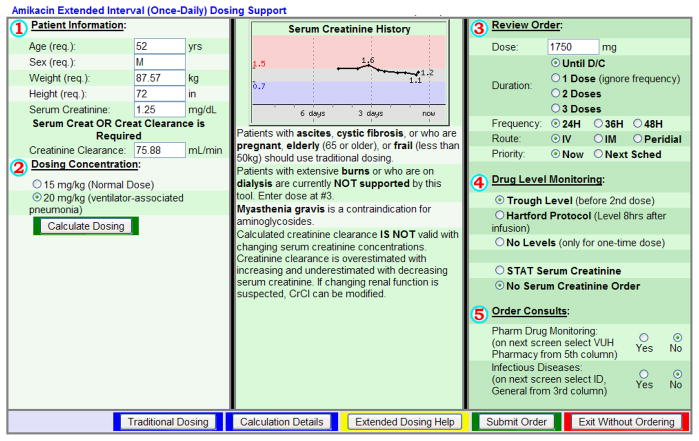
Figure I. Amikacin Extended Interval Dosing Advisor in CPOE.
The patient specific descriptive data shown in Part 1 is automatically imported from the most recent measurements in the patient’s electronic chart. The provider then selects the Dosing Concentration in Part 2. After pressing the “calculate dose” button, the suggested dose and interval appear in Part 3, which can be changed. Part 4 offers the provider several monitoring options for levels and serum creatinine. After this, the provider presses the “submit order” button and amikacin is ordered. EID was the default CDS dosing regimen unless patients were older than 65 years of age or had a creatinine clearance less than 30ml/min. Tobramycin EID advisor (not pictured) has a dosing concentration of 7mg/kg for all indications, except for cystic fibrosis (10mg/kg).
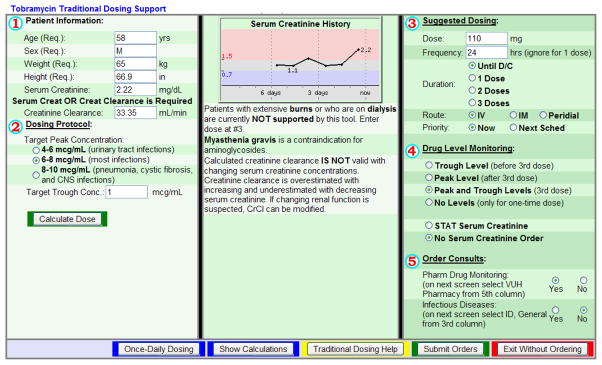
Figure II. Tobramycin Traditional Dosing Advisor in CPOE.
The patient specific descriptive data shown in Part 1 is automatically imported from the most recent measurements in the patient’s electronic chart. The provider then selects the Target Peak Concentration in Part 2 based on the indication for tobramycin. The Target Trough Concentration is set at 1mcg/ml, but may be changed. After pressing the “calculate dose” button, the suggested dose and interval appear in Part 3, which can again be changed. Part 4 offers the provider several monitoring options for levels and serum creatinine. After this, the provider presses the “submit order” button and tobramycin is ordered. Traditional dosing was recommended for all patients older than 65 years of age or with a creatinine clearance less than 30ml/min. Amikacin TD advisor (not pictured) has a goal peak of 15–20mcg/ml for urinary tract infections, 20–30mcg/ml for most infections, and 25–35mcg/ml for pneumonia, CNS infections, or cystic fibrosis patients.
Figure III. Initial Dosing Calculations.
Kel: k elimination constant, CrCl: Creatinine Clearance, Vd: Volume of Distribution
TD Dosing Weight= Ideal Body Weight (IBW) or Dosing Body Weight (DBW) if actual weight is >120% IBW
EID Dosing Weight= actual weight or DBW if actual weight is >120% IBW
Study Design
After implementation on November 1, 2008, the advisor was evaluated by prospectively selecting sequential patients, whose tobramycin or amikacin initial regimens were ordered through the advisor until February 1, 2009. A historical control group of hospitalized adults admitted to VUMC between May 1, 2008 to October 31, 2008 and prescribed amikacin or tobramycin was matched to the intervention group using a random number generator to retrospectively select pre-advisor patients. Patients were excluded if diagnosed with cystic fibrosis, pregnant, receiving hemodialysis, prescribed a single dose of amikacin or tobramycin, prescribed inhaled tobramycin, or were prescribed an aminoglycoside during one of the infrequent periods where there was no therapeutic drug monitoring pharmacist on duty. Since burn patients have a varying degree of distribution and clearance changes, and it was left to the provider’s discretion to use the advisor suggested regimen, they were included in this study. The study was approved by the Vanderbilt University Medical Center institutional review board, with waiver of informed consent due to the retrospective nature of the study.
To compare the appropriateness of initial dosing prior to and following the implementation of the advisor, a pharmacist [author ZC] reviewed the selected patient charts and, after verifying age, sex, height, weight, serum creatinine, and indication for use, calculated the extended interval dose or traditional dose. The methods to generate the recommended dose were identical to those employed by the advisor (Hartford nomogram for EID and Sawchuck-Zaske for TD). The appropriateness of EID or TD dosing was determined by comparing recorded diagnoses and laboratory data to selection guidelines established in the original Hartford and Sawchuck-Zaske publications. [4,13]
The primary outcome was the proportion of initial amikacin and tobramycin orders with a dose within ± 10% of the study reference standard for appropriate dose, which was defined as the dose adherent to the Hartford nomogram (for EID) or the Sawchuck-Zaske pharmacokinetic equations (for TD). Using an advisor as the intervention and reference standard has been used in CDS trials previously. [18] A range of 10% has previously been used when comparing computerized to provider dosing [11, 19], recognizing that a dose differing less than 10% will likely not alter clinical outcomes. Secondary outcomes were the number of final orders with dosing intervals that matched the expert recommendations, the frequency of peak and trough concentrations within the target ranges, and the incidence of nephrotoxicity. Ordered doses greater than 125% or less than 75% of the study reference standard were categorized as serious dosing errors. Nephrotoxicity was defined as new onset dialysis within 14 days of aminoglycoside initiation or an increase in serum creatinine (Scr) >50% or 0.5mg/dl from baseline, defined as the Scr measured on the day of aminoglycoside initiation. The peak Scr used to classify nephrotoxicity was the highest value drawn during or up to 14 days after aminoglycoside therapy. Trough drug levels were included only if measured ± 2 hours from the end of the dosing interval, but prior to the next dose. Peak drug levels were included if measured between 0.5 and 1.5 hours following a drug administration. Peak and trough drug levels measured outside of these time windows, as recorded by a bar-coded medication administration system, were not included in the analysis. The goal ranges for amikacin troughs were <2mcg/ml for EID and <10mcg/ml for TD. The goal ranges for tobramycin troughs were <0.5mcg/ml for EID and <2mcg/ml for TD. Peaks were only evaluated for TD, and the goal was indication dependant. Goal peaks for urinary tract infections were 4–6mcg/ml for tobramycin and 15–20mcg/ml for amikacin. Goal peaks for pneumonia or CNS infections were 8–10mcg/ml for tobramycin and 25–35mcg/ml for amikacin. For all other infections, the goal peaks were 6–8mcg/ml for tobramycin and 20–30mcg/ml for amikacin.
Statistical Analysis
Based on a preliminary pilot, we anticipated 40% or fewer initial orders for amikacin or tobramycin would be within 10% of the recommended dose. We assumed the advisor would double the rate of appropriate initial prescriptions and calculated 50 patient records would need to be reviewed before and after implementation of the study intervention for each drug to achieve 90% power. All proportions in the pilot and final analysis were compared using Fisher’s exact test with an a priori level of significance of 0.05.
Results
Ninety-seven amikacin patients and one hundred and nineteen tobramycin patients were randomly selected and included in the final analysis. Baseline characteristics between the two groups were similar (Table I). The majority of the patients included were male, and the most common indication for therapy was empiric ventilator or healthcare-associated pneumonia (VAP/HCAP), as defined by the Infectious Disease Society of America.[14] We excluded 42 patients receiving amikacin and 38 patients receiving tobramycin who met exclusion criteria. Initial Dosing and Interval
Table I.
Characteristics of Subjects and Prescribed Aminoglycoside Regimens
| Pre-Advisor Amikacin (n=51) |
Post-Advisor Amikacin (n=46) |
Pre-Advisor Tobra (n=67) |
Post-Advisor Tobra (n=52) |
Combined Pre-Advisor (n=118) |
Combined Post-Advisor (n=98) |
|
|---|---|---|---|---|---|---|
| Age Mean±SD | 49±16 | 53±17 | 55±18 | 51±17 | 53±17 | 52±17 |
| Male n(%) | 37 (72.5%) | 39 (84.8%) | 49 (73 %) | 41 (79%) | 86 (73%) | 80 (82%) |
| Indication n (%) | ||||||
| VAP/HCAP | 35 (69%) | 25 (54%) | 66 (98.5%) | 48 (92%) | 101 (86%) | 73 (74.5%) |
| Sepsis | 11 (22%) | 14 (30%) | 1 (1.5%) | 3 (6%) | 12 (10%) | 17 (17.5%) |
| Other | 5 (9%) | 7 (16%) | 0 (0%) | 1 (2%) | 5 (4%) | 8 (8%) |
| Median Duration of Therapy Days[IQR] | 3 [2,4] | 3.5 [3,6.75] | 3 [2,4] | 3 [2.75,4] | 3 [2,4] | 3 [3,5.25] |
| EID n(%) | 19 (37%) | 30 (65%) | 50 (75%) | 36 (69%) | 69 (58%) | 66 (67%) |
| TD n(%) | 32 (63%) | 16 (35%) | 17 (25%) | 16 (31%) | 49 (42%) | 32 (33%) |
Tobra: Tobramycin
EID: Extended Interval Dosing
TD: Traditional Dosing
VAP: Ventilator Acquired Pneumonia
HAP: Healthcare-associated pneumonia
IQR: 25% and 75% Inter-quartile range
The primary outcome, initial amikacin and tobramycin doses within 10% of recommended dose, increased from 40% in the pre-advisor arm to 80% in the post-advisor arm (p<0.001) (Table II). This increase was significant for both amikacin (43% vs 76%, p=0.002) and tobramycin (37% vs 83%, p<0.001) treated patients. The advisor reduced the risk of having an initial dose greater or less than 10% of the recommended dose by 65% (RR 0.35, 95% CI: 0.22–0.54). The number needed to treat to prevent one erroneous dose was 3 patients (95% CI 2–4).
Table II.
Adherence to Reference Standard for EID and TD Aminoglycoside Orders
| Amikacin | Tobramycin | Combined | |||||||
|---|---|---|---|---|---|---|---|---|---|
| pre-advisor (n=51) |
post-advisor (n=46) |
p | pre-advisor (n=67) |
post-advisor (n=52) |
P | pre-advisor (n=118) |
post-advisor (n=98) |
p | |
| Doses within 10% of Recommended* n(%) | 22 (43%) | 35 (76%) | 0.002 | 25 (37%) | 43 (83%) | <0.001 | 47 (40%) | 78 (80%) | <0.001 |
| EID: n(%) | 12 (55%) | 27 (77%) | 20 (80%) | 33 (77%) | 32 (68%) | 60 (77%) | |||
| TD: n(%) | 10 (45%) | 8 (23%) | 5 (20%) | 10 (23%) | 15 (32%) | 18 (23%) | |||
| Doses>110% of Recommended n(%) | 16 (31%) | 3 (6.5%) | 15 (22%) | 1 (2%) | 31 (26%) | 4 (4%) | |||
| Doses<90% of Recommended n(%) | 13 (25%) | 8 (17%) | 27 (40%) | 8 (15%) | 40 (34%) | 16 (16%) | |||
| Doses > ±25% of Recommended n(%) | 13 (25%) | 5 (11%) | 0.073 | 15 (22%) | 1 (2%) | 0.0009 | 28 (24%) | 6 (6%) | 0.006 |
| Doses>125% of Recommended n(%) | 7 (14%) | 2 (4%) | 2 (3%) | 0 (0%) | 9 (7.5%) | 2 (2%) | |||
| Doses<75% of Recommended n(%) | 6 (12%) | 3 (6.5%) | 13 (20%) | 1 (2%) | 19 (16%) | 4 (4%) | |||
| Interval > Recommended n(%) | 19 (37%) | 2 (4%) | 3 (4%) | 0 (0%) | 22 (18%) | 2 (2%) | |||
| Interval < Recommended n(%) | 5 (10%) | 4 (9%) | 17 (25%) | 4 (8%) | 22 (18%) | 8 (8%) | |||
Recommended doses were calculated using the same equations the advisor employs
CDS: Clinical Decision Support
EID: Extended Interval Dosing
TD: Traditional Dosing
DBW: Dosing Body Weight
The percentage of correct dosing intervals based on estimated renal function increased from pre-advisor to post-advisor for tobramycin and amikacin combined (63% vs 90%, p<0.001), amikacin alone (53% vs 87%, p=0.004), and tobramycin alone (70% vs 92%, p=0.005). Overall, the proportion of ordered regimens which matched the recommended regimen for both dose and interval increased from 31% pre-advisor to 76% post-advisor (p<0.0001).
Therapeutic Drug Monitoring and Nephrotoxicity
The rate of peak drug level measurement increased from 18% pre-advisor to 47% post-advisor when combining both amikacin and tobramycin TD orders (Table III), but the increase in measurement did not result in a statistical difference in peaks in goal range. Post- advisor patients had a higher percentage of trough concentrations in the defined goal ranges (59% vs 89%, p=0.004) for the combined group. Most of the improvement in target trough concentrations occurred in patients receiving tobramycin. Nephrotoxicity was not statistically different between the two groups respectively (25% vs. 17%, p=0.2), but the point estimate was lower following advisor implementation.
Table III.
Serum Drug Concentration Outcomes
| Amikacin | Tobramycin | Combined | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-advisor (n=51) |
Post-advisor (n=46) |
p | Pre-advisor (n=67) |
Post-advisor (n=52) |
p | Pre-advisor (n=118) |
Post-advisor (n=98) |
p | |
| Total Peaks: n(% of TD) | 8 (24%) | 7 (44%) | 1 (6%) | 8 (50%) | 9 (18%) | 15 (47%) | |||
| Peaks in Goal Range* n(%) | 5 (63%) | 5 (71%) | 1.0 | 0 (0%) | 4 (50%) | 1.0 | 5 (55%) | 9 (60%) | 0.78 |
| Total Troughs: n(%) | 15 (29%) | 30 (65%) | 29 (43%) | 34 (65%) | 44 (37%) | 64 (65%) | |||
| Troughs in goal range n(%) | 15 (100%) | 29 (97%) | 1.0 | 11 (38%) | 28 (82%) | 0.006 | 26 (59%) | 57 (89%) | 0.0004 |
| Elevated troughs – TD n(%) | 0 | 0 | 3 (10%) | 0 | 3 (2.5%) | 0 (0%) | |||
| Elevated troughs – EID n(%) | 0 | 1 (4%) | 15 (52%) | 6 (18%) | 15 (13%) | 7 (7%) | |||
EID: Extended Interval Dosing
TD: Traditional Dosing
Goal peak ranges are defined by indication for tobramycin and amikacin in the Study Design section
Provider-Advisor Interaction
Of the 11 incorrect post-advisor orders for amikacin, 9 were less than 90% of the recommended dose because the physician chose a subtherapeutic dosing concentration for VAP (Figure II). The remaining 2 were >110% of the recommended dose because a nurse, entering the order for a physician, manually changed the dose through the advisor. Of the 9 incorrect doses for post- advisor tobramycin, 4 resulted from choosing the incorrect dosing concentration for VAP, and another 4 resulted from a physician manually changing the dose recommended by the advisor. One incorrect dose was due to a pharmacist using IBW instead of the DBW to calculate the dose. In summary, 65% of doses outside the 10% window were caused by improper goal peak selection.
Discussion
The introduction of a comprehensive clinical decision support system for the initial prescription of aminoglycoside antibiotics was associated with a doubling in the rate of appropriate dosing and intervals and improved the rate that target trough levels were achieved. The initial dosing regimens improved even in a setting where aminoglycoside prescribing is closely monitored by clinical pharmacists. The advantage of CPOE-based decision support is that it can prevent inappropriate doses being ordered and administered before the regimen can be optimized by a clinical pharmacy service.
A 2006 national survey of 1,125 hospitals reported aminoglycosides were the most frequent drug class to be directly managed by pharmacists (64% of hospitals surveyed). [20] For hospitals that employ a “pharmacy to dose” protocol, our results indicate that a computerized advisor could complement clinical pharmacy services, which could free clinical pharmacists to utilize their time on more complex aminoglycoside consults. Vincent and colleagues in a 2009 study, found that the median time for pharmacists to complete a pharmacist-to-dose order for aminoglycosides with an automated pharmacist alert system was 29 minutes.[9] Much of this time is spent collecting information that can be provided at the time of ordering by a laboratory system linked to CPOE. According to the pharmacists on the therapeutic drug monitoring surveillance team, the implementation of the aminoglycoside advisor reduced the amount of time spent adjusting initial aminoglycoside regimens. Although we were unable to quantify this change in resource use, we can estimate 30 minutes per order based on previously published data. [9]
The improvement in aminoglycoside ordering is particularly significant because in the pre-advisor arm, 59% of amikacin orders and 41% of tobramycin orders were ordered for less than 90% of the recommended dose or with too long an interval. Since 86% of orders were for pneumonia, many aminoglycoside regimens prior to implementation of the advisor were likely delivering subtherapeutic serum and intrapulmonary concentrations.[5,21–24] The advisor significantly improved the percentage of orders with the correct interval based on the patient’s estimated renal function. Prescribing the correct interval is vital since too long an interval exposes the bacteria to subtherapeutic concentrations for longer than the post-antibiotic effect. Conversely, dosing too often leads to elevated trough concentrations and increased risk of nephrotoxicity.
While previous CDS trials have evaluated the effect on other high risk medications, [18,25–27] our study builds upon the limited published literature showing improvement specifically in aminoglycoside dosing with implementation of clinical decision support. The initial studies using computer-aided dosing of aminoglycosides involved administering a loading dose, drawing serial serum levels, and manually entering these values into a computer system that would recommend a subsequent regimen.[15,28] While these trials showed improvements in aminoglycoside dosing, they only evaluated traditional dosing, made no differentiation of goal levels based on indication, were labor intensive, and could not realistically be performed around-the-clock, limiting their application to when pharmacists were present. More recently, a trial of CPOE based CDS for gentamicin dosing in neonates reported an anecdotal improvement in dosing, however no statistical significance was reported.[29] The CDS supplied the provider with the infant’s weight and calculated a dose of 5mg/kg for all patients. No gentamicin levels were reported to verify that CDS dosing resulted in better pharmacokinetic outcomes. Lastly, in 2006, a trial studying TD gentamicin CDS reported an anecdotal improvement in troughs <2mcg/ml and peaks >4mcg/ml compared to a historical control, although statistical significance was not reported. [30]
Our trial is the first to study the impact of CDS on EID, which is preferred over TD because it optimizes the aminoglycoside’s concentration-dependent actions while being associated with less nephrotoxicity.[4] Our study is an important step in aminoglycoside CDS literature because it is the first to: interface multiple electronic healthcare databases, show statistically significant differences in dosing, interval, and troughs, and provide complete aminoglycoside assistance around-the-clock for both TD and EID initial dosing.
In contrast to the other reports of aminoglycoside CDS systems, [15,28,30] we found no difference in the percent of peaks at goal between pre-advisor and post-advisor dosing. The lack of difference between groups has several explanations. First, only 33% of our post-advisor orders were TD, limiting the number of regimens to evaluate. Secondly, we had very few peaks to assess because of the short duration of therapy and number of peaks drawn outside of our time window for evaluation, which is a limitation of our trial. Of the peaks evaluated in Table III, all were from regimens to treat pneumonia, except for one post-advisor amikacin regimen for a urinary tract infection. Although we were unable to prove a difference in peaks at goal, we feel confident the TD advisor, using the Sawchuk-Zaske method[13] (Figure IIIa) which has established its ability to provide predictable peaks[31], did provide efficacious peaks because it accounted for indication when calculating a dose (Figure II). Our study did find a statistically significant increase in the percent of troughs in our goal range for tobramycin, but not for amikacin. Amikacin orders prior to the implementation of the advisor were frequently underdosed (59%), and serum trough levels were correspondingly low, but it is likely that peak levels (if they were consistently measured) would be subtherapeutic.
Despite our lower troughs in the post- advisor arm, we did not see a statistically significant difference in nephrotoxicity. One explanation may be that aminoglycoside nephrotoxicity is independently associated with duration of therapy [4], and our median duration of therapy was only 3 days due to de-escalation of antibiotic coverage based on the timing of final culture and sensitivity results.
Several limitations to the advisor and study design became apparent during the evaluation. As previously stated, we were unable to show a difference in peaks in the goal ranges. One cause of low post-advisor peaks was improper goal peak selection by the provider. Our TD advisor defaulted to a moderate goal range for the peak drug level which corresponded to the goal range for most indications in our hospital (Figure II). However, 65% of dose errors within aminoglycoside regimens ordered via the advisor occurred because the provider did not alter the default goal peak range to match the actual indication for therapy. Following the study, the advisor was re-programmed to avoid defaulting to a goal range. Secondly, a blinded, consensus assessment of study outcomes was not completed during the study. However, the study pharmacist applied objective, pre-specified criteria to define outcomes, and all recommended dose outcomes were calculated with standard equations. [4,7,13–16] Finally, there was insufficient power to adequately study whether the use of the advisor improved patient outcomes, although the limited data suggested the rate of nephrotoxicity improved.
Conclusion
In conclusion, CDS embedded into a CPOE system significantly improves initial aminoglycoside doses, intervals, and trough concentrations at goal compared to standard provider dosing. This approach provides optimized initial dosing 24 hours a day, seven days a week, which may be helpful in streamlining workload for pharmacy based therapeutic drug monitoring programs. In light of the literature defining the importance of adequate aminoglycoside concentrations, clinical decision support is a valuable approach to creating individualized regimens at the time of ordering.
Acknowledgments
The authors were funded in part by National Library of Medicine R01 LM009965-02 (LRW, JFP). No authors have any financial or commercial conflicts of interest to report. This research has been presented at the Southeastern Residency Conference in Athens, GA on April 29, 2009. The authors thank Matt Marshall, PharmD, for his input and assistance with statistical calculations. We also thank Ira Phillips for his contribution in developing the gentamicin decision support dosing advisor.
References
- 1.Kuperman GJ, Bobb A, Payne TH, Avery AJ, et al. Medication-related clinical decision support in computerized provider order entry systems: A review. J Am Med Inform Assoc. 2007;14:29–40. doi: 10.1197/jamia.M2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA. 1995;274:29–34. [PubMed] [Google Scholar]
- 3.Bobb A, Gleason K, Husch M, Feinglass J, Yarnold PF, Noskin GA. Epidemiology of prescribing errors: potential impact of computerized prescriber order entry. Arch Intern Med. 2004;164:785–792. doi: 10.1001/archinte.164.7.785. [DOI] [PubMed] [Google Scholar]
- 4.Nicolau DP, Collin DF, Belliveau PP, Nightingale CH, Ross JW, Quintiliani R. Experience with a once-daily aminoglycoside program administered to 2,184 adult patients. Antimicrob Agents Chemother. 1995;39:650–655. doi: 10.1128/AAC.39.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carcas AJ, Garcia-Satue JL, Zapater P, Frias-Iniesta J. Tobramycin penetration into epithelial lining fluid of patients with pneumonia. Clin Pharmacol Ther. 1999;65:245–250. doi: 10.1016/S0009-9236(99)70103-7. [DOI] [PubMed] [Google Scholar]
- 6.Rybak MJ, Abate BJ, Kang SL, Ruffing MJ, Lerner SA, Drusano GL. Prospective evaluation of the effect of an aminoglycoside dosing regimen on rates of observed nephrotoxicity and ototoxicity. Antimicrob Agents Chemother. 1999;43:1549–1555. doi: 10.1128/aac.43.7.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drusano GL, Ambrose PG, Bhavnani SM, Bertino JS, Nafziger AN, Louie A. Back to the future: Using aminoglycosides again and how to dose them optimally. Clin Infect Dis. 2007;45:753–760. doi: 10.1086/520991. [DOI] [PubMed] [Google Scholar]
- 8.Burton ME, Ash CL, Hill DP, Jr, Handy T, Shepherd MD, Vasko MR. A controlled trial of computerized Bayesian aminoglycoside administration. Clin Pharmacol Ther. 1991;49:685–694. doi: 10.1038/clpt.1991.86. [DOI] [PubMed] [Google Scholar]
- 9.Vincent WR, Martin CA, Winstead PS, Smith KM, Gatz J, Lewis DA. Effects of a pharmacist-to-dose computerized request on promptness of antimicrobial therapy. J Am Med Inform Assoc. 2009;16:47–53. doi: 10.1197/jamia.M2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koppel R, Metlay JP, Cohen A, et al. Role of computerized physician order entry systems in facilitating medication errors. JAMA. 2005;293:1197–1203. doi: 10.1001/jama.293.10.1197. [DOI] [PubMed] [Google Scholar]
- 11.Eslami S, Abu-Hanna A, de Keizer NF, de Jonge E. Errors associated with applying decision support by suggesting default doses for aminoglycosides. Drug Saf. 2006;29:803–809. doi: 10.2165/00002018-200629090-00004. [DOI] [PubMed] [Google Scholar]
- 12.Chaffee BW, Zimmerman CR. Developing and implementing clinical decision support for use in a computerized prescriber-order-entry system. Am J Health-Syst Pharm. 2010;67:391–400. doi: 10.2146/ajhp090153. [DOI] [PubMed] [Google Scholar]
- 13.Sawchuk RJ, Zaske DE. Pharmacokinetics of dosing regimens which utilize multiple intravenous infusions: gentamicin in burn patients. J Pharmacokinet Biopharm. 1976;4:183–195. doi: 10.1007/BF01086153. [DOI] [PubMed] [Google Scholar]
- 14.American Thoracic Society and Infectious Disease Society of America. Guidelines for the management of adults with hospital-acquire, ventilator-associated, or healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 15.Begg EJ, Atkinson HC, Jeffery GM, Taylor NW. Individualized aminoglycoside dosage based on pharmacokinetic analysis is superior to dosage based on physician intuition at achieving target plasma drug concentrations. Br J Clin Pharmacol. 1989;28:137–141. doi: 10.1111/j.1365-2125.1989.tb05405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olson KM, Rudis MI, Rebuck JA, et al. Effect of once-daily dosing vs multiple daily dosing of tobramycin on enzyme markers of nephrotoxicity. Crit Care Med. 2004;32:1678–1682. doi: 10.1097/01.ccm.0000134832.11144.cb. [DOI] [PubMed] [Google Scholar]
- 17.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 18.McCluggage L, Lee K, Potter T, Dugger R, Pakyz A. Implementation and evaluation of Vancomycin nomogram guidelines in a computerized prescriber-order-entry system. Am J Health-Syst Pharm. 2010;67:70–5. doi: 10.2146/ajhp080625. [DOI] [PubMed] [Google Scholar]
- 19.Lenert LA, Klostermann H, Coleman RW, Lurie J, Blaschke TF. Practical computer-assisted dosing for aminoglycoside antibiotics. Antimicrob Agents Chemother. 1992;36:1230–1235. doi: 10.1128/aac.36.6.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bond CA, Raehl CL. 2006 national clinical pharmacy services survey: clinical pharmacy services, collaborative drug management, medication errors, and pharmacy technology. Pharmacother. 2008;28:1–13. doi: 10.1592/phco.28.1.1. [DOI] [PubMed] [Google Scholar]
- 21.Moore RD, Smith CR, Lietman PS. Association of Aminoglycoside plasma levels with therapeutic outcome in gram negative pneumonia. Am J Med. 1984;77:657–662. doi: 10.1016/0002-9343(84)90358-9. [DOI] [PubMed] [Google Scholar]
- 22.Noone P, Pattison JR, Garfield DD. The effective use of gentamicin in life-threatening sepsis. BMJ. 1974;50:9–16. [PubMed] [Google Scholar]
- 23.Moore RD, Lietman PS, Smith CR. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimum inhibitory concentration. J Infect Dis. 1987;155:93–9. doi: 10.1093/infdis/155.1.93. [DOI] [PubMed] [Google Scholar]
- 24.Chamto E, Amari EB, Rohner P, Delden CV. Effectiveness of combination antimicrobial therapy for Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother. 2003;47:2756–2764. doi: 10.1128/AAC.47.9.2756-2764.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins CD, Pedersen CA, Schneider PJ, Miller AS, Sierawski SJ, Roux RK. Effect on amphotericin B lipid complex use of a clinical decision support system for computerized prescriber order entry. Am J Health Syst Pharm. 2004;61:1395–9. doi: 10.1093/ajhp/61.13.1395. [DOI] [PubMed] [Google Scholar]
- 26.Walton R, Dovey S, Harvey E, Freemantle N. Computer support for determining drug dose: systematic review and meta-analysis. BMJ. 1999;318:984–990. doi: 10.1136/bmj.318.7189.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaushal R, Shojania KG, Bates DW. Effects of computerized physician order entry and clinical decision support systems on medication safety. Arch Intern Med. 2003;163:1409–1416. doi: 10.1001/archinte.163.12.1409. [DOI] [PubMed] [Google Scholar]
- 28.Hickling K, Begg E, Moore ML. A prospective randomized trial comparing individualized pharmacokinetic dosage prediction for aminoglycosides with prediction based on estimated creatinine clearance in critically ill patients. Intensive Care Med. 1989;15:233–237. doi: 10.1007/BF00271057. [DOI] [PubMed] [Google Scholar]
- 29.Cordero L, Kuehn L, Kumar RR, Mekhjian HS. Impact of computerized physician order entry on clinical practice in a newborn intensive care unit. J Perinatology. 2004;24:88–93. doi: 10.1038/sj.jp.7211000. [DOI] [PubMed] [Google Scholar]
- 30.Chan AF, Wang HY, Leung HW. Incorporation of a Gentamicin dosage calculator into a computerized prescriber-order-entry system. Am J Health-Syst Pharm. 2006;63:1344–1345. doi: 10.2146/ajhp050474. [DOI] [PubMed] [Google Scholar]
- 31.Sawchuk RJ, Zaske DE, Cipolle RJ, Wargin WA, Strate RG. Kinetic model for gentamicin dosing with the use of individual patient parameters. Clin Pharmacol Ther. 1977;21(3):362–369. doi: 10.1002/cpt1977213362. [DOI] [PubMed] [Google Scholar]