Abstract
Purpose
To investigate correlated factors on final visual acuity in conjunction with fluorescein angiography (FA) and optical coherence tomography (OCT) findings of chronic central serous chorioretinopathy (CSCR).
Methods
Twenty-four patients (36 eyes) with typical findings of chronic CSCR based on medical records, FA and OCT results were enrolled in this study. We investigated demographic findings, initial and final visual acuity (VA), and some typical findings of FA including the type of leakage pattern, the existence of a gravitational tract and an abnormal hyperfluorescent area centered on the fovea. We also investigated OCT findings to examine serous retinal detachment, outer photoreceptor layer (OPRL) preservation, continuity of the inner segment (IS) and the outer segment (OS) of the photoreceptor layer in case of macular attachment, and other typical findings. The converted logarithm of the minimum angle of resolution VA was used to investigate the statistical correlation with these FA and OCT findings.
Results
An abnormal hyperfluorescent area within 1 macular photocoagulation study disc area on FA and cystoid degeneration on OCT were correlated with poor final VA of less than 20 / 40. However, the preserved OPRL and the continuity of IS / OS junction were correlated with a good final VA of 0.5 or more.
Conclusions
These specific findings could be associated with recurrent or persistent subretinal fluid and could be important parameters of decision for treatment.
Keywords: Chronic central serous chorioretinopathy, Fluorescein angiographic findings, Optical coherence tomography findings
Central serous chorioretinopathy (CSCR) is characterized by serous retinal detachment (RD) with focal leakage at the level of the retinal pigment epithelium (RPE) [1,2]. Most of these cases resolve within four to six months, and a good final visual acuity of more than 20 / 40 is observed in 90% of cases, although color vision and contrast sensitivity are often disturbed [3,4]. CSCR shows bilateral involvement in 30%, recurrence in 30% to 50% and severe visual loss in 5% of patients due to its chronic course [5-9].
Chronic CSCR, which is also known as diffuse retinal pigment epitheliopathy, is generally characterized by multifocal, irregularly distributed and often widespread RPE changes associated with varying degrees of low-grade leakage. This disease is often bilateral and occurs more frequently in elderly patients. Chronic CSCR is the most severe form and involves persistent or recurrent serous retinal detachment, RPE detachment, macular pigmentary change, gravitational tract, telangiectatic change of a retinal capillary, capillary nonperfusion, subretinal fibrosis, neuroretinal degeneration, and secondary choroidal neovascularization [10-13].
CSCR can be diagnosed through biomicroscopic examination, fluorescein angiography (FA), optical coherence tomography (OCT), and indocyanine angiographic findings. These diagnostic tools supply reciprocal information and have helped further the understanding of the pathophysiology of this disease and its treatment decisions [11,14-21].
Some reports exist regarding the factors that influence the final visual outcome of CSCR [3-5,12,18,22], but few reports have been published on chronic CSCR, especially in conjunction with simultaneous findings of FA and OCT. The purpose of this study was to investigate correlated factors on final visual acuity in conjunction with the clinical, FA and OCT findings of chronic CSCR.
Materials and Methods
We enrolled 36 eyes of 24 patients with typical findings of chronic, recurrent CSCR among 121 patients who were diagnosed with CSCR based on medical records, FA and OCT from 2005 to 2007 at the Casey Eye Institute, Oregon Health and Science University.
The subgroups were defined as follows: Chronic CSCR included findings of multifocal, irregularly distributed or widespread RPE change with varying degrees of low-grade leakage on FA and an apparent history of recurrence or persistent symptoms of more than six months [23,24]. Chronic active CSCR (CACSCR) was defined as findings with apparent neurosensory RD, and chronic nonactive CSCR (CNACSCR) or sequelae of CSCR exhibited findings with no neurosensory RD. Recurrent CSCR included findings with no severe RPE change on FA compared with chronic CSCR and a leaking pattern consistent with acute CSCR but with an apparent history of recurrence (Fig. 1).
Fig. 1.
Subgroup of chronic central serous chorioretinopathy (CSCR). (A) Chronic active CSCR, (B) chronic non-active CSCR, and (C) recurrent CSCR, respectively.
We investigated demographic findings such as age, race, sex, time of first diagnosis of CSCR, initial and final visual acuity (VA), and changes of clinical findings throughout the period of this study.
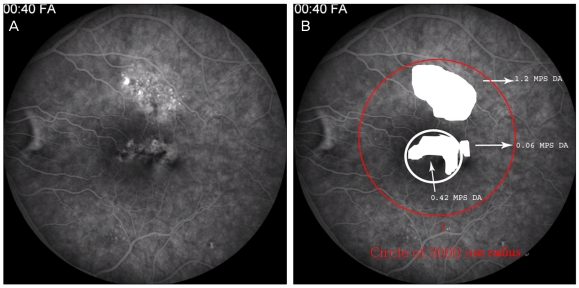
We used the Zeiss FF3 (Carl Zeiss Meditec, Dublin, CA, USA) for FA and examined the central 30 degrees and the peripheral field when necessary. We investigated the type of leakage pattern and the existence of a gravitational tract and measured the area of abnormal hyperfluorescence, which included any window defect, i.e., staining associated with low-grade leaking, except a hyperfluorescent area associated with focal leakage or RPE detachment. We also checked the foveal involvement and measured the hyperfluorescent area within the 1 Macular Photocoagulation Study Disc Area (MPS DA) and a circle with a 3,000-µm radius centered on the fovea (Fig. 2). We used ANKA-ProView software (Topcon Medical Systems, Paramus, NJ, USA), which allows the calculation of the area using pixel-size data based on image sensor specifications and the magnification of the camera system used to measure the hyperfluorescent area. The scale of the area was calculated with MPS DA.
Fig. 2.
Quantification of an abnormal hyperfluorescent lesion within the 1 Macular Photocoagulation Study Disc Area (MPS DA) and circle with a radius of 3,000 µm centered on the fovea using image analysis software.
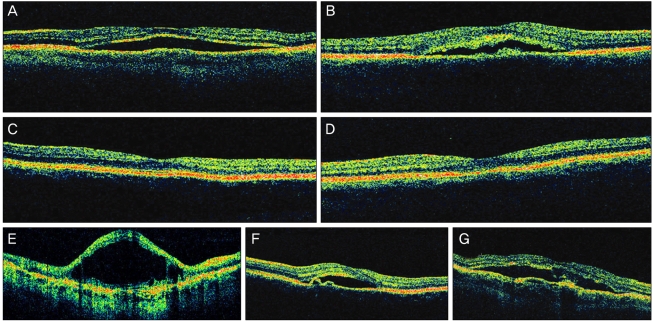
We performed OCT (Stratus OCT, Carl Zeiss Meditec) to examine serous RD or RPE detachment, outer photoreceptor layer (OPRL) preservation in case of serous RD, and continuity of the inner segment (IS) and outer segment (OS) of the photoreceptor layer in case of macular attachment. We also investigated the macular thickness, the change associated with the RPE-choroid band, and the change in the neurosensory layer (Fig. 3). We converted the final Snellen VA to logarithm of the minimum angle of resolution (logMAR) VA to investigate the statistical correlation with FA, and OCT findings of the last follow-up visit.
Fig. 3.
Factors for investigation in optical coherence tomography findings. From (A) to (G) represents serous retinal detachment, intact outer photoreceptor layer with bulging on the retinal pigment cell (RPE) layer, intact and disrupted outer segment, inner segment junction of the photoreceptor, cystoid degeneration, RPE detachment, irregularity of the RPE and choroidal band (undulation or thickness abnormality), respectively.
Statistical analysis
We used univariate and multivariate analysis with the mixed effect method for correlation of VA of less than 20 / 40 and FA factors including foveal involvement, area of abnormal hyperfluorescence within 1 MPS DA and a circle with a 3,000-µm radius centered on the fovea, existence of a gravitational tract, leakage pattern and central macula thickness by OCT. We also used Fisher's exact test to analyze the correlation of the final VA and OCT factors including existence of serous RD and PED, preservation of OPRL in serous RD, IS / OS continuity in macular attachment, abnormality of an RPE-choroid band, and existence of cystoid degeneration. Statistical significance was interpreted as p < 0.05.
Results
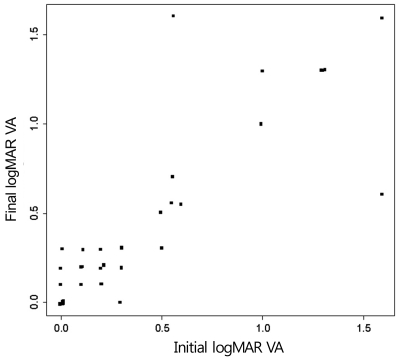
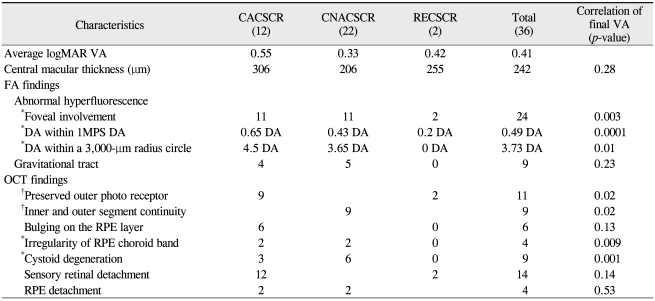
On initial examination, 15 eyes showed CACSCR; 22 of 24 patients were male (Table 1). The period of CSCR history ranged from 10 months to 20 years. The initial average logMAR VA was 0.4, and 23 out of the 36 eyes showed a Snellen VA of 20 / 40 or more; 7 eyes were 20 / 200 or less. The final average logMAR was 0.41 and showed no statistical change compared to the initial VA. At final examination, 24 of the 36 eyes showed a VA of 20 / 40 or more, and 7 eyes were 20 / 200 or less. The average follow-up period was five months, and five eyes showed a decreased VA of more than 0.2 by logMAR VA, while three eyes showed an increased VA of more than 0.2 by logMAR VA (Fig. 4). During the follow-up period, these changes were related with a new serous RD or a change of volume of serous fluid in all but two cases, one of which developed choroidal neovascularization (CNV) and the other recovered from the unexplained visual loss.
Table 1.
Initial demographic findings
CACSCR = chronic active central serous chorioretinopathy; CNACSCR = chronic nonactive central serous chorioretinopathy; RECSCR = recurrent central serous chorioretinopathy.
Fig. 4.
Relationship of initial logarithm of the minimum angle of resolution (logMAR) visual acuity (VA) and final logMAR VA.
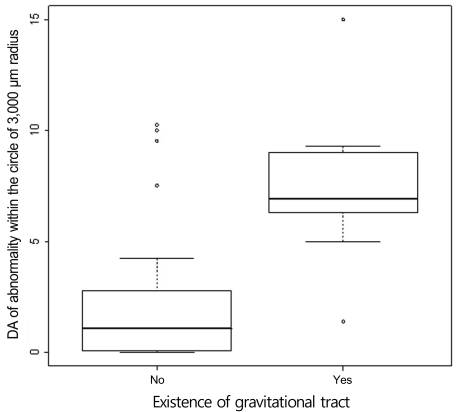
Focal-point leakage on FA occurred in 7 patients; 4 were initially diagnosed with recurrent CSCR (RECSCR) and three as CACSCR. The other 13 initially diagnosed CACSCR patients showed no apparent leaking points on FA but neurosensory RD on OCT; this leakage pattern was not statistically correlated with the final VA of less than 20 / 40 (p > 0.05). Nine patients showed a gravitational tract in the inferior retina and a positive correlation with the hyperfluorescence area in the circle of a 3,000-µm radius centered on the fovea (p < 0.05) (Fig. 5) but no correlation with the final VA of less than 20 / 40 (p > 0.05). Twenty-two eyes initially showed abnormal hyperfluorescence in the fovea, but 24 eyes showed final involvement. At the final examinations, the abnormal hyperfluorescent area on FA was 0.65 DA and 4.5 DA within 1 MPS DA and the circle of 3,000-µm radius, respectively, and differed according to the subgroup (Table 2). These three factors of foveal involvement, an abnormal hyperfluorescent area within 1 MPS DA and a circle with a 3,000-µm radius showed statistically significant correlations with a final VA of less than 20 / 40 in univariate analysis (p < 0.01). However, an abnormal hyperfluorescent area within 1 MPS DA centered on the fovea was the dominant factor having a statistically significant correlation with the final VA in multivariate analysis (p < 0.01) (Fig. 6).
Fig. 5.
The existence of a gravitational tract was compared with the area of abnormal hyperfluorescence within a circle with a 3,000-µm radius centered on the fovea using Wilcoxon's rank sum test. These two variables were statistically correlated (p < 0.05). DA = disc area.
Table 2.
Final clinical findings
CACSCR = chronic active central serous chorioretinopathy; CNACSCR = chronic nonactive central serous chorioretinopathy; RECSCR = recurrent central serous chorioretinopathy; VA = visual acuity; logMAR = logarithm of the minimum angle of resolution; FA = fluorescein angiography; DA = disc area; MPS = Macular Photocoagulation Study; OCT = optical coherence tomography; RPE = retinal pigment epithelium.
*Statistically significant correlation of final VA of less than 20 / 40; †Statistically significant correlation of final VA of 20 / 40 or more.
Fig. 6.
Area of abnormal hyperfluorescence within 1 Macular Photocoagulation Study Disc Area (MPS DA) centered on the fovea was highly correlated with final logarithm of the minimum angle of resolution (logMAR) visual acuity (VA) of more than 0.3 (equivalent to Snellen VA of less than 20 / 40) (p < 0.01).
Initially, 20 eyes showed serous RD on OCT; 8 of these eyes had extramacular involvement and 3 eyes of the remaining 12 eyes showed subtle serous fluid (SRF). During the follow-up period, 3 of 5 eyes with RECSCR and 4 of 15 eyes with CACSCR showed a disappearance of serous fluid, which indicated an increased tendency for persistent SRF in CACSCR; however, this finding was inconclusive due to different follow-up periods. One patient showed new SRF at the final visit. We were not sure whether this case of SRF was recurrent or persistent because this finding could have changed during the follow-up period. The average macular thickness in CNACSCR was 206 µm at the final examination, but there were few extremely atrophic cases. One case showed 126 um central macular thickness (thinnest among all cases), and this patient had a diffuse hyperfluorescent area in the post pole (6.9 MPS DA) with suspected subretinal fibrosis and a VA of 20 / 400. However, final central macular thickness was not statistically correlated with final VA.
The signs associated with an RPE-choroid band were small bulging on the band in six eyes and an irregular change of thickness with undulation in four eyes at final examination (Fig. 2E and 2G). The latter findings were usually combined with CNV and cystoid degeneration or subretinal fibrotic changes and were statistically correlated with a final VA of less than 20 / 40 (p < 0.05).
OPRL was seen in 17 of 20 eyes initially and in 11 of 14 eyes finally (Table 2). Initially, most of the eyes showed an even profile appearance, but a granulated change was observed in 2 eyes during the follow-up period (Fig. 2B). In contrast to the author's expectations, no eyes showed any distinctive atrophic changes of OPRL in the state of neurosensory RD, and 2 of the 3 eyes that showed no distinctive OPRL had severe cystoid degeneration. The preserved OPRL was correlated with the final VA of 0.5 or more (p < 0.05).
The continuity of IS / OS on OCT was observed in 7 of 16 eyes in CNACSCR initially and in 9 of 22 eyes finally. Among 7 eyes with initial serous RD in which SRF disappeared during the follow-up period, only 2 eyes showed the continuity of IS / OS at the final visit. This visible continuity of IS / OS was statistically correlated with a final VA of 20 / 40 or more (p < 0.05). Five patients among the 7 patients who showed a final VA of 0.1 or less had severe cystoid degeneration on OCT. This sign was highly correlated with a final VA of 20 / 40 or less (p < 0.01) (Table 2).
Discussion
The major differences between this study and previous reports [4,22-24] were the definition of the CSCR classification, follow-up period length and clinical factors evaluated for statistical analysis. We focused on clinical factors that influenced the final VA according to our defined criteria and the initial or final findings in a relatively short follow-up period.
The average age, bilaterality and predominance of male patients in our study were not different from previous epidemic reports [3,14,25-27]. The higher average age in this study compared with that of idiopathic CSCR was expected considering the chronic course of this disease. Five patients among the total 24 patients had more than a 15-year history of CSCR.
Abnormal hyperfluorecence on FA implied a previous episode of this disease, and this finding was observed in 43 to 92% of cases in previous reports. This different rate is due to the varying selection of patients and follow-up period lengths [22-24]. Loo et al. [22] reported that this change was not correlated with the final VA, and Wong et al. [28] determined that this area had a tendency for a negative correlation with the final VA but showed no statistically significant difference. Our study found a statistically significant correlation of the final VA and area of abnormal hyperfluorescence within 1 MPS DA centered on the fovea by FA. This discrepancy could be caused by the fact that the previous report did not use a detailed quantitative method and did not analyze foveal involvement, implying possible photoreceptor cell atrophy [12]. Although we could not conclude how long persistent or recurrent SRF had influenced the amount of abnormal hyperfluorescent areas on FA, we did observe that a relatively short duration of SRF caused abnormal hyperfluorescence on FA.
Inferior gravitational atrophic tract implicates previous severe persistent SRF. In contrast to a previous report that showed a statistical correlation with a poor visual outcome [10], our study showed no correlation with final VA in the absence of combined macular pathologic findings such as CNV or cystoid degeneration.
OCT can supply more information about the diagnosis and prediction of prognosis in CSCR [11,12,16-18]. In particular, OCT can show subtle SRF and cystoid degeneration that cannot be easily found upon biomicroscopic examination, as well as changes of macular thickness. Thus OCT is specifically useful in the diagnosis of chronic CSCR that has subtle SRF and in cases where identification of a leaking area on FA is difficult. In addition, OCT can show small RPE detachment and changes of the RPE-choroid layer and the outer photoreceptor layer [16-18].
Eight eyes with extramacular involvement of SRF were initially identified in this study. This finding can occur in the recovery period but can also be a manifestation of an initial finding of chronic CSCR and may indicate new or persistent SRF despite a lack of symptoms. Bujarborua et al. [29] reported that an asymptomatic eye can have SRF in 22% of patients who had history of CSCR, and Wang et al. [30] reported that persistent SRF of more than four months duration can induce photoreceptor cell atrophy and poor visual outcome even after absorption of SRF. Thus, chronic CSCR patients should be followed more frequently, despite no symptoms.
Loo et al. [22] reported that serous RD and RPE detachment are correlated with a final VA of 0.5 or less. However, our study showed no statistical correlation of VA and RPE detachment, probably because only four patients had RPE detachment. This small number of patients with RPE detachment could be explained by the limitations of six radial scans of OCT. This method can miss the all-RPE detachment unlike en face OCT or spectral domain OCT, which can scan a wide area simultaneously [17]. The present study also showed no correlation of existent serous RD with a final VA of less than 20 / 40. These discrepancies with previous reports could be due to the fact that our sample included many patients with extramacular SRF and subtle SRF in chronic cases [31]. However, preservation of OPRL on OCT in most patients with serous RD could be another explanation. Piccolino et al. [18] reported that patients with a well-preserved outer photoreceptor layer can sustain good VA despite serous RD and will have good visual prognosis after macula attachment. Our study also showed that one of the two factors with a good correlation with final VA of 20 / 40 or more was well-preserved OPRL on OCT.
In contrast to experimental retinal detachment [32], long survival of outer photoreceptor cells in persistent SRF can be explained by the high concentration of oxygen and glucose in the SRF connected with the tissue fluid of choroids [33]. Clinically there have been controversies about the duration and survival of the photoreceptor layer. Wang et al. [30]. reported that persistent SRF for more than four months can induce photoreceptor cell atrophy and decrease the macular thickness, causing poor visual outcome, but Piccolino et al. [18] found that persistent SRF for more than one year can cause photoreceptor cell atrophy and granulated changes. Our study showed granulated change of OPRL in one patient who had persistent SRF for 10 months, but this patient showed a VA of 20 / 20. Periodic examination by OCT is required because symptom duration mainly depends on vague memory recall by the patients.
Our study investigated the correlation of final VA of 20 / 40 or more and the continuity of IS and OS on OCT and showed statistical significance. Although 8 of 15 eyes having a final VA of 20 / 40 or more among the 22 patients with final macula attachment showed continuity of IS / OS, none of the eyes having a final VA of less than 20 / 40 showed continuity. This result can be explained either by the misalignment of outer segments during the recovery process of macular attachment [34,35] or by the relatively low resolution of the Stratus OCT. We did not use spectral domain OCT, which can provide more detailed information about IS and OS junction. In the future, we expect that this OCT can supply more exact information on this relationship.
Among the seven patients with final VA of 0.1 or less, one patient developed CNV, another patient had severe macular atrophy with subretinal fibrosis and the rest had cystoid degeneration, which showed a statistical correlation with VA of less than 20 / 40 and even 20 / 200 or less. The present study found four patients had irregular thickness and undulation of RPE-choroid hyperreflective band. Among these patients, apparent CNV occurred in one patient during the follow-up period, probable occult CNV was seen in another patient and cystoid degeneration was noted in two additional patients. We assumed that these OCT findings in chronic CSCR represented probable poor prognosis, suggesting the risk of CNV development.
Iida et al. [12] reported that seven eyes with a VA of 20 / 200 or less had cystoid degeneration, and this finding was correlated with poor VA. The present study also showed a correlation with poor final VA. Chronic foveal macular detachment, fluid accumulation in the inner retina and outer retina ischemia are the proposed mechanisms of this finding. In contrast to Iida's results that showed a foveal RPE abnormality on FA only in four of seven patients with cystoid degeneration, the present study showed all five patients had foveal involvement of abnormal hyperfluorescence by FA, implicating macular atrophy or RPE. The three remaining patients had a history of CSCR for more than 7 years, and two patients had a history of more than 20 years. There must be more efforts to elucidate the possible cause of cystoid degeneration aside from a long history of CSCR.
Photodynamic therapy has recently been used to treat chronic CSCR. This therapy can be used as an alternative method because conventional laser therapy cannot be applied in most cases of chronic CSCR; furthermore, this method effectively eliminates serous fluid [36-38]. Unfortunately, this therapy also has the drawbacks of possible neural retinal damage and development of choroidal neovascularization [39-41]. The ideal treatment for chronic CSCR should help promote survival of the photoreceptor layer by effective elimination of SRF without any other side effects. Neurotrophic factor [42,43] can be an adjuvant method for treatment of CSCR but requires more research.
In conclusion, preserved OPRL in cases of serous RD on OCT and continuity of IS / OS in case of macular attachment on OCT were correlated with a good final VA of 0.5 or more, and areas of abnormal hyperfluorescence within 1 MPS DA on FA and cystoid degeneration on OCT were correlated with a poor final VA of less than 20 / 40. All of these findings could be associated with recurrent or persistent SRF and could be important parameters of decision for treatment.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Gass JD. Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol. 1967;63(Suppl):1–139. [PubMed] [Google Scholar]
- 2.Schatz H, Madeira D, Johnson RN, McDonald HR. Central serous chorioretinopathy occurring in patients 60 years of age and older. Ophthalmology. 1992;99:63–67. doi: 10.1016/s0161-6420(92)32010-x. [DOI] [PubMed] [Google Scholar]
- 3.Castro-Correia J, Coutinho MF, Rosas V, Maia J. Long-term follow-up of central serous retinopathy in 150 patients. Doc Ophthalmol. 1992;81:379–386. doi: 10.1007/BF00169099. [DOI] [PubMed] [Google Scholar]
- 4.Klein ML, Van Buskirk EM, Friedman E, et al. Experience with nontreatment of central serous choroidopathy. Arch Ophthalmol. 1974;91:247–250. doi: 10.1001/archopht.1974.03900060257001. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert CM, Owens SL, Smith PD, Fine SL. Long-term follow-up of central serous chorioretinopathy. Br J Ophthalmol. 1984;68:815–820. doi: 10.1136/bjo.68.11.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu JG, Friberg TR. Idiopathic central serous retinopathy in China: a report of 600 cases (624 eyes) treated by acupuncture. Ophthalmic Surg. 1987;18:608–611. [PubMed] [Google Scholar]
- 7.Bujarborua D. Long-term follow-up of idiopathic central serous chorioretinopathy without laser. Acta Ophthalmol Scand. 2001;79:417–421. doi: 10.1034/j.1600-0420.2001.079004417.x. [DOI] [PubMed] [Google Scholar]
- 8.Bouzas EA, Karadimas P, Pournaras CJ. Central serous chorioretinopathy and glucocorticoids. Surv Ophthalmol. 2002;47:431–448. doi: 10.1016/s0039-6257(02)00338-7. [DOI] [PubMed] [Google Scholar]
- 9.Lafaut BA, Salati C, Priem H, De Laey JJ. Indocyanine green angiography is of value for the diagnosis of chronic central serous chorioretinopathy in elderly patients. Graefes Arch Clin Exp Ophthalmol. 1998;236:513–521. doi: 10.1007/s004170050114. [DOI] [PubMed] [Google Scholar]
- 10.Yannuzzi LA, Shakin JL, Fisher YL, Altomonte MA. Peripheral retinal detachments and retinal pigment epithelial atrophic tracts secondary to central serous pigment epitheliopathy. Ophthalmology. 1984;91:1554–1572. doi: 10.1016/s0161-6420(84)34117-3. [DOI] [PubMed] [Google Scholar]
- 11.Iida T, Hagimura N, Sato T, Kishi S. Evaluation of central serous chorioretinopathy with optical coherence tomography. Am J Ophthalmol. 2000;129:16–20. doi: 10.1016/s0002-9394(99)00272-x. [DOI] [PubMed] [Google Scholar]
- 12.Iida T, Yannuzzi LA, Spaide RF, et al. Cystoid macular degeneration in chronic central serous chorioretinopathy. Retina. 2003;23:1–7. doi: 10.1097/00006982-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Gomolin JE. Choroidal neovascularization and central serous chorioretinopathy. Can J Ophthalmol. 1989;24:20–23. [PubMed] [Google Scholar]
- 14.Spitznas M, Huke J. Number, shape, and topography of leakage points in acute type I central serous retinopathy. Graefes Arch Clin Exp Ophthalmol. 1987;225:437–440. doi: 10.1007/BF02334172. [DOI] [PubMed] [Google Scholar]
- 15.Yamada K, Hayasaka S, Setogawa T. Fluorescein-angiographic patterns in patients with central serous chorioretinopathy at the initial visit. Ophthalmologica. 1992;205:69–76. doi: 10.1159/000310315. [DOI] [PubMed] [Google Scholar]
- 16.Montero JA, Ruiz-Moreno JM. Optical coherence tomography characterisation of idiopathic central serous chorioretinopathy. Br J Ophthalmol. 2005;89:562–564. doi: 10.1136/bjo.2004.049403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirami Y, Tsujikawa A, Sasahara M, et al. Alterations of retinal pigment epithelium in central serous chorioretinopathy. Clin Experiment Ophthalmol. 2007;35:225–230. doi: 10.1111/j.1442-9071.2006.01447.x. [DOI] [PubMed] [Google Scholar]
- 18.Piccolino FC, de la Longrais RR, Ravera G, et al. The foveal photoreceptor layer and visual acuity loss in central serous chorioretinopathy. Am J Ophthalmol. 2005;139:87–99. doi: 10.1016/j.ajo.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 19.Prunte C. Indocyanine green angiographic findings in central serous chorioretinopathy. Int Ophthalmol. 1995;19:77–82. doi: 10.1007/BF00133176. [DOI] [PubMed] [Google Scholar]
- 20.Prunte C, Flammer J. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol. 1996;121:26–34. doi: 10.1016/s0002-9394(14)70531-8. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi K, Hasegawa Y, Tokoro T. Indocyanine green angiography of central serous chorioretinopathy. Int Ophthalmol. 1986;9:37–41. doi: 10.1007/BF00225936. [DOI] [PubMed] [Google Scholar]
- 22.Loo RH, Scott IU, Flynn HW, Jr, et al. Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina. 2002;22:19–24. doi: 10.1097/00006982-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Yap EY, Robertson DM. The long-term outcome of central serous chorioretinopathy. Arch Ophthalmol. 1996;114:689–692. doi: 10.1001/archopht.1996.01100130681007. [DOI] [PubMed] [Google Scholar]
- 24.Levine R, Brucker AJ, Robinson F, et al. Long-term follow-up of idiopathic central serous chorioretinopathy by fluorescein angiography. Ophthalmology. 1989;96:854–859. doi: 10.1016/s0161-6420(89)32810-7. [DOI] [PubMed] [Google Scholar]
- 25.Spaide RF, Campeas L, Haas A, et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology. 1996;103:2070–2079. doi: 10.1016/s0161-6420(96)30386-2. [DOI] [PubMed] [Google Scholar]
- 26.Gackle HC, Lang GE, Freissler KA, Lang GK. Central serous chorioretinopathy: clinical, fluorescein angiography and demographic aspects. Ophthalmologe. 1998;95:529–533. doi: 10.1007/s003470050310. [DOI] [PubMed] [Google Scholar]
- 27.Wang M, Sander B, la Cour M, Larsen M. Clinical characteristics of subretinal deposits in central serous chorioretinopathy. Acta Ophthalmol Scand. 2005;83:691–696. doi: 10.1111/j.1600-0420.2005.00582.x. [DOI] [PubMed] [Google Scholar]
- 28.Wong R, Chopdar A, Brown M. Five to 15 year follow-up of resolved idiopathic central serous chorioretinopathy. Eye (Lond) 2004;18:262–268. doi: 10.1038/sj.eye.6700637. [DOI] [PubMed] [Google Scholar]
- 29.Bujarborua D, Chatterjee S, Choudhury A, et al. Fluorescein angiographic features of asymptomatic eyes in central serous chorioretinopathy. Retina. 2005;25:422–429. doi: 10.1097/00006982-200506000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Wang MS, Sander B, Larsen M. Retinal atrophy in idiopathic central serous chorioretinopathy. Am J Ophthalmol. 2002;133:787–793. doi: 10.1016/s0002-9394(02)01438-1. [DOI] [PubMed] [Google Scholar]
- 31.Wang M, Munch IC, Hasler PW, et al. Central serous chorioretinopathy. Acta Ophthalmol. 2008;86:126–145. doi: 10.1111/j.1600-0420.2007.00889.x. [DOI] [PubMed] [Google Scholar]
- 32.Erickson PA, Fisher SK, Anderson DH, et al. Retinal detachment in the cat: the outer nuclear and outer plexiform layers. Invest Ophthalmol Vis Sci. 1983;24:927–942. [PubMed] [Google Scholar]
- 33.Bill A, Tornquist P, Alm A. Permeability of the intraocular blood vessels. Trans Ophthalmol Soc U K. 1980;100:332–336. [PubMed] [Google Scholar]
- 34.Anderson DH, Guerin CJ, Erickson PA, et al. Morphological recovery in the reattached retina. Invest Ophthalmol Vis Sci. 1986;27:168–183. [PubMed] [Google Scholar]
- 35.Sakai T, Calderone JB, Lewis GP, et al. Cone photoreceptor recovery after experimental detachment and reattachment: an immunocytochemical, morphological, and electrophysiological study. Invest Ophthalmol Vis Sci. 2003;44:416–425. doi: 10.1167/iovs.02-0633. [DOI] [PubMed] [Google Scholar]
- 36.Taban M, Boyer DS, Thomas EL, Taban M. Chronic central serous chorioretinopathy: photodynamic therapy. Am J Ophthalmol. 2004;137:1073–1080. doi: 10.1016/j.ajo.2004.01.043. [DOI] [PubMed] [Google Scholar]
- 37.Chung SE, Kang JH, Kang SW. Chronic central serous chorioretinopathy: photodynamic therapy. J Korean Ophthalmol Soc. 2007;48:279–284. [Google Scholar]
- 38.Lai TY, Chan WM, Li H, et al. Safety enhanced photodynamic therapy with half dose verteporfin for chronic central serous chorioretinopathy: a short term pilot study. Br J Ophthalmol. 2006;90:869–874. doi: 10.1136/bjo.2006.090282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reinke MH, Canakis C, Husain D, et al. Verteporfin photodynamic therapy retreatment of normal retina and choroid in the cynomolgus monkey. Ophthalmology. 1999;106:1915–1923. doi: 10.1016/S0161-6420(99)90401-3. [DOI] [PubMed] [Google Scholar]
- 40.Peyman GA, Kazi AA, Unal M, et al. Problems with and pitfalls of photodynamic therapy. Ophthalmology. 2000;107:29–35. doi: 10.1016/s0161-6420(99)00012-3. [DOI] [PubMed] [Google Scholar]
- 41.Zacks DN, Ezra E, Terada Y, et al. Verteporfin photodynamic therapy in the rat model of choroidal neovascularization: angiographic and histologic characterization. Invest Ophthalmol Vis Sci. 2002;43:2384–2391. [PubMed] [Google Scholar]
- 42.Paskowitz DM, Nune G, Yasumura D, et al. BDNF reduces the retinal toxicity of verteporfin photodynamic therapy. Invest Ophthalmol Vis Sci. 2004;45:4190–4196. doi: 10.1167/iovs.04-0676. [DOI] [PubMed] [Google Scholar]
- 43.Cayouette M, Behn D, Sendtner M, et al. Intraocular gene transfer of ciliary neurotrophic factor prevents death and increases responsiveness of rod photoreceptors in the retinal degeneration slow mouse. J Neurosci. 1998;18:9282–9293. doi: 10.1523/JNEUROSCI.18-22-09282.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]