Summary
Carcinoma associated fibroblasts (CAFs) that express α-smooth-muscle-actin (αSMA) contribute to cancer progression, but their precise origin and role is unclear. Using mouse models of inflammation-induced gastric cancer, we show that at least 20% of CAFs originate from bone marrow (BM) and derive from mesenchymal stem cells (MSCs). αSMA+ myofibroblasts (MF) are niche cells normally present in BM and increase markedly during cancer progression. MSC-derived CAFs that are recruited to the dysplastic stomach express IL-6, Wnt5α and BMP4, show DNA-hypomethylation, and promote tumor growth. Moreover, CAFs are generated from MSCs and are recruited to the tumor in TGF-β- and SDF-1α-dependent manner. Carcinogenesis therefore involves expansion and relocation of BM-niche cells to the tumor to create a niche to sustain cancer progression.
Keywords: Tumor microenvironment, Stem Cells, Bone Marrow-derived Cells (BMDC), MSC, CAF, MF, Carcinogenesis
Introduction
Tumors consist of cancer cells and diverse stromal cells that constitute the tumor microenvironment and contribute to tumor progression. Stem cells or their immediate progeny can give rise to various tumor-contributing cells through a dysregulated self-renewal process (Wicha et al., 2006), but the origin of many niche cells critical for tumor growth (Li and Neaves, 2006) has not been established. Much of the tumor stroma consists of cancer-associated fibroblasts (CAFs) that express αSMA. CAFs closely resemble myofibroblasts (MF) that are present in the gastrointestinal mucosa. Clinical evidence supports the contribution of stroma to the development of a variety of tumors (Coussens and Werb, 2002). In general, there is a higher incidence of tumor formation in tissues with chronically inflamed stroma, particularly Helicobacter pylori gastritis which is associated with gastric cancer (Houghton and Wang, 2005).
Phenotypically, CAFs resemble normal gastrointestinal MF in that they express αSMA and other MF markers such as vimentin or FSP1; biologically they are different, and it remains puzzling why CAFs would appear only in the setting of cancer but not in the normal adult organs. Tumors that have a desmoplastic stroma, consisting of more stromal cells disrupting the tissue homogeneity, often have a poorer prognosis (Maeshima et al., 2002). CAFs isolated from breast cancer tissue promote proliferation of cancer cell lines, increase angiogenesis to a greater extent, and have a distinct gene expression pattern compared to normal fibroblasts (Allinen et al., 2004). CAFs isolated from prostate cancer direct tumor progression of initiated prostatic epithelium and can transform nontumorigenic human prostatic epithelial cells line into tumorigenic ones (Hayward et al., 2001). CAFs express increased levels of the chemokine SDF-1 (Orimo et al., 2005) and genes such as Gremlin-1 that are not expressed in most normal tissues (Sneddon et al., 2006). Finally, we recently showed that CAFs were more hypomethylated than normal gastric stromal cells (Jiang et al., 2008).
CAFs have altered biology compared to normal MFs and seem to accumulate in tumors, a number of studies have explored the origins of CAFs, which include resident fibroblasts (Orimo et al., 2005), smooth muscle cells, endothelial cells, epithelial cells (through EMT), fibrocytes and BM-derived cells such as MSCs (Direkze et al., 2003; Karnoub et al., 2007). Chronic inflammation associated with increased cancer risk (Forbes et al., 2004) and tumor xenografts (Ishii et al., 2003) recruit BM-derived MF. In gastric tumors of patients that received gender-mismatched BM transplants, many CAFs are bone-marrow derived (Worthley et al., 2009). However, the precise BM cell type that gives rise to CAFs remains unclear.
Several studies have pointed to MSCs as a potential source of CAFs (Guo et al., 2008). MSCs, when mixed with weakly metastatic human breast carcinoma cells, increase the metastatic abilities of cancer cells (Karnoub et al., 2007). MSCs exposed to tumor-conditioned medium assume a CAF-like phenotype, including sustained expression of SDF-1 and the ability to promote tumor cell growth (Mishra et al., 2008). MSCs are defined as pluripotent stem cells that contribute to normal bone, adipose, cartilage and muscle (Pittenger et al., 1999). MSCs originate in the BM but can be found throughout the body; they are often involved in tissue remodeling after injury or chronic inflammation. BM-derived cells are often recruited to carcinogenic sites by cytokines such as IL-1β (Houghton et al., 2004; Tu et al., 2008), and indeed CAFs promote further cell recruitment through secretion of chemokines such as SDF-1 (Orimo et al., 2005). MSCs are among the BM-derived cells that have been shown to be recruited to tumors and to promote their growth. While some studies have suggested that MSCs can differentiate into CAFs, the differentiation of MSC into CAFs or MF has not been demonstrated conclusively (Stappenbeck and Miyoshi, 2009). In this study we aimed to investigate the cellular origin and role of CAFs within the BM and analyzed their function in normal BM and in the tumor microenvironment..
Results
αSMA+ MFs increase with gastric dysplasia and contribute to a desmoplastic tumor microenvironment
To understand the changes that occur in stromal cells during gastric cancer progression, we analyzed αSMA-RFP transgenic mice that express RFP under the direction of the αSMA promoter and collagen-α1-EGFP transgenic mice that express EGFP under the control of the collagen-α1 promoter (Magness et al., 2004). A tissue-specific expression pattern for the 3kb αSMA promoter fragment driven RFP relative to endogenous αSMA expression was confirmed in gastric mucosa (Figure 1D and S1A). Both sets of mice were infected with Helicobacter felis (H. felis), resulting in chronic gastritis, atrophy, metaplasia, and dysplasia (Figure S1b). In uninfected mice, few αSMA-RFP+ MF were present in gastric tissue (Figure 1a, b), whereas collagenα1/EGFP+ stromal cells were abundant (Figure 1a, b). 12 to 18 months after infection, collagenα1/EGFP+ fibroblasts and αSMA-RFP+ fibroblasts increased with chronic inflammation and cancer progression (Figure 1a–c). Although collagen-α1-EGFP+ fibroblasts were abundant at early, they increased only slightly at late stages of neoplasia; αSMA-RFP+ fibroblasts were scarce early after infection, but increased markedly at later stages, particularly during dysplasia, suggesting that αSMA-RFP+ fibroblasts contribute to the neoplastic process. They were defined as MF by their expression of endogenous αSMA and vimentin (data not shown). Similarly, αSMA/collagen-α1 double positive cells increased with dysplasia (Figure S1c, d), especially in invasive or highly dysplastic regions (Figure S1e). Additionally, we crossed the αSMA-RFP mice with IL-1β/αSMA-RFP mice,which express human IL-1β specifically in the stomach and develop spontaneous gastric inflammation and cancer (Tu et al., 2008). The expression of αSMA and RFP increased by 6 and 12 months in IL-1β/αSMA-RFP mice (Figure 1d).
Figure 1. BM-derived αSMA-expressing cells contribute to the tumor microenvironment.
(A) αSMA staining (upper panel) and endogenous αSMA-RFP (middle panel) or endogeneous collgen-α1 (lower panel) expression in stomachs of αSMA-RFP/collagen-α1-GFP double transgenic mice and following 12- or 18-month of H. felis infection.
(B) Relative number of αSMA (red) or collagen (green) expressing cells in the stomach of αSMA-RFP/collagen-α1-GFP double transgenic mice without and with 12- or 18 months of H. felis infection, (*=p<0,05 compared to WT and #=p<0,05 compared to 12 month)
(C) Western blot for αSMA and β-tubulin in gastric tissue of WT, IL-1β transgenic mice, and WT mice with 18 month of H. felis infection
(D) αSMA staining in a dysplastic region in 12-month old IL-1β transgenic mice and 95% co-localization of endogenous RFP expression and αSMA in stomachs of 12 months old IL-1β/αSMA-RFP mice
(E) FACS analysis of RFP+ gastric MF at 8 PD, isolated from uninfected αSMA-RFP mice (WT MF) or H. felis (18 mo) infected αSMA-RFP mice (18 months H. felis).
All data are represented as mean +/− SEM (See also Figure S1)
We isolated fibroblastic cells from stomachs of αSMA-RFP mice, and specifically cultured the MF using glutamine withdrawal (McKaig et al., 1999) (Figure S1f). Sorting after 4 population doublings (PD) revealed that 33% of the cells from uninfected WT mice and 70% from mice with dysplasia expressed RFP (Figure 1e), suggesting that αSMA/RFP+ CAFs have a growth advantage over αSMA/RFP- WT-MF, correlating with in vivo results. Gastric RFP+ MF of uninfected mice grew for up to 25 PD. In contrast, gastric RFP+ CAFs from long-term H. felis-infected mice grew much faster and up to 80 PD. Interestingly we observed that RFP+ cells, when cultured with sorted RFP- cells, appeared to be surrounded by RFP- cells (Figure S1g), suggesting the possibility that RFP+ cells might be providing a niche for RFP- cells. In addition, RFP+ CAFs isolated from long-term infected mice with dysplasia expressed increased amounts of TGF-β, IL-6, and TNF-α compared to WT RFP+ MF (Figure S1h).
Bone marrow-derived cells (BMDC) contribute to αSMA+ CAFs in an inflammation-related model of gastric carcinogenesis
To determine how BMDC contribute to stromal cells in an inflammation-dependent model of cancer, IL-1β mice were irradiated and transplanted with EGFP- (UBC-EGFP), RFP- (αSMA-RFP) or double-labeled (UBC-EGFP/αSMA-RFP) BM, and animals were observed for 12–14 months. Engraftment of MSC’s was initially confirmed through isolation (Figure S2a) and characteristic differentiation (Figure S2b) of GFP+ MSC isolated from BM 4 months post transplant, consistent with previously published studies (Wang et al., 2009), confirming that mouse BM MSCs can be transplanted (Simmons et al., 1987; Yokota et al., 2006). In vitro culture revealed that one third of MSC were GFP+ and thus donor derived (Figure S2a). Transplantation of αSMA-RFP BM revealed abundant engraftment in the BM of RFP positive cells after 18 months (Figure S2c). In IL-1β or H. felis infected mice that received labeled BM the development of dysplasia was preceded by the influx of a large number of labeled cells (Figure 2a and S2d). While a large proportion of the EGFP+ cells were immune cells (e.g. lymphocytes and myeloid cells), 12 months after BM transplant (BMT) in IL-β mice, and 18 month after BMT in H. felis-infected mice, approximately 20% of αSMA+ cells in regions of gastric dysplasia were EGFP+ (Figure 2a, c and S2d), whereas few if any double positive (αSMA+/EGFP+) cells were found in uninfected WT mice. To quantify the BMDCs, we transplanted BM from UBC-GFP/αSMA-RFP mice into WT H. felis-infected, IL-1β, or control mice. IHC and FACS revealed that in mice with dysplasia, 20% of the GFP+ BM-derived cells were also RFP+ (Figure 2b-f and S2e). These findings point to a role for αSMA+/EGFP+ cells in inflammation-induced carcinogenesis.
Figure 2. A significant portion of gastric CAFs originate from the bone marrow.
(A) EGFP (lower panel) or RFP (upper panel) expression and co-staining for αSMA and DAPI in stomachs of WT or 12 and 18-month mice with H. felis infection (arrows indicate αSMA and GFP or RFP co-expression) after UBC-GFP- or αSMA-RFP-labeled BMT.
(B) FACS of freshly isolated gastric MF from WT mice with double-labeled BMT after 18 months of H. felis infection.
(C) FACS data on BM cell contribution to the tumor microenvironment in WT, IL-1β, and H. felis infected WT mice harboring gastritis or dysplasia. Green: non-αSMA+ BM-derived cells, red: αSMA+ BM-derived cells, grey: all non-BM-derived cells. (*=p< 0,05)
(D, E) Endogenous RFP and GFP expression in stomach of 18 months H. felis infected WT mice after UBC-GFP/αSMA-RFP- double-labeled BM transplantation
(F) Endogenous RFP and GFP expression in in vitro culture of isolated MF from stomachs of 18 months H. felis infected WT mice after UBC-GFP/αSMA-RFP- double-labeled BM transplantation
(G) Gene expression data after FACS sorting of GFP(−) and GFP(+) CAFs from a BM-transplanted H. felis-infected IL-1β mouse. qRT-PCR for expression of GFP, CXCL1, CCL5, SSP1, CXCR4, MMP9, IL-6, IL-1β, SDF-1α, and TNFa (copies are calculated per 10000 copies of GAPDH, *=p<0.05 compared to all other cells).
All data are represented as mean +/− SEM. (See also Figure S2 and Table S1)
We isolated and cultured MF and CAFs from the stomachs of EGFP+ BM-transplanted 12-month-old WT and IL-1β mice to determine the contribution of BMDC to the MF population (Figure S2f). After 4 PD, more than 70% of CAFs from IL-1β mice (with dysplasia) were EGFP+, indicating their BM origin. Culture of RFP+/GFP+ CAFs (Figure 2f) from double transgenic donors further confirmed that aSMA+ cells were BM-derived. After sorting, the vast majority of EGFP- cells survived only a few PD whereas EGFP+ CAFs proliferated and could be cultured for at least 80 PD and expressed MF markers (αSMA, vimentin) but not epithelial markers (E-cadherin, data not shown). No cells in the MF or CAF populations were positive for CD31, CD45, CD3e, CD11b, CD45R/B220, Ly6G, Ly-6C, or TER-119 based on FACS analysis (data not shown), indicating no contamination with leukocytes. BM-derived CAFs expressed significantly higher mRNA levels of TNF-α, IL-6, SDF-1, and TGF-1β (Figure S2g, h), compared to WT MF.
These data suggest that a significant population of CAFs in the tumor microenvironment of inflammation-induced gastric cancer is BM-derived and contribute to tumor promotion. Since resident CAFs could only be cultured for 2–3 passages and thus could not be used for extensive comparison to BM-derived CAFs, we additionally isolated total RFP(+) gastric CAFs (HF-CAF) from aSMA-RFP mice and compared them to GFP(+) BM-derived gastric CAFs (BM-CAF). Only 20% of HF-CAF were BM-derived. We compared their gene expression directly after FACS sorting (Figure S2i and Table S1). BM-CAFs expressed higher levels of inflammatory genes (IL-6, IL-1β, IL-33) and a number of tumor and stem cell associated factors (CCL5, SPP1, Notch3, MMP9, CD47, CXCR4, PARP10,) compared to HF-CAFs. To confirm that this gene expression signature represents the BM-derived fraction of the CAFs, we separated BM-transplanted GFP+ gastric CAFs from resident GFP- gastric CAFs from H. felis-infected IL-1β mice after short term in vitro culture by FACS, and analyzed their gene expression. BM-CAFs demonstrated an expression profile of selected inflammatory genes similar to what we observed using microarray (Figure 2g). We compared the gene expression signature of our BM-derived CAFs with a previously reported inflammatory gene signature for skin CAFs (Erez et al., 2010). Four out of the 7 key inflammatory signature genes proposed by Erez et al. were also upregulated (p<0.05) in our gene list (CXCL1, IL-1β, IL-6, SPP1) (Figure S2i and Table S1). Additionally we identified 20 out of 60 genes with a significant (p<0.05) log fold-change within the Erez gene list that were identically up or down regulated in our comparison of BM-CAFs versus HF-CAFs. These findings suggest the possibility that this inflammatory gene signature might be largely determined by the BM-derived CAFs.
Expansion of αSMA+ MF in the BM of mice with gastric dysplasia
To characterize the BM lineage that gives rise to αSMA+ CAFs, we screened BM from αSMA-RFP mice with and without H. felis infection and BM from αSMA-RFP/IL-1β mice for αSMA+ cells. FACS analyses showed rare αSMA-RFP+ cells in the BM of uninfected control mice (0.02% +/−0.007%; Figure 3a, Table S2) but a significant stepwise increase in αSMA-RFP+ cells in the BM from aged IL-1β transgenic mice (1.2% +/−0.18%) and mice with long-term (18m) H. felis infection (0.9% +/−0.1%), both with gastric dysplasia (Figure 3a), suggesting that long-term chronic inflammation and/or dysplasia is required for remodeling of the BM. In a model of accelerated gastric cancer (H. felis in combination with N-Methyl-N-nitrosourea (MNU) (Tomita et al., 2010)) we confirmed the dependence of BM αSMA cell expansion on dysplasia (Figure 3a). Histopathological analysis confirmed a marked increase of RFP+ and αSMA+ cells in BM after 14 months of H. felis infection (Figure 3b). While RFP+/αSMA+ cells were occasionally surrounded by small groups of CD34+ hematopoietic stem cells (Figure S3a), we could not confirm any significant correlation in the localization of these cell types. Whereas undetectable in uninfected control mice, we observed in the peripheral blood (0.009%) of mice with dysplasia rare RFP+ cells (Table S2), which expressed high levels of αSMA (Figure S3b). In addition, the MSC and CAF cultures from dysplastic stomachs were morphologically very similar. Colony-forming units (CFUs) were consistently observed when we cultured gastric CAFs in MSC specific media (Figure S3c). These data indicate that accumulation of differentiated αSMA+ BM-derived cells in the BM and blood correlates temporally with the development of gastric dysplasia.
Figure 3. In a mixed population of MSCs the αSMA-RFP- cells contain the stem cells and RFP+ cells express a typical MF marker.
(A) FACS for αSMA-RFP+ cells in freshly isolated whole BM of 6, 9, 12, 15 and 18 month old αSMA-RFP+ mice and αSMA-RFP+ mice with H. felis infection (red) and 6, 12 and 15 month old IL-1β/αSMA-RFP+ transgenic mice (green) and 6, 9 and 12 month old αSMA-RFP+ mice with H. felis infection and MNU treatment (purple).
(B) αSMA and RFP staining of the BM of C57/B6 (lower row) or αSMA-RFP (upper row) mice before (control) and after 14 months of H. felis infection. Arrows indicate cells staining positive. (*=p< 0,05, data are represented as mean +/− SEM) See also Figure S3 and Table S2
(C) CFUs from 1000 plated RFP+ (red), RFP- (green) or RFP+/− (black) cells were counted after 14 days culture.
(D) Adipocyte (brown) and osteoblast (orange) differentiation of RFP+, RFP- or RFP+/− cells was measured after 14 days culture in differentiation mediua. After Oil Red-O or Alizarin Red staining % of differentiated cells was calculated.
(E) Representative pictures of RFP+/− cells after differentiation experiments (left as control) and after adipocyte (middle, with Oil Red-O staining) or osteoblast (right with Alizarin Red staining) differentiation.
(F) Quantitative expression of αSMA or collagen in RFP+ (red), RFP- (green) or RFP+/− (black) cells at passage 5 after a single RFP sorting.
(G) Representative pictures of αSMA, FSP1, collagen, and vimentin staining in RFP+, RFP− or RFP+/− cells. (*=p< 0.05, **=p<0.01 data are represented as mean +/− SEM) (See also Figure S3)
MSCs are a possible source for MF, so we established MSC cultures from whole BM from uninfected αSMA-RFP mice. The cultured, adherent cells were shown to be MSCs by their ability to form CFUs (Figure 3c) and differentiation into adipocytes and osteoblasts (Figure 3d, e). MSCs displayed longevity and grew exponentially for up to 80 days without signs of senescence or differentiation (Figure 4b) and with a normal karyotype (Figure S3d). Interestingly, MSC cultures also contained cells that expressed the RFP. Although histopathological evaluation showed a moderate number of αSMA/RFP+ cells in the BM, only 0.02% of fresh eluted whole BM expressed RFP by FACS, suggesting limitations to this extraction technique for stromal cells (Table S2). Nevertheless, 1.1% of cells after growth for several days in culture on plastic dishes and 50% or more of cells after several PD expressed RFP (Table S2), indicating that myofibroblastic differentiation is highly favored under these in vitro conditions. IHC demonstrated that the RFP+ cells expressed high levels of endogenous αSMA, FSP1, and vimentin, but low levels of collagen (Figure 3f, g). These data confirm that murine BM-derived MSCs can differentiate into αSMA+ cells that resemble CAFs.
Figure 4. αSMA-RFP- stem cells give rise to their αSMA-RFP+ niche cells.
(A) After sorting of αSMA-RFP+ MSCs after 6 PD, RFP- cells (upper panel) and RFP+ cells (lower panel) were cultured separately and RFP expression of each population was determined. Pictures below the FACS blots represent each respective population.
(B) PD of RFP+ (red), RFP- (green), RFP+/− (black) and RFP- cells after multiple sorting to eliminate RFP+ cells (light green). RFP expression status is shown in the red bar below the graph (* indicate potential tetraploidy after more than 80 days in in vitro culture).
(C) Elimination of RFP+ cells through TK-ablation: Freshly isolated MSCs (adherent BM cultures) from αSMA-RFP mice were transfected with an αSMA-TK construct and treated with 50μM gancyclovir. Remaining RFP- cells only proliferated after the treatment and gave rise to any RFP+ cells when cultured on feeder cells (lower panel). Data are represented as mean +/− SEM
(D) Schematic illustration of the results: MSC (RFP- cells) give rise to MSCs and MF (RFP+ cells). After sorting, RFP+ cells become senescent after few PDs. RFP- cells can give rise to its niche cells, RFP+ MF, and generate a heterogeneous MSC population, consisting of RFP+ and RFP- cells.
(E) Representative time-lapse microscopy images of a RFP- cell (black arrow) giving rise to RFP- (black arrow) and RFP+ (red arrow) cells in a population of RFP+/− cells. Elapsed time (from start of the microscopy, 12h after seeding) is indicated. (See also Movie S1, Table S2, and Figure S4)
In the heterogeneous MSC population, αSMA(−) MSCs give rise to αSMA+ MF which function as niche cells
With continuous culture, MSCs contained a large proportion of αSMA-RFP+ cells, indicating the heterogeneous nature, similar to the BM-derived CAF population isolated from the stomach. FACS analysis of heterogeneous MSC cultures (Table S2) revealed that the majority of cells expressed RFP (71%) at 6 PD but that this RFP+ population declined with more PD. When we compared the number of RFP+ cells after 6 PD we found significantly more RFP+ cells in MSC derived from IL-1β/αSMA-RFP mice or H. felis-infected αSMA-RFP mice, consistent with the higher number of RFP+ cells in the BM (Figure 3a).
MFs are characterized by expression of αSMA, vimentin, and FSP1, in contrast to fibroblasts, which express collagen-α1, or stem cells, which rarely express these markers. RFP+ cells purified from the heterogeneous RFP+/− MSCs by FACS expressed all three MF markers, and thus are distinct from RFP- cells which did not express αSMA (Figure 3f, g). Collagen expression was higher in the RFP- population and αSMA expression was higher in the RFP+ population (Figure 3f); consistent with the concept that the RFP+ cells represent more differentiated MF, and that the RFP- population also differentiates into, or contains, a fibroblast-like cell type.
Analysis of the BM-derived MSCs showed that the RFP- cells contained the true MSC. Up to 80% of the unsorted MSCs (which we will refer to herein as RFP+/− cells) and up to 80% of the once-sorted RFP- cells, differentiated into adipocytes and osteoblasts when grown in differentiation specific media (Figure 3d), whereas only ~20% of RFP+ cells were able to differentiate into these cell types. RFP- cells generated more CFUs (17 CFUs), whereas RFP+ cells formed very few (5 CFUs): RFP+/− cultures were even more efficient at colony formation (42 CFUs) (Figure 3c). RFP- cells were CD44+, but did not express Sca-1 and only 2.4% expressed c-kit, but upon differentiation into a mixed RFP+/− population, the cells expressed higher levels of c-kit and Sca-1; thus, these latter markers are characteristic for the RFP+ cells (Figure S3e), consistent with recent publications (Wang et al., 2009) indicating a dynamic change in cell surface marker expression upon culture of MSCs.
We next established a lineage relationship in the production of αSMA+ cells from MSCs and demonstrated that RFP+ cells were derived from RFP- cells (schematic illustration in Figure 4d). After a first sorting and isolation of RFP- cells, cultures of RFP- cells generated new RFP+ cells and RFP- cells in the same proportion with increasing numbers of RFP+ cells over time (Figure 4a). Sorted RFP+ cells generated more RFP+ cells but not RFP- cells (Figure 4a); after 10 PD they became senescent (Figure 4b). Interestingly, after 80 days of in vitro culture, RFP- and RFP+/− cells became tetraploid (Figure S3f) but maintained a normal karyotype in contrast to previous reports (Izadpanah et al., 2008). These data suggest that RFP- cells give rise to RFP+ cells but not vice versa (Figure 4e and Movie S1).
RFP- cells showed superior self-renewal properties compared to RFP+ cells (Figure 4b), consistent with the RFP- cells being the progenitor to RFP+ cells. To generate pure RFP- cells, we carried out multiple (3 to 5) sortings of RFP- cells until we could not detect any RFP/αSMA+ cells in the population. When MSC cultures were maintained in an RFP- state, the cells became senescent after a few passages, indicating that RFP+ cells are needed for RFP- cell survival. Multiple sorted RFP- cells could be prevented from senescence by the addition of RFP+ cells (data not shown), suggesting that the senescence phenotype was not due to cell damage from sorting but instead due to the absence of secreted factors by RFP+ cells. To confirm these observations, we transfected RFP+/− cells with a 3kb αSMA-thymidine kinase (TK) construct (Figure S4) in order to eliminate αSMA+ cells from the population. Freshly isolated RFP+/− MSCs, transfected with αSMA-TK, and treated for 10 days with gancyclovir (50μM GCV), showed a complete and irreversible loss of RFP+ cells followed by loss of RFP- cells (Figure 4c). In contrast, freshly isolated and αSMA-TK transfected RFP+/− cells grown on fetal fibroblasts (FEF) (Okawa et al., 2007) survived and generated new RFP+ cells 1–2 days after GCV treatment (Figures 4c, S4b). These data indicate that RFP- stem cells can only survive in a heterogeneous population of RFP- and RFP+ cells, and that the RFP+ cells probably function as niche cells for the RFP- stem cells from which they derived.
TGF-β induces αSMA expression in RFP- MSC cells through CXCR4/SDF1α and upregulates Gremlin-1
To investigate mechanisms by which BM- and MSC-derived MF are induced during progression to dysplasia, we examined the effect of growth factors on RFP+ MF production. We stimulated unsorted or sorted MSC cultures with TGF-β or PDGF, which have been strongly linked to MF differentiation. In all cell populations (RFP+, RFP- and RFP+/− cells), RFP/αSMA expression was strongly stimulated by TGF-β and to a lesser extent by PDGF, with the greatest effect on the RFP- population (Figure 5a, S5a). In addition, growth factor stimulation resulted in more rapid proliferation of RFP+ cells (Figure S5a,b).
Figure 5. Regulation of the stem cell niche in the BM and tumor.
(A) Percentages of RFP+ cells in populations of RFP+ (red), RFP- (green), and RFP+/− (black) cells after incubation with TGF-β or PDGF.
(B) Results of ELISA for SDF-1α expression in RFP+, RFP-, RFP+/−, and WT gastric MF with and without incubation with TGF-β or PDGF.
(C) Gene expression data from bone marrow MSC cultures (RFP+, RFP−, RFP+/−), CAFs and WT MF. qRT-PCR for expression of IL-6, SDF-1a, TGF-β, BMP4, Wnt5a, Shh, Dkk1, Gremlin 1 and CXCR4 from in vitro culture of RFP-, RFP+, RFP+/− cells, gastric RFP+ CAFs and WT gastric MF as a control. (copies are calculated per 10000 copies of GAPDH, * = p<0.05 compared to all other cells, # = p<0.05 compared to RFP- cells, $ = p<0.05 compared to RFP+ cells, + = p<0.05 compared to MF (Dunnett test for multiple comparisons)).
(D) Quantitative analysis of the dose-dependent effect on RFP expression in RFP+/− after 48h incubation with TGF-β and 100ng/ml (left) or 1ug/ml (right) of the CXCR4 inhibitor AMD3100.
(E) qRT-PCR for Gremlin-1 expression in RFP+, RFP-, and RFP+/− cells with (red) and without (blue) 48h incubation with TGF-β.
(F) Representative picture of Gremlin-1 staining of WT mouse stomach (left) and mouse stomach with gastric dysplasia after 14 months of H. felis infection (middle), and invasive gastric cancer in 14-month old IL-1β mice (right).
(G) Representative pictures of Gremlin-1 staining (green) of RFP+/− and RFP- cells (left, RFP [red] is endogeneous αSMA-RFP). Control stomach (lower panel, right) and stomach with gastric dysplasia after 14 months of H. felis infection (lower panel, right) are shown.
(H) Representative double staining for Gremlin-1 (red) and αSMA (green) in gastric dysplasia in 18 months H. felis infected mice (I and J) Representative picture of Gremlin1
(I) and Nestin1 (J) staining in 12 months old GFP-BM transplanted IL-1β mice (*=p< 0,05, all data are represented as mean +/− SEM) (See also Figure S5)
Next we examined factors expressed by RFP+ cells that might contribute to the BM niche and tumor microenvironment. IL-6 has been associated with the development of gastrointestinal cancer (Grivennikov et al., 2009). BM-derived gastric CAF and WT-MF expressed high levels of IL-6 compared to parental MSCs (Figure 5C, a finding confirmed by ELISA (data not shown). RFP+ cells also expressed higher levels of BMP4 and Wnt5A (factors known to contribute to the stem cell niche) and exhibited a CAF-like gene expression pattern (Figure 5C). This inflammatory gene expression profile correlated with analyses of BM-derived GFP+ gastric CAFs compared to the total gastric CAF population (Table S1). SDF-1α, which has been associated with stromal-dependent cancer progression, was highly expressed in RFP- cells compared to WT-MF or RFP+ cells (Figure 5B, C). Thus, it seems likely that SDF-1α is expressed by MSCs rather than CAFs, and may represent a mechanism by which MSCs regulate MF proliferation or recruitment to the niche.
We therefore examined the effect of TGF-β stimulation on SDF-1α production by MSC. Incubation with TGF-β increased SDF1α production 5-fold in RFP- cells (Figure 5B) but not in RFP+ cells or WT MF. TGF-β– mediated induction of proliferation and differentiation in MSCs was inhibited by the CXCR4 antagonist AD3100 (not shown), as CXCR4 was mainly expressed on RFP- and RFP+/− cells (Figure 5C). AMD3100 reduced TGF-β-induced differentiation of RFP−/+ cells into RFP+ cells in a dose-dependent matter (Figure 5D, S5c). Thus, TGF-b can induce MF differentiation of MSCs in part through upregulation of SDF1α in MSCs. Migration of cancer cells induced by BM-derived gastric IL-1β-MF was significantly increased compared to WT-MF, and this effect could be inhibited by blocking CXCR4 (Figure S5d).
The BMP antagonist Gremlin-1 was upregulated during gastric carcinogenesis (Figure 5F) but was expressed at low levels in RFP- cells and RFP+ cells (Figure 5E). Interestingly, though, Gremlin-1 was upregulated in RFP+/− cells, suggesting that Gremlin-1 is induced by an interaction between RFP+ and RFP- cells. Gremlin-1 was highly expressed in CAFs but not in WT-MF (Figure 5E and 5C) and TGFβ upregulated Gremlin-1 expression in RFP- cells but not in RFP+ cells (Figure 5E). In RFP+/− cells, Gremlin1 could only be detected in RFP- cells (Figure 5G), and αSMA and Gremlin-1 did not overlap in CAFs in gastric dysplasia (Figure 5H), indicating that Gremlin-1 is likely a marker of MSC in their niche in the BM and tumor microenvironment. IHC revealed that the majority of Gremlin-1+ cells in IL-1β-mice transplanted with EGFP-BM were GFP positive (Figure 5I), indicating that Gremlin-1+ cells were largely BM derived. A recent study (Mendez-Ferrer et al., 2010) reported Nestin1 as a marker for MSCs in the BM, where MSCs contribute to the BM niche for HSCs. We observed co-localization of a subpopulation of Nestin1+ and GFP+ BM-transplanted stromal cells in gastric dysplasia (Figure 5J), confirming that BM-derived MSC are present in the dysplastic stomach. Sonic hedgehog (Shh), which regulates stem cells, and Dickkopf-1 (DKK1), an inhibitor of the Wnt pathway, were also expressed mainly in RFP+/− cells, reflecting an active niche environment (Figure 5C).
BM-derived MF from gastric dysplasia or MSC induce invasive growth of gastric tumor cells in a 3-D organotypic model
Given the evidence that CAFs originate from BM-MSCs and are recruited to the stomach at early stages of dysplasia, we investigated interactions between tumor cells and CAFs. When co-cultured with the human gastric cancer cells AGS, RFP-, RFP+, and RFP+/− cells all increased the expression of RFP/αSMA (Figure 6a), along with the MF markers FSP1 and vimentin (not shown). In contrast, expression of collagen was down-regulated in RFP- and RFP+/− cells to approximately the level found in RFP+ MF (Figure 6a). Thus, co-culture with tumor cells induces differentiation of MSC toward the myofibroblastic lineage. In co-culture experiments, SDF-1α levels were significantly decreased in all stromal cell populations (Figure 6b), particularly in the RFP- cells, consistent with increased differentiation of MSCs into αSMA+ MF.
Figure 6. MF differentiation of MSC promotes in vitro tumor invasion.
(A) % change of αSMA and collagen expression in RFP-, RFP+, and RFP+/− cells co-cultured with AGS or MKN45 gastric tumor cells (see Figure 3d for controls, *= p< 0,05)
(B) Results of ELISA for SDF-1α expression in RFP+, RFP-, and RFP+/− cells in single or co-culture with AGS gastric tumor cells
(C) Quantification of invasions per 1cm from organotypic culture experiments of in RFP−, RFP+, and RFP+/− cells of WT MF and IL-1β-BM MF grown in a 3D collagen/Matrigel matrix with AGS on top. (*=p< 0,05 compared to WT MF and #= p<0.05 compared to RFP+/− or IL-1β MF)
(D) Representative pictures of organotypic culture with AGS cells growing on top of a 3-D collagen/Matrigel matrix that contains different cell populations. Upper panel: RFP+ cells (arrows indicate invasion sites) with representative pictures of the staining for human epithelial antigen (hEA) to detect human AGS cells (middle) or αSMA to detect the αSMA-RFP expressing cells in the matrix (arrows at right). Middle panel: AGS cells on RFP- (left) or RFP+/− (middle) cells, and on RFP+/− cells after incubation with 5-azacytidine (35ul/ml) for 10 days. Lower panel: Left panel shows IL-1β BM MF; middle panel shows isolated gastric MF from WT mice with 18 months of H. felis infection; right panel shows WT, uninfected gastric MF (arrows indicate invasion sides).
(E) Relative methylation in the cytosine extension assay of RFP+/− and RFP+ cells compared to RFP+/− cells treated with TGF-β for 48h.
(F) FACS for RFP expression in RFP+/− cells before (left) and after (right) incubation with 5-azacytidine for 10 days and 6 PD (*=p< 0,05). All data are represented as mean +/− SEM)
To study the biologic effects of BM- and MSC-derived MF on tumor cells, we used a 3-D organotypic cell culture system: AGS cells were laid onto an extracellular matrix (ECM) gel mixture with MSC-derived cell populations (RFP-, RFP+ and RFP+/− cells), normal WT gastric MF, gastric CAFs, or BM-derived gastric CAFs (Figure 6c, d). When placed on WT-MF, AGS grew with minimal signs of invasion, as expected for a well-differentiated, weak-tumorigenic cell line in organotypic culture (Figure 6d). In contrast, when cultured on pooled gastric CAFs, tumor cell invasion into ECM could be detected (Figure 6d). However, AGS cells cultured on the RFP+ cells (19 invasion sides/cm), the RFP+/− cells (8 invasion sides/cm), and the IL-1β BM-derived CAFs (7 invasion sides/cm), revealed high levels of invasion into ECM (Figure 6c, d). In contrast, nearly no invasion was observed when AGS cells were cultured on RFP- cells.
Given recent findings that widespread hypomethylation characterizes CAFs from human gastric tumors and from a transgenic mouse model of multistage gastric carcinogenesis (Jiang et al., 2008), we examined the role of methylation in development of RFP+ cells. We observed significantly (p<0.05) higher levels of DNA methylation in RFP+/− than in RFP+ cells (Figure 6e), supporting the concept that development of RFP+ MF is regulated in part by hypomethylation. Incubation of mixed RFP+/− MSCs with 5-aza-dC induced DNA demethylation, increased the proportion of RFP+ cells (Figure 6f), and increased the degree of tumor cell invasion in the organotypic model (Figure 6d). Interestingly, TGF-β also induced hypomethylation in RFP+/− cells (Figure 6e). These findings indicate that hypomethylation of RFP+/− cells from the BM can contribute to the development of a CAF phenotype, might be sufficient to further transform nontumorigenic tumor cells, and contributes to tumor invasion.
BM- and MSC-derived MFs induce tumor growth in vivo when injected locally or at a distance
To confirm the findings from the organotypic model, we analyzed the effects of BM- and MSC-derived MFs in xenograft tumor models. When 105 MKN45 cells were injected, there was only minor tumor growth after 6 weeks, but tumors were observed with 106 MKN45 cells, or when 105 MKN45 cells were co-injected with stromal cells (Figure S6A, b). Co-injection of 105 MKN45 cells with fetal fibroblasts (FEF), or RFP- MSCs resulted in small tumors. Large tumors were observed when 105 MKN45 cells were co-injected with RFP+, RFP+/−, or IL-1β-BM-CAF (Figure S7b, d). The non-tumorigenic AGS cells formed tumors only in combination with RFP+ or IL-1β-BM-CAF using same number of cells (105 or 106, Figure S6c). Preliminary experiments with mouse pancreatic cancer cells and CAFs confirmed those findings (data not shown). These in vivo data support the conclusions from organotypic assays that BM- and MSC- derived RFP+ cells contribute to tumor growth and have properties that are distinct from normal fibroblasts and MF.
To further examine the in vivo importance of MSCs to the CAF population, we injected MKN45 cells into both flanks of a mouse but injected only one flank with IL-1β-BM-CAF (GFP+), WT MF, gastric CAFs (HF-MF), RFP+, RFP− or RFP+/− cells. Flanks injected with cancer cells along with RFP- cells or WT MF developed small tumors, whereas the opposite flanks injected with only cancer cells did not develop tumors (Figure S6d). Co-injection of cancer and RFP+ cells resulted in formation of large tumors on the flank of co-injections and small tumors on the other flank. Interestingly, co-injection of cancer cells and RFP+/− cells, gastric CAFs (HF-MF), or IL-1β-BM-MF cells resulted in formation of large tumors on both flanks (Figure 7a, b). These findings indicate that CAFs that contain MSCs and myofibroblastic cells are likely able to promote tumor growth at distant sites. When we injected one flank with 105 MKN cells and IL-1β-BM-CAF (without tumor cells) on the opposite flank, we observed increased tumor growth as with injections on the same flank (Figure 7b, c). Similar results were observed with IL-1β-BM-MF injected intraperitoneally (Figure S6e). IHC for αSMA showed an increased number of MFs in the large tumors with co-injections of RFP+ or RFP+/− cells, compared to those of RFP- cells, fetal fibroblasts, or WT-MF (Figure 7a, b). RFP or GFP expression were detected in large tumors on both flanks by IHC (Figure 7a, white arrows) and PCR (Figure S6f); RFP and GFP primarily co-localized with αSMA expression in mice that received co-injections of tumor and RFP+ and RFP+/− cells, or IL-1β-BM-CAF. Interestingly, not all GFP-labeled IL-1β-BM-MF expressed αSMA (Figure 7a, b, orange arrows), consistent with the notion that these CAFs preparations were similar to the RFP+/− population, containing MF and MSC.
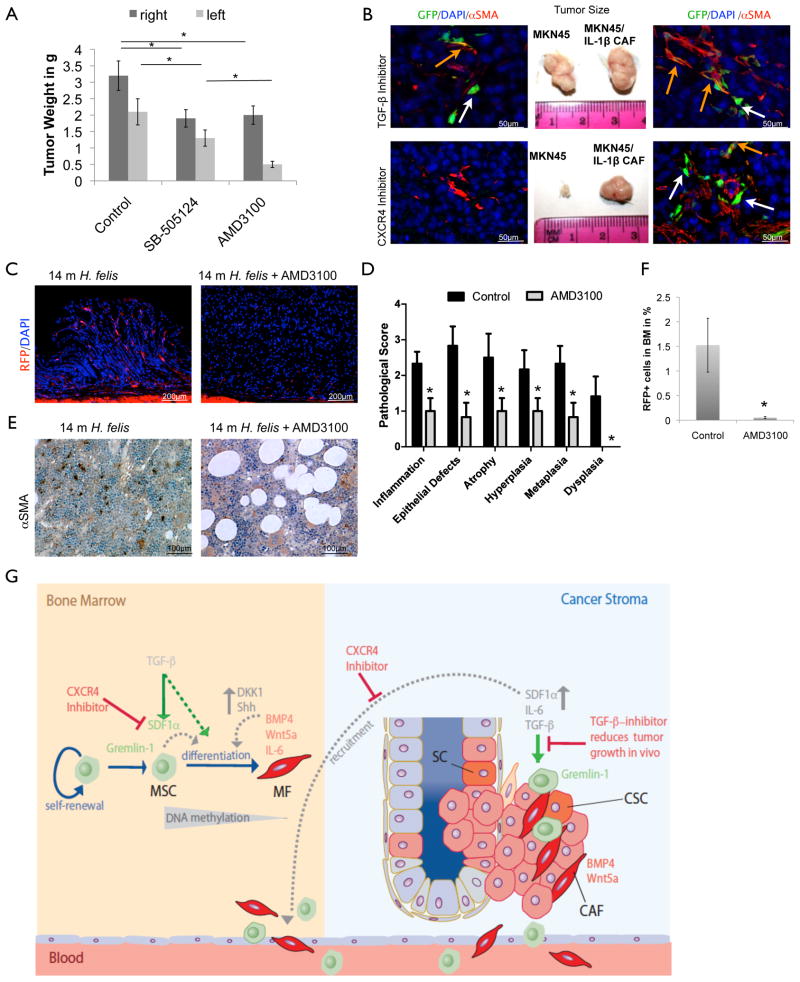
Figure 7. BM-derived gastric MF and heterogeneous MSC promote xenograft tumor growth that can be inhibited by TGF-β or CXCR4.
(A) For xenograft experiments, 105 RFP+ (upper row), RFP+/− (second row), IL-1β-BM MF (third row), and gastric RFP+ CAFs (lower row) were co-injected with 105 MKN45 cells on the right flank and 105 MKN45 cells were injected alone on the left flank of SCID mice. Tumor injection sites and tumor sizes after isolation are shown. Left or right side shows representative images of staining for αSMA and RFP (upper 2 rows). The third row shows tumors of αSMA and RFP and representative IHC for GFP, αSMA and DAPI (lower panel, orange arrows indicate double staining and white arrows indicate only GFP expression)
(B) Quantification and statistical analysis of xenograft experiment of 105 fibroblasts (FB), RFP+, RFP-, RFP+/−, and IL-1β-BM MF after co-injected with 105 MKN45 on the one side but only tumor cells on the contra lateral side. IL-1β-BM MF were injected at a distant site and 106 MKN cells were injected into the contralateral flank. (*=p<0.05 compared to MKN alone, #=p<0.05 compared to RFP+ and RFP+/− cells, and $=p< 0.005 compared to RFP- and FB, (Dunnett, All data are represented as mean and measure points).
(C) RFP+/− cells or IL-1β-BM-CAF were injected s.c. in a mouse with a tumor of 106 MKN cells growing on the left flank. IHC for αSMA (green) and endogeneous RFP show double staining (orange arrows) or only αSMA expression (white arrow) in the tumor. (See also Figure S6)
To determine if endogenous BMDC also contributed to the observed tumor growth, we performed experiments with WT C57/B6 mice transplanted with BM from αSMA-RFP mice or UBC-GFP mice (Figure S6h). Chimeric mice with the UBC-GFP BM were given injections of RFP+/− cells together with syngeneic 105 TC-1 murine cancer cells in one flank and TC-1 cells alone on the other. Mice with αSMA-RFP BM received co-injections of GFP+ IL-1β-BM-MF and TC-1 cells in one flank and only TC-1 cells in the other (Figure S6h). Again, we observed increased tumor growth with co-injections (data not shown), compared to only tumor cells; and stromal cells were recruited to the tumors. Tumors contained multiple BM-derived GFP+ or RFP+ cells, including αSMA+ MF-shaped cells (Figure S6h). Interestingly, co-injection of MSC-containing CAF preparations significantly increased the number of GFP+ BMDC and RFP+ MF in tumors, in contrast to few GFP+ cells in tumors of mice not given co-injections (Figure S6g,h). Thus, the recruitment of BM-derived CAFs can be accelerated by the presence of MSC in the tumor microenvironment. Taken together, BM- and MSC-derived αSMA+ MF can be recruited to the tumor to stimulate growth, but the combination of the RFP+ myofibroblastic cells and RFP- MSCs, which recreate the BM niche at the tumor site, is a particularly potent stimulant of tumor growth and responsible for further recruitment of BMDCs (See model: Figure 8g).
Figure 8. CXCR4/SDF1 inhibition reduced MF and MSC recruitment and tumor growth.
(A) Effects of TGF-β or CXCR4 inhibition in xenografts of co-injection with IL-1β BM-MF and MKN45 cells on one flank and MKN45 on the other flank. Tumor size (middle) after 6 weeks of tumor growth in mice given the TGF-βR2 inhibitor (upper panel) or CXCR4 inhibitor (lower panel). Representative IHC of each tumor (right, left) with staining for endogeneous GFP and αSMA (orange arrows indicate double staining and white arrows indicate expression of only GFP).
(B) Quantification and statistical analysis of xenograft experiment with mice co-injected with IL-1β BM-MF and MKN45 cells on one flank and MKN45 alone on the other flank. Tumor size in g after 6 weeks of tumor growth in mice given the TGF-βR2 inhibitor (SB-505124) or CXCR4 inhibitor (AMD3100) (*=p< 0.05 (Dunnett)) (C–F) Effect of CXCR4 inhibitor treatment on stomachs of 16 months old H. felis infected αSMA/RFP mice after 4 months of AMD3100 treatment:
(C) Representative IHC of (left) untreated control and (right) AMD3100 treated mice and
(D) histopathological scoring of the same experiment (*=p<0.05, (N=3)).
(E) Representative pictures of (left) untreated control BM and (right) BM of AMD3100 treated mice with αSMA staining and
(F) quantification of RFP+ cells in the BM by FACS (*=p<0.05, (N=3) . All data are represented as mean +/− SEM.
(G) Schematic drawing that depicts interactions between the bone marrow niche (left) and the gastric cancer stroma (right). A significant portion of CAFs (red) originate from the bone marrow and are derived from MSCs (green). The normal bone marrow niche consists of self-renewing MSCs that give rise to MF that resemble CAFs and likely contribute to the normal stem cell niche in the bone marrow. In their niche, MSC express both Gremlin-1 and SDF-1. TGF-β can induce the differentiation of MSC into MFs through an SDF1α-dependent pathway that involves DNA hypomethylation. MF express BMP4, which seems to induce Gremlin-1 in MSCs; BMP4 and Wnt5a likely induce DKK1 or Shh in the normal, heterogeneous population of MSC. With cancer progression, the number of CAFs increases markedly in the bone marrow niche and blood. These bone marrow niche cells are expanded in a TGF-β dependent matter and recruited through CXCR4/SDF1α signaling together with Gremlin-1-expressing MSC to incipient tumors where they now appear as CAFs.
CXCR4/SDF1 contributes to migration of BM-derived MF to the tumor whereas TGF-β inhibition decreases MF differentiation in vivo
Having shown that SDF-1α signaling modulated MF differentiation in vitro, we analyzed the importance of SDF-1α signaling in vivo. We co-injected GFP-labeled IL-1β-BM-MF with MKN45 cells in one flank, and MKN45 cells alone in the other flank, and then administered a TGF-β receptor 2 (R2) inhibitor (SB-505124) or CXCR4 inhibitor (AMD3100) for 6 weeks. Mice given the TGF-βR2 inhibitor, developed smaller tumors on both flanks (Figure 8a, b), compared with untreated control mice (Figure 8a). IHC revealed a decrease in total GFP-labeled and αSMA+/GFP+ MF, suggesting less differentiation of MSCs to CAFs, consistent with the in vitro results. In mice that were given the CXCR4 inhibitor, we observed a slight decrease in tumor size on the flank that received co-injection of IL-1β BM-MF and MKN45 cells, but very small tumors on the flank only injected with tumor cells (Figure 8a, b). IHC revealed no GFP+ cells with few if any αSMA-expressing MF on the contralateral side, and a reduced number of non-GFP αSMA+ cells on the co-injection side (Figure 8a). These findings indicate that SDF-1α signaling is likely required for the recruitment and migration of MSC or MF to the tumor site.
To extend these findings, we treated H. felis-infected αSMA-RFP mice (12m) with the CXCR4 inhibitor (5 mg/kg/day AMD3100 in 4 consecutive Alzet© mini pumps) for 4 months and analyzed the effects on gastric carcinogenesis and αSMA/RFP+ BMDCs. CXCR4 antagonism resulted in a marked reduction in αSMA+ cells in the BM of the treated mice, which seemed to be replaced by fat cells (Figure 8e, f), it led to a reduction in gastric αSMA+ cells (Figure 8c), and inhibited the development of gastric dysplasia compared to untreated control mice (Figure 8d). The findings are consistent with the model in which targeted inhibition of BM αSMA+ MF production reduces cancer progression, although we cannot completely exclude a direct effect of the CXCR4 antagonist on the development of dysplasia.
Discussion
Although it is widely accepted that tumorigenesis is regulated by interactions between tumor cells and CAFs, the precise origin and function of CAFs has been unclear. We show that: (1) CAFs increase during chronic inflammation and gastric cancer progression, particularly during the transition to dysplasia; (2) CAFs are derived from αSMA+ MF present in the normal BM; and (3) αSMA+ MF are generated from MSC and contribute to the normal BM niche and MSC self-renewal. During chronic inflammation and carcinogenesis, these αSMA+ BM niche cells are (4) expanded in a TGF-β–dependent matter and recruited through CXCR4/SDF1α signaling together with Gremlin-1-expressing MSC to incipient tumors where they (5) contribute to a tumor niche that promotes and sustains tumor progression.
In inflammation-dependent models of gastric cancer, progression to dysplasia is associated with accumulation of αSMA-expressing CAFs, compared to collagen-α1-expressing fibroblasts. αSMA-expressing CAFs could in theory originate from local fibroblasts, endothelial cells, mesenchymal cells, local epithelial cells during the EMT, or recruited BMDC. Studies using RIPTag transgenic mice showed that BMDC can give rise to CAFs and fibroblasts (Direkze et al., 2004), which was confirmed in other cancer models (Guo et al., 2008). More importantly, studies in patients with gastric cancer that received gender-mismatched BM transplants have confirmed the BM origin of CAFs in gastric cancer (Worthley et al., 2009). We demonstrate through BM reconstitution studies in mice that 20% of αSMA+ fibroblastic cells that accumulate in dysplasia are BM-derived, and that BM MSCs are the likely origin of these CAFs (Figure 8G). In contrast to previous reports (Simmons et al., 1987; Yokota et al., 2006), we show here that it is indeed possible to transplant BM MSCs in the mouse. CAFs recruited from the BM are functionally more active as promoters of tumor growth and invasion compared to normal or resident fibroblasts, and exhibit a previously reported NF-κB dependent inflammatory gene expression signature (Erez et al., 2010), which accounts for higher levels of pro-inflammatory cytokines in CAFs (Karnoub et al., 2007; Orimo et al., 2005).
In mouse models of inflammation induced gastric dysplasia, αSMA+ CAF were recruited to the stomach after first accumulating in the peripheral blood and in the BM. Indeed, the accumulation of αSMA+ cells in the BM appeared to correlate with the development of dysplasia, and suggests that carcinogenesis involves an early stage of BM remodeling. In the normal BM, MSCs are able to give rise to αSMA+ cells that morphologically and functionally behave identically to CAFs isolated from the dysplastic stomach, and these αSMA+ MF cells appear to be expanded in cancer.
Previous studies have suggested that MSCs can be induced to differentiate into CAFs, particularly when exposed to tumor-conditioned media (Mishra et al., 2008). However, we show that differentiation of MSC into αSMA+ cells is a normal pattern of differentiation in vitro and in vivo, compatible with asymmetric stem cell division in a broad definition, although more specification regarding an intrinsic or extrinsic mechanism would be needed (Morrison and Kimble, 2006). Moreover, that αSMA+ myofibroblastic cells represent normal niche cells within the BM that maintain the self-renewal properties of MSCs. In culture, MSC lose their proliferative potential and ability to self-renew when αSMA+ cells are specifically eliminated, and these capabilities are restored if αSMA+ cells are added back to the MSC. The αSMA-RFP transgene has allowed us to sort the heterogeneous, adherent population of BM mesenchymal cells, revealing that the true MSC is αSMA- but can give rise to αSMA+ MF.
Most adult stem cells are extremely difficult to culture in vitro in the absence of supportive niche cells or defined growth factors, and the fact that MSCs generate their own αSMA+ niche cells clarifies the self-renewal abilities of BM MSC cultures. The concept that a stem cell is able to generate its own niche cell has precedence (Mathur et al., 2010; Snippert et al., 2010). In addition, the presence of the αSMA+ cells in the BM raises interesting questions regarding the possible role of these cells beyond support of the MSC. The BM niche is thought to consist of osteoblasts (Lo Celso et al., 2009), which only exist in the bone, but further studies are required to determine if CAF-like cells support the growth of stem cells beyond the MSC.
The development of dysplasia and tumors in the stomachs of mice was associated temporally with an expansion of αSMA+ cells in the BM, which was reproduced by co-culture of MSCs with gastric cancer cells, indicating the effect of a soluble, secreted factor. Previous studies have shown that co-culture of MSCs with cancer cells could result in differentiation into CAFs (Mishra et al., 2008), and that DNA hypomethylation induced by 5-aza-dC promoted the differentiation of pooled human MSC cultures into CAFs. We had shown that CAFs become markedly hypomethylated during early stages of human gastric carcinogenesis (Jiang et al., 2008). Here, we confirm that hypomethylation is sufficient to induce differentiation of CAFs and that the effect is specific for the RFP- MSCs. However, we also demonstrate that a soluble growth factor often secreted by tumors, TGF-β, may contribute to the development of CAFs from MSCs during tumorigenesis. Incubation of RFP- BM-derived MSCs with TGF-β induced DNA hypomethylation and accelerated their differentiation into αSMA+ CAFs. The promotion by TGF-β of myofibroblastic differentiation occurs in part through an SDF-1α dependent pathway, since inhibition of CXCR4 blocked this differentiation process. Thus, although global hypomethylation is a well-known feature of malignant tumors, we demonstrate that TGF-β can induce both fibroblastic differentiation and global DNA hypomethylation rapidly in vitro.
The αSMA+ MF niche cells express a number of factors that likely contribute to the stem cell niche and also maintain the self-renewal properties of incipient tumors (Figure 8G). Factors such as Wnt5a, BMP4 and IL-6 were highly expressed by RFP+ cells, as well as BM-derived CAFs isolated from gastric dysplasia. Wnt signaling has been shown in the gut to be important both for the maintenance of tissue stem cells and the generation of cancer stem cells (CSC) (Brabletz et al., 2009) and recently was associated with maintaining or inducing stemness in CSC (Vermeulen et al.). BMPs have also been linked to intestinal and hematopoietic stem cell maintenance, controlling the stem cell number through regulation of the niche size (Zhang and Li, 2005). IL-6 appears to be required for the survival of intestinal epithelial cells and the development of inflammation-associated cancer of the gut, probably through activation of STAT-3 pathways in intestinal progenitors (Grivennikov et al., 2009). However, although the RFP+ CAFs promoted in vitro proliferation of cancer cells in organotypic models, the RFP+/− cells, which contained MSCs and CAFs, appeared to be very important in promoting in vivo tumor growth, particularly when injected at a distance from the tumor site. These findings indicate that MSCs are required for the production or migration of CAFs over time. Our results suggest that SDF-1α is likely produced by the MSC-containing RFP- population, rather than the RFP+ CAFs; SDF-1α seems to function in an autocrine function to stimulate more niche cells, and a paracrine function to attract and maintain CAFs in close proximity to tumors. Thus, CXCR4 antagonism - in our xenograft studies and our gastric carcinogenesis studies - appeared to inhibit stromal cell recruitment to the tumors and also block the production of CAFs in the BM of mice with cancer.
The two cell types (RFP- MSC and RFP+ MF) function together as stem cell and niche cell and communicate with each other. A cross-talk between the MSC and MF results in a unique pattern of gene expression characteristic of the niche, compared to those of each individual cell population. For example, DKK1 and Shh are significantly upregulated in heterogeneous RFP+/− cultures but not in sorted RFP- and RFP+ cells, indicating that these factors can only be expressed in a functioning stem cell niche. Gremlin-1 was expressed on RFP- cells, presumably the true MSC, but mainly under the conditions when the MSCs were cultured together in a mixed RFP+/RFP- population. Interestingly, Gremlin-1, an antagonist of the BMP pathway, is not expressed by normal adult fibroblasts or MF from the skin or most solid organs; expression of Gremlin-1 has been reported to be unique to stromal cells in the setting of cancer (Sneddon et al., 2006) and was consistently upregulated in our model of gastric carcinogenesis. Since Gremlin-1 expression was observed only in cultures that contain MSCs and MFs/CAFs, it is possible that MSC expression of Gremlin-1 occurs in response to BMP signals from the αSMA+ myofibroblastic cells. Consistently, high levels of Gremlin-1 have been observed in mouse embryonic fibroblast cells that are capable of maintaining human embryonic stem cells in culture (Pera et al., 2004).
In summary, we show that chronic inflammation and epithelial dysplasia lead to remodeling of the BM and expansion of αSMA+ MFs in a manner that promotes cancer growth and progression. The CAF-like MFs contribute to the niche for the MSCs, and it is this fully intact MSC niche that is recruited to the tumor site and that stimulates malignant progression. The recruitment of the niche to the tumor site can be blocked by CXCR4 inhibition and the differentiation of MSCs can be abrogated by TGF-β inhibition. We propose a model in which during the earliest stages of inflammation-induced tumor development, the BM undergoes remodeling, mediated in part by TGF-β, and then the MSC-CAF stem cell niche promotes tumor progression through SDF-1α signaling (Figure 8G). These observations have implications for the early diagnosis and treatment of cancer.
Experimental procedures
Isolation and culture of cells
BM cells were collected by flushing femurs of αSMA-RFP mice. MSC were cultured in murine mesenchymal medium with supplements (MesenCult). Serial cultures of the different MSC were performed in 10-cm dishes by plating 105 cells, refeeding the cells every other day, and subculturing every 4–5 days. The doubling number of each passage was calculated with the formula PD=(nf/n0)/log2. WT (WT-MF), BM derived (BM-MF) and αSMA-RFP MF (RFP-MF) were isolated from the stomach of C57BL/6, IL-1β/αSMA-RFP and αSMA-RFP reporter mice. Stomachs were cut into small pieces which were incubated with collagenase I at 37ºC for 1 hour. Cells were filtered with a cell strainer and washed with PBS. Harvested cells were cultured in RPMI medium without Glutamine, supplemented with 10% FBS (Hyclone) and penicillin-streptomycin at 37ºC in 5% CO2. Characteristic features of MF (abundant myofibrils with dense bodies, indented nucleus, basal lamina-like structure, capacity to express αSMA, vimentin and laminin) were demonstrated in both primary and secondary cultures.
Cultured cells were incubated with 35ug/ml of 5-aza-2-deoxycitidine (Sigma) for hypomethylation experiments or 10ng/ml of murine recombinant TGF-β1 (Invitrogen) or murine recombinant PDGF (Sigma) for growth factor stimulation experiments.
Xenograft studies in SCID mice
All mice studies and breeding were carried out under the approval of IACUC of Columbia University. Six- to eight-week-old SCID mice were used for subcutaneous injection with a mixture of tumor cells and either RFP+, RFP- or RFP+/− αSMA-MSC (1x105) or GFP labeled BM-derived MF (1x105) isolated from the stomach. Human gastric cancer cell lines MKN45 and AGS or the mouse Lung Cancer cell line TC-1 were used. Mice were followed for 6 weeks after injection of tumor cells. TGF-β was inhibited in mice with 5mg/kg of SB-505124 (hydrochloride hydrate, Sigma); CXCR4 was inhibited in mice with 5mg/kg of AMD3100 (Sigma), as previously described (Azab et al., 2009).
Gene Analysis Accession Number
Micro Array data were deposited on the Gene Expression Omnibus (GEO) and can be found under the following accession number: GSE23548
Significance.
Tumorigenesis is driven by alterations in tumor cells but also changes in the stromal microenvironment. We demonstrate that a significant percentage of CAFs in inflammation-associated gastric cancer originate from MSCs and that MFs contribute to the normal stem cell niche in the BM. MSC give rise to their own niche cells, which might apply to other stem cell niches. In inflammation-induced cancer progression MFs increases in the BM niche and blood during progression to dysplasia. Therefore, we propose a model in which the earliest stages of tumor development are characterized by remodeling of the BM, followed by relocation of the MSC-CAF stem cell niche to the tumor site where it promotes tumor progression.
Supplementary Material
Acknowledgments
We would like to thank D. Brenner and S. Magness for the generous gift of αSMA-RFP and Collagen-α1-GFP mice, H. Nakagawa for instructing in organotypic culture, P. Good for excellent technical assistance, A. Whelan and J. DeGrazia for managing the mouse colony, R. Chen for help with IHC, K. Novak for critical reading and editing of the manuscript, and all members of the T.C.W. lab for helpful comments and discussion. T.C.W. is supported by National Institutes of Health grants 1U54CA126513, RO1CA093405 and R01CA120979. M.Q. is supported by a grant from the Mildred-Scheel-Stiftung, Deutsche Krebshilfe, Germany. W.S. is supported by the Japan Society for the Promotion of Science 2009, ST is supported a NIH grant R21CA149865.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, Porter D, Hu M, Chin L, Richardson A, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Azab AK, Runnels JM, Pitsillides C, Moreau AS, Azab F, Leleu X, Jia X, Wright R, Ospina B, Carlson AL, et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009;113:4341–4351. doi: 10.1182/blood-2008-10-186668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabletz S, Schmalhofer O, Brabletz T. Gastrointestinal stem cells in development and cancer. J Pathol. 2009;217:307–317. doi: 10.1002/path.2475. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Direkze NC, Forbes SJ, Brittan M, Hunt T, Jeffery R, Preston SL, Poulsom R, Hodivala-Dilke K, Alison MR, Wright NA. Multiple organ engraftment by bone-marrow-derived myofibroblasts and fibroblasts in bone-marrow-transplanted mice. Stem Cells. 2003;21:514–520. doi: 10.1634/stemcells.21-5-514. [DOI] [PubMed] [Google Scholar]
- Direkze NC, Hodivala-Dilke K, Jeffery R, Hunt T, Poulsom R, Oukrif D, Alison MR, Wright NA. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res. 2004;64:8492–8495. doi: 10.1158/0008-5472.CAN-04-1708. [DOI] [PubMed] [Google Scholar]
- Erez N, Truitt M, Olson P, Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell. 2010;17:135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- Forbes SJ, Russo FP, Rey V, Burra P, Rugge M, Wright NA, Alison MR. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology. 2004;126:955–963. doi: 10.1053/j.gastro.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Oshima H, Kitmura T, Taketo MM, Oshima M. Stromal fibroblasts activated by tumor cells promote angiogenesis in mouse gastric cancer. J Biol Chem. 2008;283:19864–19871. doi: 10.1074/jbc.M800798200. [DOI] [PubMed] [Google Scholar]
- Hayward SW, Wang Y, Cao M, Hom YK, Zhang B, Grossfeld GD, Sudilovsky D, Cunha GR. Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer Res. 2001;61:8135–8142. [PubMed] [Google Scholar]
- Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–1571. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- Houghton J, Wang TC. Helicobacter pylori and gastric cancer: a new paradigm for inflammation-associated epithelial cancers. Gastroenterology. 2005;128:1567–1578. doi: 10.1053/j.gastro.2005.03.037. [DOI] [PubMed] [Google Scholar]
- Ishii G, Sangai T, Oda T, Aoyagi Y, Hasebe T, Kanomata N, Endoh Y, Okumura C, Okuhara Y, Magae J, et al. Bone-marrow-derived myofibroblasts contribute to the cancer-induced stromal reaction. Biochem Biophys Res Commun. 2003;309:232–240. doi: 10.1016/s0006-291x(03)01544-4. [DOI] [PubMed] [Google Scholar]
- Izadpanah R, Kaushal D, Kriedt C, Tsien F, Patel B, Dufour J, Bunnell BA. Long-term in vitro expansion alters the biology of adult mesenchymal stem cells. Cancer Res. 2008;68:4229–4238. doi: 10.1158/0008-5472.CAN-07-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Gonda TA, Gamble MV, Salas M, Seshan V, Tu S, Twaddell WS, Hegyi P, Lazar G, Steele I, et al. Global hypomethylation of genomic DNA in cancer-associated myofibroblasts. Cancer Res. 2008;68:9900–9908. doi: 10.1158/0008-5472.CAN-08-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res. 2006;66:4553–4557. doi: 10.1158/0008-5472.CAN-05-3986. [DOI] [PubMed] [Google Scholar]
- Lo Celso C, Fleming HE, Wu JW, Zhao CX, Miake-Lye S, Fujisaki J, Cote D, Rowe DW, Lin CP, Scadden DT. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–96. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeshima AM, Niki T, Maeshima A, Yamada T, Kondo H, Matsuno Y. Modified scar grade: a prognostic indicator in small peripheral lung adenocarcinoma. Cancer. 2002;95:2546–2554. doi: 10.1002/cncr.11006. [DOI] [PubMed] [Google Scholar]
- Magness ST, Bataller R, Yang L, Brenner DA. A dual reporter gene transgenic mouse demonstrates heterogeneity in hepatic fibrogenic cell populations. Hepatology. 2004;40:1151–1159. doi: 10.1002/hep.20427. [DOI] [PubMed] [Google Scholar]
- Mathur D, Bost A, Driver I, Ohlstein B. A transient niche regulates the specification of Drosophila intestinal stem cells. Science. 2010;327:210–213. doi: 10.1126/science.1181958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKaig BC, Makh SS, Hawkey CJ, Podolsky DK, Mahida YR. Normal human colonic subepithelial myofibroblasts enhance epithelial migration (restitution) via TGF-beta3. Am J Physiol. 1999;276:G1087–1093. doi: 10.1152/ajpgi.1999.276.5.G1087. [DOI] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, Ganesan S, Glod JW, Banerjee D. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008;68:4331–4339. doi: 10.1158/0008-5472.CAN-08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441:1068–1074. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- Okawa T, Michaylira CZ, Kalabis J, Stairs DB, Nakagawa H, Andl CD, Johnstone CN, Klein-Szanto AJ, El-Deiry WS, Cukierman E, et al. The functional interplay between EGFR overexpression, hTERT activation, and p53 mutation in esophageal epithelial cells with activation of stromal fibroblasts induces tumor development, invasion, and differentiation. Genes Dev. 2007;21:2788–2803. doi: 10.1101/gad.1544507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Pera MF, Andrade J, Houssami S, Reubinoff B, Trounson A, Stanley EG, Ward-van Oostwaard D, Mummery C. Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist noggin. J Cell Sci. 2004;117:1269–1280. doi: 10.1242/jcs.00970. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Simmons PJ, Przepiorka D, Thomas ED, Torok-Storb B. Host origin of marrow stromal cells following allogeneic bone marrow transplantation. Nature. 1987;328:429–432. doi: 10.1038/328429a0. [DOI] [PubMed] [Google Scholar]
- Sneddon JB, Zhen HH, Montgomery K, van de Rijn M, Tward AD, West R, Gladstone H, Chang HY, Morganroth GS, Oro AE, Brown PO. Bone morphogenetic protein antagonist gremlin 1 is widely expressed by cancer-associated stromal cells and can promote tumor cell proliferation. Proc Natl Acad Sci U S A. 2006;103:14842–14847. doi: 10.1073/pnas.0606857103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, Clevers H. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Stappenbeck TS, Miyoshi H. The role of stromal stem cells in tissue regeneration and wound repair. Science. 2009;324:1666–1669. doi: 10.1126/science.1172687. [DOI] [PubMed] [Google Scholar]
- Tomita H, Takaishi S, Menheniott TR, Yang X, Shibata W, Jin G, Betz K, Kawakami K, Minamoto T, Tomasetto C, et al. Inhibition of gastric carcinogenesis by the hormone, gastrin, is mediated by suppression of TFF1 epigenetic silencing. Gastroenterology. 2010 doi: 10.1053/j.gastro.2010.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, Betz KS, Penz-Oesterreicher M, Bjorkdahl O, Fox JG, Wang TC. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408–419. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen L, De Sousa EMF, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- Wang SS, Asfaha S, Okumura T, Betz KS, Muthupalani S, Rogers AB, Tu S, Takaishi S, Jin G, Yang X, et al. CFU-F Forming Bone Marrow Cells Delay Progression To Gastric Dysplasia In A Helicobacter Model of Gastric Tumorigenesis. Stem Cells. 2009 doi: 10.1002/stem.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea--a paradigm shift. Cancer Res. 2006;66:1883–1890. doi: 10.1158/0008-5472.CAN-05-3153. discussion 1895–1886. [DOI] [PubMed] [Google Scholar]
- Worthley DL, Ruszkiewicz A, Davies R, Moore S, Nivison-Smith I, Bik To L, Browett P, Western R, Durrant S, So J, et al. Human gastrointestinal neoplasia-associated myofibroblasts can develop from bone marrow-derived cells following allogeneic stem cell transplantation. Stem Cells. 2009;27:1463–1468. doi: 10.1002/stem.63. [DOI] [PubMed] [Google Scholar]
- Yokota T, Kawakami Y, Nagai Y, Ma JX, Tsai JY, Kincade PW, Sato S. Bone marrow lacks a transplantable progenitor for smooth muscle type alpha–actin-expressing cells. Stem Cells. 2006;24:13–22. doi: 10.1634/stemcells.2004-0346. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li L. BMP signaling and stem cell regulation. Dev Biol. 2005;284:1–11. doi: 10.1016/j.ydbio.2005.05.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.