Abstract
Osteoporosis is a systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase in bone fragility and susceptibility to fracture. In order to improve the treatment of osteoporosis, identification of anabolic and orally available agents with minimal side effects is highly desirable. Psoralen is a coumarin-like derivative extracted from Chinese herbs, which have been used to treat bone diseases for thousands of years. However, the role of Psoralen in osteoblast function and the underlying molecular mechanisms remain poorly understood. In this study, we found that Psoralen promoted osteoblast differentiation in primary mouse calvarial osteoblasts in a dose-dependent manner, demonstrated by up-regulation of expressions of osteoblast-specific marker genes including type I collagen, osteocalcin and bone sialoprotein and enhancement of alkaline phosphatase activity. We further demonstrated that Psoralen up-regulated the expression of Bmp2 and Bmp4 genes, increased the protein level of phospho-Smad1/5/8, and activated BMP reporter (12xSBE-OC-Luc) activity in a dose-dependent manner, as well as enhanced the expression of Osx, the direct target gene of BMP signaling. Deletion of the Bmp2 and Bmp4 genes abolished the stimulatory effect of Psoralen on expression of osteoblast marker genes, such as Col1, Alp, Oc and Bsp. Our results suggest that Psoralen acts through activation of BMP signaling to promote osteoblast differentiation and demonstrate that Psoralen could be a potential anabolic agent to treat patients with bone loss-associated diseases such as osteoporosis.
Keywords: Psoralen, BMP-2, BMP-4, Osteoblast, Osteoporosis
Introduction
Osteoporosis is a systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with consequent increases in bone fragility and susceptibility to fracture [1]. There are about 44 million people in the United States affected by osteoporosis and low bone mass [2]. The clinical complications include fractures, disability and chronic pain. It is estimated that 54% of women age 50 and older will sustain an osteoporotic fracture during their lifetime [3]. Further, approximately 24% of patients who experience a hip fracture will die within a year [4]. Osteoporosis has been a major public health threat for the elderly, particularly for postmenopausal women.
The current clinical treatment regimens for osteoporosis are anti-resorptive drugs, which maintain bone mass by inhibiting osteoclast function, such as estrogen, estrogen receptor analogues, calcitonin and bisphosphates. The effect of these drugs in increasing bone mass or recovering bone loss is relatively minor, probably no more than 2% per year [5]. The potential complications, including breast cancer, uterine bleeding and cardiovascular events, also restrict their usage for osteoporosis. It is desirable, therefore, to identify better and safe anabolic agents with low cyto-toxicity. Since new bone formation is primarily mediated by osteoblasts, agents that act by either increasing the osteoblast proliferation or inducing osteoblast differentiation can enhance bone formation [6,7].
Chinese herbal medicine has been widely used to prevent and treat diseases for thousands of years. Psoralea corylifolia fruit (Buguzhi) is one of the commonly used herbs in formulas that are prescribed for the treatment of fractures, bone and joint diseases. Psoralen (molecular formula: C3H6O3, molecular weight: 186.16) is the main active ingredients extracted from the fruits of Psoralea corylifolia L.. It has been reported that Psoralen when applied with collagen matrix had stimulatory effect on local new bone formation in vivo [8]. However, effects of Psoralen on osteoblast function and the underlying molecular mechanisms remain poorly unknown. In this study, we clarified the detailed molecular mechanisms of Psoralen on osteoblast function using primary osteoblast cells. The results demonstrated that Psoralen induced osteoblast differentiation through activation of BMP signaling in osteoblasts.
Materials and Methods
Materials and Reagents
Psoralen was obtained from the company of Sigma (St. Louis, MO) and the purity of this compound is more than 99%. Stock solutions of Psoralen were prepared in dimethyl sulfoxide (DMSO; Sigma, St.Louis, MO) and stored at −20°C.
Cell Culture
Calvariae from neonatal mice were removed, washed with sterile PBS solution, digested in 0.2% collagenase A (Roche, Indianapolis, IN) for 1 hour at 37°C with shaking, then pooled and collected cells. Cells were cultured on 25 cm2 flasks in a-Minimum Essential Medium (a-MEM; GibcoBRL, GrandIsland, NY) supplemented with 10% fetal bovine serum (FBS; GibcoBRL, GrandIsland, NY) and antibiotics (100U/ml of penicillin-streptomycin; Invitrogen, Carlsbad, CA) Cell culture medium was replaced every three days. When osteoblast cells reached 80% confluence, they were harvested with 0.25% trypsin-EDTA solution. The cells were seeded in 96-well plates and 6-well plates at a density of 1×104 and 1×106 cells/well respectively and cultured in a humidified atmosphere of 5% CO2 and 95% air, at 37°C. All animal protocols were approved by the University Committee on Animal Resources of the University of Rochester.
Cell Viability Assay
Primary calvarial osteoblasts were seeded in 96-well plates at a density of 1×104 cells/well. After 2-day culture, cells were treated with Psoralen at concentrations of 0, 1, 10, 100, and 1000 µM for 48 hours. The supernatant was then removed and 100 µl of Cell-Titer Blue buffer (Promega, Madison, WI) was added to each well. After incubation at 37°C for 4 hours, the cell viability assay was performed and the fluorescence activity was detected at excitation/emission wavelengths of 560/590 nm in a FLUO-STAR plate reader (Promega, Madison, WI).
Cell Proliferation Assay
Inhibition of cell proliferation by Psoralen was measured by 3-[4,5-dimethylthiazol]-2,5-diphenylterazolium bromide assay (MTT assay). Osteoblasts cells were plated in 96-well plates at a density of 1×104 cells/well. After 2-day culture, the cells were treated with Psoralen (0, 1, 10, and 100 µM) for 48 hours. The supernatant was then removed and 20 µl of 0.5% MTT (Sigma, St. Louis, MO) was added to each well. After 4-h incubation at 37°C, the supernatant was again removed and acid-ethanol (100 ml of 0.04N HCl in ethanol) was added to all wells and mixed thoroughly to dissolve the dark blue crystals. The plates were read on an ELISA reader (Multiskan EX; Thermo Electron Corporation, Waltham, MA) at a wavelength of 490 nm and a reference wavelength of 690 nm was also used.
Quantitative PCR (qPCR)
Osteoblasts cells were seeded in 6-well plates at a density of 1×106 cells/well. After 2-day culture, Cells were treated with Psoralen at concentrations of 0, 10, 50 and 100 µM for 48 hours. Total RNA from the cells of each well was isolated respectively using RNeasy Mini Kit (Qiagen, Valencia, CA). One µg of total RNA was reverse-transcribed separately into cDNA using the iScriptcDNA synthesis kit (Bio-Rad, Hercules, CA). Quantitative PCR (qPCR) analysis was carried out using Absolute QPCR SYBR Green Master Mix (Thermo Scientific, Waltham, MA) in a total volume of 20 µl of buffered solution containing 1 µl of the diluted (1:5) reverse transcription product in the presence of 10 pM of sense and antisense primers specific for the genes listed in Table 1. The cycling conditions were 15-min polymerase activation at 95 °C followed by 45 cycles, 95 °C for 20 s, 58°C for 20 s and 72°C for 30 s. All reactions were performed in triplicate.
Table 1.
Mouse primers for real-time quantitative PCR assays
| Genes | Forward primer | Reverse Primer |
|---|---|---|
| β-actin | 5′-TGTTACCAACTGGGACGACGACA-3′ | 5′-CTGGGTCATCTTTTCACGGT-3′ |
| Alp | 5′-TGACCTTCTCTCCTCCATCC-3′ | 5′-CTTCCTGGGAGTCTCATCCT-3′ |
| Bmp2 | 5′-ACTTTTCTCGTTTGTGGAGC-3′ | 5′-GAACCCAGGTGTCTCCAAGA-3′ |
| Bmp4 | 5′-GAGGAGGAGGAAGAGCAGAG-3′ | 5′-TGGGATGTTCTCCAGATGTT-3′ |
| Osx | 5′-AAAGGAGGCACAAAGAAG-3′ | 5′-CACCAAGGAGTAGGTGTGTT-3′ |
| Col1a1 | 5′-CCTGGTAAAGATGGGCC-3′ | 5′-CACCAGGTTCACCTTTCGCACC-3′ |
| Oc | 5′-CTTGAAGACCGCCTACAAAC-3′ | 5′-GCTGCTGTGACATCCATAC-3′ |
| Bsp | 5′-AGGACTGCCGAAAGGAAGGTTA-3′ | 5′-AGTAGCGTGGCCGGTACTTAAA-3′ |
ALP Activity/Staining Assay
After cultured in 6-well plates with Psoralen (0, 1, 10, and 100 µM) for 48 hours, osteoblast cells were lysed with Passive Lysis Buffer (Promega, Madison, WI). Protein concentrations were determined by Coomassie Protein Assay Kit (BIORAD, Hercules, CA) and ALP activities were analyzed with solutions containing 0.5 mg/ml of p-nitrophenyl-phosphate (Sigma, St. Louis, MO) in AMP buffer (0.5 M 2-methyl-1, 2-aminopropanol and 2 mM magnesium chloride, pH 10.3) at 37°C for 15 minutes. The reaction was stopped by a solution containing 0.3M sodium phosphate (pH=12.3). The collected medium was determined by reading the absorption value at 405 nm and the enzyme activity were normalized by the protein concentration. For the ALP staining, the cells were fixed in 10% Neutral Buffered Formalin for 15 min, washed, and then incubated with ALP staining buffer, NBT-BCIP (BIORAD, Hercules, CA) at 37°C for 30 min. All reactions were performed in triplicate independently.
Western Blot Analysis
Osteoblasts cells were cultured in 6-well plates with Psoralen at concentrations of 0, 1, 10 and 100 µM. After 2-h and 48-h culture, cells lysates were respectively extracted with E-PER protein extraction reagents (Thermo Scientific, Waltham, MA) according to the manufacturer’s protocol. Proteins were transferred onto a PVDF membrane (BIORAD, Hercules, CA) and the membrane was blocked with 5% non-fat milk in PBST solution for 1 hour at room temperature (RT). After incubation with the primary antibody overnight at 4°C and the HRP-conjugated secondary antibodies (Thermo Scientific, Waltham, MA) for 1 hours at RT, the protein expression was detected using a SuperSignal West Femto Maximum Sensitivity Substrate Kit (Thermo Scientific, Waltham, MA).
The anti-β-Actin polyclonal mouse antibody (Sigma, St. Louis, MO), anti-osterix polyclonal rabbit antibody (Abcam, Cambridge, MA), anti-phospho-Smad1(Ser463/465)/Smad5(Ser463/465)/Smad8(Ser426/428) and anti-total-Smad1 polyclonal rabbit antibodies (Cell Signaling Technologies, Beverly, MA) were used as primary antibodies.
Dual Luciferase Assay
Osteoblast cells were seeded at a density of 1×104 cells/well in 96-well plates. The BMP signaling reporter construct, 12xSBE-OC-Luc,(29) and the SV40-Renilla luciferase construct was co-transfected into the cells using FuGENE HD Transfection Reagent (Invitrogen, Carlsbad, CA). Cells were incubated with Psoralen at concentrations of 0, 10, 50 and 100 µM for 48 hours. Cell lysates were then extracted and the relative amounts of Renilla and firefly luciferase were measured using a dual luciferase assay kit (Promega, Madison, WI). The Renilla/firefly luciferase ratio was calculated and normalized against the control.
Rescue assay by in vitro deletion of the Bmp2 and Bmp4 Gene
The Bmp2fx/fx;Bmp4fx/fx mice were genotyped and identified by PCR using the following primers: wild-type allele: the upper primer, 5’-AGGGTTTCAGGTCAGTTTCCG-3’ and the lower primer, 5’-TCCGAAGGTAAGTGTGCTTGG-3’ ; and BMP2-floxed allele: upper primer, 5’-AGGGTTTCAGGTCAGTTTCCG-3’ and the lower primer, 5’-GATGATGAGGTTCTTGGCGG-3’; Bmp4-floxed allele: upper primer, 5′-AGACTCTTTAGTGAGCATTTTCAAC-3′ and the lower primer, 5′-AGCCCA ATTTCCACAACTTC-3′.
Primary calvarial osteoblasts isolated from 3-day-old neonatal Bmp2fx/fx;Bmp4fx/fx mice were cultured in αMEM supplemented with 10% FBS. Cells were infected with Ad-GFP or Ad-Cre (titer: 4×108 pfu/ml, Baylor College of Medicine, Houston, TX) for 3 days. After recovery for 2 days, cells were treated with or without Psoralen (100 µM) for 48 hours. Changes in expression of Bmp2, Bmp4, type I collagen (Col1), osteocalcin (Oc), alkaline phosphatase (Alp) and bone sialoprotein (Bsp) were examined using qPCR analysis.
Statistical Analysis
All exprements were repeated three times independently and all the data were expressed as means ± standard deviation (SD). Statistical comparisons with results of multiple groups were analyzed using one-way ANOVA followed by Dunnett’s test. For experiments involving two groups, unpaired Student’s t-test was performed. A value of p<0.05 was considered statistically significant.
Results
Psoralen Had No Effect on Osteoblast Proliferation
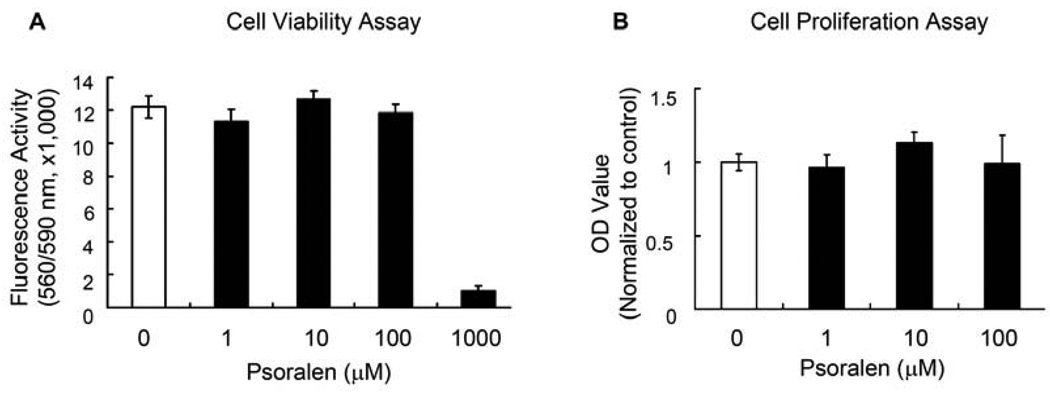
Assessment of cell viability did not detect obvious toxicity when Psoralen was used at a dose up to 100 µM. However, when the dose reached 1000 µM, Psoralen started to show toxic effect (Fig. 1A). Based on these observations, 1, 10 and 100 µM of Psoralen was used in the following experiments. We first measured the effect of Psoralen on cell proliferation of primary mouse calvarial osteoblasts by MTT assay. As shown in Fig. 1B, Psoralen did not have significant effects on cell growth at the concentrations of 1–100 µM after 48-h treatment in primary osteoblasts.
Fig. 1.
Psoralen has no effect on osteoblast proliferation. A) Cell viability assays showed that Psoralen had no cell toxicity when used at concentrations ranging from 0 to 100 µM. B) MTT assays showed that Psoralen did not have significant effects on cell growth at the concentrations used (1–100 µM) after 2-d treatment in primary mouse calvarial osteoblasts.
Psoralen Stimulates Osteoblast Differentiation
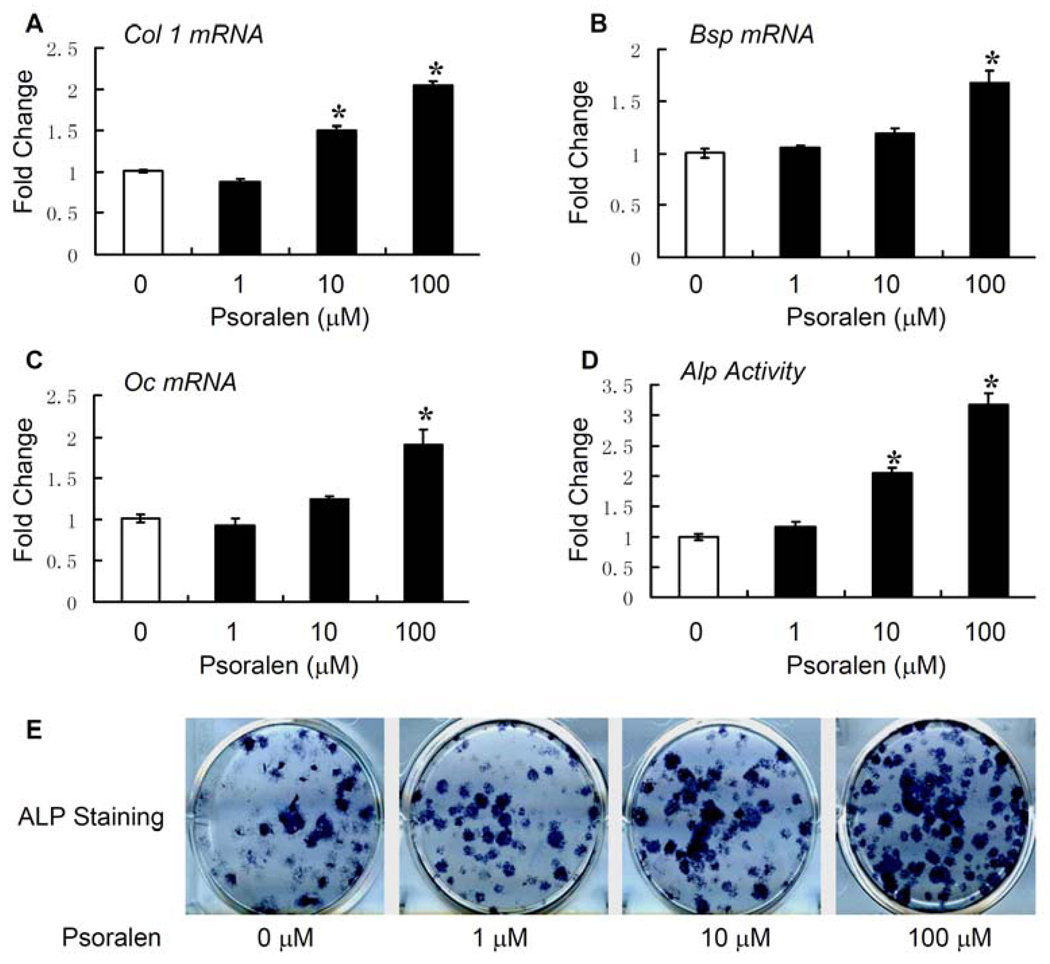
To determine if Psoralen has effects on osteoblast differentiation, the qPCR assay was performed. Psoralen promoted osteoblast differentiation in a dose-dependent manner, demonstrated by the up-regulation of osteoblast-specific marker genes such as Col1, Bsp and Oc (Fig. 2A–C) after 48-h treatment in primary osteoblasts. Since an increase of ALP activity is another important indicator of osteoblast differentiation, we also measured ALP activity and performed ALP staining assay. We found that Psoralen enhanced ALP activity dose-dependently in primary osteoblasts. Psoralen had the most significant effects (3.2-fold increase of ALP acticvity) at the concentration of 100 µM (Fig. 2D, E). Taken together, these results suggest that Psoralen significantly stimulates osteoblast differentiation.
Fig. 2.
Psoralen stimulates osteoblast differentiation. Psoralen (0, 1, 10 and 100 µM) was added to the cell culture of primary mouse calvarial osteoblasts for 2 days. Quantitative PCR (qPCR) assay was performed to examine the expression of osteoblast-specific genes after treatment with Psoralen. A) Psoralen increased the mRNA expression of Col1 in osteoblasts in a dose-dependent manner, with more pronounced effect at the concentration of 100 µM. B, C) Treatment with Psoralen at the concentration of 100 µM also significantly increased the mRNA expression of Oc and Bsp in osteoblasts. D) Psoralen dose-dependently enhanced the alkaline phosphatase (ALP) activity in osteoblasts, especially at the concentration of 100 µM. E) ALP staining also showed treatment with Psoralen at the concentrations of 10 and 100 µM increased the ALP activity in osteoblasts. *p<0.05 when compared to the group without Psoralen.
Psoralen Activates BMP Signaling
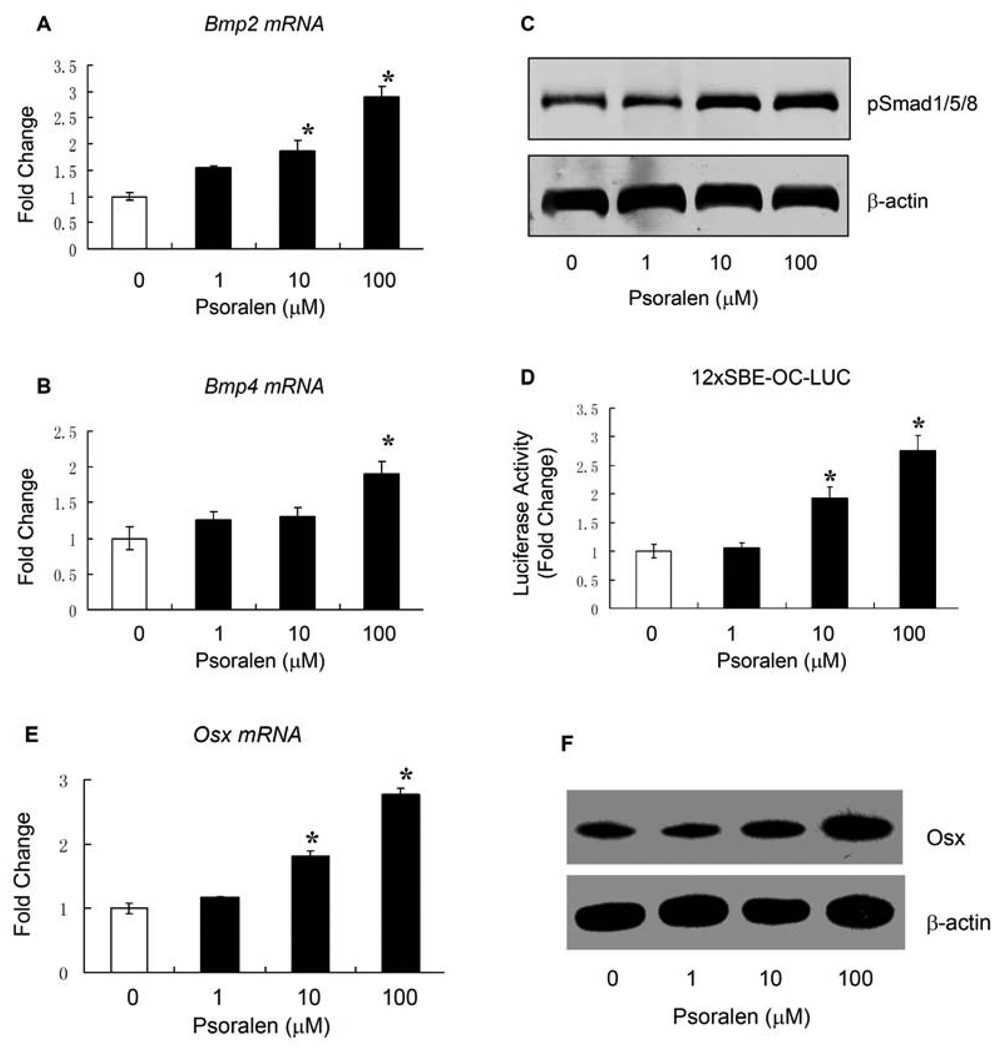
BMPs have been shown to play an important role in osteoblast differentiation. To determine the mechanism of Psoralen on osteoblast differentiation, we examined the effect of Psoralen on the expression of Bmp2 and Bmp4 and the activity of BMP signaling. Our qPCR data showed that Psoralen significantly enhanced mRNA expressions of Bmp2 and Bmp4 in primary osteoblasts. As shown in Fig. 3A and Fig. 3B, cells treated with Psoralen at the concentration of 100 µM had the most significant effect (2.9-fold increase for Bmp2 and 1.9-fold increase for Bmp4, respectively). To determine if Psoralen activates the downstream molecules of BMP signaling, we measured the protein levels of phosphorylated Smad1/5/8 and found that Psalen significantly enhanced phosphorylated Smad1/5/8 levels in primary osteoblasts at the concentrations of 10 and 100 µM (Fig. 3C), although had no significant effect on the total Smad1 protein level (Data now shown). To further confirm the role of Psoralen on BMP signaling, we also examined the effect of Psoralen on the activity of a BMP signaling reporter, 12xSBE-OC-Luc. Consistently, Psoralen stimulated the luciferase activity of this BMP signaling reporter in primary osteoblasts, especially at the concentration of 100 µM (a 2.8-fold increase of luciferase activity) (Fig. 3D). We finally examined the effect of Psoralen on the expression of Osx, the direct target gene of BMP signaling and found that treatment with Psoralen at the concentration of 100 µM significantly increased the expression of Osx, including the mRNA and protein levels. Taken together, these results demonstrate that Psoralen may regulate osteoblast differentiation through activation of BMP signaling.
Fig. 3.
Psoralen activates BMP signaling in osteoblast cells. Primary mouse calvarial osteoblasts were treated with Psoralen (0, 1, 10 and 100 µM) for 2 days. A) qPCR results showed that Psoralen dose-dependently increased the mRNA levels of Bmp2 in osteoblasts, more pronounced effect was observed at the concentration of 100 µM. B) qPCR results also showed that treatment with Psoralen at the concentration of 100 µM significantly increased the mRNA level of Bmp4 in osteoblasts. C) Western blot analysis showed that Psoralen stimulated the phosphorylation of Smad1/5/8 at the concentrations of 10 and 100 µM in osteoblast cells. D) Dual luciferase assay showed that the activity of BMP signaling reporter (12xSBE-OC-Luc) in osteoblasts were significantly increased after treated with Psoralen at the concentration of 10 or 100 µM. E–F) In addition, treatment of primary osteoblasts with Psoralen at the concentration 100 µM, significantly enhanced the mRNA and protein expression of Osx, the direct target gene of BMP signaling. *p<0.05 when compared to the group without Psoralen.
Psoralen-Induced Osteoblast Differentiation Is BMP-2- and BMP4-Dependent
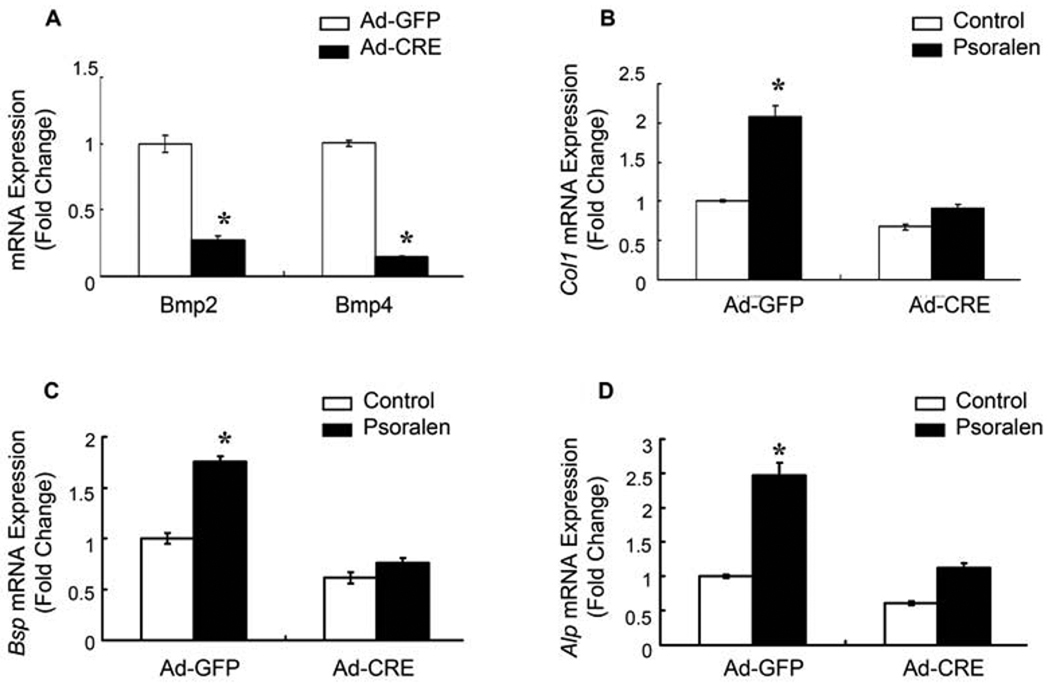
To further determine if Psoralen-induced osteoblast differentiation is dependent on its activation of BMP signaling, primary calvarial osteoblasts were isolated from Bmp2fx/fx;Bmp4fx/fx mice and infected with adenovirus expressing Cre recombinase (Ad-Cre). Infection of Ad-Cre efficiently deleted the BMP-2 and BMP-4 gene in primary osteoblast cells (Fig. 4A). Deletion of the Bmp2 and Bmp4 genes significantly inhibited Psoralen-induced expression of Col1, Alp and Bsp in primary osteoblasts (Fig. 4B–D). Thus, our results suggest that Psoralen may induce osteoblast differentiation in a BMP-2- and BMP-4-dependent manner.
Fig. 4.
Psoralen-induced osteoblast differentiation is BMP-2- and BMP-4-dependent. Primary calvarial osteoblasts isolated from 3-day-old neonatal Bmp2fx/fx;Bmp4fx/fx mice were infected with either Ad-GFP or Ad-Cre and then treated with or without 100 µM Psoralen for 2 days. A) qPCR data indicated that infection of Ad-Cre efficiently deleted the Bmp2 and Bmp4 gene in primary osteoblasts. B–D) Deletion of the Bmp2 and Bmp4 genes partially reversed Psoralen-induced expression of Col1, Alp and Bsp in osteoblasts. *p<0.05 when compared to the group without Psoralen.
Discussion
Psoralen is a tricyclic aromatic compound, which is present in medicinal herbs such as Psoralea corylifolia L., Glehnia littoralis Fr., Schmidr ex Miq., Heracleum lanatum Michx., Ruta grveolens L. and Ficus carica. Previous studies showed that Prosalen had cardiovascular improved effect, smooth muscle diastolic effect, bacteriostatic effect, anti-neoplastic effect, hemostatic effect, photosensitivity effect and effect of stimulating bone formation [8,9,10]. In the present studies, we demonstrated that Psoralen induced osteoblast differentiation without affecting cell growth. Treatment of primary osteoblasts with Psoralen increased the expression of Col1a1, Alp, Oc and Bsp (markers of osteoblast differentiation), suggesting that Psoralen is a potent agent regulating osteoblast differentiation.
BMPs play an important role in postnatal bone formation and bone remodeling [11]. A genetic study using a combined linkage and association analyses has linked the Bmp2 gene to a phenotype of low BMD and high fracture risk [12]. Polymorphisms in the Bmp4 gene are also demonstrated to be associated with senile osteoporosis [13]. BMP-2 and BMP-4 have stimulatory effect on the expression of Col1, Alp and Oc [14]. They act on bone cells by binding to their cell surface receptors and subsequently inducing phosphorylation of Smads1/5/8. Phosphorylated Smad1/5/8 forms a complex with Smad4 and translocates into the nucleus whereby it activates the transcription of bone-specific genes and then stimulates bone formation [15–19]. In fact, several small molecular weight compounds have been identified by their abilities to activate BMP signaling. It has been reported that statins stimulated bone formation by up-regulation of Bmp2 activity [20]. A similar effect was also observed with proteasome inhibitors and microtubule inhibitors that increased Bmp2 expression through inhibition of Gli2 and Gli3 degradation and induced osteoblast differentiation [21–23]. Our previous study demonstrated that Osthole, a coumarin derivative present in many herbs such as Cnidium monnieri and Angelica pubescens, induced Bmp2 expression through activation of Wnt/β-catenin signaling and stimulated osteoblast differentiation [24]. To clarify the mechanism by which Psoralen promotes osteoblast differentiation, we examined its effects on expression of Bmp genes. Our data showed that treatment of primary osteoblasts with Psoralen enhanced the Bmp2 and Bmp4 expression. We further demonstrated that Psoralen enhanced phosphorylation of Smad1/5/8, the central molecules in BMP signaling, and increased the expression of Osx, the downstream target gene of BMP signaling.
To further determine if Psoralen-induced osteoblast differentiation is BMP-2- and BMP-4-dependent, we have used an in vitro gene deletion approach by infecting osteoblasts derived from Bmp2fx/fx;Bmp4fx/fx mice with Ad-Cre. We found that deletion of the Bmp2 and Bmp4 genes in osteoblasts blocked Psoralen-induced osteoblast differentiation, suggesting that Psoralen may stimulate osteoblast differentiation by activation of BMP signaling pathway. Targeted deletion of the Bmp2 and Bmp4 genes only partially reverses the effects of Psoralen on Col1, Alp and Bsp expression in osteoblasts. This observation could be attributed by several potential reasons. First, in vitro deletion of the Bmp2 and Bmp4 genes in osteoblasts may not be complete. Second, other BMP genes may also play a role in Psoralen-induced osteoblast differentiation. Indeed, our qPCR results indicated that Psoralen at a concentration of 100 µM also up-regulated the expression of Bmp7 in primary osteoblasts with 3.8-fold increase (data not shown), although it had no significant effect on the expression of BMP-6 (data not shown). Third, BMP signaling may not be the only pathway that Psoralen acts on to stimulate osteoblast differentiation. In summary, our study indicates that Psoralen stimulates osteoblast differentiation by activation of BMP signaling. Psoralen may have a potential application in the treatment of osteoporosis and non-union fracture.
Acknowledgments
We would like to thank Dr. Stephen Harris (University of Texas Health Science Center, San Antonio, TX) for providing us the Bmp2fx/fx;Bmp4fx/fx mice. This work was supported in part by the National Basic Research Program of China (973 Program,2010CB530400 to YW), the Key Program of Natural Science Foundation of China (30930111 to YW), the International Cooperation Programs of National Natural Science Foundation of China (30710103904 to YW), the Key Basic Research Program of Shanghai Natural Science Foundation of China (07JC14050 to QS), and by the National Institute of Health (NIH) Grants (R01 AR055915, R01 AR051189 and R01 AR054465 to DC).
Abbreviations
- OP
osteoporosis
- BMP
bone morphogenetic protein
- Smad
signaling mothers against decapentaplegic
- Oc
osteocalcin
- Col1
collagen type 1
- Bsp
bone sialoprotein
- Alp
alkaline phosphatase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: All authors have no conflicts of interest
References
- 1.Christiansen C. Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94:646–650. doi: 10.1016/0002-9343(93)90218-e. [DOI] [PubMed] [Google Scholar]
- 2.Lindsay R. A guide to Diagnosis, Prevention, and Treatment. New York: Raven Press; 1992. Osteoporosis. [Google Scholar]
- 3.Chrischilles EA, Butler CD, Davis CS, et al. A model of lifetime osteoporosis impact. Arch Intern Med. 1991;151:2026–2032. [PubMed] [Google Scholar]
- 4.US Congress OoTA. Washington, DC: US Government Printing Office; Hip Fracture Outcomes in People Age 50 and Over-Background Paper. 1994
- 5.Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–1514. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 6.Ducy P, Schinke T, Karsenty G. The osteoblast: a sophisticated fibroblast under central surveillance. Science. 2000;289:1501–1504. doi: 10.1126/science.289.5484.1501. [DOI] [PubMed] [Google Scholar]
- 7.Lane NE, Kelman A. A review of anabolic therapies for osteoporosis. Arthritis Res Ther. 2003;5:214–222. doi: 10.1186/ar797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong RWK, Rabie ABM. Effect of psoralen on bone formation. J Orthop Res. 2010;3:1–5. doi: 10.1002/jor.21124. [DOI] [PubMed] [Google Scholar]
- 9.Miura H, Nishida H, Linuma M. Effect of crude fraction of Psoralea corylifolia seed extract on bone calcification. Planta Med. 1996;62:150–153. doi: 10.1055/s-2006-957839. [DOI] [PubMed] [Google Scholar]
- 10.Wong RWK, Rabie ABM. Effect of Buguzhi (Psoralea corylifolia fruit) extract on bone formation. Phytother Res. 2010;24 Suppl 2:S233–S234. doi: 10.1002/ptr.3049. [DOI] [PubMed] [Google Scholar]
- 11.Sykaras N, Opperman LA. Bone morphogenetic proteins (BMPs): how do they function and what can they offer the clinician? J Oral Sci. 2003;45:57–73. doi: 10.2334/josnusd.45.57. [DOI] [PubMed] [Google Scholar]
- 12.Styrkarsdottir U, Cazier JB, Kong A, et al. Linkage of osteoporosis to chromosome 20p12 and association to BMP2. PLoS Biol. 2003;1:E69. doi: 10.1371/journal.pbio.0000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramesh Babu L, Wilson SG, Dick IM, et al. Bone mass effects of a BMP4 gene polymorphism in postmenopausal women. Bone. 2005;36:555–561. doi: 10.1016/j.bone.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Xiao Y, Haase H, Young WG, Bartold PM. Development and transplantation of a mineralized matrix formed by osteoblasts in vitro for bone regeneration. Cell Transplant. 2004;13:15–25. doi: 10.3727/000000004772664851. [DOI] [PubMed] [Google Scholar]
- 15.Bilican B, Fiore-Heriche C, Compston A, et al. Induction of Olig2 precursors by FGF involves BMP signalling blockade at the Smad level. PLoS ONE. 2008;3:e2863. doi: 10.1371/journal.pone.0002863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao X, Chen D. The BMP signaling and in vivo bone formation. Gene. 2005;357:1–8. doi: 10.1016/j.gene.2005.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 18.Miyazono K, Maeda S, Imamura T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005;16:251–263. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Wan M, Cao X. BMP signaling in skeletal development. Biochem Biophys Res Commun. 2005;328:651–657. doi: 10.1016/j.bbrc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 20.Mundy G, Garrett R, Harris S, et al. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286:1946–1949. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 21.Garrett IR, Chen D, Gutierrez G, et al. Selective inhibitors of the osteoblast proteasome stimulate bone formation in vivo and in vitro. J Clin Invest. 2003;111:1771–1782. doi: 10.1172/JCI16198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao M, Ko SY, Liu JH, et al. Inhibition of microtubule assembly in osteoblasts stimulates bone morphogenetic protein 2 expression and bone formation through transcription factor Gli2. Mol Cell Biol. 2009;29:1291–1305. doi: 10.1128/MCB.01566-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao M, Qiao M, Harris SE, et al. The zinc finger transcription factor Gli2 mediates bone morphogenetic protein 2 expression in osteoblasts in response to hedgehog signaling. Mol Cell Biol. 2006;26:6197–6208. doi: 10.1128/MCB.02214-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang D, Hou W, Zhou Q, et al. Osthole stimulates osteoblast differentiation and bone formation by activation of β-Catenin-BMP signaling? Journal of Bone and Mineral Research. 2010;6:1234–1245. doi: 10.1002/jbmr.21. [DOI] [PMC free article] [PubMed] [Google Scholar]