Abstract
Because tumors develop resistance to chemotherapeutic agents, the cancer research community continues to search for effective chemosensitizers. One promising possibility is to use dietary agents that sensitize tumors to the chemotherapeutics. In this review, we discuss that the use of resveratrol can sensitize tumor cells to chemotherapeutic agents. The tumors shown to be sensitized by resveratrol include lung carcinoma, acute myeloid leukemia, promyelocytic leukemia, multiple myeloma, prostate cancer, oral epidermoid carcinoma, and pancreatic cancer. The chemotherapeutic agents include vincristine, adriamycin, paclitaxel, doxorubicin, cisplatin, gefitinib, 5-Fluorouracil (5-FU), velcade, and gemcitabine. The chemosensitization of tumor cells by resveratrol appears to be mediated through its ability to modulate multiple cell signaling molecules, including drug transporters, cell survival proteins, cell proliferative proteins, and members of the NF-κB and STAT-3 signaling pathways. Interestingly, however, this nutraceutical has also been reported to suppress apoptosis induced by paclitaxel, vincristine, and daunorubicin in some tumor cells. The potential mechanisms underlying this dual effect are discussed. Overall, studies suggest that resveratrol can be used to sensitize tumors to standard cancer chemotherapeutics.
Keywords: apoptosis, cancer therapy, chemoresistance, chemosensitization, resveratrol, tumor
Introduction
Since the inception of national war on cancer in 1971, significant progress has been made in understanding risk factors, treatments, and prognosis of the disease. Despite this fact, the overall cancer death rate has not decreased appreciably, and the disease remains one of the leading causes of mortality worldwide. The therapies available to date for cancer treatment are surgery, radiotherapy, and chemotherapy. Chemotherapy is often used as a main regimen in the overall treatment of most cancers. However, the development of tumor resistance to chemotherapy (chemoresistance) presents a major hurdle in cancer therapy.1 Further, complications emerges when cancer cells develop chemoresistance by multiple mechanisms. Therefore, there is an urgent need to identify a strategy that can overcome chemoresistance and sensitize tumor cells to chemotherapeutic agents.
Chemosensitization is one strategy to overcome chemoresistance. It is based on the use of one drug to enhance the activity of another by modulating one or more mechanisms of resistance. Among the potential chemosensitizers are natural agents such as resveratrol. Over the years, more and more natural products have beeen discovered to be effective against cancer drugs because of their mutitargeting property, low cost, low toxicity, and immediate availability. More than 60% of the anticancer drugs available to date in the market are of natural origin.2, 3
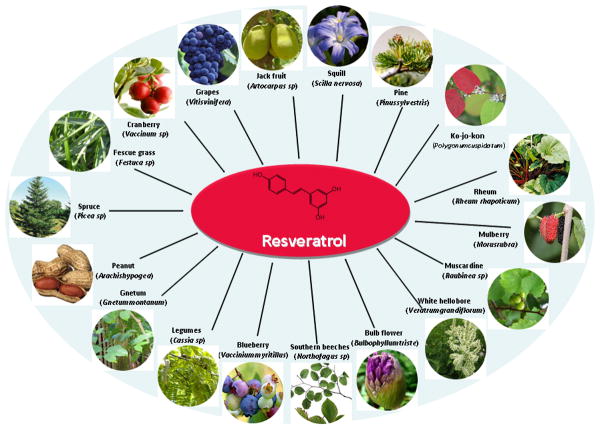
Resveratrol (3,4′,5-trihydroxy-trans-stilbene) was first isolated in 1940 as an ingredient of the roots of white hellebore (Veratrum grandiflorum O. Loes) and since then it has been identified in extracts from more than 70 other plant species (Fig 1).4-7 It is one of the main constituents of red wine, the consumption of which has been associated with 40% lower incidence of heart infarction in France than in other comparable countries, the so-called French paradox.8 Resveratrol is a phytoalexin (antimicrobial) that has broad-spectrum beneficial health effects including anti-infective, anti-oxidant, and cardioprotective functions.5 Since Jang and colleagues published the first article on the anti-cancer potential of resveratrol in 1997, a great interest from cancer researchers has continued in this molecule.9
Figure 1.
Molecular structure and sources of resveratrol. The sources of resveratrol include fruits, vegetables, legumes, and weeds.
The anti-cancer activities of resveratrol are mediated through modulation of several cell signaling molecules regulating cell cycle progression, inflammation, proliferation, apoptosis, invasion, metastasis, and angiogenesis of tumor cells.6, 10, 11 It has been shown that resveratrol can sensitize resistant cells to chemotherapeutic agents by overcoming one or more mechanisms of chemoresistance. In some tumor cells, however, resveratrol has been shown to act as chemoprotector. The potential mechanisms underlying this dual effect are described in this review.
Mechanisms of chemoresistance in tumor cells
The mechanisms of chemoresistance in tumor cells can be intrinsic (cells are resistant before the treatment), or acquired (resistance is developed during the treatment). The most important mechanisms of chemoresistance as listed in Table 1 are described below:
Table 1. Mechanism of Chemoresistance of Tumor Cells.
| Mechanism | Effect |
|---|---|
| Increased drug efflux | |
| P-glycoprotein ↑ | Transports lipophilic and natural compounds out of the resistant tumor cells13 |
| MRP ↑ | Transports chemotherapeutic drugs out of the cells in association with cellular glutathione14 |
| LRP ↑ | Transports compounds out of the cells by exocytosis15 |
| BRCP ↑ | Resistant to mitoxantrone, daunorubicin, doxorubicin and topoisomerase I inhibitors16 |
| Increased drug inactivation | |
| GSH/GST ↑ | Detoxification of drugs to an inactive compound17 |
| DPD ↑ | Catabolism of 5-FU in the liver19 |
| Altered target molecules | An increase, an inactivation, or a decrease in the target molecules20, 21 |
| DNA repair ↑ | Removal of drug-DNA adduct and repair of drug-induced DNA lesions through the action of DNA repair enzymes66 |
| Altered growth factor signaling | |
| EGFR ↑ | Activation of EGFR that leads to activation of intracellular cell survival pathways such as PI3-K/Akt, ERK1/2 andSTAT-325 |
| Altered cell death signaling | |
| p53 ↓ | Enhanced tumor cell survival 67 |
| Bcl-2, Bcl-xL ↑ | Enhancement of cell survival30 |
| Bax ↓ | An increase in cell survival68 |
| c-FLIP ↑ | Inactivation of caspase-831 |
| XIAP ↑ | Inhibition of activities of caspases32, 69 |
| Survivin ↑ | Inhibition of caspase-9 activity70 |
| NF-κB ↑ | Up-regulation of genes involved in growth regulation, inflammation, carcinogenesis, and apoptosis34, 35 |
| STAT-3 ↑ | Upregulation of Bcl-xL, Mcl-1, survivin, and cyclins71 |
Bax, Bcl-2-associated X protein; Bcl-2, B cell lymphoma-2; Bcl-xL, B cell lymphoma extra large; BRCP, breast cancer resistance protein; c-FLIP, FLICE/caspase 8 inhibitory protein; GSH/GST, glutathione/glutathione S-transferase; DPD, dihydropyrimidine dehydrogenase; 5-FU, 5-fluorouracil; EGFR, epidermal growth factor receptor; ERK, extracellular signal-regulated kinase; LRP, lung-resistance-related protein; Mcl-1, myeloid cell leukemia 1; MRP, multi-drug resistance associated protein; NF-κB, nuclear factor kappa B, PI3-K, phosphoionositide 3 kinase; Akt, AKT8 virus oncogene cellular homolog; STAT-3, signal transducers and activators of transcription protein-3; XIAP, X-linked inhibitor of apoptosis.
Drug influx and efflux
Since the anti-tumor agent must reach into the cancer cell in a concentration sufficient to exert its effect, alterations in the uptake or efflux of the drug could be responsible for the acquisition of chemoresistance.12 Drug efflux from cells is mediated by the activity of transporter proteins called ATP-dependent multidrug transporters. The most important drug transporters associated with chemoresistance are multidrug resistance protein (MDR; P-glycoprotein, P-gp),13 multi-drug resistance associated protein (MRP1),14 lung resistance-related protein (LRP), 15 and breast cancer resistance protein (BRCP). 16
Inactivation of chemotherapeutic agents
Another chemoresistance mechanism is mediated by the glutathione/glutathione S-transferase (GSH/GST) system.17 A number of reports have shown that the resistance of tumor cells to chemotherapeutic drugs is associated with an increase in the expression of GSH or GST.18 Dihydropyrimidine dehydrogenase (DPD), which is responsible for catabolizing 5-Fluorouracil (5-FU) primarily in the liver, has been shown to confer resistance to 5-FU in colorectal cancer cells through this mechanism.19
Alterations in target molecules
During the course of chemotherapy, the target may be modified or decreased to a level where it ceases to have any significant cellular influence. The best example of a molecular target susceptible to modifications is topoisomerase II. Modifications in the topoisomerase II contribute to the development of resistance to various classes of drugs. 20, 21
Enhanced DNA repair
Direct or indirect DNA alteration is the basis of the mechanism of action of many cytostatic drugs. Tumor cells have gained enhanced ability to repair damaged DNA by multiple mechanisms viz., direct repair, mismatch repair (MMR), base excision repair (BER), and nucleotide excision repair (NER). For example, BER appears to be important in resistance to some lesions induced by chemotherapy.22 One of the key elements in the NER process is the “excision repair cross-complementing 1 protein” (ERCC1).23 Up-regulation of ERCC1 has been associated with chemoresistance of various tumors including colon cancer cells.23
Growth factor signaling
The epidermal growth factor receptor (EGFR) activates molecular pathways involved in a variety of cellular processes, such as differentiation, proliferation, survival, and transformation.24 Overexpression of EGFR in tumor cells has been associated with an increase in resistance to chemotherapeutic agents.25 It has been proposed that constitutive activation of pathways downstream of EGFR such as the PI3K/Akt and MAPK pathways can also confer resistance to EGFR-targeted therapy.26
Altered cell death regulation
Acquisition of resistance to cell death mechanisms in particular apoptotic cell death is one of the hallmarks of tumor cells and plays an important role in the development of resistance to anticancer agents.27 Tumor cells acquire resistance to apoptosis by such mechanisms as down-regulating pro-apoptotic proteins (p53 and Bax), up-regulating anti-apoptotic proteins (Bcl-2; Bcl-xL), and activating survival pathways.
A key factor in the induction of apoptosis in response to chemotherapy is the tumor suppressor protein p53. In approximately 50% of cancer tumors p53 is found to be mutated.28 A large study carried out in 1997 by the NCI revealed a positive correlation between p53 status and sensitivity to cytotoxic drugs.29
Bcl-2 family proteins regulate both pro-apoptotic and anti-apoptotic proteins. Bcl-2 itself is an anti-apoptotic protein that is inappropriately over-expressed in a number of tumors and has been reported to contribute to chemoresistance.30 Some other cell survival proteins that have been associated with development of chemoresistance include c-FLIP,31 X-linked inhibitor of apoptosis (XIAP),32 and survivin.33
NF-κB activation pathway
The transcription factor nuclear factor (NF)-κB, originally identified in 1986, is a ubiquitously expressed transcription factor that regulates over 500 genes involved in immunoregulation, growth regulation, inflammation, carcinogenesis, and apoptosis.34, 35 Various in vitro and in vivo studies have shown that constitutive activation of NF-κB inhibits chemotherapy-induced apoptosis in a number of tumor types.36-38
Signal transducer and activator of transcription-3 (STAT-3) activation pathway
STAT3 is a ubiquitously expressed member of the STAT family of transcription factors that is activated by tyrosine phosphorylation via upstream receptors such as epidermal growth factor (EGF) and platelet-derived growth factor and cytokines such as interleukin-6 (IL-6).39 Work from our laboratory as well as others have shown that STAT-3 can confer tumor resistance to chemotherapeutic agents.40-43
Chemosensitization of tumor cells by resveratrol
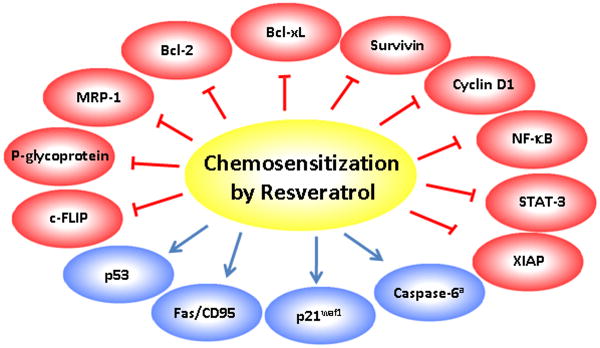
Resveratrol exerts its sensitization effect by modulating one or more mechanisms of chemoresistance (Table 2, Fig 2). Research from in vitro and in vivo studies indicate that resveratrol can overcome chemoresistance in tumor cells by modulating apoptotic pathways, down-regulating drug transporters, and down-modulating proteins involved in tumor cell proliferation. Additionally, resveratrol has also been shown to overcome chemoresistance by inhibiting NF-κB and STAT-3 pathway.
Table 2. Chemosensitization of Tumors by Resveratrol.
| Chemotherapeutic agent | Tumor type | Mechanism |
|---|---|---|
| Cytotoxic drugs | Tumor cells | Cell cycle arrest, Surviving↓ 44 |
| Cisplatin, Gefitinib, Paclitaxel | NSCLC | Survivin ↓ 45 |
| Paclitaxel | NHL and MM | Bcl-xL ↓ 46 |
| 5-FU | Colon cancer cells | Centrosome amplification56 Caspase-6 ↑a 47 |
| Velcade | MM and T cell leukemia | Fas/CD95 recruitment 48 |
| Doxorubcin | Melanoma | Cyclin D1 ↓ and p53 ↑ 49 |
| Vincristine, adriamycin, | Oral carcinoma | p-glycoprotein ↓, Bcl-2 ↓51 |
| Paclitaxel | KBv200 cells | |
| Doxorubicin | AML cells | MRP1 ↓52 |
| Paclitaxel | Lung cancer cells | p21waf1 ↑ 53 |
| Velcade, thalidomide | MM | NF-κB ↓ and STAT-3 ↓ 42 |
| Gemcitabine | Pancreatic cancer | NF-κB ↓ 57 |
| Anticancer agents | HL60/VCR58 | |
| Doxorubicin, cisplatin | Ovarian and uterine cancer cells59 | |
| Gemcitabine | Pancreatic cancer cells60 |
AML, acute myeloid leukemia; Bcl-2, B cell lymphoma-2; Bcl-xL, B cell lymphoma extra large; 5-FU, 5-fluorouracil; MM, multiple myeloma; MRP, multi-drug resistance associated protein; NF-κB, nuclear factor kappa B; NHL, Non-Hodgkin Lymphoma; NSCLC, non small cell lung cancer; STAT-3, signal transducers and activators of transcription protein-3; ↑, up-regulation; ↓, down-regulation; ↑a, activation.
Figure 2.
Mechanism of chemosensitization of tumors by resveratrol. Resveratrol sensitizes tumor cells to chemotherapeutic agents by targeting proteins involved in cell survival, cell proliferation and drug transport.
Most of the reports indicate that resveratrol sensitizes tumor cells to chemotherapeutic agents by modulating cell survival proteins. For example, it has been shown to sensitize human cancer cell lines (neuroblastoma, glioblastoma, breast carcinoma, prostate carcinoma, leukemia, and pancreatic carcinoma) to such chemotherapeutic agents as doxorubicin, cytarabine (AraC), actinomycin D, taxol, and methotrexate by downregulating survivin expression and increasing apoptosis.44 Similarly, in another study, resveratrol potentiated apoptosis induced by chemotherapeutic agents such as cisplatin, gefitinib, and paclitaxel in multidrug-resistant non-small cell lung cancer cells that was associated with a decrease in survivin expression.45 In non-Hodgkin's lymphoma (NHL) and multiple myeloma cells, inhibition of Bcl-xL expression by resveratrol was critical for its sensitization effect on paclitaxel-induced apoptosis.46 Resveratrol has also been shown to sensitize tumor cells by inducing caspase activation. For example, in a study using HCT116 human colon cancer cells, resveratrol exerted synergistic effects with 5-FU in a caspase-6 dependent manner. Furthermore, the nature of resveratrol's effect was dependent on concentration. At higher concentrations, resveratrol synergistically promoted 5-FU-mediated apoptosis, while it inhibited 5-FU-triggered apoptosis at lower concentrations.47 In one study, resveratrol was shown to enhance the apoptotic potential of perifosine and velcade in multiple myeloma and T-cell leukemia cells by enhancing recruitment of Fas/CD95 death receptor in the extrinsic pathway of apoptosis.48
In a few cases, resveratrol has been shown to sensitize tumor cells to chemotherapeutic agents through modulation of the tumor suppressor gene p53. For example, it was shown to enhance the chemotherapeutic potential of doxorubicin in chemoresistant B16 melanoma cells through up-regulation of p53.49 Interestingly, in another study an enhancement in the sensitization effect of resveratrol on apoptosis induced by various drugs in cancer cell lines was found to be p53 independent.44
In some tumor cells, resveratrol has been shown to eliminate chemoresistance by decreasing the activity of membrane transporters such as P-gp and multi-drug resistance-associated protein (MRP). One study showed that resveratrol induced increased accumulation of daunorubicin in human multidrug-resistant carcinoma KB-C2 cells in a concentration-dependent manner. Although the authors did not examine the effect of resveratrol on chemosensitivity, they observed that the increase in daunorubicin accumulation was associated with a decrease in P-gp level.50 In a recent study, Quan et al., examined the potential of resveratrol to overcome chemoresistance of oral epidermoid carcinoma KBv200 cells to vincristine, adriamycin, and paclitaxel.51 Resveratrol produced a synergistic effect when combined with the chemotherapeutic agents and reversed the multidrug-resistant phenotype of KBv200 cells. The reversal of chemoresistance phenotype was associated with a decrease in the expression of P-gp. Using three doxorubicin-resistant acute myeloid leukemia cells (AML-2/DX30, AML-2/DX100, AML-2/DX300), Kweon et al., recently investigated the potential of resveratrol to reverse the resistance phenotype of tumor cells.52 They found an enhanced expression of the MRP1 gene in chemoresistant cells. When resveratrol was administered, the expression of MRP1 was down-regulated, while cellular uptake of doxorubicin into the resistant cells was increased. Based on these observations, the group concluded that resveratrol may facilitate the cellular uptake of doxorubicin via an induced downregulation of MRP1 expression, and that resveratrol may prove useful in overcoming doxorubicin resistance, or in sensitizing doxorubicin-resistant AML cells to anti-leukemic agents.
Resveratrol has also been shown to enhance chemosensitivity of tumor cells by arresting cells at different stages of the cell cycle and by down-regulating the genes involved in cell proliferation. For example, in chemoresistant B16 melanoma, resveratrol enhanced doxorubicin induced cytotoxicity that was accompanied by a down-regulation of cyclin D1.49 Combined treatment with resveratrol was also associated with an increase in the cell cycle arrest at the G(1)-S phase. In another study, when resveratrol was administered prior to paclitaxel treatment in lung cancer cells (A549, EBC-1, Lu65), a significant enhancement in the anti-proliferative potential of paclitaxel was observed. Resveratrol also induced the expression of cell cycle regulator p21/waf1 expression and lowered the paclitaxel threshold required for killing tumor cells.53
In a few cases, resveratrol has been shown to overcome chemoresistance through downregulation of NF-κB and STAT-3 pathways. In our laboratory, resveratrol was shown to enhance the apoptotic and anti-proliferative potential of velcade and thalidomide in multiple myeloma cells.42 Such an enhancement was associated with inhibition of NF-κB and STAT-3 activation pathways. Resveratrol administration was also associated with accumulation of sub-G(1) population, increase in Bax release, and activation of caspase-3. This was further correlated with down-regulation of various proliferative and antiapoptotic gene products, including cyclin D1, cIAP-2, XIAP, survivin, Bcl-2, Bcl-xL, Bfl-1/A1, and TRAF2. Investigation of the mechanism revealed that resveratrol inhibited NF-κB activation through inhibition of IκBα phosphorylation and IKK activation. These observations were further supported by an inhibition of NF-κB and STAT-3 in multiple myeloma patients.
Centrosome amplification, defined as the presence of three or more centrosomes in a cell, is a common feature in cancer cells. Centrosome amplification is often associated with genetic instability, resulting in the accumulation of deleterious genomic stresses in the cells. Therefore, cells with centrosome amplification are likely to be more efficiently eliminated by cell death mechanisms.54 This has been appropriately described as “apoptosis limits centrosome amplification and genomic instability”.55 Recently, Lee et al. investigated the relationship between centrosome amplification and apoptosis sensitivity in colon cancer cells. They found that centrosome amplification is a spontaneous process in cancer cells and could also be induced by subtoxic concentrations of 5-FU. The group further found that cancer cells with centrosome amplification, whether spontaneous or 5-FU-induced, were more sensitive to apoptosis induction by resveratrol.56 Based on their observations, the group concluded that centrosome amplification might be a novel chemosensitization approach to cancer therapy.
In some cases, resveratrol has been shown to overcome chemoresistance by more than one mechanism. For example, the reversal of chemoresistace of KBv200 cells to vincristine, adriamycin, and paclitaxel by reseveratrol was shown not to be only due to down-regulation of P-gp but also due to a decrease in Bcl-2 expression.51 Similarly, in a number of cancer cell lines, resveratrol was shown to overcome chemoresistance by inducing cell cycle arrest at S phase and downregulating survivin to enhance the effect of a variety of chemotherapeutic agents.44 Another study using lung cancer cells showed that resveratrol has potential to enhance chemotherapeutic potential of paclitaxel by enhancing both pro-apoptotic and anti-proliferative potential.53
While most of the reports are based on the in vitro data, some in vivo studies also support the chemosensitizing potential of resveratrol. Zhao and group examined the efficacy of resveratrol in a nude mice bearing multidrug-resistant human non-small cell lung cancer cells (NSCLC). The cells were treated with resveratrol at a concentration of 25, 50, or 100 μM in in vitro studies, and nude mice were implanted with NSCLC and fed a special diet that included resveratrol at a dose of either 1 g/kg/day or 3 g/kg/day. The rate of cell proliferation, apoptosis ratio, cell cycle phase distribution, IC50 values of cisplatin, gefitinib, and paclitaxel, implanted tumor volume, and expression of survivin in resveratrol-treated and control mice were determined. Resveratrol significantly inhibited the proliferation of cancer cells, induced apoptosis, arrested the cell cycle between G0-G1 and S phase or at the G2/M phase, decreased the IC50 values of chemotherapeutic drugs, and showed anti-tumor effects in nude mice that were implanted with cancer cells. In addition, resveratrol affected the proliferation of cancer cells in a dose- and time-dependent manner. Expression of survivin in cancer cells decreased even at low doses of resveratrol, but the effects were dose-dependent.45
Whether resveratrol can sensitize pancreatic cancer (PaCa) cells to gemcitabine in vitro and in vivo was investigated recently in our laboratory.57 In in vitro, resveratrol inhibited the proliferation of human PaCa cell lines, synergized the apoptotic effects of gemcitabine, inhibited the constitutive activation of NF-κB and expression of Bcl-2, Bcl-xL, COX-2, cyclin D1, MMP-9 and VEGF. We then established PaCa xenografts in nude mice, randomized them into 4 groups, and treated them with vehicle, gemcitabine, resveratrol, or a combination. Resveratrol significantly suppressed the growth of the tumor, and this effect was further enhanced by gemcitabine. The markers of proliferation index, Ki-67, and micro vessel density, CD31, were significantly downregulated in tumor tissue by the combination of gemcitabine and resveratrol. As compared to vehicle control, resveratrol also suppressed NF-κB activation and expression of cyclin D1, COX-2, ICAM-1, MMP-9 and survivin. Overall the results demonstrated that resveratrol can potentiate the effects of gemcitabine through suppression of markers of proliferation, invasion, angiogenesis, and metastasis.
In a few cases, resveratrol has been shown to sensitize tumor cells to chemotherapy, but the molecular mechanism is not known. For example, resveratrol exhibited diverse anti-cancer activities with doxorubicin, cycloheximide, busulfan, gemcitabine, and paclitaxel in multidrug-resistant variant HL60/VCR (P-gp positive) cells.58 Similarly, in human ovarian (OVCAR-3) and uterine (Ishikawa) cancer cells, resveratrol was shown to enhance the growth inhibitory/anticancer activity of cisplatin and doxorubicin.59 A polymethoxylated resveratrol analogue, N-hydroxy-N′-(3,4,5-trimethoxphenyl)-3,4,5-trimethoxy-benzamidine (KITC), also showed synergistic effect with gemcitabine as an anti-cancer agent in the human pancreatic cancer cell lines AsPC-1 and BxPC-3.60 In these studies, the molecular mechanism of resveratrol action was not elucidated.
Resveratrol as a chemoprotector
Chemosensitization strategy aims at using one drug to enhance the activity of another selectively in the tumor cells, while limiting any undesired toxicity or side effects in normal cells. While resveratrol has been shown to increase the efficacy of chemotherapeutic agents; under certain circumstances, in some cell types, it has also been reported to reduce the efficacy of chemotherapeutic agents. Similarly, evidence is also emerging showing that such normal cells as endothelial cells, lymphocytes, and chondrocytes are vulnerable to resveratrol.10 These contradictory reports present a major caveat for use of resveratrol as a chemosensitizer.
The potential of resveratrol as an anti-apoptotic agent was investigated in human neuroblastoma cell line SH-SY5Y.61 When neuroblastoma cells were treated with paclitaxel, a significant increase in apoptosis was observed. The induction in paclitaxel-induced apoptosis was abrogated by resveratrol treatment. Investigation of the mechanism revealed that resveratrol was able to inhibit the activation of caspase 7 and PARP cleavage that occurred in SH-SY5Y cells exposed to paclitaxel. In a subsequent study, the group evaluated the anti-apoptotic effect of resveratrol by studying its effect on cell cycle progression in neuroblastoma cells.62 Resveratrol was found to induce S-phase cell cycle arrest that was associated with an increase of cyclin E and cyclin A, a downregulation of cyclin D1 with no alteration in cyclin B1, and cdk 1 activation. The resveratrol-induced S-phase block prevented neuroblastoma cells from entering mitosis, the phase of the cell cycle in which paclitaxel exerts its activity.
In another study, resveratrol negatively affected paclitaxel-induced apoptosis in 5637 bladder cancer cells.63 The antagonism was associated with a decrease in paclitaxel-induced caspase-3 activation, PARP cleavage, and G2/M cell cycle arrest. The antagonism of paclitaxel-induced apoptosis by resveratrol seemed to involve several intracellular signal pathways including Akt, MAPK and NF-κB.63 When human leukemia cells were exposed to low concentrations of resveratrol (4-8 μM), a decrease in apoptosis induced by hydrogen peroxide or the anticancer drugs vincristine, and daunorubicin was observed.64 Resveratrol exerted its effect by acting as anti-oxidant and reduced caspase activation, DNA fragmentation, and translocation of cytochrome c induced by the agents.64 The authors showed that NADPH oxidase-dependent elevation of intracellular superoxide that blocks mitochondrial hydrogen peroxide production was essential for apoptosis inhibition by resveratrol.
The sensitization effect of resveratrol on paclitaxel-induced cell death in breast cancer cells was investigated.65 Resveratrol strongly diminished the susceptibility of MDA-MB-435s, MDA-MB-231 and SKBR-3 cells to paclitaxel-induced cell death in culture, although the effect was not observed in MCF-7 cells. Using MDA-MB-435s cells as a representative model, the authors made a similar observation in athymic nude mice. Mechanistically, the modulating effect of resveratrol was attributable to its inhibition of paclitaxel-induced G(2)/M cell cycle arrest, together with an accumulation of cells in the S-phase. In addition, resveratrol was also found to suppress paclitaxel-induced accumulation of reactive oxygen species (ROS) and subsequently the inactivation of anti-apoptotic Bcl-2 family proteins. The group concluded that concomitant use of resveratrol with paclitaxel is detrimental in certain types of human cancers.
Summary, conclusions, and future perspective
Acquisition of chemoresistance remains one of the promising problems of chemotherapy failure in cancer patients. Cancer cells are very intelligent: they will do whatever they need to survive. The fact that tumor cells develop multiple chemoresistance mechanisms and that more than one mechanism may work simultaneously complicates the success of chemotherapy. In this regard, resveratrol seems to be an ideal candidate as an adjunct to reverse chemoresistance. However, additional is required to clarify the following issues:
First, despite more than 1100 publications on cancer chemotherapeutic potential of resveratrol with around 20 reports on its chemosensitization potential, only a few studies using clinically relevant animal models have been done. Second, combinations may be synergistically cytotoxic to normal cells as well. Most of the studies done to date have focused on tumor cells only. Whether and how resveratrol discriminates between normal and cancer cells is largely unknown. Third, resveratrol has been shown to act as chemoprotector in some cancer cells. Fourth, it remains to be seen whether the expected chemosensitization will be reproduced in humans. Would such a combination increase the patient's life?
Therefore, future studies should focus on careful and accurate characterization of molecular mechanisms of chemosensitization, determination of efficacy of resveratrol combinations by clinically relevant in vivo studies and finally demonstration of safety and effectiveness of combinations in patients.
Table 3. Chemoprotection of Tumors by Resveratrol.
| Chemotherapeutic agent | Tumor type | Mechanism |
|---|---|---|
| Paclitaxel | SH-SY5Y neuroblastoma cells | Caspase-7 ↓, PARP cleavage ↓61 |
| Paclitaxel | Neuroblastoma cells | S-phase block62 |
| Paclitaxel | Bladder cancer cells | Caspase-3 activation ↓, PARP cleavage ↓, G2/M cell cycle arrest ↓63 |
| Vincristine, Daunorubicin H2O2 | Leukemia cells | NADPH oxidase ↑a 64 |
| Paclitaxel | Breast cancer cells | Bcl-2 active, ROS ↓65 |
Bcl-2, B cell lymphoma-2; NADPH, nicotinamide adenine dinucleotide phosphate reduced; PARP, poly (ADP-ribose) polymerase; ROS, reactive oxygen species; ↓, down-regulation; ↑a, activation.
Acknowledgments
We thank Walter Pagel from the Department of Scientific Publications for carefully proofreading the manuscript and providing valuable comments. Dr. Aggarwal is the Ransom Horne, Jr., Professor of Cancer Research. This work was supported by a grant from the Clayton Foundation for Research (B.B.A.), a core grant from the National Institutes of Health (CA-16 672), a program project grant from National Institutes of Health (NIH CA-124787-01A2), and grant from Center for Targeted Therapy of M.D. Anderson Cancer Center.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
References
- 1.Higgins CF. Multiple molecular mechanisms for multidrug resistance transporters. Nature. 2007;446:749–57. doi: 10.1038/nature05630. [DOI] [PubMed] [Google Scholar]
- 2.Gupta SC, et al. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 29:405–34. doi: 10.1007/s10555-010-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981-2002. J Nat Prod. 2003;66:1022–37. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 4.Takaoka M. Of the phenolic substances of white hellebore (Veratrum grandiflorum Loes. Fil.) J Faculty Sci Hokkaido Imperial University. 1940;3:1–16. [Google Scholar]
- 5.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 6.Aggarwal BB, et al. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 2004;24:2783–840. [PubMed] [Google Scholar]
- 7.Aggarwal BB, Shishodia S. Resveratrol in Health and Disease. CRC Press Taylor & Francis Group; Los Angeles: 2006. [Google Scholar]
- 8.Richard JL. Coronary risk factors. The French paradox. Arch Mal Coeur Vaiss. 1987;80 Spec No: 17-21. [PubMed] [Google Scholar]
- 9.Jang M, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–20. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 10.Shakibaei M, Harikumar KB, Aggarwal BB. Resveratrol addiction: to die or not to die. Mol Nutr Food Res. 2009;53:115–28. doi: 10.1002/mnfr.200800148. [DOI] [PubMed] [Google Scholar]
- 11.Harikumar KB, Aggarwal BB. Resveratrol: a multitargeted agent for age-associated chronic diseases. Cell Cycle. 2008;7:1020–35. doi: 10.4161/cc.7.8.5740. [DOI] [PubMed] [Google Scholar]
- 12.Huang Y, et al. Membrane transporters and channels: role of the transportome in cancer chemosensitivity and chemoresistance. Cancer Res. 2004;64:4294–301. doi: 10.1158/0008-5472.CAN-03-3884. [DOI] [PubMed] [Google Scholar]
- 13.Endicott JA, Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–71. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- 14.Deeley RG, Cole SP. Function, evolution and structure of multidrug resistance protein (MRP) Semin Cancer Biol. 1997;8:193–204. doi: 10.1006/scbi.1997.0070. [DOI] [PubMed] [Google Scholar]
- 15.Izquierdo MA, et al. Drug resistance-associated marker Lrp for prediction of response to chemotherapy and prognoses in advanced ovarian carcinoma. J Natl Cancer Inst. 1995;87:1230–7. doi: 10.1093/jnci/87.16.1230. [DOI] [PubMed] [Google Scholar]
- 16.Lee JS, et al. Reduced drug accumulation and multidrug resistance in human breast cancer cells without associated P-glycoprotein or MRP overexpression. J Cell Biochem. 1997;65:513–26. [PubMed] [Google Scholar]
- 17.Batist G, et al. Overexpression of a novel anionic glutathione transferase in multidrug-resistant human breast cancer cells. J Biol Chem. 1986;261:15544–9. [PubMed] [Google Scholar]
- 18.Chao CC, et al. Overexpression of glutathione S-transferase and elevation of thiol pools in a multidrug-resistant human colon cancer cell line. Mol Pharmacol. 1992;41:69–75. [PubMed] [Google Scholar]
- 19.Diasio RB, Harris BE. Clinical pharmacology of 5-fluorouracil. Clin Pharmacokinet. 1989;16:215–37. doi: 10.2165/00003088-198916040-00002. [DOI] [PubMed] [Google Scholar]
- 20.Cole SP, et al. Non-P-glycoprotein-mediated multidrug resistance in a small cell lung cancer cell line: evidence for decreased susceptibility to drug-induced DNA damage and reduced levels of topoisomerase II. Cancer Res. 1991;51:3345–52. [PubMed] [Google Scholar]
- 21.Webb CD, et al. Attenuated topoisomerase II content directly correlates with a low level of drug resistance in a Chinese hamster ovary cell line. Cancer Res. 1991;51:6543–9. [PubMed] [Google Scholar]
- 22.Liu L, et al. Pharmacologic disruption of base excision repair sensitizes mismatch repair-deficient and -proficient colon cancer cells to methylating agents. Clin Cancer Res. 1999;5:2908–17. [PubMed] [Google Scholar]
- 23.Youn CK, et al. Oncogenic H-Ras up-regulates expression of ERCC1 to protect cells from platinum-based anticancer agents. Cancer Res. 2004;64:4849–57. doi: 10.1158/0008-5472.CAN-04-0348. [DOI] [PubMed] [Google Scholar]
- 24.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 25.Chen X, Yeung TK, Wang Z. Enhanced drug resistance in cells coexpressing ErbB2 with EGF receptor or ErbB3. Biochem Biophys Res Commun. 2000;277:757–63. doi: 10.1006/bbrc.2000.3731. [DOI] [PubMed] [Google Scholar]
- 26.Magne N, et al. Influence of epidermal growth factor receptor (EGFR), p53 and intrinsic MAP kinase pathway status of tumour cells on the antiproliferative effect of ZD1839 (“Iressa”) Br J Cancer. 2002;86:1518–23. doi: 10.1038/sj.bjc.6600299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 28.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–31. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 29.O'Connor PM, et al. Characterization of the p53 tumor suppressor pathway in cell lines of the National Cancer Institute anticancer drug screen and correlations with the growth-inhibitory potency of 123 anticancer agents. Cancer Res. 1997;57:4285–300. [PubMed] [Google Scholar]
- 30.Chun E, Lee KY. Bcl-2 and Bcl-xL are important for the induction of paclitaxel resistance in human hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2004;315:771–9. doi: 10.1016/j.bbrc.2004.01.118. [DOI] [PubMed] [Google Scholar]
- 31.Longley DB, et al. c-FLIP inhibits chemotherapy-induced colorectal cancer cell death. Oncogene. 2006;25:838–48. doi: 10.1038/sj.onc.1209122. [DOI] [PubMed] [Google Scholar]
- 32.Sasaki H, et al. Down-regulation of X-linked inhibitor of apoptosis protein induces apoptosis in chemoresistant human ovarian cancer cells. Cancer Res. 2000;60:5659–66. [PubMed] [Google Scholar]
- 33.Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3:46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- 34.Gupta SC, et al. Inhibiting NF-kappaB activation by small molecules as a therapeutic strategy. Biochim Biophys Acta. doi: 10.1016/j.bbagrm.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46:705–16. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- 36.Bharti AC, Aggarwal BB. Chemopreventive agents induce suppression of nuclear factor-kappaB leading to chemosensitization. Ann N Y Acad Sci. 2002;973:392–5. doi: 10.1111/j.1749-6632.2002.tb04671.x. [DOI] [PubMed] [Google Scholar]
- 37.Bharti AC, Aggarwal BB. Nuclear factor-kappa B and cancer: its role in prevention and therapy. Biochem Pharmacol. 2002;64:883–8. doi: 10.1016/s0006-2952(02)01154-1. [DOI] [PubMed] [Google Scholar]
- 38.Wang CY, Mayo MW, Baldwin AS., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–7. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 39.Levy DE, Darnell JE., Jr Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–62. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 40.Bharti AC, et al. Nuclear factor-kappaB and STAT3 are constitutively active in CD138+ cells derived from multiple myeloma patients, and suppression of these transcription factors leads to apoptosis. Blood. 2004;103:3175–84. doi: 10.1182/blood-2003-06-2151. [DOI] [PubMed] [Google Scholar]
- 41.Real PJ, et al. Resistance to chemotherapy via Stat3-dependent overexpression of Bcl-2 in metastatic breast cancer cells. Oncogene. 2002;21:7611–8. doi: 10.1038/sj.onc.1206004. [DOI] [PubMed] [Google Scholar]
- 42.Bhardwaj A, et al. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-kappaB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood. 2007;109:2293–302. doi: 10.1182/blood-2006-02-003988. [DOI] [PubMed] [Google Scholar]
- 43.Ahn KS, et al. Guggulsterone, a farnesoid X receptor antagonist, inhibits constitutive and inducible STAT3 activation through induction of a protein tyrosine phosphatase SHP-1. Cancer Res. 2008;68:4406–15. doi: 10.1158/0008-5472.CAN-07-6696. [DOI] [PubMed] [Google Scholar]
- 44.Fulda S, Debatin KM. Sensitization for anticancer drug-induced apoptosis by the chemopreventive agent resveratrol. Oncogene. 2004;23:6702–11. doi: 10.1038/sj.onc.1207630. [DOI] [PubMed] [Google Scholar]
- 45.Zhao W, et al. Resveratrol down-regulates survivin and induces apoptosis in human multidrug-resistant SPC-A-1/CDDP cells. Oncol Rep. 23:279–86. [PubMed] [Google Scholar]
- 46.Jazirehi AR, Bonavida B. Resveratrol modifies the expression of apoptotic regulatory proteins and sensitizes non-Hodgkin's lymphoma and multiple myeloma cell lines to paclitaxel-induced apoptosis. Mol Cancer Ther. 2004;3:71–84. [PubMed] [Google Scholar]
- 47.Chan JY, et al. Resveratrol displays converse dose-related effects on 5-fluorouracil-evoked colon cancer cell apoptosis: the roles of caspase-6 and p53. Cancer Biol Ther. 2008;7:1305–12. doi: 10.4161/cbt.7.8.6302. [DOI] [PubMed] [Google Scholar]
- 48.Reis-Sobreiro M, Gajate C, Mollinedo F. Involvement of mitochondria and recruitment of Fas/CD95 signaling in lipid rafts in resveratrol-mediated antimyeloma and antileukemia actions. Oncogene. 2009;28:3221–34. doi: 10.1038/onc.2009.183. [DOI] [PubMed] [Google Scholar]
- 49.Gatouillat G, et al. Resveratrol induces cell-cycle disruption and apoptosis in chemoresistant B16 melanoma. J Cell Biochem. 110:893–902. doi: 10.1002/jcb.22601. [DOI] [PubMed] [Google Scholar]
- 50.Nabekura T, Kamiyama S, Kitagawa S. Effects of dietary chemopreventive phytochemicals on P-glycoprotein function. Biochem Biophys Res Commun. 2005;327:866–70. doi: 10.1016/j.bbrc.2004.12.081. [DOI] [PubMed] [Google Scholar]
- 51.Quan F, et al. Reversal effect of resveratrol on multidrug resistance in KBv200 cell line. Biomed Pharmacother. 2008;62:622–9. doi: 10.1016/j.biopha.2008.07.089. [DOI] [PubMed] [Google Scholar]
- 52.Kweon SH, Song JH, Kim TS. Resveratrol-mediated reversal of doxorubicin resistance in acute myeloid leukemia cells via downregulation of MRP1 expression. Biochem Biophys Res Commun. 395:104–10. doi: 10.1016/j.bbrc.2010.03.147. [DOI] [PubMed] [Google Scholar]
- 53.Kubota T, et al. Combined effects of resveratrol and paclitaxel on lung cancer cells. Anticancer Res. 2003;23:4039–46. [PubMed] [Google Scholar]
- 54.Nigg EA. Centrosome aberrations: cause or consequence of cancer progression? Nat Rev Cancer. 2002;2:815–25. doi: 10.1038/nrc924. [DOI] [PubMed] [Google Scholar]
- 55.Cuomo ME, et al. p53-Driven apoptosis limits centrosome amplification and genomic instability downstream of NPM1 phosphorylation. Nat Cell Biol. 2008;10:723–30. doi: 10.1038/ncb1735. [DOI] [PubMed] [Google Scholar]
- 56.Lee SC, Chan JY, Pervaiz S. Spontaneous and 5-fluorouracil-induced centrosome amplification lowers the threshold to resveratrol-evoked apoptosis in colon cancer cells. Cancer Lett. 288:36–41. doi: 10.1016/j.canlet.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 57.Harikumar KB, et al. Resveratrol, a multitargeted agent, can enhance antitumor activity of gemcitabine in vitro and in orthotopic mouse model of human pancreatic cancer. Int J Cancer. 127:257–68. doi: 10.1002/ijc.25041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duraj J, et al. Diverse resveratrol sensitization to apoptosis induced by anticancer drugs in sensitive and resistant leukemia cells. Neoplasma. 2006;53:384–92. [PubMed] [Google Scholar]
- 59.Rezk YA, et al. Use of resveratrol to improve the effectiveness of cisplatin and doxorubicin: study in human gynecologic cancer cell lines and in rodent heart. Am J Obstet Gynecol. 2006;194:e23–6. doi: 10.1016/j.ajog.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 60.Bernhaus A, et al. Antitumor effects of KITC, a new resveratrol derivative, in AsPC-1 and BxPC-3 human pancreatic carcinoma cells. Invest New Drugs. 2009;27:393–401. doi: 10.1007/s10637-008-9183-7. [DOI] [PubMed] [Google Scholar]
- 61.Nicolini G, et al. Anti-apoptotic effect of trans-resveratrol on paclitaxel-induced apoptosis in the human neuroblastoma SH-SY5Y cell line. Neurosci Lett. 2001;302:41–4. doi: 10.1016/s0304-3940(01)01654-8. [DOI] [PubMed] [Google Scholar]
- 62.Rigolio R, et al. Resveratrol interference with the cell cycle protects human neuroblastoma SH-SY5Y cell from paclitaxel-induced apoptosis. Neurochem Int. 2005;46:205–11. doi: 10.1016/j.neuint.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 63.Mao QQ, et al. Resveratrol confers resistance against taxol via induction of cell cycle arrest in human cancer cell lines. Mol Nutr Food Res. doi: 10.1002/mnfr.200900392. [DOI] [PubMed] [Google Scholar]
- 64.Ahmad KA, et al. Resveratrol inhibits drug-induced apoptosis in human leukemia cells by creating an intracellular milieu nonpermissive for death execution. Cancer Res. 2004;64:1452–9. doi: 10.1158/0008-5472.can-03-2414. [DOI] [PubMed] [Google Scholar]
- 65.Fukui M, Yamabe N, Zhu BT. Resveratrol attenuates the anticancer efficacy of paclitaxel in human breast cancer cells in vitro and in vivo. Eur J Cancer. 46:1882–91. doi: 10.1016/j.ejca.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heim MM, et al. Differential modulation of chemosensitivity to alkylating agents and platinum compounds by DNA repair modulators in human lung cancer cell lines. J Cancer Res Clin Oncol. 2000;126:198–204. doi: 10.1007/s004320050033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miyashita T, et al. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994;9:1799–805. [PubMed] [Google Scholar]
- 68.Hayward RL, et al. Enhanced oxaliplatin-induced apoptosis following antisense Bcl-xl down-regulation is p53 and Bax dependent: Genetic evidence for specificity of the antisense effect. Mol Cancer Ther. 2004;3:169–78. [PubMed] [Google Scholar]
- 69.Li J, et al. Human ovarian cancer and cisplatin resistance: possible role of inhibitor of apoptosis proteins. Endocrinology. 2001;142:370–80. doi: 10.1210/endo.142.1.7897. [DOI] [PubMed] [Google Scholar]
- 70.Salvesen GS, Duckett CS. IAP proteins: blocking the road to death's door. Nat Rev Mol Cell Biol. 2002;3:401–10. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- 71.Aoki Y, Feldman GM, Tosato G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood. 2003;101:1535–42. doi: 10.1182/blood-2002-07-2130. [DOI] [PubMed] [Google Scholar]