Abstract
Degradation of different cargo by macroautophagy is emerging as a highly selective process which relies upon specific autophagy receptors and adapter molecules that link the cargo with the autophagic molecular machinery. We have recently reported that the large phsophatidylinositol-3-phosphate (PtdIns(3)P)-binding protein Alfy (Autophagy-linked FYVE protein) is required for selective degradation of aggregated proteins. Although depletion of Alfy inhibits Atg5-dependent aggregate degradation, overexpression of Alfy results in Atg5-dependent aggregate clearance and neuroprotection. Alfy-mediated degradation requires the ability of Alfy to directly interact with Atg5. This ability to interact with the core autophagic machinery may cause Alfy to diminish the responsiveness to nonselective autophagic degradation as measured by long-lived protein degradation. Thus, increasing Alfy-mediated protein degradation may be beneficial in some organs, but may be detrimental in others.
Key words: autophagy, protein aggregates, neurodegeneration, Alfy, aggregation, degradation
Introduction
Macroautophagy is the process whereby cytoplasmic constituents become sequestered by expansion of a de novo synthesized phagophore membrane, which closes to form a double-membrane autophagosome. To achieve degradation of the sequestered cargo, the autophagosome must fuse with the lysosome either directly or after fusing with vesicles of the endocytic pathway. Long considered a nonselective pathway induced in response to cellular stresses such as starvation, there is growing evidence that autophagic processes are also highly selective quality control mechanisms whose basal levels are important to maintain cellular health.1–3
In mammalian systems, the mechanisms that drive cargo selection for selective degradation by macroautophagy are currently an area of intense study. Overall, the emerging model very much echoes a pathway previously described in yeast known as cytoplasm to vacuole targeting (Cvt).4,5 Briefly, Cvt is the means by which the vacuolar enzymes aminopeptidase I and alpha-mannosidase are trafficked to the vacuole after being sequestered into small autophagosome-like vesicles. Sequestration involves an interplay between the core autophagic machinery, the specific cargo receptor Atg19 and the specificity adaptor Atg11, which together, connect the cargo to the molecular machinery that builds the autophagosome.4 In mammalian systems, the list of autophagy receptors for different cargo continues to grow, and currently includes p62/SQSTM1, NBR1, NIX and NDP52.6–8 The autophagy receptors all contain two key domains: an LC3-interacting region (LIR) or LC3-recognition sequence (LRS) that permits interaction with the Atg8 family members MAP1LC3, GABARAP, GEC1/GABARAPL1 and GATE-16/GABARAPL2, and a cargo recognition domain. For example, the best studied autophagy receptor, p62, contains a LIR and a ubiquitin-binding UBA domain,9,10 Through its UBA domain, p62 interacts with various ubiquitinated cargoes including protein aggregates,11 the midbody remnants formed after cytokinesis,12 peroxisomes,13,14 mitochondria,15 intracellular bacteria16,17 and ribosomal proteins.18 In contrast to autophagy receptors, the mammalian specificity adaptors have remained largely unidentified. Specificity adaptors function as scaffolding proteins that bring the cargo-receptor complex in contact with the core autophagic machinery to allow the autophagic membrane to form around the cargo. In Cvt, Atg11 transports the cargo-receptor complex to the preautophagosomal structure (PAS) prior to binding to Atg8. Atg11 also interacts with the core Atg proteins Atg9 and Atg1–13 kinase complex.4 We propose that Alfy may be a critical specificity adaptor for the elimination for ubiquitinated protein aggregates.
Alfy/WDFY3
Alfy (also known as WDFY3) is a 400 kDa protein that is ubiquitously expressed in mammalian tissue although most abundantly in the brain. Alfy predominantly localizes to the nucleus and nuclear membrane under basal conditions, but is recruited to cytoplasmic ubiquitin-positive protein aggregates under stress conditions.19,20 Alfy is also recruited to nuclear protein aggregates such as mutant Ataxin-1 and to promyelocytic leukemia (PML) nuclear bodies.20,21
A role for Alfy in macroautophagy-mediated protein aggregate degradation (aggrephagy for short) was suggested by its colocalization with the autophagic markers Atg5 and Atg8/LC3,19 and from studies of Drosophila lacking the Alfy homologue Blue Cheese (bchs), which are adult viable, but have a reduced life span accompanied by an accelerated accumulation of ubiquitinated proteins and neuronal degeneration.22 Recently, we reported that Alfy is required for the macroautophagic degradation of cytoplasmic protein aggregates associated with Huntington disease (HD) and Parkinson disease (PD), but is not required for bulk macroautophagic degradation under starvation conditions.20 Co-immunoprecipitation and colocalization studies indicate that Alfy scaffolds a complex containing the p62-positive, ubiquitinated, aggregation-prone protein and the core autophagy proteins Atg5, Atg12, Atg16 and LC3. Most interestingly, overexpression of Alfy (or bchs) decreases the number of protein inclusions and protects cells from expanded polyglutamine toxicity in an autophagy-dependent manner in both a primary neuronal HD model and a Drosophila eye model of polyglutamine toxicity.
Although there are no known functional domains in the N-terminal two thirds of Alfy, its C terminus contains several domains including a PH-BEACH domain assemblage, five WD40 repeats and a PtdIns(3)P-binding FYVE domain.19 The BEACH domain, first identified in the mouse beige and the human Chediak-Higashi syndrome proteins (beige and CHS), is highly conserved and generally found in large proteins (greater than 2,000 amino acids) involved in vesicle trafficking, membrane dynamics and receptor signaling.23,24 The functional role of the BEACH domain is unclear, however studies indicate that its presence is required to maintain the functionality of BEACH-domain containing proteins. The BEACH domain is associated with a PH (pleckstrin homology)-like domain, and structural and functional studies suggest that a prominent groove at the interface between these domains may be used to recruit their binding partners.25,26 We have recently found that the PH-BEACH region in Alfy is involved in the direct interaction with the autophagy receptor p62.21 Similar to Atg11, Alfy seems to facilitate interaction of cargo-bound p62 with the membrane bound LC3.20 It will be interesting to determine whether other BEACH family proteins might interact with p62.
The WD40 repeats of Alfy are essential for its colocalization and interaction with Atg5.20 The Atg12-5 complex is required for an early stage of autophagosome formation, and together with the membrane-bound Atg16L, may act as an E3-like enzyme to facilitate an essential step for autophagosome formation, the conjugation of Atg8/LC3 proteins to phosphatidylethanolamine.27,28 Depletion of Alfy in primary and stable cells prevents recruitment of not only Atg5 but also LC3 to the expanded polyglutamine protein. Moreover, although overexpression of Alfy enhances the clearance of protein aggregates, mutations within the WD40 repeats abrogate this effect, indicating that the interaction between Alfy and Atg5 is critical for formation of autophagic membranes around the inclusion.20 It is not clear if Alfy mediates recruitment of LC3 to the inclusion through a direct interaction or whether its role in LC3 recruitment is indirect via its binding to Atg5 or p62.
The FYVE domain of Alfy binds specifically to PtdIns(3)P,19 a product of the phosphatidylinositol kinases including Vps34, a class III phosphatidylinositol 3-kinase (PtdIns3K). PtdIns(3)P is a critical lipid required for all forms of macroautophagy,29 and how modulating the interaction of Alfy with PtdIns(3)P-containing membranes affects Alfy function is an interesting area for investigation. Since Alfy is not involved in starvation-induced macroautophagy, competition with other autophagic PtdIns(3)P effectors may be an important means by which aggrephagy is regulated.
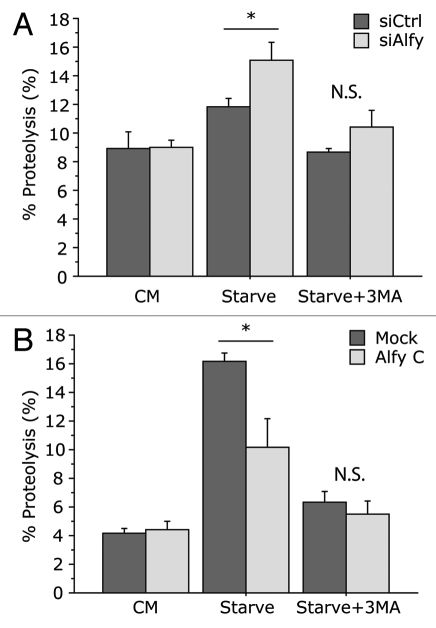
Although it is clear that Alfy shuttles between the nucleus and cytoplasm, the functional significance of this nuclear shuttling remains elusive. In light of the increased clearance observed upon Alfy overexpression,20 it is tempting to speculate that the cytoplasmic levels of Alfy may be the rate-limiting step in aggrephagy. As Alfy interacts with the core autophagic machinery, increased cytoplasmic Alfy levels may lead to toxicity under conditions of starvation or other stressors during which general macroautophagy may be necessary,30 and keeping Alfy in the nucleus would be a way of avoiding this. If this is the case, then our recent study indicating that p62 is required for Alfy to leave the nucleus21 suggests that this autophagic receptor may signal when aggrephagy should be activated. The fact that tissue expression levels of Alfy (highest in brain and lowest in liver) inversely correlate with responsiveness to starvation further supports a potential need to regulate the availability of Alfy to avoid competition for the macroautophagic machinery. To determine if such competition may exist, we examined long-lived protein degradation upon both Alfy depletion and Alfy overexpression (Fig. 1). As shown previously, reducing the levels of Alfy did not interfere with a macroautophagy-dependent degradation of long-lived proteins upon starvation. Interestingly, however, when Alfy levels were depleted, the degree of proteolysis that occurred in response to starvation was significantly higher than in the presence of Alfy (Fig. 1A). In contrast, overexpression of the C terminus fragment of Alfy that leads to increased aggregate clearance blunted the starvation response (Fig. 1B). Since Alfy can directly bind to Atg5, these data suggest that the presence of Alfy during starvation may partially corral the macroautophagic machinery away from nonselective degradation of cytosolic proteins. Nonetheless, in light of the FYVE and PH-BEACH domains, it is also possible that competition is occurring at yet unrecognized steps since there is still much to uncover about Alfy function and how it influences autophagosome maturation. Further studies of overexpression of Alfy that lacks the signals for nucleocytoplasmic shuttling, WD40 domain, FYVE domain or PH-BEACH domain may better our understanding of this process.
Figure 1.
Modulation of Alfy levels permits starvation-mediated macroautophagy, but modulates the degree of proteolysis in response to starvation. (A) Long-lived protein degradation (LLPD) upon depletion of Alfy by siRNA. HeLa cells were transfected with a scramble siRN A sequence (siCtrl) or a sequence against Alfy (siAlfy). 48 h later, long-lived proteins were labeled with [14C]-valine. Cells were placed in complete media (CM), Hank's Basic Salt Solution (HBSS) with 10 mM HEPES (Starve) or HBSS with 10 mM HEPES plus 10 mM 3-methyladenine (Starve+3MA). Under all conditions 10 mM cold valine was also supplemented. After 4 to 6 h, cells were collected and processed by TCA precipitation and measured for proteolysis as previously described in reference 20. ANOVA revealed that upon Alfy depletion there was a significant increase of proteolysis under starvation conditions (Starve, F(1,22) = 5.430; *p = 0.0294). There was no significant difference under nonstarved (CM, F(1,22) = 0.101; p = 0.9782) or starvation + 3MA treated (Starve+3MA, F(1,6) = 1.999; p = 0.2071) conditions (N.S. = ‘not significant’). In both siCtrl and siAlfy transfected cells, starvation led to a significant increase in % proteolysis (siCtrl, p = 0.0207; siAlfy, p = 0.0001) but not in the presence of 3MA (siCtrl, p = 0.8878; siAlfy, p = 0.45). (B) LLPD upon overexpression of Alfy. HeLa cells were transfected with an empty vector (Mock) or a vector encoding the p62- and Atg5-binding C terminus of Alfy (amino acids 2,285–3,526) that was previously shown to increase aggregate-clearance (Alfy C).20 LLPD was monitored as described in (A). ANOVA revealed that overexpression of Alfy leads to significantly less proteolysis upon starvation (Starve, F(1,4) = 8.047; *p = 0.0470). There was no significant difference under unstarved (CM, F(1,4) = 0.115; p = 0.7511) or starvation + 3MA treated (Starve+3MA, F(1,6) = 0.510; p = 0.5145) conditions. In both Mock and Alfy C transfected cells, starvation led to a significant increase in % proteolysis (Mock, p = 0.0001; Alfy C, p = 0.0221) which was significantly inhibited by 3MA (Mock, p = 0.001; Alfy C, p = 0.0473).
Taken together, our data indicate that Alfy functions as a scaffolding adaptor protein (Fig. 2): Alfy connects the receptor-bound cargo to the core autophagic machinery and permits the autophagic membrane to form closely to the cargo, excluding bulk cytoplasm. In Cvt, Atg11 overexpression leads to an increased capacity for sequestration of the Cvt cargo precursor aminopeptidase I.4 Similarly, overexpression of Alfy or its C-terminal p62-, Atg5- and PtdIns(3)P-binding region can stimulate aggrephagy as indicated by increased aggregate clearance and cytoprotection.20 This suggests that in the brain, which does not acutely respond to starvation, increasing Alfy levels may be beneficial and drive the elimination of the accumulating proteins that may underlie neurodegeneration. In contrast, in organs which acutely respond to starvation such as liver, increasing Alfy levels may be detrimental, as it will compete with the core machinery during the starvation response. To complement the ongoing cell-based and biochemical studies, in vivo studies will help us determine the importance of Alfy under these different physiological conditions.
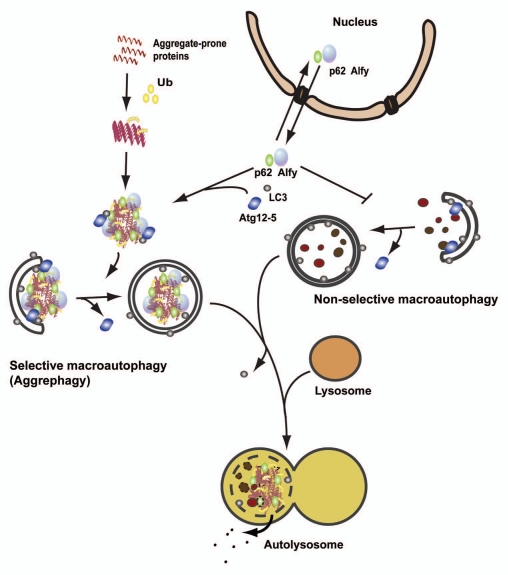
Figure 2.
Schematic comparison of selective macroautophagy of aggregate-prone proteins (aggrephagy) and nonselective macroautophagy. Aggregate-prone proteins become ubiquitinated and start to oligomerize. The ubiquitin-binding autophagy receptor p62, together with the large scaffolding protein Alfy, drives the formation of larger aggregates that are targeted for autophagic degradation through interaction of p62 and Alfy with the core autophagic machinery (LC3 and the Atg12-5 complex, respectively). Alfy also binds to PtdIns(3)P in the autophagic membrane and may facilitate binding of Atg12-5 to the membrane-associated Atg16, creating the Atg12-5-Atg16 complex, which might work as an E3-like ligase to permit LC3 conjugation to PE in the membrane, and autophagosomes to form closely around the inclusion. Alfy becomes recruited from the nucleus to cytoplasmic protein aggregates formed upon cellular stress in a p62-dependent manner. Whereas overexpression of Alfy or its p62-, Atg5- and PtdIns(3)P-binding C terminus leads to increased aggrephagy and decreased nonselective starvation-induced macroautophagy, depletion of Alfy has the opposite effect. Thus, nucleocytoplasmic shuttling of Alfy might be a way to regulate the level of aggrephagy versus nonselective macroautophagy.
Acknowledgements
We would like to acknowledge the following foundations for their generous support: NINDS RO1NS050199, RO1NS063973 and Parkinson's disease foundation (A.Y.); and the Norwegian Council of Research and the Norwegian Cancer Society (A.S.).
References
- 1.Tooze SA, Yoshimori T. The origin of the autophagosomal membrane. Nat Cell Biol. 2010;12:831–835. doi: 10.1038/ncb0910-831. [DOI] [PubMed] [Google Scholar]
- 2.Mizushima N, Levine B. Autophagy in mammalian development and differentiation. Nat Cell Biol. 2010;12:823–830. doi: 10.1038/ncb0910-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Z, Klionsky DJ. Eaten alive: a history of macro-autophagy. Nat Cell Biol. 2010;12:814–822. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lynch-Day MA, Klionsky DJ. The Cvt pathway as a model for selective autophagy. FEBS Lett. 2010;584:1359–1366. doi: 10.1016/j.febslet.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamoto A, Simonsen A. The elimination of accumulated and aggregated proteins: A role for aggrephagy in neurodegeneration. Neurobiol Dis. 2010 doi: 10.1016/j.nbd.2010.08.015. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kraft C, Peter M, Hofmann K. Selective autophagy: ubiquitin-mediated recognition and beyond. Nat Cell Biol. 2010;12:836–841. doi: 10.1038/ncb0910-836. [DOI] [PubMed] [Google Scholar]
- 7.Kirkin V, McEwan DG, Novak I, Dikic I. A role for ubiquitin in selective autophagy. Mol Cell. 2009;34:259–269. doi: 10.1016/j.molcel.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 8.Lamark T, Kirkin V, Dikic I, Johansen T. NBR1 and p62 as cargo receptors for selective autophagy of ubiquitinated targets. Cell Cycle. 2009;8:1986–1990. doi: 10.4161/cc.8.13.8892. [DOI] [PubMed] [Google Scholar]
- 9.Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 10.Vadlamudi RK, Joung I, Strominger JL, Shin J. p62, a phosphotyrosine-independent ligand of the SH2 domain of p56lck, belongs to a new class of ubiquitin-binding proteins. J Biol Chem. 1996;271:20235–20237. doi: 10.1074/jbc.271.34.20235. [DOI] [PubMed] [Google Scholar]
- 11.Bjørkøy G, Lamark T, Brech A, Outzen H, Perander M, Øvervatn A, et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171:603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pohl C, Jentsch S. Midbody ring disposal by autophagy is a post-abscission event of cytokinesis. Nat Cell Biol. 2009;11:65–70. doi: 10.1038/ncb1813. [DOI] [PubMed] [Google Scholar]
- 13.Platta HW, Erdmann R. Peroxisomal dynamics. Trends Cell Biol. 2007;17:474–484. doi: 10.1016/j.tcb.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Kim PK, Hailey DW, Mullen RT, Lippincott-Schwartz J. Ubiquitin signals autophagic degradation of cytosolic proteins and peroxisomes. Proc Natl Acad Sci USA. 2008;105:20567–20574. doi: 10.1073/pnas.0810611105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geisler S, Holmstrom KM, Skujat D, Fiesel FC, Rothfuss JC, Kahle PJ, et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 16.Zheng YT, Shahnazari S, Brech A, Lamark T, Johansen T, Brumell JH. The adaptor protein p62/SQSTM1 targets invading bacteria to the autophagy pathway. J Immunol. 2009;183:5909–5916. doi: 10.4049/jimmunol.0900441. [DOI] [PubMed] [Google Scholar]
- 17.Deretic V. Autophagy in infection. Curr Opin Cell Biol. 2010;22:252–262. doi: 10.1016/j.ceb.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ponpuak M, Davis AS, Roberts EA, Delgado MA, Dinkins C, Zhao Z, et al. Delivery of cytosolic components by autophagic adaptor protein p62 endows autophagosomes with unique antimicrobial properties. Immunity. 2010;32:329–341. doi: 10.1016/j.immuni.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simonsen A, Birkeland HC, Gillooly DJ, Mizushima N, Kuma A, Yoshimori T, et al. Alfy, a novel FYVE-domain-containing protein associated with protein granules and autophagic membranes. J Cell Sci. 2004;117:4239–4251. doi: 10.1242/jcs.01287. [DOI] [PubMed] [Google Scholar]
- 20.Filimonenko M, Isakson P, Finley KD, Anderson M, Jeong H, Melia TJ, et al. The selective macroautophagic degradation of aggregated proteins requires the PI3P-binding protein Alfy. Mol Cell. 2010;38:265–279. doi: 10.1016/j.molcel.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clausen TH, Lamark T, Isakson P, Finley K, Larsen KB, Brech A, et al. p62/SQSTM1 and ALFY interact to facilitate the formation of p62 bodies/ALIS and their degradation by autophagy. Autophagy. 2010;6:330–344. doi: 10.4161/auto.6.3.11226. [DOI] [PubMed] [Google Scholar]
- 22.Finley KD, Edeen PT, Cumming RC, Mardahl-Dumesnil MD, Taylor BJ, Rodriguez MH, et al. blue cheese mutations define a novel, conserved gene involved in progressive neural degeneration. J Neurosci. 2003;23:1254–1264. doi: 10.1523/JNEUROSCI.23-04-01254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang N, Wu WI, De LA. BEACH family of proteins: phylogenetic and functional analysis of six Dictyostelium BEACH proteins. J Cell Biochem. 2002;86:561–570. doi: 10.1002/jcb.10254. [DOI] [PubMed] [Google Scholar]
- 24.De LA. The role of BEACH proteins in Dictyostelium. Traffic. 2003;4:6–12. doi: 10.1034/j.1600-0854.2003.40102.x. [DOI] [PubMed] [Google Scholar]
- 25.Jogl G, Shen Y, Gebauer D, Li J, Wiegmann K, Kashkar H, et al. Crystal structure of the BEACH domain reveals an unusual fold and extensive association with a novel PH domain. EMBO J. 2002;21:4785–4795. doi: 10.1093/emboj/cdf502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gebauer D, Li J, Jogl G, Shen Y, Myszka DG, Tong L. Crystal structure of the PH-BEACH domains of human LRBA/BGL. Biochemistry. 2004;43:14873–14880. doi: 10.1021/bi049498y. [DOI] [PubMed] [Google Scholar]
- 27.Hanada T, Noda NN, Satomi Y, Ichimura Y, Fujioka Y, Takao T, et al. The Atg12-Atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. J Biol Chem. 2007;282:37298–37302. doi: 10.1074/jbc.C700195200. [DOI] [PubMed] [Google Scholar]
- 28.Fujita N, Hayashi-Nishino M, Fukumoto H, Omori H, Yamamoto A, Noda T, et al. An Atg4B mutant hampers the lipidation of LC3 paralogues and causes defects in autophagosome closure. Mol Biol Cell. 2008;19:4651–4659. doi: 10.1091/mbc.E08-03-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simonsen A, Tooze SA. Coordination of membrane events during autophagy by multiple class III PI3-kinase complexes. J Cell Biol. 2009;186:773–782. doi: 10.1083/jcb.200907014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scarlatti F, Granata R, Meijer AJ, Codogno P. Does autophagy have a license to kill mammalian cells? Cell Death Differ. 2009;16:12–20. doi: 10.1038/cdd.2008.101. [DOI] [PubMed] [Google Scholar]