Abstract
Fibroblast growth factor-23 (FGF23), a hormone central to phosphate and vitamin D metabolism, reduces renal absorption of phosphate by downregulating the sodium-phosphate cotransporter Npt2a. However, the mechanisms of FGF23 action in the kidney are unclear, as Npt2a localizes to the proximal tubule (PT) and the FGF23 coreceptor α-Klotho (KL) localizes to the distal convoluted tubule (DCT). Immunofluorescent analyses following FGF23 injection in mice showed robust staining for phospho-ERK1/2, a marker of FGF23 bioactivity, only within the DCT in a subset of KL-positive cells. This activity colocalized with the FGF23 receptor FGFR1 and was present in DCT cells that were adjacent to Npt2a-expressing PT segments. Although KL is expressed as both secreted and membrane-bound isoforms, only the membrane-bound isoform was capable of mediating FGF23 bioactivity. These findings provide novel insight into the mechanisms of hormone-regulated phosphate metabolism by identifying an intrarenal signaling axis for FGF23.
The hormone fibroblast growth factor-23 (FGF23) plays a central role in phosphate metabolism, as demonstrated by several genetic disorders characterized by increased FGF23 serum levels, including autosomal dominant hypophosphatemic rickets,1 X-linked hypophosphatemic rickets,2 and autosomal recessive hypophosphatemic rickets.3 Studies in animal models have shown that increased serum concentrations of FGF23 lead to renal phosphate wasting through downregulation of the proximal tubule (PT) apical membrane Type II (Npt2a and Npt2c) sodium-phosphate cotransporters.4,5 The reciprocal disorder, familial hyperphosphatemic tumoral calcinosis (TC), caused by mutations in FGF23 and the glycosylating enzyme GALNT3, is characterized by decreased serum levels of active FGF23, normal parathyroid hormone levels, increased or normal 1,25(OH)2 vitamin D levels, and often severe hyperphosphatemia due to excessive renal phosphate reabsorption.6
The coreceptor α-Klotho (KL) was recently identified as necessary for FGF23 bioactivity.7,8 KL is produced as two isoforms due to alternative splicing of the same five-exon gene. Membrane-bound KL (mKL) is a 130-kD single-pass transmembrane protein comprised of all five exons and is characterized by a large extracellular domain with a short cytoplasmic region of 11 residues that does not contain signaling capabilities.9 The secreted form of KL (sKL) is 80 kD and is alternatively spliced within exon 3, producing a KL protein species that possesses the extracellular region, but not the transmembrane domain, and is secreted into the circulation.9 A third isoform is produced by cleavage of mKL in proximity to the extracellular face of the plasma membrane, referred to as “cut mKL” (cKL), resulting in a protein that is also found in the circulation.10 The circulating forms of KL have led to interpretations that KL itself may act as a hormone.10
In vitro evidence supports associations between fibroblast growth factor receptor 1c and KL as part of a receptor complex to elicit FGF23 signaling through the mitogen activated protein kinase (MAPK) cascade and phospho-ERK1/2 (p-ERK1/2).7,8 Underlying the importance of the formation of a KL-fibroblast growth factor receptor complex, high levels of FGF23 signaling in vitro occur when KL and fibroblast growth factor receptor 1c are both present.7 In support of FGF23-KL interactions, the Fgf23- and KL-null animals have identical hyperphosphatemic phenotypes.11–14 Additionally, a novel recessive, inactivating mutation in the human KL gene resulted in impaired KL expression and a severe tumoral calcinosis phenotype, most likely due to end-organ resistance to FGF23.15 Although it has been shown that KL permits FGF23 signaling in vitro, the mechanisms underlying FGF23 bioactivity in the kidney are unclear, because KL localizes to the distal convoluted tubule (DCT) and FGF23 mediates its effects on Npt2a, Npt2c, and vitamin-D metabolizing enzymes within the PT.4,16 We therefore undertook studies to test the dynamics of FGF23 function in vivo, which has revealed multistaged FGF23 bioactivity within the kidney nephron.
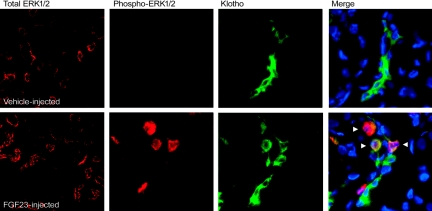
To identify FGF23-dependent MAPK activity in vivo, wild-type mice were injected with 10 μg of recombinant human FGF23 or vehicle and sacrificed at 5 and 10 min, time points associated with p-ERK1/2 signaling from FGF23-injected animals,7 as well as at 30 and 60 min. After sacrifice, an immunofluorescent approach was used to assess the spatial distribution of FGF23 activity within the kidney. The FGF23-injected animals demonstrated robust nuclear p-ERK1/2 reactivity limited to a subset of KL-positive cells only within the DCT at both the 5- and 10-min time points (Figure 1), which was completely dissipated by 30 to 60 min (not shown). The control animals were negative for DCT expression of p-ERK1/2, but had strong KL immunoreactivity (Figure 1). Total ERK1/2 staining was equally distributed in all nephron segments for both vehicle- and FGF23-injected animals (Figure 1).
Figure 1.
FGF23-dependent p-ERK1/2 signaling in kidney. Results from vehicle-injected animals are shown in the upper panels, and FGF23-injected animals in the lower panels at 5 min postinjection (10-min results were identical). Total ERK1/2 staining was equally positive in all nephron segments for vehicle- and FGF23-injected animals. Phospho-ERK1/2 staining was only observed in the FGF23-injected animals; KL was positive in both vehicle- and FGF23-injected animals. p-ERK1/2 staining (red) localized to the nucleus in the same nephron segment as KL (green) in FGF23-injected mice, as shown by p-ERK1/2 and KL costaining (Merge column; arrows show positive nuclear p-ERK1/2 colocalized with KL). Nuclei were stained blue using DAPI in the Merge column.
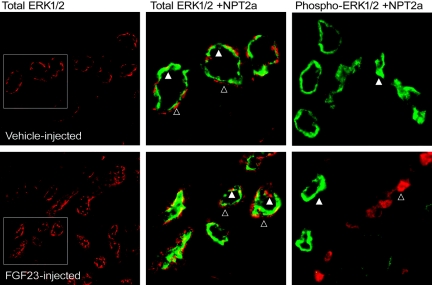
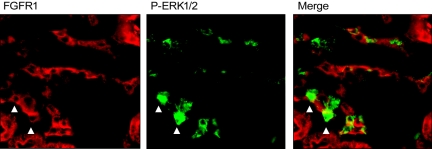
Next, to test FGF23 activity in nephron segments expressing known downstream targets of FGF23, Npt2a localization was assessed in parallel with FGF23 signaling. In these analyses p-ERK1/2 activity was positive within DCT nephron segments adjacent to, but clearly distinct from Npt2a expression in the PT (Figure 2), indicating that initial FGF23 bioactivity through MAPK corresponds with the expression of KL and not with the phosphate transporters required for maintaining blood phosphorus levels. In vitro, FGF23, KL, and fibroblast growth factor receptor 1 (FGFR1) form a heteromeric complex to elicit a signaling cascade through p-ERK1/2.7,8 In the FGF23-injected animals, p-ERK1/2 colocalized with FGFR1 staining (Figure 3), indicating the formation of heteromeric interactions between KL and FGFR1 in vivo. Distinct apical membrane expression of KL and FGFR1 was not detected, thus KL and FGFR1 expression is likely localized to the basolateral membrane. It has been shown that both FGFR1 and KL are glycosylated within the TGN before residence in the cell membrane, consistent with the fact that a portion of the FGFR1 and KL staining also appears as intracellular.10,17 In agreement with the above results from vehicle-injected animals, there was no p-ERK1/2 and FGFR1 colocalization in this experimental group (not shown).
Figure 2.
Distribution of FGF23 activity and Npt2a. Total ERK1/2 (red) was detected widely throughout the kidney (left panels; low magnification). Total ERK1/2 (red, open arrowheads) colocalized with Npt2a (green, closed arrowhead) in the PT (boxed area from left panels are shown at high magnification in the center panels). Npt2a was detected in vehicle-injected animals; however, p-ERK1/2 staining was not observed in control mice (right panel, open arrowhead). In contrast, p-ERK1/2 staining (red) was readily detectable and spatially separated from Npt2a in the PT (green, closed arrowhead) in the FGF23-injected mice (right panel).
Figure 3.
Localization of p-ERK1/2 and FGFR1. FGFR1 (red) staining was detected in the kidney (left panel). Staining for p-ERK1/2 (green, center panel) colocalized with a subset of FGFR1-positive cells in FGF23-injected mice. Arrows highlight cells that are FGFR1 and p-ERK1/2 positive in the same kidney section.
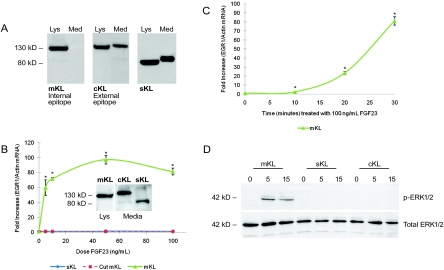
Through alternative splicing and protease activity, α-KL can arise as distinct isoforms (mKL, sKL, and cKL);10,18 therefore, the isoform(s) that mediates FGF23 signaling is unclear. To test KL activity, HEK293 stable cell lines were developed that expressed either mKL (and therefore cKL in the conditioned media) or sKL (Figure 4A). The mKL protein carries an intracellular C-terminal V5 tag that is removed after protease cleavage of mKL at the external cell surface (mKL is then soluble as “cKL” in the media). The presence of cKL in the media was confirmed by Western blot using an anti-KL antibody to an external epitope, in tandem with an anti-V5 antibody that recognizes the intracellular tag (present only in the cell lysate; Figure 4A).
Figure 4.
FGF23-dependent KL activity. (A) Full-length mKL was detected in the lysate (Lys), but not in the cell media (Med) using an anti-V5 antibody to a C-terminal tag (left panels). cKL was detectable in the cell media using an anti-human KL antibody to an extracellular KL epitope (center). sKL was detected in the cell lysate and media using anti-V5 (right). (B) HEK293 cells stably expressing mKL or sKL, and native HEK293 cells treated with cKL were treated with vehicle or 5, 10, 50, and 100 ng/ml of FGF23 for 30 min. EGR1 mRNA, assessed by quantitative PCR, showed a dose-dependent increase in the mKL cells (*P < 0.05). There was no difference in EGR1 mRNA compared with control in either sKL or cKL. (Inset) Western blot shows one-half of the total mKL, cKL, and sKL protein present per well for the qPCR experiments graphed in Figure 4B. (C) A time-dependent increase in EGR1 mRNA compared with control was observed when mKL cells were treated with 100 ng/ml of FGF23 for 10, 20, and 30 min (*P < 0.05). (D) HEK293 cells stably expressing mKL or sKL, and native HEK293 cells treated with cKL, were treated with 100 ng/ml of FGF23 for 0, 5, and 15 min. p-ERK1/2 expression, as assessed by Western blot analyses, was only detected in the mKL cells (upper panel). Total ERK1/2 was similar for all cells across treatments (lower panel).
To test the KL isoforms that mediate FGF23-dependent bioactivity, HEK293 cells stably expressing the proteins were treated with 5 to 100 ng/ml of FGF23. Transcription of the early growth response-1 (EGR1) gene mRNA, measured as an established marker of FGF23 activity,7,19 increased with FGF23 doses in the presence of mKL, with a maximum increase of 90-fold compared with vehicle-treated cells (P < 0.05; Figure 4B). EGR1 mRNA expression also increased (P < 0.05) with time-dependent FGF23 treatment in the mKL cell line (Figure 4C). In contrast to the robust signaling of the mKL isoform, there was no increase in EGR1 mRNA levels in the presence of FGF23 and cKL or sKL versus control (Figure 4B), although protein levels for these isoforms were similar to mKL as assessed by Western analyses (Figure 4B, inset). Additionally, sKL and cKL still showed no FGF23-dependent increases in EGR1 when 10-fold more recombinant KL protein was added (not shown).
In parallel experiments, FGF23-dependent p-ERK1/2 activity was tested. Consistent with the findings above for EGR1 mRNA induction (Figure 4B), only cells stably expressing mKL had increased p-ERK1/2 activity, as assessed by Western blot analyses (Figure 4D). These results indicate that the isoform of α-KL that acts to mediate FGF23-dependent intracellular signaling through ERK1/2 is the mKL species.
The biologic actions of FGF23 are in part mediated by the coreceptor α-KL, which forms a heteromeric complex with FGFR1 to elicit MAPK signaling.7 Within the kidney, KL is expressed in the DCT; however, FGF23 regulates Npt2a and Npt2c in the PT, creating a paradox for FGF23 bioactivity. The studies presented here demonstrate that after FGF23 delivery in vivo, p-ERK1/2 staining colocalized with KL (Figure 1 and 2) and FGFR1 (Figure 3) in the DCT, spatially separated from Npt2a staining in the PT (Figure 2). The relatively short time course (5 to 10 min) required to detect p-ERK1/2 in the DCT after FGF23 delivery indicates that initial actions of FGF23 occur in the DCT, followed by reduced Npt2a in the PT through a secondary step that does not require p-ERK1/2 in a process that can take 30 min to hours.20 FGF23 also regulates NPT2c expression in vivo.4 In contrast to the broad localization of NPT2a within the proximal convoluted and proximal straight tubules, NPT2c expression is limited to the proximal convoluted tubule.21,22 Because NPT2c expression is more restricted than NPT2a expression, it is likely that in a similar manner to NPT2a, NPT2c does not colocalize with FGF23-dependent p-ERK1/2 activity. Although p-ERK1/2 reactivity localizes to the DCT and not with NPT2a after FGF23 delivery, it is possible that an alternative signaling event independent of p-ERK1/2 could occur within the PT. Finally, it is intriguing that p-ERK1/2 was detected in a subset of KL-positive cells. The molecular nature of this observation remains to be explored, but we cannot rule out the possibility that because p-ERK1/2 detection is transient, subsets of cells may rapidly downregulate this signaling after FGF23 delivery and would thus appear to be KL- and FGFR1 positive and p-ERK1/2 negative.
KL is expressed as both secreted and membrane-bound isoforms. Our results demonstrated that although multiple KL isoforms exist, only the mKL isoform was capable of mediating FGF23 bioactivity (Figure 4), as measured by increased EGR1 mRNA and p-ERK1/2 levels. Taken together with the parallel mapping of p-ERK1/2 activity in vivo (Figure 1), these findings indicate that FGF23-mediated signaling occurs within the DCT through the membrane-bound form of KL. Our results revealed that positive p-ERK1/2 staining in the DCT can be adjacent to nephron segments expressing Npt2a, supporting the possibility that a paracrine factor or process may be produced in the DCT that mediates local, but downstream, effects in the PT.
In summary, we have demonstrated that initial FGF23-mediated signaling in the kidney through MAPK occurs in the DCT and is colocalized with KL expression. This activity is spatially separated from Npt2a in the PT, which may allow for fine control of FGF23 bioactivity through nephron segment-specific events.
CONCISE METHODS
Animal Studies
Animal studies were performed according to the Institutional Animal Care and Use Committee for Indiana University, and comply with the National Institutes of Health guidelines for the use of animals. Wild-type C57Bl6 mice (Jackson Laboratories, Bar Harbor, ME) were injected with either vehicle or 10 μg of recombinant human FGF23 intravenously. Human FGF23 used in the analyses was produced in insect cells (d-Mel-2) and is therefore fully glycosylated (generous gift from Susan C. Schiavi, Ph.D., Genzyme Corp.). Animals were sacrificed after 5, 10, 30, and 60 min and the kidneys were removed.
Immunofluorescence
Kidneys were fixed with 4% paraformaldehyde, mounted in optimal cutting temperature mounting medium (Sakura Finetek), and sliced into 10-μm sections. Sections were treated with pepsin (Biocare Medical) for antigen retrieval and probed with either anti-mouse KL (Santa Cruz Biotechnology); phospho-ERK1/2 (p-ERK1/2, Cell Signaling); total ERK1/2 (Promega); FGFR1 (Santa Cruz Biotechnology); or Npt2a (antibody L697)23 followed by incubation with the appropriate fluorescence secondary antibodies (Alexa Fluor, Invitrogen). Imaging was performed using a Leica DM5000B fluorescence microscope (Leica Microsystems, Inc.), SPOT camera, and computer program (RTKE Diagnostic Instruments, Inc.).
Cell Culture
HEK293 cells (American Type Culture Collection) were cultured in D-MEM/F-12 (Invitrogen) supplemented with 10% FBS (Hyclone), 1 mM sodium pyruvate, 25 mM l-glutamine, and 25 mM penicillin-streptomycin at 37°C and 5% carbon dioxide. For deriving stable cell lines, cells were transfected with 2 μg of plasmid DNA (either human mKL or sKL cDNAs) using the Fugene 6 (Roche) transfection reagent following manufacturer's protocol. Cells stably expressing either mKL or sKL were selected using 800 μg/ml of G418. To produce conditioned media containing the cut membrane form of KL (cKL), serum-free conditioned media from the mKL stable cell line was collected. For KL isoform bioactivity assays, HEK293 cells stably expressing mKL or sKL (1.5 × 105) were seeded on 12-well plates. The mKL and sKL cell lines were serum starved for 16 h, and for cKL activity, conditioned media containing cKL was added to native HEK293 cells during serum starvation overnight. The cell lines were treated with vehicle or FGF23 at the doses and times specified in the Results section for each experiment, and cell lysates, conditioned media, or RNA was then collected. To further test cKL and sKL activity, 10 ml of sKL and cKL conditioned media was concentrated to 1 ml using an YM-30 Centricon (Millipore). The 10× concentrated sKL and cKL media was added to either sKL or native HEK293 cells, respectively, for serum starvation overnight. For 10× sKL and cKL activity, cells were treated with either vehicle or 100 ng/ml of FGF23 for 30 min and the RNA was harvested for analysis.
Western Blot Analysis
Western blot analysis was performed as described previously.24 The blots were incubated with 1:1000 primary antibody (anti-V5 conjugated to HRP, Invitrogen, Inc.; anti-human KL, generous gift from Immutopics, Inc.; anti p-ERK1/2, Cell Signaling Technologies, Inc.; or total-ERK, Promega) and then incubated with the appropriate secondary antibody at 1:3000 (anti-goat HRP, Santa-Cruz; anti-rabbit HRP, Biorad). Detection was performed using the ECL Plus western blotting detection reagents (Amersham GE Healthcare).
Quantitative RT-PCR
RNA was harvested from cellular lysates using the RNeasy kit (Qiagen, Inc.) according to manufacturer's protocols. The RNA samples were then tested with intron-spanning primers specific for human EGR1 mRNA, and human β-actin was used as an internal control (primer sequences available upon request). The TaqMan One-Step RT-PCR kit was used to perform quantitative PCR. Data were collected and analyzed with a 7500 Real-Time PCR system and software (Applied Biosystems). The expression of EGR1 and actin mRNAs was calculated relative to vehicle-treated control. Each RNA sample was analyzed in triplicate, and each experiment was performed independently at least three times. The 2−ΔΔCT method described by Livak was used to analyze the data.25
Statistical Analysis
Statistical analyses of the data were performed by t test and significance for all tests was set at P < 0.05. Data are presented as means ± SEM.
DISCLOSURES
K.E.W. receives royalties for licensing the FGF23 gene to Kirin Pharma.
Acknowledgments
The authors acknowledge support by National Institutes of Health grant KD063934 (K.E.W.) and the Indiana Genomics Initiative of Indiana University, supported in part by the Lilly Endowment, Inc. The authors are grateful to Susan C. Schiavi, Ph.D., Genzyme Corporation, Framingham, Massachusetts, for supplying FGF23 and to Harry C. Dietz, Johns Hopkins, for supplying the KL plasmids.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.ADHR Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 26: 345–348, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H, Ljunggren O, Oba K, Yang IM, Miyauchi A, Econs MJ, Lavigne J, Juppner H: Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med 348: 1656–1663, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE: Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet 38: 1310–1315, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larsson T, Marsell R, Schipani E, Ohlsson C, Ljunggren O, Tenenhouse HS, Juppner H, Jonsson KB: Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology 145: 3087–3094, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Shimada T, Urakawa I, Yamazaki Y, Hasegawa H, Hino R, Yoneya T, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T: FGF-23 transgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type IIa. Biochem Biophys Res Commun 314: 409–414, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Larsson T, Yu X, Davis SI, Draman MS, Mooney SD, Cullen MJ, White KE: A novel recessive mutation in fibroblast growth factor-23 causes familial tumoral calcinosis. J Clin Endocrinol Metab 90: 2424–2427, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T: Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444: 770–774, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Kuro-o, M: Klotho as a regulator of fibroblast growth factor signaling and phosphate/calcium metabolism. Curr Opin Nephrol Hypertens 15: 437–441, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o, M, Nabeshima Y: Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun 242: 626–630, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y: Secreted Klotho protein in sera and CSF: Implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett 565: 143–147, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T: Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113: 561–568, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI: Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390: 45–51, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Segawa H, Yamanaka S, Ohno Y, Onitsuka A, Shiozawa K, Aranami F, Furutani J, Tomoe Y, Ito M, Kuwahata M, Imura A, Nabeshima Y, Miyamoto K: Correlation between hyperphosphatemia and type II Na-Pi cotransporter activity in klotho mice. Am J Physiol Renal Physiol 292: F769–F779, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Sitara D, Razzaque MS, Hesse M, Yoganathan S, Taguchi T, Erben RG, Juppner H, Lanske B: Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol 23: 421–432, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ichikawa S, Imel EA, Kreiter ML, Yu X, Mackenzie DS, Sorenson AH, Goetz R, Mohammadi M, White KE, Econs MJ: A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest 117: 2684–2691, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T: Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A 98: 6500–6505, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duchesne L, Tissot B, Rudd TR, Dell A, Fernig DG: N-glycosylation of fibroblast growth factor receptor 1 regulates ligand and heparan sulfate co-receptor binding. J Biol Chem 281: 27178–27189, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o, M: Suppression of aging in mice by the hormone Klotho. Science 309: 1829–1833, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goetz R, Beenken A, Ibrahimi OA, Kalinina J, Olsen SK, Eliseenkova AV, Xu C, Neubert TA, Zhang F, Linhardt RJ, Yu X, White KE, Inagaki T, Kliewer SA, Yamamoto M, Kurosu H, Ogawa Y, Kuro-o M, Lanske B, Razzaque MS, Mohammadi M: Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol Cell Biol 27: 3417–3428, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamazaki Y, Tamada T, Kasai N, Urakawa I, Aono Y, Hasegawa H, Fujita T, Kuroki R, Yamashita T, Fukumoto S, Shimada T: AntiFGF23 neutralizing antibodies show the physiological role and structural features of FGF23. J Bone Miner Res 23: 1509–1518, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Custer M, Lotscher M, Biber J, Murer H, Kaissling, B: Expression of Na-P (i) cotransport in rat kidney: Localization by RT-PCR and immunohistochemistry. Am J Physiol 266: F767–F774, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Segawa H, Kaneko I, Takahashi A, Kuwahata M, Ito M, Ohkido I, Tatsumi S, Miyamoto K: Growth-related renal type II Na/Pi cotransporter. J Biol Chem 277: 19665–19672, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Kim GH, Martin SW, Fernandez-Llama P, Masilamani S, Packer RK, Knepper MA: Long-term regulation of renal Na-dependent cotransporters and ENaC: Response to altered acid-base intake. Am J Physiol Renal Physiol 279: F459–F467, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Larsson T, Davis SI, Garringer HJ, Mooney SD, Draman MS, Cullen MJ, White KE: Fibroblast growth factor-23 mutants causing familial tumoral calcinosis are differentially processed. Endocrinology 146: 3883–3891, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]