Abstract
To accommodate functional demands, the composition and organization of the skeleton differ among species. Microcomputed tomography has improved our ability markedly to assess structural parameters of cortical and cancellous bone. The current study describes differences in cortical and cancellous bone structure, bone mineral density, and morphology (geometry) at the proximal femur, proximal femoral diaphysis, lumbar vertebrae, and mandible in mice, rats, rabbits, dogs, and nonhuman primates. This work enhances our understanding of bone gross and microanatomy across lab animal species and likely will enable scientists to select the most appropriate species and relevant bone sites for research involving skeleton. We evaluated the gross and microanatomy of the femora head and neck, lumbar spine, and mandible and parameters of cancellous bone, including trabecular number, thickness, plate separation, and connectivity among species. The skeletal characteristics of rabbits, including a very short femoral neck and small amounts of cancellous bone at the femoral neck, vertebral body, and mandible, seem to make this species the least desirable for preclinical research of human bone physiology; in comparison, nonhuman primates seem the most applicable for extrapolation of data to humans. However, rodent (particularly rat) models are extremely useful for conducting basic research involving the skeleton and represent reliable and affordable alternatives to dogs and nonhuman primates. Radiology and microcomputed tomography allow for reliable evaluation of bone morphology, microarchitecture, and bone mineral density in preclinical and clinical environments.
Abbreviations: mCT, microcomputed tomography
The skeleton is a mechanically optimized biological system and its composition and organization accommodate to the functional demands placed on it. In general, the mechanical properties of bone are determined by bone geometry, mineral density, and structure.6,9,16,64,69 Hydroxyl apatite provides most of the strength and stiffness to the skeleton and permits the use of radiologic technologies to assess bone mass and structure. New technologies including dual-energy X-ray absorbtiometry, quantitative computed tomography, and microcomputed tomography (mCT) have been developed to assess the gross and microanatomy of the skeleton during health and disease. Using noninvasive methodology to describe bone anatomy and structure has broad application in several scientific disciplines, including medical and pharmaceutical research, animal health and meat industry, and biologic and forensic anthropology.22,46,52 The current study focuses on using radiography and mCT to describe major skeletal differences between several commonly used laboratory animal species. These differences manifest in the physical characteristics of their skeletons, including their morphology and dimensions, but differences in growth rates and laboratory conditions might also influence cortical and cancellous bone structures as well as the biochemical composition of bone.1,3,43 Using Wessler's definition70 of an animal model, Kalu37 has suggested that the convenience, relevance, and appropriateness (compatibility to humans) of particular animal models should be considered when deciding what laboratory animals to use to study the skeletal effects of novel therapeutics aimed to prevent or cure osteoporosis. Kalu's suggestions36 likely can be applied to all areas of pharmaceutical research involving animals. Regardless of the animal model or species is used to study intended or undesired skeletal effects of novel compounds, the decreased physical activity of animals under laboratory conditions and differences in skeletal mechanical properties between humans (bipeds) and lab animals (quadrupeds) should always be taken into account.12,18,49,51,55,61 Along these lines, the Food and Drug Administration requires that novel therapies in osteoporosis research must be tested both in rodents (preferably rats) as well as a large animal model.61 This requirement is based on the fact that growth plates in rodents remain open throughout their life span, allowing bone growth and modeling to continue, resembling immature skeleton.28,71 However, the reason for using a second species in preclinical skeletal research is the lack of the Haversian system in rodents; therefore, other species, including dogs, nonhuman primates, pigs, and sheep should be considered.36,49,55 Because the skeletal system is highly responsive to mechanical loads that influence bone geometry and structure as well as the rate of bone remodeling, the structural properties of cancellous and cortical bone at several skeletal sites with different mechanical properties, such as the femoral neck, vertebrae, and mandible, should be assessed.5,9,10,17,53,63,67
By using radiology and mCT to describe major skeletal differences among common laboratory animals, we hope to help investigators to pick the right species when conducting animal studies aiming to address issues associated with skeletal health. In the present study, we compared bone properties at 3 skeletal sites with different mechanical properties—the proximal femur, lumbar vertebrae, and mandible—in laboratory mice, rats, rabbits, dogs, and cynomolgus macaques.
Materials and Methods
Bone samples.
Experimentation using laboratory animals including mice (Mus musculus, C57BL6), rats (Rattus norvegicus, CD/SD), rabbits (Oryctolagus cuniculus, New Zealand white), dogs (Canis familiaris, beagle), and nonhuman primates (Macaca fascicularis, Mauritian cynomolgus macaque) is a mandatory step regulated by the Food and Drug Administration for each Investigational New Drug Application that is aimed to minimize potential harm of novel drug candidates to patients that participate in the clinical trials. The safety preclinical studies in laboratory species require only small samples of bone tissue from that used for pathologic examination; the vast majority of the skeleton frequently is discarded without use in further research. To evaluate the bone morphology and structure of cancellous and cortical bone at the proximal femur, lumbar vertebrae, and mandible, we collected the bones from euthanized animals that were used in various preclinical studies. We used only animals that received vehicle only in these previous studies to minimize the chance of drug-associated changes in bone structure and bone mineral density. All animals in the original studies were maintained in an AAALAC-accredited research facility, and the original animal procedures were IACUC-approved and conformed to the Guide for the Care and Use of Laboratory Animals.26 The right femurs, lumbar vertebrae (L4, L5), and mandibles were collected immediately after necropsy, cleaned of soft tissues, and placed in 10% formalin for 24 h, transferred to 70% alcohol, and stored at 4 °C before being used for X-ray and mCT analyses. The numbers of animals used in the current study were 6 male and 6 female mice (age, 5 mo); 6 male and 6 female rats (age, 5 mo); 4 male and 4 female rabbits (age, 16 mo old); 4 male and 3 female dogs (age, 3.5 to 4 y old); 2 male and 3 female cynomolgus macaques (age, 4 y) and 1 male cynomolgus macaque (age, 5 y). Therefore, all animals from which bones were collected had reached sexual maturity before they were used in the original studies, and radiography confirmed sealed growth plates in macaques, dogs, and rabbits.
X-ray and mCT measurements.
Femurs.
The femurs used for mCT analysis in this study were collected from animals that were euthanized according to their respective study protocols. Whole femurs were harvested at necropsy, cleaned of soft tissue, and fixed in 10% formalin. Proximal femurs were radiographed by using a digital X-ray system (Faxitron X-ray, Wheeling, IL) with the following manufacturer-recommended settings according to the size of the bone samples: mouse: 10 s at 25 kV; rat: 12 s at 30 kV; rabbit: 12 s at 35 kV; dog: 12 s at 35 kV and macaques: 12 s at 35 kV. Radiographic images were used to assess the gross anatomy of the region of interest for every animal used in this study. Because large differences in size and morphology of the skeletal areas between species prevented use of the same region of interest across species, we first ran ‘scout’ mCT images for each anatomic site and for each species to ensure that we could consistently and reproducibly scan and analyze particular target regions in each species and that size of the target picked permitted meaningful analysis of cancellous bone structure. At the same time, the optimal thickness and resolution parameters were determined for each species and each anatomic location that conformed to the limits of the imaging system and study goals.20 This optimization ensured that trabecular thickness and number could be captured, measured, and analyzed when the number and thickness of slices chosen to capture bone structure at the target region in each species assessed in this study were applied. Results from the pilot studies then were applied to capture the exact cortical or cancellous bone areas that we attempted to measure by using the optimal mCT settings.20
Femurs were analyzed by using the VivaCT 40 mCT system (Scanco Medical, Bassersdorf, Switzerland) with the following settings: mouse, 45 kVp at 88 µA at high resolution (10 µm); rat, 55 kVp at 109 µA at high resolution (12.5 µm); rabbit, 55 kVp at 109 µA at high resolution (15 µm); dog, 70 kVp at 85 µA at high resolution (19 µm); and NHP, 70 kVp at 85 µA at high resolution (19 µm). Cancellous bone parameters at the femoral neck and head were analyzed; these parameters included tissue volume (bone and bone marrow combined), bone volume, bone volume: tissue volume ratio, trabecular number, trabecular thickness, trabecular separation (distance between individual trabeculae), trabecular connectivity (the number of times per unit area that adjacent trabeculae touch each other) and bone mineral density (g Ca2+/cm2). Cortical bone parameters were analyzed at femoral proximal diaphysis and included tissue volume, bone volume, bone volume:tissue volume ratio, marrow volume, and cortical thickness. A single sample of human proximal femur was obtained through a tissue bank and was used only as a standard for comparison. Because of large differences in the size and shape of the proximal femur among species, the setup presented in Table 1 was applied to analyze cancellous and cortical bone parameters by using mCT.
Table 1.
mCT parameters used to collect and analyze cortical and cancellous bone structure and bone mineral density
| Mouse | Rat | Rabbit | Canine | Cynomolgus macaque | ||
| Mid-diaphysis | ||||||
| No. of slices | 20 | 20 | 20 | 20 | 20 | |
| Thickness (mm) | 0.21 | 0.25 | 0.30 | 0.38 | 0.38 | |
| Resolution (µm) | 10.5 | 12.5 | 15.0 | 19.0 | 19.0 | |
| Femoral head | ||||||
| No. of slices | 25 | 50 | 25 | 50 | 50 | |
| Thickness (mm) | 0.03 | 0.65 | 0.30 | 0.95 | 0.95 | |
| Resolution (µm) | 10.5 | 12.5 | 15.0 | 19.0 | 19.0 | |
| Femoral neck | ||||||
| No. of slices | 25 | 50 | 25 | 50 | 50 | |
| Thickness (mm) | 0.03 | 0.65 | 0.30 | 0.95 | 0.95 | |
| Resolution (µm) | 10.5 | 12.5 | 15.0 | 19.0 | 19.0 |
Lumbar vertebrae.
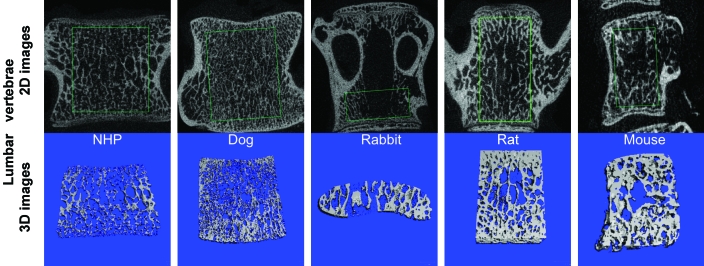
L4 and L5 were collected at necropsy and carefully cleaned of soft tissue; 3 to 5 d later, only L4 vertebra from each animal enrolled in the study was radiographed by using a digital X-ray system (Faxitron X-Ray LLC, Wheeling, IL) at the same settings described earlier for femurs. Only cancellous bone parameters were analyzed at vertebral bodies and included tissue volume, bone volume, tissue volume: bone volume ratio, trabecular number, trabecular thickness, trabecular separation, trabecular connectivity, and bone mineral density. Due to their very thin vertebral bodies which contains very little to no cancellous bone, the region of interest for rabbit lumbar vertebrae differed from that of other species and was placed at the bottom of the lumbar vertebral body, which provides sufficient cancellous bone for mCT measurements.
Mandibles.
The right mandibles were collected at necropsy and carefully cleaned of soft tissue; 3 to 5 d later the mandibles were radiographed by using a digital X-ray system (Faxitron X-Ray, Wheeling, IL) at the same settings described earlier for femurs. Cancellous bone parameters were analyzed at mandibular bodies and included only the area below the first and second molars. Connective tissue surrounding roots of the first and second molars and cortical bone shell were not included in the analyses. The following parameters were evaluated by mCT: tissue volume, bone volume, bone volume: tissue volume ratio, trabecular number, trabecular thickness, trabecular separation, trabecular connectivity, and bone mineral density.
Statistical analysis
Statistical analyses of interspecies differences were not carried out due to large variations in size and shape of the bones between species, relatively small sample size, and use of both genders in each species analyzed. The results obtained from all subjects of a particular species were used (regardless of sex) to generate summary data, reported as mean ± 1 SD.
Results
Femur.
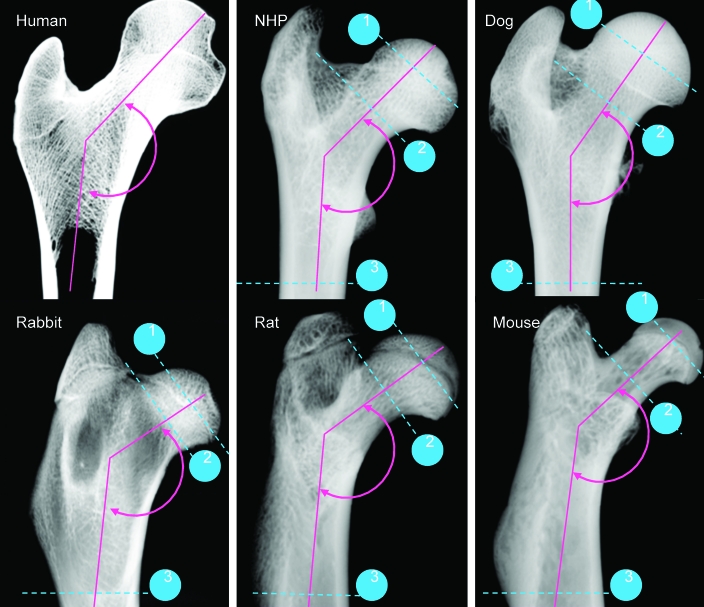
The gross anatomy of the human proximal femur has distinct dissimilarity from the laboratory species. The most obvious distinctions included the angle between the diaphyseal shaft and femoral neck, the length and width of the femoral neck, and size and shape of the femoral head (Figure 1).
Figure 1.
Radiographic images of the femoral proximal diaphysis captured by digital radiography from human, nonhuman primate, dog, rabbit, rat, and mouse. The human sample was used for reference only. The angle between the femoral shaft and the femoral neck is shown in green. Dotted blue lines and associated numbers indicate the sites at the proximal femur where structural parameters were measured by mCT: 1, femoral head; 2, femoral neck; and 3, proximal femoral diaphysis.
Femoral head.
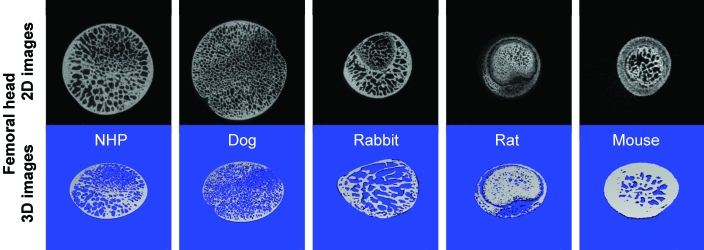
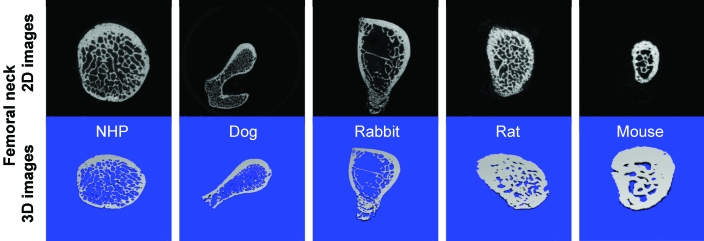
Results from mCT analyses of the cancellous bone at the femoral head provide values for cancellous bone volume (in mm3) in cynomolgus macaques (43.96 ± 5.03), dogs (59.72 ± 10.87), rabbits (3.22 ± 0.58), rats (2.59 ± 0.90) and mice (0.19 ± 0.06; Tables 2 to 6, Figures 2 to 6). The ratio of bone volume to tissue volume ranged from 0.77 ± 0.12 and 0.74 ± 0.06 in mice and rats, as compared with 0.58 ± 0.04 in rabbits, 0.37 ± 0.05 in dogs, and 0.36 ± 0.05 in cynomolgus macaques. Trabecular number (per mm) was 2.70 ± 0.27 in cynomolgus macaques, 3.58 ± 0.14 in dogs, 3.83 ± 0.65 in rabbits, 5.58 ± 0.54 in rats, and 6.16 ± 1.55 in mice. Trabecular thickness was fairly consistent among species and ranged from 0.10 μm in dogs to 0.15 μm in rabbits. As a result, trabecular connectivity was high in mice, followed by rats and rabbits, dogs, and cynomolgus macaques. Mineral density of cancellous bone at the femoral head was similar among all species involved in this study.
Table 2.
Cancellous and cortical bone parameters obtained from cynomolgus macaques by using mCT
| Femoral head | Femoral neck | Proximal diaphysis | Lumbar vertebrae | Mandible | |
| Tissue volume (mm3) | 124.67 ± 33.22 | 59.25 ± 21.84 | 355.9 ± 83.4 | 72.42 ± 34.3 | 8.95 ± 0.31 |
| Bone volume (mm3) | 43.96 ± 5.03 | 17.93 ± 4.25 | 176.0 ± 37.7 | 8.13 ± 1.43 | 5.30 ± 1.20 |
| Bone volume:tissue volume (ratio) | 0.36 ± 0.05 | 0.32 ± 0.08 | 0.50 ± 0.03 | 0.12 ± 0.03 | 0.59 ± 0.14 |
| No. of trabeculae (per mm) | 2.70 ± 0.27 | 2.04 ± 0.23 | not done | 1.89 ± 0.39 | 2.36 ± 0.39 |
| Trabecular thickness (μm) | 0.14 ± 0.02 | 0.15 ± 0.02 | not done | 0.06 ± 0.004 | 0.25 ± 0.05 |
| Trabecular separation (μm) | 0.24 ± 0.04 | 0.34 ± 0.08 | not done | 0.49 ± 0.1 | 0.18 ± 0.09 |
| Trabecular connectivity (per mm3) | 63.44 ± 19.05 | 23.4 ± 6.8 | not done | 80.6 ± 34.3 | 31.69 ± 13.16 |
| Bone mineral density (g/cm2) | 816.8 ± 14.8 | 823.6 ± 17.4 | 19.5 ± 2.8 | 814.2 ± 19.8 | 844.3 ± 29.3 |
| Cortical thickness (mm) | not done | not done | 1041.0 ± 17.8 | not done | not done |
| Marrow volume (mm3) | not done | not done | 179.9 ± 47.7 | not done | not done |
Table 6.
Cortical and cancellous bone parameters obtained from mice by using mCT
| Femoral head | Femoral neck | Proximal diaphysis | Lumbar vertebrae | Mandible | |
| Tissue volume (mm3) | 0.25 ± 0.06 | 0.08 ± 0.02 | 0.56 ± 0.07 | 0.77 ± 0.15 | 0.11 ± 0.00 |
| Bone volume (mm3) | 0.19 ± 0.06 | 0.05 ± 0.01 | 0.22 ± 0.04 | 0.17 ± 0.03 | 0.08 ± 0.01 |
| Bone volume:tissue volume (ratio) | 0.77 ± 0.12 | 0.62 ± 0.05 | 0.38 ± 0.03 | 0.22 ± 0.03 | 0.69 ± 0.11 |
| No. of trabeculae (per mm) | 6.16 ± 1.55 | 8.53 ± 0.76 | not done | 5.98 ± 0.49 | 5.44 ± 0.47 |
| Trabecular thickness (μm) | 0.13 ± 0.05 | 0.13 ± 0.05 | not done | 0.04 ± 0.003 | 0.13 ± 0.02 |
| Trabecular separation (μm) | 0.04 ± 0.01 | 0.04 ± 0.003 | not done | 0.13 ± 0.02 | 0.06 ± 0.02 |
| Trabecular connectivity (per mm3) | 247.5 ± 127.5 | 482.5 ± 127.5 | not done | 541.5 ± 91.0 | 62.76 ± 33.36 |
| Bone mineral density (g/cm2) | 898.5 ± 85.4 | 973.7 ± 36.9 | 1206.4 ± 24.5 | 845.3 ± 10.7 | 1286.9 ± 62.6 |
| Cortical thickness (mm) | not done | not done | 0.23 ± 0.03 | not done | not done |
| Marrow volume (mm3) | not done | not done | 0.35 ± 0.3 | not done | not done |
Figure 2.
2D (black) and 3D (blue) images of the femoral head obtained by mCT.
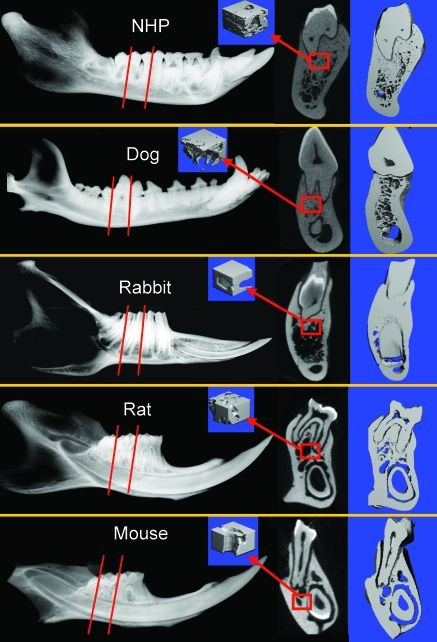
Figure 6.
Radiographs of the mandible captured by (A) digital radiography and (B) 2D and (C) 3D mCT images of the cross-sectional areas made through the first or second molar as indicated by the dotted line. (D) Cancellous bone parameters were measured in the areas beneath the first or second molar indicated by the red squares.
Table 4.
Cancellous and cortical bone parameters obtained from rabbits by using mCT
| Femoral head | Femoral neck | Proximal diaphysis | Lumbar vertebrae | Mandible | |
| Tissue volume (mm3) | 5.60 ± 1.19 | 11.79 ± 1.56 | 156.8 ± 9.8 | 6.96 ± 0.46 | 1.00 ± 0.03 |
| Bone volume (mm3) | 3.22 ± 0.58 | 3.44 ± 1.02 | 59.7 ± 2.9 | 0.67 ± 0.26 | 0.46 ± 0.17 |
| Bone volume:tissue volume (ratio) | 0.58 ± 0.04 | 0.29 ± 0.07 | 0.38 ± 0.02 | 0.24 ± 0.04 | 0.46 ± 0.17 |
| No. of trabeculae (per mm) | 3.83 ± 0.65 | 2.28 ± 0.35 | not done | 2.68 ± 0.21 | 2.15 ± 0.53 |
| Trabecular thickness (μm) | 0.15 ± 0.02 | 0.15 ± 0.02 | not done | 0.09 ± 0.01 | 0.21 ± 0.04 |
| Trabecular separation (μm) | 0.11 ± 0.02 | 0.32 ± 0.07 | not done | 0.29 ± 0.03 | 0.30 ± 0.22 |
| Trabecular connectivity (per mm3) | 138.2 ± 48.1 | 39.2 ± 10.3 | not done | 68.9 ± 22.2 | 6.74 ± 5.48 |
| Bone mineral density (g/cm2) | 718.9 ± 27.1 | 785.1 ± 12.5 | 1167.5 ± 17.5 | 718.9 ± 10.4 | 973.6 ± 44.7 |
| Cortical thickness (mm) | not done | not done | 9.5 ± 0.4 | not done | not done |
| Marrow volume (mm3) | not done | not done | 97.2 ± 8.6 | not done | not done |
Table 5.
Cancellous and cortical bone parameters obtained from rats by using mCT
| Femoral head | Femoral neck | Proximal diaphysis | Lumbar vertebrae | Mandible | |
| Tissue volume (mm3) | 3.51 ± 1.17 | 1.49 ± 0.20 | 2.66 ± 0.12 | 3.35 ± 0.20 | 0.97 ± 0.06 |
| Bone volume (mm3) | 2.59 ± 0.90 | 0.99 ± 0.15 | 1.01 ± 0.03 | 1.10 ± 0.11 | 0.60 ± 0.14 |
| Bone volume:tissue volume (ratio) | 0.74 ± 0.06 | 0. 66 ± 0.07 | 0.38 ± 0.02 | 0. 33 ± 0.03 | 0. 61 ± 0.15 |
| No. of trabeculae (per mm) | 5.58 ± 0.54 | 5.32 ± 0.18 | not done | 4.77 ± 0.31 | 3.33 ± 0.29 |
| Trabecular thickness (μm) | 0.13 ± 0.02 | 0.13 ± 0.02 | not done | 0.07 ± 0.003 | 0.19 ± 0.06 |
| Trabecular separation (μm) | 0.05 ± 0.01 | 0.06 ± 0.01 | not done | 0.14 ± 0.01 | 0.11 ± 0.04 |
| Trabecular connectivity (per mm3) | 227.6 ± 39.1 | 171.7 ± 33.7 | not done | 178.6 ± 29.2 | 23.65 ± 8.33 |
| Bone mineral density (g/cm2) | 788.1 ± 11.6 | 928.1 ± 15.9 | 1117.6 ± 9.5 | 760.4 ± 8.0 | 1024.3 ± 42.5 |
| Cortical thickness (mm) | not done | not done | 0.44 ± 0.03 | not done | not done |
| Marrow volume (mm3) | not done | not done | 1.64 ± 0.12 | not done | not done |
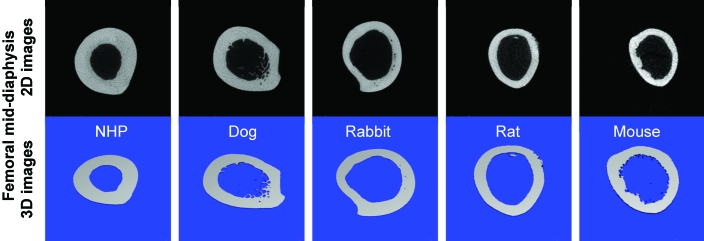
Figure 4.
2D (black) and 3D (blue) images of the proximal femoral diaphysis obtained by mCT.
Femoral neck.
The morphology of the femoral neck and adjoining area, including the greater trochanter and intertrochanteric region, showed a great deal of variability among the lab animal species evaluated (Figure 3). The tissue volume parameter in the femoral neck varied between compared species. The cancellous bone volume (in mm3) at the femoral neck was 17.93 ± 4.25 in cynomolgus macaques, 8.83 ± 1.50 in dogs, 3.44 ± 1.02 in rabbits, 0.99 ± 0.15 in rats, and 0.50 ± 0.01 in mice (Tables 2 to 6). Trabecular number at the femoral neck was 0.32 ± 0.08 in cynomolgus macaques, 0.22 ± 0.04 in dogs, 0.29 ± 0.07 in rabbits, 0.66 ± 0.07 in rats, and 0.62 ± 0.05 in mice. Trabecular thickness was fairly consistent among all species, ranging from 0.09 to 0.15 μm. The mineral density of cancellous bone at the femoral neck was also fairly consistent among all laboratory species involved in this study (Table 3).
Figure 3.
2D (black) and 3D (blue) mCT images of the femoral neck with cancellous and cortical bone and intertrochanteric region in dog. The intertrochanteric region in dog was captured because the gross anatomy of the proximal femur and limitations of the mCT holder did not allow for capturing only the femoral neck region.
Table 3.
Cancellous and cortical bone parameters obtained from dogs by using mCT
| Femoral head | Femoral neck | Proximal diaphysis | Lumbar vertebrae | Mandible | |
| Tissue volume (mm3) | 160.14 ± 12.23 | 39.68 ± 2.20 | 422.4 ± 54.0 | 72.65 ± 22.9 | 8.83 ± 0.34 |
| Bone volume (mm3) | 59.72 ± 10.87 | 8.83 ± 1.50 | 201.1 ± 17.0 | 9.65 ± 1.95 | 3.13 ± 1.16 |
| Bone volume:tissue volume (ratio) | 0.37 ± 0.05 | 0.22 ± 0.04 | 0.48 ± 0.02 | 0.14 ± 0.02 | 0.35 ± 0.12 |
| No. of trabeculae (per mm) | 3.58 ± 0.14 | 2.44 ± 0.21 | not done | 2.54 ± 0.08 | 1.93 ± 0.27 |
| Trabecular thickness (μm) | 0.10 ± 0.02 | 0.09 ± 0.01 | not done | 0.05 ± 0.01 | 0.18 ± 0.05 |
| Trabecular separation (μm) | 0.18 ± 0.01 | 0.32 ± 0.04 | not done | 0.34 ± 0.003 | 0.35 ± 0.10 |
| Trabecular connectivity (per mm3) | 92.30 ± 14.42 | 52.2 ± 11.2 | not done | 112.7 ± 20.0 | 31.94 ± 7.71 |
| Bone mineral density (g/cm2) | 850.3 ± 7.0 | 871.2 ± 9.5 | 1058.5 ± 13.3 | 855.9 ± 12.8 | 904.39 ± 14.2 |
| Cortical thickness (mm) | not done | not done | 14.3 ± 2.7 | not done | not done |
| Marrow volume (mm3) | not done | not done | 221.3 ± 37.0 | not done | not done |
Proximal diaphysis.
The ratio of cortical bone volume to tissue volume was 0.50 ± 0.03 in cynomolgus macaques, 0.48 ± 0.04 in dogs, 0.38 ± 0.02 in rabbits, 0.38 ± 0.02 in rats, and 0.38 ± 0.03 in mice. The cortical bone thickness (in mm) was 19.5 ± 2.8 in cynomolgus macaques, 14.3 ± 2.7 in dogs, 9.5 ± 0.4 in rabbits, 0.44 ± 0.03 in rats, and 0.23 ± 0.03 in mice. Cortical bone mineral density (g/cm2) was 1041.0 ± 17.8 in cynomolgus macaques, 1058.5 ± 13.3 in dogs, 1167.5 ± 17.5 in rabbits, 1117.6 ± 9.5 in rats, and 1206.4 ± 24.5 in mice. (Tables 2 to 6, Figures 2 to 6).
Lumbar vertebrae.
Radiographs and mCT images revealed large differences in bone morphology and microanatomic structure of the cancellous bone of lumbar vertebrae (Figures 2 to 6). The ratio between bone volume and tissue volume ranged from 0.22 ± 0.03 to 0.33 ± 0.03 in mice and rats, as compared with 0.24 ± 0.04 in rabbits, 0.14 ± 0.02 in dogs, and 0.12 ± 0.03 in cynomolgus macaques. Trabecular number (per mm) at the lumbar vertebral bodies was 1.89 ± 0.39 in cynomolgus macaques, 2.54 ± 0.08 in dogs, 2.68 ± 0.21 in rabbits, 4.77 ± 0.31 in rats, and 5.98 ± 0.49 in mice. Trabecular thickness (in mm) at the lumbar vertebral was 0.060 ± 0.004 in cynomolgus macaques, 0.05 ± 0.01 in dogs, 0.09 ± 0.01 in rabbits, 0.070 ± 0.003 in rats, and 0.040 ± 0.003 in mice (Tables 2 to 6). Relative to other species, rabbits exhibited a very narrow mid-portion of the vertebral body (Figures 5).
Figure 5.
2D (black) and 3D (blue) images of lumbar vertebrae obtained by mCT. The green box in the 2D images indicates the region of interest where concellous bone structural analyses was performed. Note that the target region in rabbit lumbar spine is different from that of other species because of the very thin vertebral body in rabbits, which contains very little to no cancellous bone. Therefore, the region of interest for rabbit lumbar vertebrae was placed at the bottom of the lumbar vertebral body to obtain sufficient cancellous bone for mCT measurements.
Mandible.
The morphology of the mandible was very different among the lab animal species studied (Figure 6). Mice and rats have very similar mandibular bones: the incisors extend throughout the entire mandible. Incisor teeth in rabbits are much shorter and extend only through the rostral third of the mandible. The morphologic features of the premolar and molar teeth of rabbits are also very different from those of all other evaluated species. Because of differences in root morphology, obtaining precise measurements in the identical area of trabecular bone at the site of the second molar was difficult. The assembly of the trabecular network and cortico-cancellous morphology was qualitatively different among species due to presence of the long incisors underneath the molars in mice and rats, as well as the depth, number, and position of the molar roots (Tables 2 to 6, Figures 6).
Discussion
The majority of skeletal research in laboratory animals is focused on metabolic bone diseases because osteoporoses of various etiologies are recognized as some of the most important public problems today.35 The entire region of the proximal femur, including the intertrochanteric area, femoral neck, and femoral head, is of enormous importance for human pathophysiology because hip fractures result in considerable morbidity and mortality with massive socio-economic consequences.39 In addition to that in the proximal femur, bone loss in the spine and jaws is associated with osteoporosis and aging.23,37,41,42 For example, there is a strong correlation between mandibular bone mass and the decline in bone mass at other skeletal sites.3,20,29 Therefore, the primary focus of our study was to compare anatomic and structural bone features in the proximal femur, lumbar vertebrae, and mandible among different laboratory animals because these anatomic sites are involved in the pathophysiology of human osteoporoses and are used most frequently in preclinical research involving the skeleton. In addition to their obvious value for studies involving osteoporoses, the imaging techniques used in this study have the potential to be applied to any research involving skeleton. Because bone strength is difficult to assess in clinic, radiologic methods typically are used to assess 2 primary determinants of bone strength: mineralization and structure.45 Even though researchers agree that bone mineral density is the single most important predictor of bone health and strength, recent studies have shown that this measure alone may be insufficient to determine the strength of cancellous bone and that trabecular architecture plays a crucial role in determining bone mechanical properties and risk of fracture.32,34,46,52,58,68
The size and gross anatomy of the proximal femur varied substantially among the laboratory species considered in this study. The features with most obvious variation include the size of the femoral head, length and angle of the femoral neck, size and morphology of the greater trochanter, and shape and size of the intertrochanteric area. Variation among species in the 3D orientation of the femoral neck relative to the diaphysis created difficulties in consistently positioning bone specimens and accurately scanning the regions of interest. Consequently, the cross-sectional area (20 to 50 slices per anatomic site) of cancellous bone chosen for mCT evaluation was not identical among individual measurements in the same species or among the different species used in our analysis. The results obtained by mCT revealed that trabecular bone volume, number, and separation measured at the femoral head and neck vary greatly among laboratory animals. Similar to the proximal femur of humans, the femoral head and neck areas of the proximal femur from dogs and cynomolgus macaques contain a considerable quantity of cancellous bone.2,30,40,73 Results from the present study indicate that the trabecular network in the proximal femur has fairly constant thickness across all species, leading us to conclude that trabecular separation and connectivity contributes to variation in trabecular number among species. Results in dogs and cynomolgus macaques from this study support earlier findings that at proximal femur where cancellous bone predominates, the combination of volumetric bone mineral density should be measured in concert with trabecular structural parameters, particularly trabecular thickness and number, because these 2 parameters used in combination better predict biomechanical properties of the proximal femur rather than does any single parameter alone.34,57 Despite subtle variation in the mineral density of the cancellous and cortical bone at femoral neck and head, density values were similar among species. The somewhat higher values in mice and rats could be due to fewer, but thicker, trabeculae in rodents relative to dogs and nonhuman primates.31 Similarly, the cortical bone mineral density at the proximal femoral diaphysis was slightly higher in mice and rat) and rabbits relative to dog and cynomolgus macaques, possibly due to the presence of Haversian systems and intracortical remodeling that is seen in dogs and nonhuman primates but far less in rabbits and rodents.15,30,40 Rodents, dogs, and cynomolgus macaques had similar ratios of bone to tissue volume. As expected, larger species had thicker cortex to maintain organ dimensionality in terms of bone length and thickness and to ensure that the mechanical demands imposed on the skeleton are met. As depicted in Figure 1, the proximal femur of dogs and cynomolgus macaques more closely resemble the gross and microanatomy of the human proximal femur than does that of rabbits, rats, and mice and therefore likely are better models for studying the physiology of the human proximal femur and hip joint. Even though the mechanical loads in the proximal femur differ between humans and quadrupeds, and the reduced physical activity of laboratory animals has an effect on biomechanical studies, the anatomic and structural similarities of the proximal femur and hip joint between humans, dogs, and nonhuman primates allow for biomedical research under laboratory conditions using dogs and nonhuman primates.8,31,45,46,47,60,66 However, it should be remembered that bone mass, gross and microanatomy are optimally designed to ensure maximal safety of the skeleton during natural activities that are unique for each species.5,63 Our study of skeletal variations among laboratory animals indicates that absolute values recorded for bone parameters cannot be directly compared between species. Our data do not allow the conclusion that based on, for example, higher bone mineral density or better connected cancellous structure, a skeleton in one species is superior to that in others.
The gross and microanatomic structure of the lumbar vertebrae varies considerably among common laboratory species. Because bone mineral density was fairly similar among all species, we believe that measurements of volumetric bone mineral density and cancellous bone structure should be used for prediction of bone strength at the lumbar spine.34,58 Because the mechanical loads on the horizontal spine in quadrupeds differ from mechanical loads in humans, in which the spine maintains an upright (vertical) position, biomechanical results and data describing structural bone changes in the vertebral column of laboratory animals require careful rationalization when extrapolating to the biomechanics in humans.7,25,64,64 However, rat models can be used successfully to provide valuable information regarding the physiology of long bones and the spine, and diverse rat models and laboratory conditions can be modeled further to better resemble the consequences of human osteoporoses on the skeleton.19,27,28,50,72
Although mechanical forces provide critical signals for bone modeling and remodeling events throughout the skeleton, the biomechanics of the jaws is unique and differs from that of weight-bearing bones in the axial skeleton.4,67 During biting, a complex pattern of stress and strains (compressive, tensile, shear, and torsional) occurs in the jaws. The range and distribution of mechanical loads varies among species but always depends on the nature of the loads applied and the material properties and geometry of the jaws.13,24 The occlusal force during biting is transferred from the teeth to the cancellous and cortical bone of the jaws; even though its volume of trabecular bone is considerably lower than that of cortical bone, the cancellous bone surrounding the tooth socket plays a key role in tooth grafting and load distribution. Moreover, trabecular structural change (damage) reflects the fragility of bone tissue more directly than does a decrease in bone volume; therefore, it is of the utmost importance to use animal models whose jaws reflect a cancellous bone distribution that is similar to that of the human mandible.54,56,57
Numerous investigators have emphasized the lack of animal models as a major impediment to supporting dental research associated with bone loss in the jaws, which is known for its complex pathogenesis and etiology.21,59 Attributes of the ideal model for dental research would include anatomic and physiologic features that are comparable to those in humans, systemic skeletal and craniofacial disease progression resembling human conditions, and an opportunity to study both systemic and local factors.59 Existing animal models all have advantages and disadvantages. Rodents and rabbits are readily available and could be used to answer some basic questions. However, they have substantial physiologic and anatomic differences from humans and do not present a good model for dental research, mainly due to the presence of incisors and relatively small areas with cancellous bone.14,33,44 Of all the species we investigated, rabbits seem to be the least desirable model to study oral mechanics and pathophysiology for extrapolation of data to humans because the incisors and rootless teeth of rabbits are embedded deep into the bony parts of the mandible, which contains very small areas of cancellous bone. Furthermore, the presence of the incisors and small cancellous bone area greatly limit the use of rodents to study madibular physiology. On the basis of results from the current study, cynomolgus macaques and dogs seem to be the models of choice for preclinical studies of periodontal disease and the dental effects associated with bone loss because the bony structure of jaws and the anatomy of molars mimic human anatomy and physiology; however, other species including pigs might also be used in dental research.48 The size and shape of the mandibular corpus are linked functionally to the biomechanics of chewing and biting and therefore are specific to species.11 In addition to bone mass and structure, other relevant differences in form and function, including forces that act on the mandible during mastication, exist between laboratory animals and humans, and those differences should be weighed when extrapolating data from animal studies to humans.
Taken together, the results of this study emphasize key parameters of the gross anatomy and microanatomy of the proximal femur, lumbar spine, and mandible of commonly used laboratory species. Combination of radiology and mCT allows for quick, reliable, and noninvasive evaluation of bone morphology, the complex microarchitecture of cortical and cancellous bone, and bone mineral density, with great translational value from the preclinical to clinical environment. The described skeletal characteristics and human relevance suggest that rabbit seems to be the least desirable species to conduct preclinical research of bone physiology due to morphology of the proximal femur, including a very short femoral neck and small amounts of cancellous bone at femoral neck, vertebral body, and mandible, whereas cynomologus macaques likely are the most appropriate among the species we studied. However, rodent models, particularly rat models, are extremely useful for conducting basic research involving the skeleton and represent a good and affordable alternative to dogs and nonhuman primates. Even though direct comparisons between species are difficult and extrapolating the data obtained to humans can be complex, we believe that a better depiction of anatomic and structural characteristics of skeletal sites in the laboratory species presented here will assist scientists in choosing the appropriate animal models and skeletal sites that best support their particular research. This choice may help researchers to reduce unnecessary work involving animals.
Acknowledgments
We thank all of our colleagues from Comparative Medicine and Drug Safety Research and Development at Pfizer (Groton, Connecticut) for their help when collecting the bones from laboratory animals.
References
- 1.Aerssens J, Boonen S, Lowet G, Dequeker J. 1998. Interspecies differences in bone composition, density, and quality: potential implications for in vivo bone research. Endocrinology 139: 663–670 [DOI] [PubMed] [Google Scholar]
- 2.Bagi CM, Wilkie D, Georgelos K, Williams D, Bertolini D. 1997. Morphological and structural characteristics of the proximal femur in human and rat. Bone 21:261–267 [DOI] [PubMed] [Google Scholar]
- 3.Banu J, Orchii PB, Okafor MC, Wang L, Kalu DK. 2001. Analysis of the effects of growth hormone, exercise and food restriction on cancellous bone in different bone sites in middle-aged female rats. Mech Ageing Dev 122:849–864 [DOI] [PubMed] [Google Scholar]
- 4.Bidez MW, Misch CE. 1992. Issues in bone mechanics related to oral implants. Implant Dent 1:289–294 [DOI] [PubMed] [Google Scholar]
- 5.Biewener AA. 1991. Musculoskeletal design in relation to body size. J Biomech 24:19–29 [DOI] [PubMed] [Google Scholar]
- 6.Boskey AL. 2003. Biomineralization: an overview. Connect Tissue Res 44 Suppl 1:5–9 [PubMed] [Google Scholar]
- 7.Bromley RG, Dockum NL, Arnold JS, Jee WS. 1966. Quantitative histological study of human lumbar vertebrae. J Gerontol 21:537–543 [DOI] [PubMed] [Google Scholar]
- 8.Brommage R. 2001. Perspectives on using nonhuman primates to understand the etiology and treatment of postmenopausal osteoporosis. J Musculoskelet Neuronal Interact 1:307–325 [PubMed] [Google Scholar]
- 9.Currey JD. 1999. The design of mineralized hard tissues for their mechanical functions. J Exp Biol 202:3285–3294 [DOI] [PubMed] [Google Scholar]
- 10.Currey JD. 2003. The many adaptations of bone. J Biomech 36:1487–1495 [DOI] [PubMed] [Google Scholar]
- 11.Daegling DJ, Hylander WL. 1997. Occlusal forces and mandibular bone strain: is the primate jaw “overdesigned”? J Hum Evol 33:705–717 [DOI] [PubMed] [Google Scholar]
- 12.Davison KS, Siminoski K, Adachi JD, Hanley DA, Goltzman D, Hodsman AB, Josse R, Kaiser S, Olszynski WP, Papaioannou A, Ste-Marie LG, Kendler DL, Tenenhouse A, Brown JP. 2006. Bone strength: the whole is greater than the sum of its parts. Semin Arthritis Rheum 36:22–31 [DOI] [PubMed] [Google Scholar]
- 13.Drozdzowska B, Pluskiewicz W. 2002. Longitudinal changes in mandibular bone mineral density compared with hip bone mineral density and quantitative ultrasound at calcaneus and hand phalanges. Br J Radiol 75:743–747 [DOI] [PubMed] [Google Scholar]
- 14.Elovic RP, Hipp JA, Hayes WC. 1995. Ovariectomy decreases the bone area fraction of the rat mandible. Calcif Tissue Int 56:305–310 [DOI] [PubMed] [Google Scholar]
- 15.Enlow DH. 1962. Functions of the Haversian system. Am J Anat 110:269–305 [DOI] [PubMed] [Google Scholar]
- 16.Felsenberg D, Boonen S. 2005. The bone quality framework: determinants of bone strength and their interrelationships, and implications for osteoporosis management. Clin Ther 27:1–11 [DOI] [PubMed] [Google Scholar]
- 17.Frost HM. 1990. Structural adaptation to mechanical usage (SATMU). 1. Redefining Wolff's law: The bone remodeling problem. Anat Rec 226:414–422 [DOI] [PubMed] [Google Scholar]
- 18.Frost HM. 1996. Perspectives: a proposed general model of the “mechanostat” (suggestions from a new skeletal-biologic paradigm). Anat Rec 244:139–147 [DOI] [PubMed] [Google Scholar]
- 19.Frost HM, Jee WS. 1992. On the rat model of human osteopenias and osteoporoses. Bone Miner 18:227–236 [DOI] [PubMed] [Google Scholar]
- 20.Gasser JA, Ingold P, Grosios K, Laib A, Hämmerle S, Koller B. 2005. Non-invasive monitoring of changes in structural cancellous bone parameters with a novel prototype microCT. J Bone Miner Metab 23:90–96 [DOI] [PubMed] [Google Scholar]
- 21.Geurs NC, Lewis CE, Jeffcoat MK. 2003. Osteoporosis and periodontal disease progression. Periodontol 2000 32:105–110 [DOI] [PubMed] [Google Scholar]
- 22.Hillier ML, Bell LS. 2007. Differentiating human bone from animal bone: a review of histological methods. J Forensic Sci 52:249–263 [DOI] [PubMed] [Google Scholar]
- 23.Hordon LD, Itoda M, Shore PA, Shore RC, Heald M, Brown M, Kanis JA, Rodan GA, Aaron JE. 2006. Preservation of thoracic spine microarchitecture by alendronate: comparison of histology and microCT. Bone 38:444–449 [DOI] [PubMed] [Google Scholar]
- 24.Horner K, Devlin H, Alsop CW, Hodgkinson IM, Adams JE. 1996. Mandibular bone mineral density as a predictor of skeletal osteoporosis. Br J Radiol 69:1019–1025 [DOI] [PubMed] [Google Scholar]
- 25.Hotchkiss CE, Stavisky R, Nowak J, Brommage R, Lees CJ, Kaplan J. 2001. Levormeloxifene prevents increased bone turnover and vertebral bone loss following ovariectomy in cynomolgus monkeys. Bone 29:7–15 [DOI] [PubMed] [Google Scholar]
- 26.Institute for Laboratory Animal Research 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 27.Jee WS, Ma Y. 1999. Animal models of immobilization osteopenia. Morphologie 83:25–34 [PubMed] [Google Scholar]
- 28.Jee WS, Yao W. 2001. Overview: animal models of osteopenia and osteoporosis. J Musculoskelet Neuronal Interact 1:193–207 [PubMed] [Google Scholar]
- 29.Jeffcoat MK, Chesnut CH., 3rd 1993. Systemic osteoporosis and oral bone loss: evidence shows increased risk factors. J Am Dent Assoc 124:49–56 [DOI] [PubMed] [Google Scholar]
- 30.Jerome CP. 1998. Primate models of osteoporosis. Lab Anim Sci 48:618–622 [PubMed] [Google Scholar]
- 31.Jerome CP, Peterson PE. 2001. Nonhuman primate models in skeletal research. Bone 29:1–6 [DOI] [PubMed] [Google Scholar]
- 32.Jiang C, Giger ML, Kwak SM, Chinander MR, Martell JM, Favus MJ. 2000. Normalized BMD as a predictor of bone strength. Acad Radiol 7:33–39 [DOI] [PubMed] [Google Scholar]
- 33.Jiang G, Matsumoto H, Yamane J, Kuboyama N, Akimoto Y, Fujii A. 2004. Prevention of trabecular bone loss in the mandible of ovariectomized rats. J Oral Sci 46:75–85 [DOI] [PubMed] [Google Scholar]
- 34.Jiang Y, Zhao J, Augat P, Ouyang X, Lu Y, Majmudar S, Genant HK. 1998. Trabecular bone mineral and calculated structure of human bone specimens scanned by peripheral quantitative computed tomography: relation to biomechanical properties. J Bone Miner Res 13:1783–1790 [DOI] [PubMed] [Google Scholar]
- 35.Johnell O, Kanis JA. 2006. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17:1726–1733 [DOI] [PubMed] [Google Scholar]
- 36.Jowsey J. 1966. Studies of Haversian systems in man and some animals. J Anat 100:857–864 [PMC free article] [PubMed] [Google Scholar]
- 37.Kalu DN. 1991. The ovariectomized rat model of postmenopausal bone loss. Bone Miner 15:175–191 [DOI] [PubMed] [Google Scholar]
- 38.Kanis JA. 2002. Assessing the risk of vertebral osteoporosis. Singapore Med J 43:100–105 [PubMed] [Google Scholar]
- 39.Kanis JA, Borgstrom F, De Laet C, Johansson H, Johnell O, Jonsson B, Oden A, Zethraeus N, Pfleger B, Khaltaev N. 2005. Assessment of fracture risk. Osteoporos Int 16:581–589 [DOI] [PubMed] [Google Scholar]
- 40.Kimmel DB, Jee WS. 1982. A quantitative histologic study of bone turnover in young adult beagles. Anat Rec 203:31–45 [DOI] [PubMed] [Google Scholar]
- 41.Krall EA, Garcia RI, Dawson-Hughes B. 1996. Increased risk of tooth loss is related to bone loss at the whole body, hip, and spine. Calcif Tissue Int 59:433–437 [DOI] [PubMed] [Google Scholar]
- 42.Kribbs PJ. 1990. Comparison of mandibular bone in normal and osteoporotic women. J Prosthet Dent 63:218–222 [DOI] [PubMed] [Google Scholar]
- 43.Kuhn LT, Grynpas MD, Rey CC, Wu Y, Ackerman JL, Glimcher MJ. 2008. A comparison of the physical and chemical differences between cancellous and cortical bovine bone mineral at two ages. Calcif Tissue Int 83:146–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lerouxel E, Libouban H, Moreau MF, Basle MF, Audran M, Chappard D. 2004. Mandibular bone loss in an animal model of male osteoporosis (orchidectomized rat): a radiographic and densitometric study. Osteoporos Int 15:814–819 [DOI] [PubMed] [Google Scholar]
- 45.Lloyd RD, Taylor GN, Miller SC, Bruenger FW, Jee WS. 2006. Ancestry of beagles in lifespan studies of radionuclide toxicity at the University of Utah. Health Phys 90:580–582 [DOI] [PubMed] [Google Scholar]
- 46.Majumdar S, Genant HK, Grampp S, Newitt DC, Truong VH, Lin JC, Mathur A. 1997. Correlation of trabecular bone structure with age, bone mineral density, and osteoporotic status: In vivo studies in the distal radius using high resolution magnetic resonance imaging. J Bone Miner Res 12:111–118 [DOI] [PubMed] [Google Scholar]
- 47.Martin RK, Albright JP, Jee WS, Taylor GN, Clarke WR. 1981. Bone loss in the beagle tibia: influence of age, weight, and sex. Calcif Tissue Int 33:233–238 [DOI] [PubMed] [Google Scholar]
- 48.Martinez-Gonzales JM, Cano-Sanchez J, Campo-Trapero J, Gronzalo-Lafuente JC, Diaz-Reganon J, Vasquez-Pineiro MT. 2005. Evaluation of minipigs as an animal model for alveolar distraction. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 99:11–16 [DOI] [PubMed] [Google Scholar]
- 49.Miller SC, Bowman BM, Jee WS. 1995. Available animal models of osteopenia–small and large. Bone 17:117S–123S [DOI] [PubMed] [Google Scholar]
- 50.Mo A, Yao W, Li C, Tian X, Su M, Ling Y, Zhang Q, Setterberg RB, Jee WS. 2002. Bipedal stance exercise and prostaglandin E2 (PGE2) and its synergistic effect in increasing bone mass and in lowering the PGE2 dose required to prevent ovariectomized-induced cancellous bone loss in aged rats. Bone 31:402–406 [DOI] [PubMed] [Google Scholar]
- 51.Mosekilde L, Danielsen CC, Sogaard CH, Thorling E. 1994. The effect of long-term exercise on vertebral and femoral bone mass, dimensions, and strength–assessed in a rat model. Bone 15:293–301 [DOI] [PubMed] [Google Scholar]
- 52.Nielsen DH, McEvoy FJ, Madsen MT, Jensen JB, Svalastoga E. 2007. Relationship between bone strength and dual-energy X-ray absorptiometry measurements in pigs. J Anim Sci 85:667–672 [DOI] [PubMed] [Google Scholar]
- 53.Pearson OM, Lieberman DE. 2004. The aging of Wolff's “law”: ontogeny and response to mechanical loading in cortical bone. Am J Phys Anthropol 39:63–99 [DOI] [PubMed] [Google Scholar]
- 54.Recker RR. 1989. Low bone mass may not be the only cause of skeletal fragility in osteoporosis. Proc Soc Exp Biol Med 191:272–274 [DOI] [PubMed] [Google Scholar]
- 55.Rodgers JB, Monier-Faugere MC, Malluche H. 1993. Animal models for the study of bone loss after cessation of ovarian function. Bone 14:369–377 [DOI] [PubMed] [Google Scholar]
- 56.Seeman E. 2003. Bone quality. Osteoporos Int 14 Suppl 5:S3–S7 [DOI] [PubMed] [Google Scholar]
- 57.Seeman E. 2005. Loading and bone fragility. J Bone Miner Metab 23 Suppl:23–29 [DOI] [PubMed] [Google Scholar]
- 58.Stenström M, Olander B, Lehto-Axtelius D, Madsen JE, Nordsletten L, Carlsson GA. 2000. Bone mineral density and bone structure parameters as predictors of bone strength: an analysis using computerized microtomography and gastrectomy-induced osteopenia in the rat. J Biomech 33:289–297 [DOI] [PubMed] [Google Scholar]
- 59.Takaishi Y, Okamoto Y, Ikeo T, Morii H, Takeda M, Hide K, Arai T, Nonaka K. 2005. Correlations between periodontitis and loss of mandibular bone in relation to systemic bone changes in postmenopausal Japanese women. Osteoporos Int 16:1875–1882 [DOI] [PubMed] [Google Scholar]
- 60.Taylor GN, Christensen WR, Jee WS, Rehfeld CE, Fisher W. 1962. Anatomical distribution of fractures in beagles injected with Pu-239. Health Phys 8:609–613 [DOI] [PubMed] [Google Scholar]
- 61.Thompson DD, Simmons HA, Pirie CM, Ke HZ. 1995. FDA Guidelines and animal models for osteoporosis. Bone 17:125S–133S [DOI] [PubMed] [Google Scholar]
- 62.Turner CH. 1991. Homeostatic control of bone structure: an application of feedback theory. Bone 12:203–217 [DOI] [PubMed] [Google Scholar]
- 63.Turner CH. 1998. Three rules for bone adaptation to mechanical stimuli. Bone 23:399–407 [DOI] [PubMed] [Google Scholar]
- 64.Turner CH. 2002. Biomechanics of bone: determinants of skeletal fragility and bone quality. Osteoporos Int 13:97–104 [DOI] [PubMed] [Google Scholar]
- 65.Turner CH, Burr DB, Hock JM, Brommage R, Sato M. 2001. The effects of PTH (1-34) on bone structure and strength in ovariectomized monkeys. Adv Exp Med Biol 496:165–179 [DOI] [PubMed] [Google Scholar]
- 66.Vajda EG, Kneissel M, Muggenburg B, Miller SC. 1999. Increased intracortical bone remodeling during lactation in beagle dogs. Biol Reprod 61:1439–1444 [DOI] [PubMed] [Google Scholar]
- 67.van der Meulen MC, Jepsen KJ, Mikic B. 2001. Understanding bone strength: size isn't everything. Bone 29:101–104 [DOI] [PubMed] [Google Scholar]
- 68.van Eijden TM. 2000. Biomechanics of the mandible. Crit Rev Oral Biol Med 11:123–136 [DOI] [PubMed] [Google Scholar]
- 69.Wallach S, Feinblatt J, Avioli L. 1992. The bone quality problem. Calcif Tissue Int 51:169–172 [DOI] [PubMed] [Google Scholar]
- 70.Wessler S. 1976. Introduction: what is a model?, p xi–xvi In: Committee on Animal Models for Thrombosis and Hemorrhagic Diseases; Institute of Laboratory Animal Resources. Animal models of thrombosis and hemorrhagic disease. Bethesda (MD): National Institutes of Health [Google Scholar]
- 71.Wronski TJ, Cintron M, Dann LM. 1988. Temporal relationship between bone loss and increased bone turnover in ovariectomized rats. Calcif Tissue Int 43:179–183 [DOI] [PubMed] [Google Scholar]
- 72.Yao W, Jee WS, Chen J, Liu H, Tam CS, Cui L, Zhou H, Setterberg RB, Frost HM. 2000. Making rats rise to erect bipedal stance for feeding partially prevented orchidectomy-induced bone loss and added bone to intact rats. J Bone Miner Res 15:1158–1168 [DOI] [PubMed] [Google Scholar]
- 73.Zebaze RM, Jones A, Welsh F, Knackstedt M, Seeman E. 2005. Femoral neck shape and the spatial distribution of its mineral mass varies with its size: clinical and biomechanical implications. Bone 37:243–252 [DOI] [PubMed] [Google Scholar]