Abstract
Cardiovascular disease in general, and cardiac arrhythmias specifically, is common in great apes. However, the clinical significance of arrhythmias detected on short-duration electrocardiograms is often unclear. Here we describe the use of an implantable loop recorder to evaluate cardiac rhythms in 4 unanesthetized adult chimpanzees (Pan troglodytes), 1 with a history of possible syncope and 3 with the diagnosis of multiform ventricular ectopy (ventricular premature complexes) and cardiomyopathy. The clinical significance of ventricular ectopy was defined further by using the implantable loop recorder. Arrhythmia was ruled out as a cause of collapse in the chimpanzee that presented with possible syncope because the implantable loop recorder demonstrated normal sinus rhythm during a so-called syncopal event. This description is the first report of the use of an implantable loop recorder to diagnose cardiac arrhythmias in an unanesthetized great ape species.
Abbreviations: ECG, electrocardiography; ILR, implantable loop recorder
Cardiovascular disease is an important cause of morbidity and mortality in captive chimpanzees (Pan troglodytes).3,11,13,22,24 Specifically, the species is known to be at risk of sudden cardiac death, which is associated with ventricular arrhythmias.11 Electrocardiography (ECG) is used to evaluate cardiac arrhythmias, conduction disturbances, cardiac chamber enlargement, and some noncardiac disorders in chimpanzees.3 However, because of the size and strength of the chimpanzee, most veterinary care procedures must be completed under anesthesia for safety reasons. ECG have previously been obtained only from sedated animals, necessitating brief recordings. Holter monitoring, the most commonly used method for obtaining long-term (usually 24 h) ECG in other species, is not possible in chimpanzees because of their ability to remove and damage the equipment.
Ventricular arrhythmias are common findings in chimpanzees and may be associated with the presence of underlying heart disease and an increased risk of sudden cardiac death.3,11,13 The only therapy that has been effective in reducing the risk of sudden cardiac death in humans with significant ventricular ectopy is implantable defibrillators.17 Although antiarrhythmic therapy has been ineffective in preventing fatal arrhythmias, it can reduce the clinical signs associated with the ectopy.26 Ventricular ectopy has been seen with some frequency in the chimpanzee,3,11,13 but most often single ventricular premature complexes of unknown significance are noted. Often ambulatory electrocardiography (Holter or event monitoring) is used in other species to assess the frequency of arrhythmias in animals with infrequent arrhythmias or when it is unclear whether the arrhythmia is causing clinical signs. In addition, telemetry, either implantable or external (which often is used in other laboratory animal species) is infeasible in chimpanzees. Although chimpanzee behavior is not amenable to Holter or event monitoring, an implantable loop recorder (ILR) would allow similar ECG data collection and might enable the clinician to better define arrhythmia significance (that is, the presence of intermittent malignant ventricular tachycardia).
ILR have been used extensively in humans for the investigation of syncope.1,5,7,16,20 Typically patients activate the ILR with a handheld activator after experiencing syncope or weakness (similar to an event monitor). However, the device can be programmed to self-activate and store data if the heart rate is above or below a programmed threshold. ILR have been used in veterinary medicine, but only 2 reports have been published to date.10,25 Currently, no reports describe the clinical use of loop recorders in captive chimpanzees or any other nonhuman primate. The hypothesis of the current investigation was that use of an ILR would enable more thorough evaluation of sporadic arrhythmias and result in improved assessment of their clinical significance.
Materials and Methods
All chimpanzees at the Alamogordo Primate Facility (Holloman Air Base, Alamogordo, New Mexico) are fed a commercial primate diet (Purina Lab Diet Monkey Diet, PMI Nutrition International, St Louis, MO). No more than 6 animals are maintained in each indoor den (180 ft2, 9.5 ft high) with radiant-heated floor and air conditioning. Chimps have 24-h access to a 242-ft2 outdoor area (12 ft high) as well as weekly access to a 802-ft2 outdoor area (Primadome, Brandes Brothers Constructors, Bee Cave, TX). Same-sex housing is maintained to ensure the NIH policy that no government-owned chimpanzees are to breed for research purposes. Chimpanzees at the Alamogordo Primate Facility are maintained in accordance with the Guide for the Care and Use of Animals.9 The facility and its program are fully AAALAC-accredited, and all procedures were IACUC-approved. At the time of this study, the colony population consisted of 202 research veteran chimpanzees.
Each chimpanzee participates in enrichment programs and is observed every 2 h by experienced, AALAS-certified animal care technicians or clinical veterinarians. The enrichment program involves daily fruits and vegetables plus biweekly forage opportunities. Novelty items such as blankets, magazines, and simulated termite mound feeders are provided also. The animals are observed for general health, activity levels, elimination, exercise tolerance, and recovery rates. Each chimpanzee receives a complete physical examination annually under anesthesia (3.0 mg/kg tiletamine hydrochloride–zolazepam), CBC, clinical chemistry, ECG, abdominal ultrasonography, tuberculosis testing, dental prophylaxis, and blood pressure assessment. Blood pressure measurements, ECG, O2 saturation, and core body temperature are recorded (Passport 2, Datascope, Mahwah, NJ). Chimpanzees with clinical signs or physical examination findings consistent with underlying heart disease undergo evaluation (physical examination, ECG [6 frontal plane leads], and echocardiography) by a board-certified veterinary cardiologist. Echocardiograms are performed (Prosound 5000, Aloka, Tokyo, Japan) by using a 2.5-mHz transducer.
Four chimpanzees that were considered to be at risk of significant, sporadic arrhythmias based on their history and physical examination, ECG, and echocardiographic findings were identified for the current study. One female chimp had a history of multiple, possible syncopal events, and 3 male chimpanzees had multiform ventricular premature complexes noted on the ECG obtained while they were anesthetized. ILR (Reveal Plus model 9526, Medtronic, Minneapolis, MN) were inserted subcutaneously in these 4 chimpanzees while they were under sedation with tiletamine hydrochloride-zolazepam (3.0 mg/kg) and after sterile surgical preparation of the skin. The device contains positive and negative poles, and subcutaneous placement on the thorax produces a bipolar ECG lead. The incision was closed with 2-0 Vicryl (Ethicon, Somerville, NJ). Ibuprofen (200 to 400 mg PO 3 times daily) was given for pain management when deemed appropriate by the veterinary staff. No other medications were administered postoperatively.
The Reveal device (Medtronic) is an ILR that can record and store ECG in unanesthetized patients. The device was developed so that long-term cardiac rhythm assessment would be possible for human patients with extremely rare syncope (1 episode annually or fewer). Intracardiac electrodes are not required, and the ILR can store up to 42 min of the ECG surrounding its activation, or multiple, shorter periods of the ECG. The device is 8 cm in length and weighs 17 g, and stored information is retrieved noninvasively by radiotelemetry. ECG data are stored continually on the device, but the information is replaced continually (therefore, the term ‘loop recorder’) unless the device is activated to save the data.
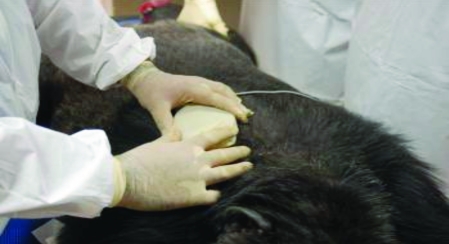
A programmer and Reveal Device Plus software (both from Medtronic) were used to program and retrieve data after implantation. Figure 1 demonstrates the use of radiotelemetry to program the device after implantation.
Figure 1.
Obtaining stored data from the implantable loop recorder.
Results
The first ILR was implanted into a chimpanzee in April 2005. The ILR was placed subcutaneously directly ventral to the heart, the anatomical location previously described for human and veterinary patients.5,25 However, after several weeks, the ILR was removed either by the chimpanzee or a denmate. The device was not recovered, and its data were lost. For subsequent animals, the ILR was implanted dorsal to the heart, subcutaneously, and between the shoulder blades.
In September 2006, the ILR device for the first chimpanzee was replaced, and ILR were implanted in the other 3 animals. The devices were read, and data were obtained in November 2006 (3 mo after implantation). Data from 2 chimpanzees were downloaded a second time, in April 2007. In addition, data were retrieved from the device of 1 chimpanzee in January 2008 (a total of 3 interrogations). All downloaded data were evaluated by a board-certified veterinary cardiologist (MMS). Each device was set initially to be activated if an animal's heart rate fell below 40 bpm or accelerated above 180 bpm. According to one study,4 the average normal resting heart rate for a sedated chimpanzee is 84 bpm, similar to the normal heart range for dogs. The average age of the male chimpanzees in our study was 33 y (n = 3), and the female chimp was 22 y at the time of the investigation.
Other than the possible syncopal events of the female chimpanzee, none of the chimpanzees exhibited clinical signs suggestive of cardiovascular disease either before or after implantation of ILR. The female chimpanzee had 3 possible syncopal events during the 3 mo period that the ILR was implanted. These events were characterized by loss of consciousness after collapse, with duration of less than 1 min. Despite the loss of consciousness, no abnormal arrhythmias were present on the corresponding ECG downloaded from the ILR, and the clinicians were able to rule out cardiogenic syncope. All 3 male chimpanzees had multiform ventricular premature complexes among the data stored by the ILR, a similar finding to the results from the ECG obtained while they were anesthetized (Figure 2). In addition, 1 of these 3 chimps was diagnosed with single supraventricular premature complexes, and another had paroxysmal atrial fibrillation. Recordings of sinus tachycardia, a normal physiologic rhythm, were recorded from all 3 male chimps. The number of ECG tracings recorded from each animal and stored by the ILR (automatically or manually) ranged from 2 to 10. Signalment, rhythm, and cardiac disease data is presented in Table 1.
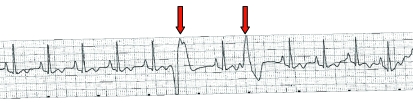
Figure 2.
Multiform ventricular premature complexes from a 31-y-old male chimpanzee sedated with tiletamine–zolazepam (lead II, 1 cm/mV, 25 mm/s).
Table 1.
Signalment, rhythm, and cardiac disease data in the 4 chimpanzees presented
| Animal no. | Sex | Age (y) | Average body weight (kg) | Hepatitis C virus status | Cardiovascular disease | ILR findings | no. of events stored |
| 1 | F | 22 | 54.95 | – | Possible syncope | Normal sinus rhythm (manually activated) | 3 |
| 2 | M | 33 | 56.89 | + | Left ventricular hypertrophy, systemic hypertension | Multiform ventricular premature complexes, sinus tachycardia, paroxysmal atrial fibrillation | 10 |
| 3 | M | 32 | 57.81 | – | Dilated cardiomyopathy | APC and multiform ventricular premature complexes, sinus tachycardia | 8 |
| 4 | M | 31 | 93.71 | – | Left ventricular hypertrophy, systemic hypertension | Multiform ventricular premature complexes, sinus tachycardia | 10 |
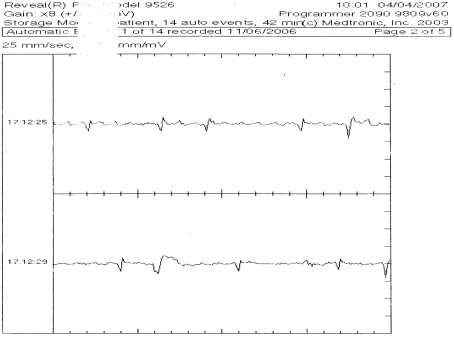
The ILR self-activated when the heart rate crossed the programmed threshold in all cases, except for 3 additional, manual activations when the female chimpanzee was observed during a period of collapse. Of the stored ECG events, 21 were normal (18 sinus tachycardia and 3 sinus rhythm [Figure 3], which were technician-activated), and 10 were pathologic (1 contained atrial premature complexes, 8 contained ventricular premature complexes [Figure 4], and 1 contained atrial fibrillation [Figure 5]). Two episodes of R-on-T were noted in one animal; R-on-T occurs when a ventricular premature complex is coupled closely to the T wave of the preceding complex.19
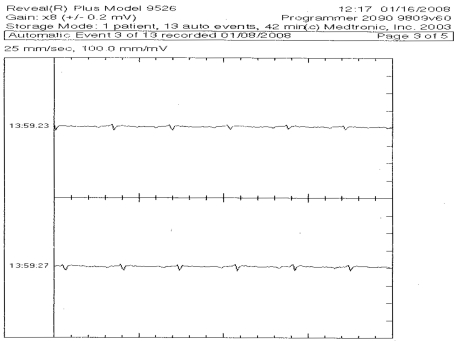
Figure 3.
ECG demonstrating sinus rhythm downloaded from an ILR.
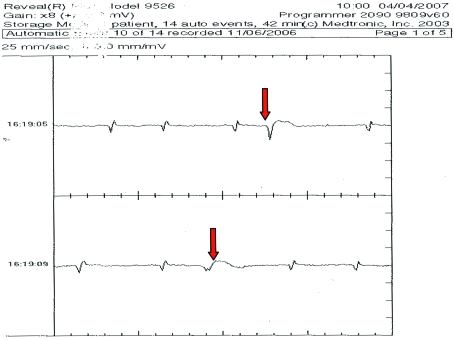
Figure 4.
ECG showing ventricular premature complexes (arrows) downloaded from an ILR implanted in a 31-y-old male chimpanzee.
Figure 5.
ECG showing atrial fibrillation with ventricular premature complexes downloaded from an ILR implanted in a 33-y-old male chimpanzee.
Discussion
Despite a paucity of information on the natural history of disease in great apes, heart disease appears to be the most common cause of death in captive populations.8,11,13,18,21,22 Cardiomyopathy appears to be the most common form of structural heart disease noted in captive chimpanzees,6,23 and cardiac arrhythmias in chimpanzees have been associated with underlying structural cardiac disease and an increased probability of sudden cardiac death.3,11,13 However, a short ECG could miss significant intermittent arrhythmias. This limitation of standard electrocardiography has led to the widespread use of ambulatory ECG in patients of other species, which are considered to be at risk of clinically significant arrhythmias. Ambulatory ECG enables assessment of the cardiac rhythm during normal activities and allows the clinician to assess a much longer period of the cardiac rhythm. Moreover, most examinations and diagnostic procedures on captive chimpanzees are performed under anesthesia, which might affect heart rate or rhythm. Various anesthetic regimens have been used to provide sedation of captive chimpanzees for evaluation. Ketamine, xylazine, and diazepam have been used to sedate chimpanzees at zoos and research facilities.4,14 Currently, tiletamine–zolazepam is the anesthetic agent used most often for the sedation of chimpanzees.3,11-14,23 This anesthetic regimen was not associated with arrhythmogenesis in macaques and lowland gorillas;2,15 however, the cardiodynamic and arrythmogenic effects of tiletamine–zolazepam on captive chimpanzees are unknown. As far as we are aware, the information obtained from the ILR in these cases represents the first electrocardiographic tracings from unanesthetized captive chimpanzees during normal activities.
Use of an ILR allows the identification of intermittent arrhythmias that occur during the chimpanzees’ normal daily activities. More importantly, the causative arrhythmia or lack thereof can be identified in animals with clinical signs such as collapse. The use of ILR in these 4 animals allowed us to rule out cardiogenic syncope in 1 and identified paroxysmal atrial fibrillation in another. With this diagnosis, as far as we know the first identification of paroxysmal atrial fibrillation in this species, appropriate medical management could be initiated. Atrial fibrillation has previously been diagnosed in a chimpanzee with severe right heart enlargement secondary to pulmonary hypertension;12 however, intermittent atrial fibrillation has not previously been recognized in this species, although it is common in humans. Furthermore, we were able to discern that the multiform ventricular premature complexes noted in the other 2 chimpanzees while under anesthesia were present while they were awake. However, ventricular tachycardia (even nonsustained) did not occur over the 6 mo that the ILR were implanted, suggesting medical management was unlikely to be clinically beneficial in these 2 chimpanzees.
Two limitations affect the ECG data obtained with ILR devices. First, an ILR cannot store 24 h of data and therefore cannot ascertain the number of abnormal beats an animal has in a 24-h period, as would be possible with a 24-h Holter monitor. However, the use of external ambulatory ECG monitoring capable of storing 24 h of data is not possible unless the animal will leave the device in place (unlikely in most chimpanzees). Second, ILR only store periods of the ECG that are outside of the programmed range. Therefore, abnormal rhythms with a normal rate (for example, accelerated idioventricular rhythm) will not be captured. Despite these limitations, use of ILR in these 4 chimpanzees resulted in clinically useful information that guided therapy.
Cardiovascular disease remains the primary cause of mortality and morbidity in captive chimpanzees,11,22,24 and cardiac arrhythmias are frequent findings in captive chimpanzee populations.3,11,13 However, in some cases the clinical significance of an arrhythmia detected on a baseline ECG may be difficult to ascertain. With a minimally invasive procedure, the use of an ILR can help diagnose intermittent arrhythmias. The information obtained from ILR provides valuable data that can be used in the medical management of captive chimpanzees.
Acknowledgments
This study was supported by NIH contract no. NO2-RR-1-209. We also thank Mr Terry Fennel (Medtronic) for his interest and support.
References
- 1.Armstrong VL, Lawson J, Kamper A, Newton J, Kenny R. 2003. The use of an implantable loop recorder in the investigation of unexplained syncope in older people. Age Ageing 32:185–188 [DOI] [PubMed] [Google Scholar]
- 2.Booker JL, Erickson HH, Fitzpatrick EL. 1982. Cardiodynamics in the rhesus macaque during dissociative anesthesia. Am J Vet Res 43:671–675 [PubMed] [Google Scholar]
- 3.Doane CJ, Lee DR, Sleeper MM. 2006. Electrocardiogram abnormalities in captive chimpanzees (Pan troglodytes). Comp Med 56: 512–518 [PubMed] [Google Scholar]
- 4.Erickson H, Olsen S. 1985. Electrocardiogram, heart rate, and blood pressure in the chimpanzee. Journal of Zoo Animal Medicine 16:89–97 [Google Scholar]
- 5.Farwell DJ, Freemantle N, Sulke N. 2006. The clinical impact of implantable loop recorders in patients with syncope. Eur Heart J 27:351–356 [DOI] [PubMed] [Google Scholar]
- 6.Hansen JF, Alford PL, Keeling ME. 1984. Diffuse myocardial fibrosis and congestive heart failure in an adult male chimpanzee. Vet Pathol 21:529–531 [DOI] [PubMed] [Google Scholar]
- 7.Houyel L, Fournier A, Centazzo S, Davignon A. 1992. Use of transtelephonic electrocardiographic monitoring in children with suspected arrhythmias. Can J Cardiol 8:741–744 [PubMed] [Google Scholar]
- 8.Hubbard GB, Lee DR, Eichberg JW. 1991. Diseases and pathology of chimpanzees at the Southwest Foundation for Biomedical Research. Am J Primatol 24:273–282 [Google Scholar]
- 9.Institute for Laboratory Animal Research 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 10.James R, Summerfield N, Loureiro J, Swift S, Dukes-McEwan J. 2008. Implantable loop recorders: a viable diagnostic tool in veterinary medicine. J Small Anim Pract 49:564–570 [DOI] [PubMed] [Google Scholar]
- 11.Lammey ML, Baskin GB, Gigliotti A, Lee DR, Ely JJ, Sleeper MM. 2008. Interstitial myocardial fibrosis in a captive chimpanzee (Pan troglodytes) population. Comp Med 58:389–391 [PMC free article] [PubMed] [Google Scholar]
- 12.Lammey ML, Lee DR, Doane CJ, Gigglioti A, Sleeper MM. 2008. Diagnosis and treatment of arterial pulmonary hypertension and atrial fibrillation in an adult chimpanzee (Pan troglodytes). J Am Assoc Lab Anim Sci 47:56–60 [PMC free article] [PubMed] [Google Scholar]
- 13.Lammey ML, Lee DR, Ely JJ, Sleeper MM. 2008. Sudden cardiac death in 13 captive chimpanzees. J Med Primatol 37 Suppl 1:39–43 [DOI] [PubMed] [Google Scholar]
- 14.Lamperez AJ, Rowell TJ. 2005. Normal C-reactive protein values for captive chimpanzees (Pan troglodytes). Contemp Top Lab Anim Sci 44:25–26 [PubMed] [Google Scholar]
- 15.Lee RV, Orlick AO, Dolensek EP, Doherty JG. 1981. The electrocardiogram of the lowland gorilla (Gorilla gorilla). J Zoo Anim Med 12:73–80 [Google Scholar]
- 16.Lombardi F, Calosso E, Mascioli G, Marangoni E, Donato A, Rossi S, Pala M, Fonti F, Lunati M. 2005. Utility of implantable loop recorder (Reveal Plus) in the diagnosis of unexplained syncope. Europace 7:19–24 [DOI] [PubMed] [Google Scholar]
- 17.Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. 1996. Improved survival with an implantable defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med 335:1933–1940 [DOI] [PubMed] [Google Scholar]
- 18.Munson L, Montali RJ. 1990. Pathology and disease of great apes at the National Zoological Park. Zoo Biol 9:99–105 [Google Scholar]
- 19.Nelson RW, Couto CW. 1998. Small animal internal medicine, p 74 St Louis (MO): Mosby [Google Scholar]
- 20.Sanatani S, Peirone A, Chiu C, Human DG, Gross GJ, Hamilton RM. 2002. Use of an implantable loop recorder in the evaluation of children with congenital heart disease. Am Heart J 143:366–372 [DOI] [PubMed] [Google Scholar]
- 21.Schulman FY, Farb A, Virmani R, Montali RJ. 1995. Fibrosing cardiomyopathy in captive Western lowland gorillas (Gorilla gorilla gorilla) in the United States: a retrospective study. J Zoo Wildl Med 26:139–143 [Google Scholar]
- 22.Seiler BM, Dick EJ, Guardado-Medoza R, VandeBerg JL, Williams JT, Mubiru JN, Hubbard GB. 2009. Spontaneous disease in the adult chimpanzee (Pan troglodytes). J Med Primatol 38:51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sleeper MM, Doane CJ, Langner PH, Curtis SK, Avila K, Lee DR. 2005. Successful treatment of idiopathic dilated cardiomyopathy in an adult chimpanzee (Pan troglodytes). Comp Med 55:80–84 [PubMed] [Google Scholar]
- 24.Varki N, Anderson D, Herndon J, Pham T, Gregg C, Cheriyan M, Murphy J, Strobert E, Fritz J, Else J, Varki A. 2009. Heart disease is common in humans and chimpanzee but is caused by different pathological processes. Evol Appl 2:101–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willis R, McLeod K, Cusack J, Wotton P. 2003. Use of an implantable loop recorder to investigate syncope in a cat. J Small Anim Pract 44:181–183 [DOI] [PubMed] [Google Scholar]
- 26.Woosley RL. 2004. Antiarrhythmic drugs, p 341–346 In: Goldman L, Ausiello D. Ceil textbook of medicine. Philadelphia (PA): Saunders [Google Scholar]