Abstract
Ulcerative dermatitis (UD) is a genetically linked syndrome that affects the neck, torso, and facial regions of C57BL/6 mice and strains with C57BL/6 background. In this study, 96 mice with skin ulcerations in 3 different regions of the body and 40 control animals without ulcerated lesions were evaluated histologically for the presence of hair-induced inflammation in the oronasal cavity. We found that 73.5% (100 of 136) of the mice had hair-induced periodontitis, glossitis, or rhinitis regardless of the presence or absence of UD. Of those mice with UD, 93.9% had hair-induced oronasal inflammation. The mandibular incisors were the most commonly affected site (64.6%), followed by the maxillary molars (20.8%), maxillary incisors (16.7%), tongue (16.7%), nasal cavity (10.4%), and mandibular molars (7.3%). In addition, oronasal hair-induced inflammation occurred in 25% (10 of 40) of the control mice. Here we show a significant association between UD and hair-induced inflammatory lesions of the oronasal cavities.
Abbreviations: Th, T helper; UD, ulcerative dermatitis
A poster presented at the national meeting of American Association of Laboratory Animal Science in 200715 and a recent publication29 both reported that C57BL/6 mice are prone to the development of hair-induced periodontitis. Both studies15,29 showed that the penetration of hair into the gingival sulcus, secondary to barbering, results in gingivitis and gingival ulceration as well as alveolar bone loss. Periodontitis is defined as inflammation of the tissues that surround and support the teeth,19 including the periodontal ligament, tooth root, and alveolar bone. Periodontitis is common in several species including humans,38,39,52 sheep,3,7,14,46,47 cows,47 cats,21,45 dogs,20,24 and rodents such as rice rats (Oryzomys palustris)22 and laboratory rats (Rattus norvegicus).49 Several factors, including mechanical-37 and chemical-induced gingivitis,41 nutritional deficiencies,18,32 systemic diseases,59 and bacterial plaque-induced gingivitis have been implicated as causes of periodontitis.13,24,52,58 If left untreated, gingivitis may become chronic, leading to the degeneration of periodontal ligaments and alveolar bone, tooth root abscessation, and the eventual shedding of the teeth.46 Clinically, weight loss can occur secondary to masticatory pain, bacteremia,40,48 and coronary heart disease of odontogenic origin.5,43,54
Ulcerative dermatitis (UD) is a genetically linked syndrome that affects the skin of C57BL/6 mice and strains with C57BL/6 background.27 UD has been associated with gender,2,27 age,33,34 percentage of dietary fat, vitamin A, ad libitum feeding,33,35 and vasculitis caused by immune complex.2 However, the exact etiology remains undetermined. Lesions are severely pruritic and commonly present on the dorsal scapulae but can develop on the torso, shoulder, and head and may be single or multifocal in distribution.30 The lesions progress from excoriation to deep ulceration secondary to severe pruritus-induced self-trauma.
As noted previously, C57BL mice are prone to development of hair-induced periodontitis. In light of this information, we hypothesize that UD is associated with hair-induced oronasal lesions secondary to excessive grooming activities.
Materials and Methods
C57BL/6 mice and mice with C57BL/6 background (n = 96) with spontaneous, untreated ulcerative dermatitis were used in this study. Three groups were established according to the anatomic location of the ulcerated lesion: 1, 40 animals with skin ulceration rostral to the occipital crest and to the ramus of the mandible (Figure 1); 2, 40 animals with ulcerated lesions of the dorsal cervical skin; and 3, 16 animals with skin ulceration in other parts of the body including torso, extremities, and ventral abdomen. Because all of these mice were clinical cases, age, sex, and specifics on any genetic alteration were not always recorded. However, the age, sex, and specific strain information was available on 40 chronically and excessively barbered, 1-y-old, C57BL/6J mice that were used as controls. The control mice were housed 4 per cage, were of the same age and sex, and all exhibited barbered fur. None of the control mice had dermal ulcerative lesions.
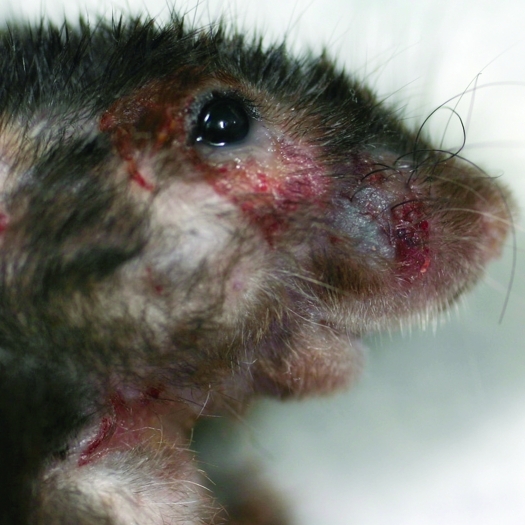
Figure 1.
Typical gross appearance of facial ulcerative dermatitis in a mouse.
All mice examined were housed in a SPF, AAALAC-accredited facility where sentinel mice were tested quarterly for mouse parvovirus (types 1 and 2 and NS1), minute virus of mice, mouse norovirus, mouse hepatitis virus, Sendai virus, lymphocytic choriomeningitis virus, polyomavirus, K virus, pneumonia virus of mice, mouse adenovirus, epizootic diarrhea of infant mice (rotavirus), mouse encephalomyelitis virus, reovirus, ectromelia virus, Mycoplasma pulmonis, and endo- and ectoparasites. All mice were fed irradiated or nonirradiated NIH31 diet (Harlan Teklad, Madison, WI) ad libitum and nonacidified filtered or reverse-osmosis water, depending on the SPF standards of the housing facilities. Macroenvironmental conditions within all rooms were maintained at 69 to 74 °F (20.6 to 23.3 °C), relative humidity of 30% to 70%, 10 to 15 air changes hourly, and 12:12-h photoperiod. The use of the mice in this study was approved by the Institutional Animal Care and Use Committee of the University of California—Los Angeles.
All animals were euthanized by using compressed carbon dioxide. A necropsy was performed on each mouse, and data collection included strain, gross pathology, and histologic evaluation of the structures of the head. The head of each mouse was fixed in 10% formalin (10% Formalin w/v 4% Formaldehyde Solution, Fischer Scientific, Fair Lawn, NJ) for 24 h, followed by 24 h in formic acid for decalcification (Protocol Decalcifier B, Fischer Scientific, Kalamazoo, MI). The heads then were sliced transversely, forming multiple thin (approximately 2 to 3 mm) sections progressing from the incisors to an area just caudal to the eyes or midcerebrum. All tissues were processed for paraffin embedding, and 5-μm sections were stained with hematoxylin and eosin (Mass Histology Service, Worcester, MA). Sections of the head were examined for the presence of lesions within the oral, nasal cavities, and sinuses. A grading scale from 0 to 4.5 was established to quantify the hair induced inflammatory reaction, tissue damage, and severity and extensiveness of the lesions: 0, no lesions; 1, mild inflammatory reaction; 1.5, same as 1 but in multiple areas; 2, moderate inflammatory reaction; 2.5, same as 2 but in multiple areas; 3, severe inflammatory reaction with or without bone resorption and remodeling; 3.5, same as 3 but in multiple areas; 4, massive changes to the architecture of the tissues; 4.5, same as 4 but in multiple areas. Histologic tissue sections were scored blindly by 2 different reviewers. Statistical significance was determined by t test (Excel 2010, Microsoft, Redmond, WA). A P value of less than 0.05 was regarded as statistically significant.
Results
To demonstrate an association between UD and oronasal hair-induced inflammation, the heads of 136 mice with and without ulcerative dermatitis were examined histologically.
A strong association between hair-induced oronasal lesions and the presence of ulcerative dermatitis was determined by t test. No significant difference was noted between the animals with UD regardless of the anatomical location of the skin lesions; however, when we compared each UD-affected group with the control, statistically significant differences emerged (mice with UD of the face: P = 1.53 × 10-11; mice with neck UD: P = 5.75 × 10-13; mice with torso UD: P = 1.59 × 10-7; mice with UD regardless of anatomic location of lesions: P = 5.20 × 10-16).
Of the 40 animals with facial UD, 92.5% (37 of 40) had hair-induced periodontitis, glossitis, or rhinitis or multiple lesions. The remaining 3 animals with facial UD had neither hair nor other inflammatory lesions in the oral or nasal cavities. Hair- and nonhair-induced oronasal inflammation was found in 97.5% (39 of 40) of the mice with ulcerative dermatitis of the neck. Specifically, 36 of the 39 mice with oronasal inflammation had hair-induced lesions with or without the presence of other oronasal pathologies, whereas the remaining 3 mice had nonhair-related lesions. Among animals with UD of the torso and extremities, 87.5% (14 of 16) of the mice had hair-induced oronasal lesions, and an additional mouse (6.3%) had lesions unrelated with hair. Although none of the 40 control animals had UD, 25% (10 of 40) did have histologic evidence of hair-induced oronasal inflammation. Overall 93.75% (90 of 96) of the population of mice with UD had hair-induced oronasal inflammation. Of those hair-induced lesions, 64.58% (62 of 96) were associated with the mandibular incisors (Figure 2), 20.8% (20 of 96) with the maxillary molars (Figure 3), 16.7% (16 of 96) with the maxillary incisors, 16.7% (16 of 96) with the tongue, 10.4% (10 of 96) with the nasal cavity, and 7.3% (7 of 96) with the mandibular molars. Of those lesions from which hair was absent, inflammatory lesions included arteritis, otitis externa and media, dentin dysplasia, tooth pulp abscess, and proliferative and ulcerative keratitis. Some of the animals had hair-induced inflammation in more than one of the structures previously mentioned (Table 1) as well as inflammatory lesions that were not associated with hair. All 10 (100%) of the control mice with hair-induced inflammation had lesions associated with the mandibular incisors, and 20% (2 of 10) also had lesions associated with the maxillary incisors.
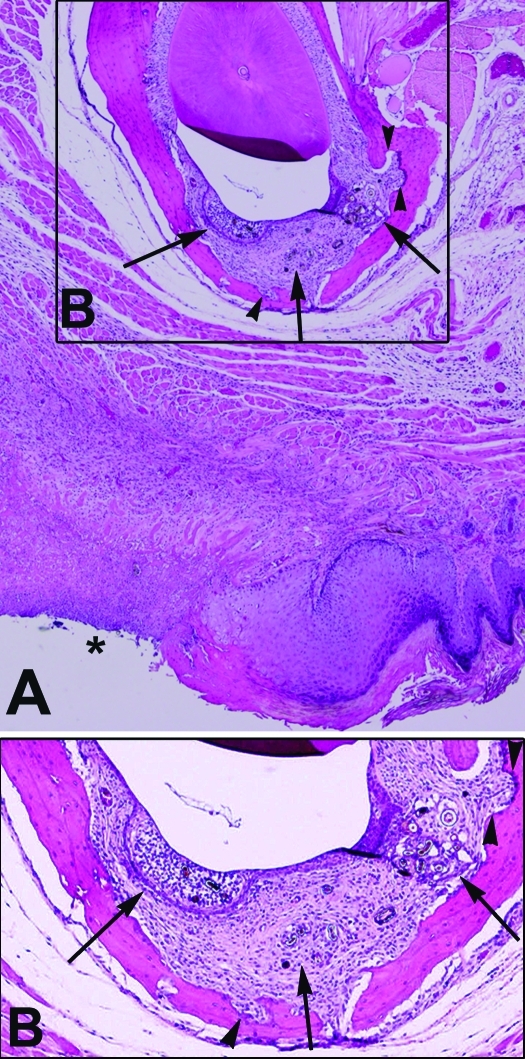
Figure 2.
(A) Cross-section of mandibular incisor hair-induced periodontitis with ipsilateral ulcerative dermatitis (asterisk). Magnification, ×4. (B) Higher magnification of the boxed region of panel A shows expansion of the periodontal space with inflammation, fibrosis, and hair fragments (arrows). Also note the scalloping of mandibular bone due to resorption and reformation (arrowheads). Magnification, ×10.
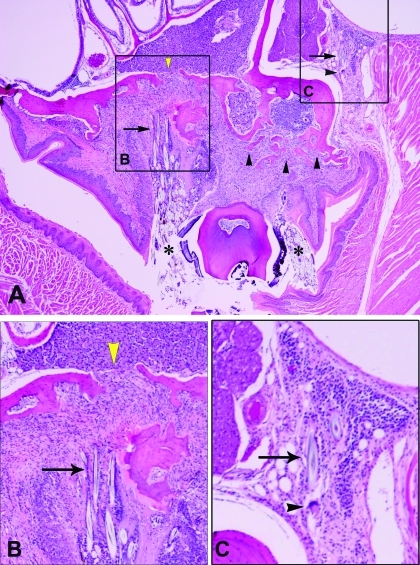
Figure 3.
(A) Cross-section of maxillary molar with extreme, bilateral expansion of the gingival sulcus. Massive amount of hair fragments (asterisk) surrounded by granulomatous inflammation and fibrosis. Also note the scalloping and discontinuity of the maxillary bone (yellow arrowheads) as well as the amount of bone resorption and new formation (arrowheads). Magnification, ×4. (B) Higher magnification of the boxed area labeled B in panel A, showing hair-induced fibrosis and bone resorption and reformation (arrow). Magnification, ×40. (C) Higher magnification of the boxed area labeled C in panel A, showing hair-induced inflammation with multinucleated giant cells (arrowhead). Magnification, ×40.
Table 1.
Mice (%) in each scoring category according to severity of hair -induced lesions
| Scoring category |
|||||||||
| 0 | 1 | 1.5 | 2 | 2.5 | 3 | 3.5 | 4 | 4.5 | |
| Ulcerative dermatitis of the face | 7.5 | 12.5 | 5.0 | 15.0 | 15.0 | 15.0 | 10.0 | 2.5 | 17.5 |
| Ulcerative dermatitis of the neck | 2.5 | 17.5 | 5.0 | 15.0 | 12.5 | 10.0 | 25.0 | 5.0 | 7.5 |
| Ulcerative dermatitis of the torso | 12.5 | 12.5 | 0.0 | 6.3 | 18.8 | 25.0 | 12.5 | 6.3 | 6.3 |
| All cases of ulcerative dermatitis | 6.3 | 15.6 | 5.7 | 15.6 | 17.2 | 17.7 | 20.3 | 8.3 | 16.1 |
| Controls | 75.0 | 7.5 | 2.5 | 7.5 | 0.0 | 5.0 | 2.5 | 0.0 | 0.0 |
Scoring categories: 0, no lesions; 1, mild inflammatory reaction; 1.5, same as 1 but in multiple areas; 2, moderate inflammatory reaction; 2.5, same as 2 but in multiple areas; 3, severe inflammatory reaction with or without bone resorption and remodeling; 3.5, same as 3 but in multiple areas; 4, massive changes to the architecture of the tissues; 4.5, same as 4 but in multiple areas.
A grading scale was established to quantify histologically the hair-induced inflammatory reaction, tissue damage, and severity and extensiveness of the lesions (Table 1). The inflammatory response was not always consistent with the number of hair fragments, varied from mild to severe, and was composed of neutrophils and macrophages, with fewer numbers of lymphocytes or mast cells or both. The amount of intralesional hair varied in fragment number from 1 to too numerous to count, resulting in varying degrees of expansion of the gingival sulcus and variation in the inflammatory cell or fibroblast response (or both). Marked periodontal alveolar bone resorption and new alveolar bone formation appeared to be associated with a moderate to severe inflammatory reaction.
Glossitis most typically occurred when hair fragments entered the duct of the lingual glands at the base of the tongue and was characterized by the presence of neutrophils, mast cells, fibrosis, and muscle atrophy (Figure 4). Rhinitis was associated with hair fragments most typically within the submucosa of the rostral ventral conchae that were surrounded by macrophages and neutrophils (Figure 5). The amount of hair varied from few to numerous fragments, and in cases where large numbers of fragments were identified, hairs often extended into the lumen. The presence of hair in the submucosa highly suggests their entry as being ‘from inside out’ rather than from the nasal lumen to the submucosa or ‘from outside in.’
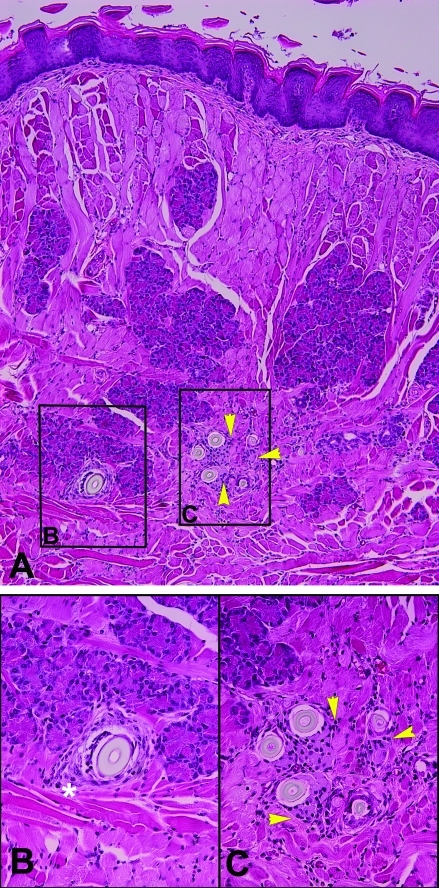
Figure 4.
(A) Cross-section of tongue with hair-induced inflammation at the level of the salivary ducts. Magnification, ×10. (B) Higher magnification of boxed area labeled B in panel A, showing hair fragment with concentric lamination of connective tissue (white asterisk). Magnification, ×40. (C) Higher magnification of boxed area labeled C in panel A, showing hair-induced glossitis with multiple hair fragments surrounded by macrophages (arrowheads). Magnification, ×40.
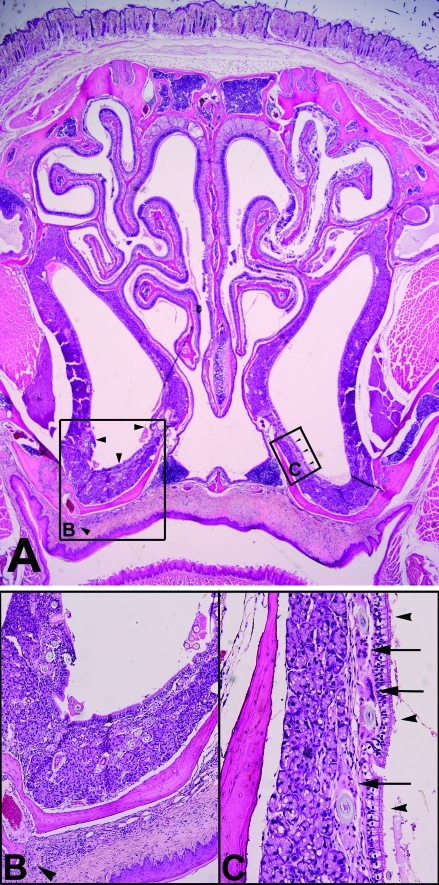
Figure 5.
(A) Cross-section of nasal cavity with bilateral hair-induced rhinitis of the nasal meatus and unilateral hair-induced palatitis. Magnification, ×2. (B) Higher magnification of boxed area labeled B in panel A, showing hair-induced rhinitis with multifocal hair fragments (arrowheads) surrounded by granulomatous inflammation and fibrosis. Also note the individual hair fragment within the palate. Magnification, ×20. (C) Higher magnification of boxed area labeled C in panel A, showing hair-induced granulomatous rhinitis with hair fragments (arrowheads) and multinucleated giant cells (arrows). Magnification, ×40.
In more than 90% (33 of 37) of the cases of hair-induced inflammation, facial UD was localized ipsilateral to the intraoral or intranasal lesions (Figure 2).
Discussion
Several authors2,27,30,29,33 have shown that UD lesions in mice are pruritic and often located cranial to the abdomen, involving the interscapular area, dorsal and ventral neck, base and pinnae of the ears, and muzzle. The cause of UD has not been established, and it is considered a multifactorial disease.25,27 In general, UD has a poor and variable response to therapeutic agents, regardless of its mechanism of action. Topical, systemic, and environmental treatments have been established without a curative option.2,27 A recent report29 suggests that hair-induced inflammation is a cause of facial abscesses as well as periodontitis and alveolar bone loss in mice. From this report, we hypothesized that the inflammatory response secondary to the presence of hair in the periodontium, tongue, or nose is associated with ulcerative dermatitis. We speculate that the pain and discomfort from the inflammation results in compulsive scratching behavior that then leads to self-trauma and ulceration of the skin, with a subsequent cascade of local inflammatory mediators.
Although the pathogenesis of UD has not been clarified, the numerous clinical reports on treatment regimens suggest that the lesions are induced by an inflammatory incident. Treatments have included vitamin E,30 cyclosporine,16 caladryl lotion,11 and ibuprofen. The pharmacodynamics of each of these treatments are as follows: vitamin E is a free-radical scavenger; ibuprofen is an inhibitor of the arachidonic acid–cyclooxygenase cascade; cyclosporine suppresses the immune system by a specific, reversible inhibition of the cell cycle in T-helper (Th) lymphocytes and suppresses IL2 synthesis;44 and the pramoxine HCl of caladryl is a local anesthetic that interferes with the transmission of impulses along the sensory nerve fibers.60 Therefore, all of these drugs and topical treatments are directed toward the modulation of inflammation or directly acting on topical nerve transmission. C57BL/6 mice have a dominant Th1 inflammatory response, with increased levels of IL12 and INFγ and decreased production of IL4 and IL13.58 INFγ activates phospholipase A2 to synthesize arachidonic acid from cell membrane phospholipids, for the downstream production of prostaglandins, thromboxanes, and leukotrienes during inflammatory events. In addition, the bactericidal activity of C57BL/6 macrophages is greater when compared with other strains, resulting in a more effective local response and a less aggressive systemic reaction, thus supporting the reliance of C57BL/6 mice on a strong Th1-driven immunity.58
Authors studying the inflammatory component of ulcerative dermatitis17 showed that E-selectin–P-selectin–Rag1 triple-knockout mice had a significantly lower incidence of spontaneous UD than did E-selectin–P-selectin double-knockout mice. The Rag1 and Rag2 genes are essential for the generation of mature B and T cells, and as a consequence, Rag1 knockout mice have severe combined immunodeficiency,53 which is potentiated when the genes for E- and P-selectins are knocked out. A severe impairment of the immune system, secondary to a reduction in Th1 lymphocytes, is protective for the development of spontaneous UD, suggesting that the condition is affected by inflammatory events. These findings are consistent with a previous study,10 in which E-selectin–P-selectin–L-selectin triple-knockout mice had a lower incidence of ulcerative dermatitis than E-selectin–P-selectin double-knockouts. Selectins are cell-adhesion molecules, exclusively involved in the binding of leukocytes to endothelial cells during margination and diapedesis.56 L-selectin is expressed on the surface of all leukocytes and is essential for leukocyte rolling at sites of inflammation42 and it is the only selectin involved in the migration of lymphocytes into lymph nodes during lymphocyte recirculation, recruitment and maturation. The lack of expression of L-selectin compromises the circulation, migration, and activation of lymphocytes during inflammatory and noninflammatory events as well as neutrophil recruitment.23,26 E- and P-selectins are cytokine-inducible endothelial adhesion molecules for neutrophils and monocytes.57 The effect on leukocyte rolling in E-selectin–P-selectin double-knockout mice was insufficient to block leukocyte recruitment notably under inflammatory conditions.8,26
Leukocyte adhesion deficiency in humans,12 canine leukocyte adhesion deficiency in Irish Setter dogs,28 and bovine leukocyte adhesion deficiency in Holstein cattle36 all are characterized by the lack of expression of adhesive molecules of the CD11/CD18 family in neutrophils, preventing them from adhering to blood vessel walls at the site of infection. In addition,129/Sv × C57BL/6J CD18-knockout mice were prone to infection and developed chronic skin ulcerations histologically characterized by erosions and ulcerations with superficial bacteria-laden crusts.51 The investigators described a dermal inflammatory infiltrate consisting of lymphocytes, histiocytes, plasma cells, and few neutrophils, suggesting poor neutrophil extravasation. The ulcerated lesions in those mice were limited to the face and the ventral chin, lesions typical of those noted in C57BL/6 mice with facial UD. However, the heads of those mice were not evaluated histologically.
Clinically UD is characterized by excessive pruritus with secondary self-induced trauma and has been treated by some by covering or trimming the nails.27 The itch–scratch–itch cycle is known to be induced by the local production of free radicals in the skin.1 In cases of human chronic periodontitis, levels of nitric oxide and endothelin are elevated, are associated with vasoconstriction9 and free radical production, and stimulate scratching behavior.3,52,55 In addition to pruritus, human patients with severe inflammation-induced periodontal bone dissolution report considerable pain,6 another factor that could be associated with self-induced trauma in mice with UD. The fact that the source of the inflammatory response in mice (in this case, hair) cannot be removed perpetuates the inflammation and thus the production of free radicals, pruritus, and pain. In the current study, more than 90% of the mice with hair-induced inflammation and facial UD had ipsilateral lesions. This observation, as well as the statistical significance between the presence of UD and hair-induced oronasal inflammation, supports our hypothesis of an association between hair-induced intraoral or intranasal lesions and UD.
Hair-induced periodontitis composed of large numbers of neutrophils, macrophages and hair fragments in BDF1 and B6C3F1 mice has been reported.50 However, in that study, the mandible was not sectioned, and only 3 sites of the maxillary arcade were sampled, thus greatly decreasing the potential for lesion identification. Data obtained in our study demonstrated that mandibular incisors are the site affected most frequently in hair-induced periodontitis.
The inflammatory response mounted against hair fragments leads to bone resorption and remodeling. In some cases, we noted porous dissolution of the maxillary bone with migration of hair fragments into the retroorbital space, with subsequent Harderian adenitis or necrosis with or without ocular proptosis. The amount and quality of bone tissue present during physiologic growth and remodeling is determined by the balance between the rates of formation and resorption.31 Osteoclasts are multinucleated cells derived from monocyte–macrophage lineage and are considered to be the principal cell responsible for bone resorption.4 IL1, cFms (the receptor for colony-stimulating factor 1), TNFα, prostaglandin E2, and TGFβ are known to activate osteoclast surface receptors and potentiate osteoclastogenesis in vivo,4 suggesting that systemic or local inflammation around alveolar bone can stimulate resorption. IL1 directly activates monocytes to differentiate into macrophages and preosteoclasts and, when present in conjunction with the receptor activator of the NFκβ ligand, cytokines and intracellular factors then can act directly on preosteoclasts, resulting in the maturation to active osteoclasts.4 Although tissue levels of inflammatory cytokines and interleukins have not been evaluated in the oronasal tissues of mice with hair-induced lesions, the presence of chronic inflammatory cells, including macrophages and multinucleated giant cells, suggests that similar increases in cytokines and interleukins occur with the lesions identified in the present study.
Here we identified several cases of hair-induced nasal inflammation in mice. The involvement of the ventral conchae, submucosal, and mucosa and the frequent lack of intraluminal hair strongly suggested the presence of an oronasal fistula. Oronasal fistulation in rats has been described,49 in which rhinitis or maxillary sinusitis was present as a consequence of food particle penetration from the gingival sulcus into the nasal cavity. In our study, we found that 10.42% of mice with UD also had hair-induced rhinitis. Although we were unable to identify direct fistula on either gross or histologic examination, these mice did have bone dissolution and numerous hair fragments scattered between the hard palate and nasal mucosa. In this study, we have shown a significant association between UD and hair-induced inflammation of the incisive and molar periodontium as well as the tongue and nasal cavity. The variability between the amount of hair and the severity of the inflammatory response could be attributed to tissue sectioning, drainage of inflammatory cells through the surface ulceration, and/or the time when foreign body, in these cases hair fragments, impacted the gingiva.
The fact that C57BL/6 mice are prone to Th1 inflammatory responses and that the local cytokine, leukotriene, and free-radical milieu produced by this chronic inflammation results in pain and discomfort, it would be expected that mice would scratch in response and promote an itch–scratch–itch cycle.
Nonhair-induced inflammatory processes, including dental dysplasia, pulpitis, tooth root abscesses, sinusitis, and rhinitis, may contribute to the development of UD. If a valuable breeding or study animal presents with UD, treatment with antiinflammatory agents as well as free radical scavengers may be beneficial, as long as this treatment has no effect on the study. Although vitamin E treatment is not 100%-effective for UD, some partial success has been reported,30 as previously mentioned.
References
- 1.Andoh T, Kuraishi Y. 2003. Nitric oxide enhances substance- P-induced itch-associated responses in mice. Br J Pharmacol 138:202–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews AG, Dysko RC, Spilman SC, Kunkel RG, Brammer DW, Johnson JK. 1994. Immune complex vasculitis with secondary ulcerative dermatitis in aged C57BL/6NNia mice. Vet Pathol 31:293–300 [DOI] [PubMed] [Google Scholar]
- 3.Andrews AH. 1981. Clinical signs and treatment of aged sheep with loose mandibular or maxillary cheek teeth. Vet Rec 108:331–333 [DOI] [PubMed] [Google Scholar]
- 4.Bartold PM, Cantley MD, Haynes DR. 2010. Mechanisms and control of pathologic bone loss in periodontitis. Periodontol 2000 53:55–69 [DOI] [PubMed] [Google Scholar]
- 5.Beck JD, Offenbacher S. 2005. Systemic effects of periodontitis: epidemiology of periodontal disease and cardiovascular disease. J Periodontol 76:2089–2100 [DOI] [PubMed] [Google Scholar]
- 6.Bodet Agusti E, Figerola R, Martinez VV. 2009. [Foreign bodies in maxillary sinus]. Acta Otorrinolaringol Esp 60:190–193 [Article in Spanish] [PubMed] [Google Scholar]
- 7.Britt DP, Baker JR. 1990. Causes of death and illness in the native sheep of North Ronaldsay, Orkney I. Adult sheep. Br Vet J 146:129–142 [DOI] [PubMed] [Google Scholar]
- 8.Bullard DC, Kunkel EJ, Kubo H, Hicks MJ, Lorenzo I, Doyle NA, Doerschuk CM, Ley K, Beaudet AL. 1996. Infectious susceptibility and severe deficiency of leukocyte rolling and recruitment in E-selectin and P-selectin double-mutant mice. J Exp Med 183:2329–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S, Wu J, Song Z, Zhang J. 2000. An investigation of immunocompetence substances in normal gingival and periodontitis tissue. Chin Med J (Engl) 113:844–847 [PubMed] [Google Scholar]
- 10.Collins RG, Jung U, Ramirez M, Bullard DC, Hicks MJ, Smith CW, Ley K, Beaudet AL. 2001. Dermal and pulmonary inflammatory disease in E-selectin and P-selectin double-null mice is reduced in triple-selectin-null mice. Blood 98:727–735 [DOI] [PubMed] [Google Scholar]
- 11.Crowley ME, Delano ML, Kirchain SM. 2008. Successful treatment of C57BL/6 ulcerative dermatitis with caladryl lotion. J Am Assoc Lab Anim Sci 47:109–110 [Google Scholar]
- 12.Dababneh R, Al-wahadneh AM, Hamadneh S, Khouri A, Bissada NF. 2008. Periodontal manifestation of leukocyte adhesion deficiency type I. J Periodontol 79:764–768 [DOI] [PubMed] [Google Scholar]
- 13.Darby I, Curtis M. 2001. Microbiology of periodontal disease in children and young adults. Periodontol 2000 26:33–53 [DOI] [PubMed] [Google Scholar]
- 14.Duncan WJ, Persson GR, Sims TJ, Braham P, Pack ARC, Page RC. 2003. Ovine periodontitis as a potential model for periodontal studies. J Clin Periodontol 30:63–72 [DOI] [PubMed] [Google Scholar]
- 15.Ezell P, Lawson GW, Nishimura I. 2007. µCT and histopathology of Mus musculus hair-induced mandibulofacial abscess demostrates severe periodontitis and alveolar bone destruction. J Am Assoc Lab Anim Sci 46:104 [Google Scholar]
- 16.Feldman S, McVay L, Kessler MJ. 2006. Resolution of ulcerative dermatitis of mice by treatment with topical 0.2% cyclosporine. J Am Assoc Lab Anim Sci 45:92–93 [Google Scholar]
- 17.Forlow SB, White EJ, Thomas KL, Bagby GJ, Foley PL, Ley K. 2002. T Cell requirement for development of chronic ulcerative dermatitis in E- and P-selectin-deficient mice. J Immunol 169:4797–4804 [DOI] [PubMed] [Google Scholar]
- 18.Fox JG, Anderson LC, Loew FM, Quimby FW. 2002. Laboratory animal medicine, 2nd ed, p 773 Orlando (FL): Academic Press [Google Scholar]
- 19.Friel JP. 1974. Dorland's ilustrated medical dictionary. Philadelphia (PA): WB Saunders [Google Scholar]
- 20.Genco CA, Van Dyke T, Amar S. 1998. Animal models for Porphyromonas gingivalis-mediated periodontal disease. Trends Microbiol 6:444–449 [DOI] [PubMed] [Google Scholar]
- 21.Girard N, Biourge V, Hennet P. 2009. Periodontal health status in a colony of 109 cats. J Vet Dent 26:147–155 [DOI] [PubMed] [Google Scholar]
- 22.Gupta OP, Shaw JH. 1956. [Periodontal disease in the rice rat. I. Anatomic and histopathologic findings]. Oral Surg Oral Med Oral Pathol 9:592–603 [Article in French] [DOI] [PubMed] [Google Scholar]
- 23.Hamann A, Rebstock S. 1993. Migration of activated lymphocytes. Curr Top Microbiol Immunol 184:109–124 [DOI] [PubMed] [Google Scholar]
- 24.Hardham J, Dreier K, Wong J, Sfintescu C, Evans RT. 2005. Pigmented-anaerobic bacteria associated with canine periodontitis. Vet Microbiol 106:119–128 [DOI] [PubMed] [Google Scholar]
- 25.Jacoby R, Fox JG, Davisson M. 2002. Biology and diseases of mice, p 107 In: Fox JG, Anderson LC, Loew FM, Quimby FW. Laboratory animal medicine, 2nd ed Orlando (FL): Academic Press [Google Scholar]
- 26.Jung U, Ley K. 1999. Mice lacking 2 or all 3 selectins demonstrate overlapping and distinct functions for each selectin. J Immunol 162:6755–6762 [PubMed] [Google Scholar]
- 27.Kastenmayer RJ, Fain MA, Perdue KA. 2006. A retrospective study of idiopathic ulcerative dermatitis in mice with a C57BL/6 background. J Am Assoc Lab Anim Sci 45:8–12 [PubMed] [Google Scholar]
- 28.Kobayashi S, Sato R, Abe Y, Inanami O, Yasui H, Omoe K, Yasuda J, Hankanga C, Oda S, Sasaki J. 2009. Canine neutrophil dysfunction caused by downregulation of β2-integrin expression without mutation. Vet Immunol Immunopathol 130:187–196 [DOI] [PubMed] [Google Scholar]
- 29.Lawson GW. 2010. Etiopathogenesis of mandibulofacial and maxillofacial abscesses in mice. Comp Med 60:200–204 [PMC free article] [PubMed] [Google Scholar]
- 30.Lawson GW, Sato A, Fairbanks LA, Lawson PT. 2005. Vitamin E as a treatment for ulcerative dermatitis in C57BL/6 mice and strains with a C57BL/6 background. Contemp Top Lab Anim Sci 44:18–21 [PubMed] [Google Scholar]
- 31.Lerner UH. 2000. Osteoclast formation and resorption. Matrix Biol 19:107–120 [DOI] [PubMed] [Google Scholar]
- 32.Li R, Byers K, Walvekar RR. 2008. Gingival hypertrophy: a solitary manifestation of scurvy. Am J Otolaryngol 29:426–428 [DOI] [PubMed] [Google Scholar]
- 33.Myers DD. 1996. The effect of weaning age and diets. In: Users of JAX mice. Bar Harbor (ME): The Jackson Laboratory [Google Scholar]
- 34.Myers DD. 1996. Notice to JAX Mice users. In: Users of JAX mice. Bar Harbor (ME): The Jackson Laboratory [Google Scholar]
- 35.Myers DD. 1997. C57BL/6J Skin lesion problem eliminated. In: Users of JAX mice. Bar Harbor (ME): The Jackson Laboratory [Google Scholar]
- 36.Nagahata H. 2004. Bovine leukocyte adhesion deficiency (BLAD): a review. J Vet Med Sci 66:1475–1482 [DOI] [PubMed] [Google Scholar]
- 37.Ngassapa DNMJC, Freihofer HP. 1986. The reaction of the periodontium to different types of splints. II. Histological aspects. Int J Oral Maxillofac Surg 15:250–258 [DOI] [PubMed] [Google Scholar]
- 38.O'Hehir TE. 1995. Check for popcorn hulls, fingernails after spotting abscesses in children. RDH 15:32. [PubMed] [Google Scholar]
- 39.O'Hehir TE, Eber R, Wang HL. 2002. Periodontal diseases in the child and adolescent. J Clin Periodontol 29:400–410 [DOI] [PubMed] [Google Scholar]
- 40.Olsen I. 2008. Update on bacteraemia related to dental procedures. Transfus Apher Sci 39:173–178 [DOI] [PubMed] [Google Scholar]
- 41.Palmer RM, Wilson RF, Hasan AS, Scott DA. 2005. Mechanism of action of environmental factors—tobacco smoking. J Clin Periodontol 32:180–195 [DOI] [PubMed] [Google Scholar]
- 42.Pavalko F. 2009. Cytoskeletal interactions with leukocyte and endothelial cell adhesion molecules. Curr Top Membr 64:133–156 [Google Scholar]
- 43.Pavlica Z, Petelin M, Juntes P, Erzen D, Crossley DA, Skaleric U. 2008. Periodontal disease burden and pathological changes in organs of dogs. J Vet Dent 25:97–105 [DOI] [PubMed] [Google Scholar]
- 44.Plumb DC. 2005. Veterinary drug handbook. Hoboken (NJ): Wiley–Blackwell [Google Scholar]
- 45.Prescott CW. 1971. Some oral lesions in the cat. Aust Vet J 47:41–45 [DOI] [PubMed] [Google Scholar]
- 46.Pugh DG. 2002. Sheep and goat medicine. Philadelphia (PA): WB Saunders [Google Scholar]
- 47.Radostits OM, Gay CC, Blood DC, Hinchcliff KW. 2000. Veterinary medicine: a textbook of the diseases of cattle, sheep, pigs, goats, and horses, 9th ed Philadelphia (PA): WB Saunders [Google Scholar]
- 48.Roberts GJ, Jaffray EC, Spratt DA, Petrie A, Greville C, Wilson M, Lucas VS. 2006. Duration, prevalence, and intensity of bacteremia after dental extractions in children. Heart 92:1274–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robinson M. 1985. Dietary-related periodontitis and oronasal fistulation in rats. J Comp Pathol 95:489–498 [DOI] [PubMed] [Google Scholar]
- 50.Sakura Y. 1997. Periodontal inflammatory and cystlike lesions in BDF1 and B6C3F1 mice. Vet Pathol 34:460–463 [DOI] [PubMed] [Google Scholar]
- 51.Scharffetter-Kochanek K, Lu H, Norman K, van Nood N, Munoz F, Grabbe S, McArthur M, Lorenzo I, Kaplan S, Ley K, Wayne Smith C, Montgomery CA, Rich S, Beaudet AL. 1998. Spontaneous skin ulceration and defective T-cell function in CD18-null mice. J Exp Med 188:119–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schaudinn C, Gorur A, Keller D, Sedghizadeh PP, Costerton JW. 2009. Periodontitis: an archetypical biofilm disease. J Am Dent Assoc 140:978–986 [DOI] [PubMed] [Google Scholar]
- 53.Sobacchi C, Marrella V, Rucci F, Vezzoni P, Villa A. 2006. RAG-dependent primary immunodeficiencies. Hum Mutat 27:1174–1184 [DOI] [PubMed] [Google Scholar]
- 54.Stein JM, Kuch B, Conrads G, Fickl S, Chrobot J, Schulz S, Ocklenburg C, Smeets R. 2009. Clinical periodontal and microbiologic parameters in patients with acute myocardial infarction. J Periodontol 80:1581–1589 [DOI] [PubMed] [Google Scholar]
- 55.Tsukumo Y, Andoh T, Yamagichi T, Nojima H, Kuraishi Y. 1999. [Involvement of nitric oxide in itch–scratch response of NC mice]. Nippon Yakurigaku Zasshi 114:17P–21P [Article in Japanese]. [DOI] [PubMed] [Google Scholar]
- 56.Vestweber D. 1993. The selectins and their ligands. Curr Top Microbiol Immunol 184:65–78 [DOI] [PubMed] [Google Scholar]
- 57.Watanabe H, Numata K, Ito T, Takagi K, Matsukawa A. 2004. Innate immune response in Th1- and Th2-dominant mouse strains. Shock 22:460–466 [DOI] [PubMed] [Google Scholar]
- 58.Ximénez-Fyvie LA, Haffajee AD, Socransky SS. 2000. Microbial composition of supra- and subgingival plaque in subjects with adult periodontitis. J Clin Periodontol 27:722–732 [DOI] [PubMed] [Google Scholar]
- 59.Xiong X, Elkind-Hirsch KE, Vastardis S, Delarosa RL, Pridjian G, Buekens P. 2009. Periodontal disease is associated with gestational diabetes mellitus: a case-control study. J Periodontol 80:1742–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhai H, Simion FA, Abrutyn E, Koehler AM, Maibach HI. 2002. Screening topical antipruritics: a histamine-induced itch human model. Skin Pharmacol Appl Skin Physiol 15:213–217 [DOI] [PubMed] [Google Scholar]