Abstract
Chronic inflammation is highly prevalent in maintenance hemodialysis (MHD) patients and associates with increased mortality. IL-1β, a pro-inflammatory cytokine, is elevated in MHD patients. A balance between IL-1β and its naturally occurring antagonist may determine the inflammatory response and its consequences in this population. We performed a pilot randomized placebo-controlled trial to evaluate the efficacy of the administration of recombinant human IL-1 receptor antagonist (IL-1ra) on biomarkers of inflammation and nutrition in MHD patients with three consecutive high sensitivity C-reactive protein (hsCRP) measurements >5 mg/L. We randomly assigned 22 patients to placebo or IL-1ra (1:1) for 4 weeks; 14 completed the trial. Patients in the IL-1ra arm had a 53% reduction in mean hsCRP compared with 1% in the placebo arm (P = 0.008), a 40% reduction in mean IL-6 levels compared with a 20% increase in the placebo arm (P = 0.03), and a 23% increase in mean prealbumin compared with 6% in the placebo arm (P = NS). In conclusion, the administration of IL-1ra in MHD patients can lower biomarkers of inflammation. Whether IL-1ra administration improves survival in this population requires additional long-term studies.
Despite all of the technical advances in dialysis therapy over the last several decades, there has been minimal improvement in the death rate of maintenance hemodialysis (MHD) patients in the United States.1 A significant portion of the MHD patients die because of cardiovascular events. Randomized controlled trials targeting traditional2 and nontraditional3–6 cardiovascular risk factors have had no significant impact in improving survival in this patient population. Systemic chronic inflammation is highly prevalent in MHD patients,7 and it has been shown to be a strong predictor of morbidity8 and mortality.9–11
Chronic inflammation in ESRD is highly prevalent and multifactorial.7 Some of the most commonly postulated etiologies include dialysis-related factors such as the extracorporeal circulation,12 the quality of the dialysate,13 the biocompatibility of the hemodialysis membranes, acute and chronic access thrombosis, and dialysis catheters.7 Non–dialysis-related factors include decreased clearance and increased production of cytokines, retention of uremic solutes, increased oxidative stress burden,14 genetic factors, and high prevalence of comorbidities associated with inflammation.14,15 Nevertheless, a significant percent of the patients have no identifiable preventable or treatable cause of persistent inflammation.16 It has been suggested that these patients are at increased risk for cardiovascular mortality and morbidity and that ameliorating inflammatory response could potentially improve their survival.17
Certain pro-inflammatory cytokines, such as IL-1, IL-6, and TNF-α, are considered early drivers of the inflammatory response.18 Other important pro-inflammatory cytokines are also up-regulated, such as IFN-γ produced by T cells.19,20 Whereas anti-cytokine therapies are proposed as potential therapies, there are very limited studies using this strategy in MHD patients.21 High levels of IL-1β, a highly active pro-inflammatory cytokine, and its naturally occurring receptor antagonist (IL-1ra) have been long documented in chronic kidney disease (CKD)22 and ESRD.23,24 Blocking IL-1β has been shown to be effective in improving gout,25 insulin secretion by pancreas in type 2 diabetes,26 and the severity of joint erosions in rheumatoid arthritis. We designed a pilot double-blind randomized placebo controlled trial to evaluate the short-term efficacy of the administration of recombinant human IL-1ra (Anakinra) on biomarkers of inflammation and nutrition in 22 MHD patients with chronic inflammation (ClinicalTrial.gov NCT00420290). We hypothesized that the administration of 100 mg of recombinant human IL-1ra three times a week would effectively decrease markers of systemic inflammatory response in MHD patients as measured by high sensitivity C-reactive protein (hsCRP) and IL-6 levels. Additionally, we evaluated the impact of therapy on biomarkers of nutrition (serum prealbumin and serum albumin) and body composition (lean body mass [LBM]).
We screened 239 subjects, 22 of whom were randomly assigned to receive either IL-1ra or placebo. Of those individuals, 14 completed the study (Figure 1 and Table 1). Reasons for drop-outs are shown in Figure 1. In the IL-1ra group, three patients dropped out because of adverse reactions, and one was found to have history of prostate cancer at week 1. In the placebo arm, two subjects dropped out before any intervention (one moved out of town and one decided to join another study), one was found to be a hepatitis C carrier, and one subject had an asthma attack requiring steroids during the study. In subjects who completed the study, ones in the placebo arm were in general more inflamed, had lower body mass index, lower serum prealbumin, lower percentage of LBM, and had more frequent fistulas at baseline (Tables 1 and 2), although none of these characteristics were statistically different. The only characteristic that was different between groups was diabetes, with no patients with a history of diabetes in the IL-1ra arm (P = 0.03), but three of seven patients had a history of diabetes in the placebo arm (Table 1). None of the baseline characteristics in Table 2 were different between groups.
Figure 1.
Patient enrollments, randomization, and completion flow diagram.
Table 1.
Inflammation and nutritional biomarkers at baseline by randomization group
| Variables | All (n = 14) | IL-1ra (n = 7) | Placebo (n = 7) | P |
|---|---|---|---|---|
| Age, years (mean ± SD) | 49 ± 13 | 48 ± 12 | 51 ± 14 | 0.8 |
| Race, % (n) African Americans | 71.4% (10) | 86% (6) | 57% (4) | 0.3 |
| Gender, % (n) males | 71.4% (10) | 71.4% (5) | 71.4% (5) | 0.99 |
| Body mass index (mean ± SD) | 31 ± 8 | 34 ± 6 | 29 ± 10 | 0.2 |
| Diabetes, % (n) | 21.4% (3) | 0% (0) | 42.9% (3) | 0.03a |
| KTV (mean ± SD) | 1.61 ± 0.33 | 1.55 ± 0.19 | 1.66 ± 0.44 | 0.9 |
| Vintage, months (median, IQR) | 35 (8 to 90) | 43 (15 to 104) | 27 (5 to 88) | 0.3 |
| Arteriovenous fistula, (%) (n) | 50% (7) | 28.6% (2) | 71.4% (5) | 0.1 |
aSignificant at α < 0.05.
Table 2.
Inflammation and nutritional biomarkers at baseline and after intervention by randomization group
| Variable | IL-1ra |
Placebo |
Pa | ||||
|---|---|---|---|---|---|---|---|
| Median (IQR) | Mean ± SD | Mean Percent Change | Median (IQR) | Mean ± SD | Mean Percent Change | ||
| Baseline CRP, mg/dl | 9.50 (6.80, 12.60) | 9.57 ± 3.95 | −53% | 19.50 (5.20, 21.30) | 15.13 ± 8.36 | −1% | 0.008 |
| Post-CRP, mg/dl | 4.50 (2.50, 6.80) | 4.67 ± 2.87 | 16.00 (4.00, 20.30) | 15.11 ± 10.18 | |||
| Baseline IL-6, pg/ml | 4.62 (4.35, 8.40) | 7.77 ± 7.48 | −40% | 6.15 (1.83, 17.21) | 8.21 ± 8.28 | 20% | 0.03 |
| Post-IL-6, pg/ml | 2.15 (0.77, 5.77) | 3.34 ± 3.09 | 8.31 (2.21, 14.55) | 8.14 ± 6.04 | |||
| Baseline albumin, g/dl | 4.05 (3.73, 4.30) | 4.03 ± 0.37 | 3% | 3.80 (3.60, 4.50) | 3.93 ± 0.55 | 0% | NS |
| Post-albumin, g/dl | 4.20 (4.00, 4.80) | 4.26 ± 0.40 | 3.85 (3.65, 4.35) | 3.95 ± 0.38 | |||
| Baseline prealbumin, mg/dl | 40.0 (31.0, 47.0) | 38.2 ± 8.8 | 23% | 31.0 (25.0, 41.0) | 31.9 ± 11.1 | 6% | NS |
| Post-prealbumin, mg/dl | 49.0 (40.0, 54.0) | 46.43 ± 11.3 | 33.0 (30.0, 44.0) | 33.6 ± 12.2 | |||
| Baseline LBM, g | 54.00 (47.1, 65.5) | 55.73 ± 9.9 | 1.70% | 50.6 (44.1, 55.1) | 50.6 ± 6.7 | −0.11% | NS |
| Post-LBM, g | 59.0 (50.6, 67.6) | 59.1 ± 8.7 | 50.0 (43.1, 53.8) | 50.4 ± 7.9 | |||
| Baseline % body fat | 40.0 (35.9, 50.7) | 39.8 ± 9.3 | −1% | 29.3 (26.7, 46.2) | 33.3 ± 12.6 | 1% | NS |
| Post % body fat | 37.6 (33.3, 42.7) | 37.6 ± 8.0 | 29.8 (24.4, 47.3) | 33.6 ± 12.3 | |||
CRP and IL-6 were log-transformed to achieve normality for the analysis. Comparison of baseline characteristics was done using nonparametric methods. None of the comparisons between groups for baseline CRP, IL-6, albumin, prealbumin, LBM, or % body fat were significant at α < 0.05.
aComparison for the percent change from baseline to 4 weeks for each marker between groups was done using ANCOVA.
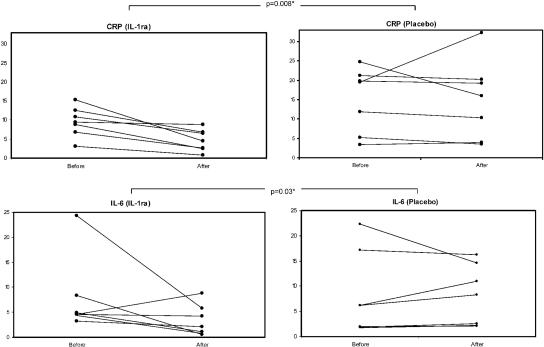
At 4 weeks, the percentage change in hsCRP concentrations was highly statistically significantly different between groups in favor of IL-1ra (P = 0.008; Figure 2 and Table 2). Specifically, compared with baseline, the IL-1ra group showed a mean drop of 53% versus a mean drop of 1% in the placebo arm for hsCRP. A similar decline was observed in IL-6 levels, with a mean percent drop of 40% in the IL-1ra arm versus a mean increase of 20% in the placebo arm (P = 0.03). Serum prealbumin increased 23% in the IL-1ra arm versus a 6% increase in the placebo arm (P = 0.1). Serum albumin increased by 3% in the intervention arm, and there was no change in the placebo arm (Table 2). There was no appreciable change in LBM in either arm (increase by 1.7% in the IL-1ra arm and decrease of −0.11% in the placebo arm). Individual data on each subject are provided in Supplemental Table 1.
Figure 2.
Pre- and poststudy levels of hsCRP (mg/dl) and IL-6 (pg/ml) in individual study subjects. hsCRP decreased in all but one individual in the IL-1ra arm, whereas all placebo subjects were either stable or increased their hsCRP. A similar trend was observed for IL-6 levels. *Comparison for the percent change from baseline to 4 weeks for each marker between groups was done using ANCOVA.
There were no serious adverse events during the study (Table 3). Injection site reaction was more frequent in the patients in the intervention arm (two events each occurring around day 14) versus none for the placebo arm. One of these subjects also had an injection site hematoma. Both subjects were withdrawn from the study. One patient in the intervention arm had a soft tissue infection in his dialysis access site at day 5 and was also withdrawn from the study. No other infections occurred during the study.
Table 3.
Adverse events
| Events | IL-1ra | Placebo |
|---|---|---|
| Any adverse event | 4 | 3 |
| Discontinuation of medication because of an adverse effect | 3a | 1b |
| Injection site reaction | 2 | 0 |
| Hemodialysis access site infection | 1 | 0 |
| Injection site hematoma | 1 | 0 |
| Thrombocytopenia | 0 | 1 |
| Fatigue | 1 | 1 |
| Asthma | 0 | 1 |
aTwo discontinued because of injection site reactions around day 14 and one because of access infection at day 5.
bDiscontinuation because of asthma attack needing steroids.
The results of this study showed for the first time that the administration of an anti-cytokine therapy, specifically human recombinant IL-1 receptor antagonist, significantly improves the chronic inflammatory response in hemodialysis patients. This improvement was observed in the significant majority of the subjects receiving the intervention for both hsCRP and IL-6 and was greater compared with any other previously examined anti-inflammatory intervention in MHD patients.27–31
Our results have important clinical and research implications. It has been consistently shown that MHD patients have an unacceptably high morbidity and mortality and that biomarkers of inflammation are robust predictors of these outcomes.1 Accordingly, a number of different potential anti-inflammatory interventions have been proposed in this patient population with only mild to moderate effect. These include but are not limited to resistance training,28 pentoxifylline (TNF-α blocker),27 angiotensin converting enzyme inhibition,30 hydroxy-methyl-glutaryl-CoA reductase inhibitors,31 and vitamin E.29 In terms of anti-cytokine therapies, a recent randomized controlled trial of a TNF-α blocker in 10 MHD patients showed no effect on biomarkers of inflammation (hsCRP or IL-6). Although the reasons for the lack of response to the TNF-α blocker are not clear, this particular study was limited by number of patients because of drop-outs, and the effects were examined over 44 weeks rather than 1 month as performed in our study. It is also possible that inflammation of CKD could be mainly IL-1β and IL-6 driven, as observed in other chronic conditions such as gout25 and diabetes,26 in which the administration of IL-1β produces a fast and sustained response, whereas there is no response to TNF-α blockers.32
Chronic inflammation plays a pivotal role in atherosclerotic cardiovascular disease both in the general33 and in the maintenance dialysis population.11 It has been reported that IL-1ra administration prevents adverse cardiac remodeling after acute myocardial infarction in patients with normal kidney function.34 It will be important in the future to determine whether IL-1 blockade will lower cardiovascular morbidity and mortality in the MHD population, especially in specific circumstances such as acute coronary syndrome or after percutaneous coronary interventions.
In addition to their role in CVD, pro-inflammatory cytokines are known to be primary mediators of protein wasting in many disease conditions, including advanced CKD. IL-6 has been shown to activate the ubiquitin proteosome system and, by selectively targeting myosin,35 plays a key role in the protein energy wasting observed in MHD patients.36 Although our study was not adequately powered to assess the changes in biomarkers of nutritional status, we did observe some encouraging results. Specifically, prealbumin concentration improved in all IL1-ra–treated subjects, with a mean increase of 23%, although this effect was not statistically significant, which could be either to the lack of power to detect changes in this specific outcome or because of the short duration of the study. On the other hand, we did not observe any notable changes in other biomarkers such as serum albumin and LBM. Although our results are preliminary, they do provide the rationale to examine the effects of IL-1ra blockade on nutritional parameters in MHD patients with protein energy wasting over a longer period of time.
Although this is the first study using an IL-1ra blocker in chronically inflamed MHD patients, it has certain limitations. Most importantly, the sample size is small, and the intervention is of short duration. Nevertheless, the response in improving inflammation was seen in all subjects, supporting that the effect is real. Indeed, the study was terminated at the first interim analysis by the data safety and monitoring board (DSMB) because of reaching efficacy boundaries (vide infra). On the other hand, our inclusion criteria were stringent, resulting in a high drop-out rate. For example, patients with catheters and history of recent infection were excluded because of the potential risk of predisposing to a new or recurrent infection. This approach resulted in a selected patient population, and the results cannot be generalized to all MHD patients.
In summary, our results suggest that the administration of IL-1ra over a period of 4 weeks successfully controls the inflammatory response in MHD patients as reflected by significant decreases in hsCRP and IL-6 levels. The treatment is generally well tolerated and safe. It should also be noted that the study sample size is small, and the follow-up was relatively short. Future long-term studies with larger and more heterogeneous patient populations are needed to examine the effects of administration of IL-1ra on cardiovascular and other outcomes in chronically inflamed MHD patients.
CONCISE METHODS
Study Participants
This study was conducted at the Vanderbilt University Medical Center and Nashville Veterans Affairs Outpatient Dialysis units between January 2008 and May 2010. Prevalent MHD patients 18 to 75 years old on MHD three times a week were eligible to participate. Inclusion criteria included the average of three consecutive serum CRP levels >5 mg/L and adequate dialysis delivery Kt/V ≥1.2. Patients were excluded if they had hemodialysis catheters, had any active or chronic infection (HIV, hepatitis B or C, or tuberculosis/tuberculin test positive), had a history of malignancy in the previous 5 years, were hospitalized within 1 month needed any immune suppression therapy, or had received any investigational study drug within 1 month before the study. The study was approved by the Institutional Review Boards from Vanderbilt University Medical Center and from the Nashville Veterans Affairs hospital, and signed informed consent was obtained from all patients.
Study Design
This was a placebo-controlled, double-blinded study (ClinicalTrials.gov number, NCT00420290.) Of 239 screened patients from two centers, 22 prevalent MHD patients were randomized in a 1:1 ratio to receive recombinant 100 mg of human recombinant IL-1ra or placebo by subcutaneous injection at each dialysis session for 4 weeks. The medication was administered by the study coordinator or the principal investigator in all subjects.
Procedures
Blood samples were drawn at baseline and at the end of the study (4 weeks). Vacutainer (Becton Dickinson, Franklin Lakes, NJ) tubes containing EDTA were used for plasma separation. Samples were transported on ice and centrifuged at 20°C at 3000 rpm for 15 minutes. Supernatants were stored in aliquots at −80°C until further use. Dual energy x-ray absorptiometry scans were performed 1 to 2 hours after dialysis (at dry weight) at baseline and at 4 weeks to evaluate changes in body composition.
Study Endpoints
The primary endpoint was the percent change in serum hsCRP from baseline to 4 weeks. Secondary outcomes were the percent changes in IL-6, serum prealbumin, serum albumin, and LBM from baseline to 4 weeks. Covariates included demographics, vintage, access type, KT/V, body mass index, CVD, and diabetes.
Statistical Analysis
Data are presented as mean ± SD or as median with interquartile range depending on their distribution. Baseline characteristics were compared using Mann-Whitney U or χ2 tests when appropriate. Analysis of covariance (ANCOVA) was used to compare percent change from baseline to 4 weeks for the primary and secondary outcomes between the groups. Outcome variables were log-transformed to improve normality in residuals, and the log-transformed baseline value of the outcome variable was adjusted as a covariate. Regression coefficients from the ANCOVA model were exponentiated, which indicates the ratio of percent change from baseline to 4 weeks. ANCOVA assesses change by adjusting baseline as a regression coefficient rather than using change directly as an outcome variable.37–39 Because of the small number of participants, no adjustment of other variables was performed. The study was overseen by a DSMB, which reviewed the adverse events on a yearly basis and reviewed the interim analyses of efficacy. A sequential analysis was planned at the time when one half of the study subjects in each arm had completed the first phase of the project. Boundaries were calculated using the Pocock method with two-sided overall significance level at 5% to conclude whether the study drug is protective or harmful on the main outcome variable (hsCRP) if test statistics reached a two-sided significance level of 0.031 at the first interim analysis with 7 patients in each arm or 0.019 at the final analysis with 14 patients in each arm.
Sample Size
The primary outcome was change in hsCRP concentration from baseline to 4 weeks. Using data from 128 MHD patients,7 CRP data were found to be highly skewed, and data were log-transformed to achieve normality. Mean (SD) transformed hsCRP was 1.22 (0.166). With 14 patients in each group (a total of 28), it was predicted that the minimum detectable difference between control and intervention would be 15% (1.22 for control group and 1.00 for the intervention group), with an 80% power and an α of 0.05 using the t test approach. Thus, the originally planned sample size was 30 individuals to complete the trial. Enrollment was terminated on June 15, 2010, after the planned interim analysis at one half of the sample size. The recommendation for termination by DSMB was based on the prespecified stopping rule for efficacy of the intervention on reduction of hsCRP with P < 0.031.
DISCLOSURES
The sponsors had no influence on the design, execution, and analysis of the results of the study.
Acknowledgments
This study was supported in part by Grants R21 DK077373 and K24 DK 062849 from the National Institute of Diabetes and Digestive and Kidney Diseases, Clinical Translational Science Award 1UL-1RR024975 from the National Center for Research Resources, and a National Kidney Foundation Young Investigator Award. A.H. is supported by VA Career Development Award 2-031-09S. Study drug and matching placebo were kindly provided by Amgen (Thousand Oaks, CA).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Collins AJ, Foley RN, Herzog C, Chavers BM, Gilbertson D, Ishani A, Kasiske BL, Liu J, Mau LW, McBean M, Murray A, St Peter W, Guo H, Li Q, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Dalleska F, Daniels F, Dunning S, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers PW, Agodoa L: Excerpts from the US Renal Data System 2009 Annual Data Report. Am J Kidney Dis 55: S1–S420, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, Ritz E: Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 353: 238–248, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA: The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 339: 584–590, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Cano NJ, Fouque D, Roth H, Aparicio M, Azar R, Canaud B, Chauveau P, Combe C, Laville M, Leverve XM: Intradialytic parenteral nutrition does not improve survival in malnourished hemodialysis patients: A 2-year multicenter, prospective, randomized study. J Am Soc Nephrol 18: 2583–2591, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Jamison RL, Hartigan P, Kaufman JS, Goldfarb DS, Warren SR, Guarino PD, Gaziano JM: Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end-stage renal disease: A randomized controlled trial. JAMA 298: 1163–1170, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Suki WN, Zabaneh R, Cangiano JL, Reed J, Fischer D, Garrett L, Ling BN, Chasan-Taber S, Dillon MA, Blair AT, Burke SK: Effects of sevelamer and calcium-based phosphate binders on mortality in hemodialysis patients. Kidney Int 72: 1130–1137, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Hung A, Pupim L, Yu C, Shintani A, Siew E, Ayus C, Hakim RM, Ikizler TA: Determinants of C-reactive protein in chronic hemodialysis patients: Relevance of dialysis catheter utilization. Hemodial Int 12: 236–243, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Ikizler TA, Wingard RL, Harvell J, Shyr Y, Hakim RM: Association of morbidity with markers of nutrition and inflammation in chronic hemodialysis patients: A prospective study. Kidney Int 55: 1945–1951, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Stenvinkel P, Wanner C, Metzger T, Heimburger O, Mallamaci F, Tripepi G, Malatino L, Zoccali C: Inflammation and outcome in end-stage renal failure: Does female gender constitute a survival advantage? Kidney Int 62: 1791–1798, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Yeun JY, Levine RA, Mantadilok V, Kaysen GA: C-Reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis 35: 469–476, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C: Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int 55: 648–658, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Caglar K, Peng Y, Pupim LB, Flakoll PJ, Levenhagen D, Hakim RM, Ikizler TA: Inflammatory signals associated with hemodialysis. Kidney Int 62: 1408–1416, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Arizono K, Nomura K, Motoyama T, Matsushita Y, Matsuoka K, Miyazu R, Takeshita H, Fukui H: Use of ultrapure dialysate in reduction of chronic inflammation during hemodialysis. Blood Purif 22[Suppl 2]: 26–29, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM: The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int 62: 1524–1538, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Stenvinkel P, Alvestrand A: Inflammation in end-stage renal disease: Sources, consequences, and therapy. Semin Dial 15: 329–337, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Hung AM, Ikizler TA: Hemodialysis central venous catheters as a source of inflammation and its implications. Semin Dial 21: 401–404, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Carrero JJ, Stenvinkel P: Persistent inflammation as a catalyst for other risk factors in chronic kidney disease: A hypothesis proposal. Clin J Am Soc Nephrol [4 Suppl 1]: S49–S55, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Tripepi G, Mallamaci F, Zoccali C: Inflammation markers, adhesion molecules, and all-cause and cardiovascular mortality in patients with ESRD: Searching for the best risk marker by multivariate modeling. J Am Soc Nephrol [16 Suppl 1]: S83–S88, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Weiss G, Goodnough LT: Anemia of chronic disease. N Engl J Med 352: 1011–1023, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Macdougall IC, Cooper AC: Erythropoietin resistance: The role of inflammation and pro-inflammatory cytokines. Nephrol Dial Transplant [17 Suppl 11]: 39–43, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Don BR, Kim K, Li J, Dwyer T, Alexander F, Kaysen GA: The effect of etanercept on suppression of the systemic inflammatory response in chronic hemodialysis patients. Clin Nephrol 73: 431–438, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Descamps-Latscha B, Herbelin A, Nguyen AT, Roux-Lombard P, Zingraff J, Moynot A, Verger C, Dahmane D, de Groote D, Jungers P, et al. : Balance between IL-1 beta, TNF-alpha, and their specific inhibitors in chronic renal failure and maintenance dialysis. Relationships with activation markers of T cells, B cells, and monocytes. J Immunol 154: 882–892, 1995 [PubMed] [Google Scholar]
- 23. Dinarello CA, Koch KM, Shaldon S: Interleukin-1 and its relevance in patients treated with hemodialysis. Kidney Int Suppl 24: S21–S26, 1988 [PubMed] [Google Scholar]
- 24. Kimmel PL, Phillips TM, Simmens SJ, Peterson RA, Weihs KL, Alleyne S, Cruz I, Yanovski JA, Veis JH: Immunologic function and survival in hemodialysis patients. Kidney Int 54: 236–244, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J: Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440: 237–241, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY: Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 356: 1517–1526, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Biolo G, Ciocchi B, Bosutti A, Situlin R, Toigo G, Guarnieri G: Pentoxifylline acutely reduces protein catabolism in chronically uremic patients. Am J Kidney Dis 40: 1162–1172, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Castaneda C, Gordon PL, Parker RC, Uhlin KL, Roubenoff R, Levey AS: Resistance training to reduce the malnutrition-inflammation complex syndrome of chronic kidney disease. Am J Kidney Dis 43: 607–616, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Himmelfarb J, Phinney S, Ikizler TA, Kane J, McMonagle E, Miller G: Gamma-tocopherol and docosahexaenoic acid decrease inflammation in dialysis patients. J Renal Nutr 17: 296–304, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Stenvinkel P, Andersson P, Wang T, Lindholm B, Bergstrom J, Palmblad J, Heimburger O, Cederholm T: Do ACE-inhibitors suppress tumour necrosis factor-alpha production in advanced chronic renal failure? J Intern Med 246: 503–507, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Stenvinkel P, Rodriguez-Ayala E, Massy ZA, Qureshi AR, Barany P, Fellstrom B, Heimburger O, Lindholm B, Alvestrand A: Statin treatment and diabetes affect myeloperoxidase activity in maintenance hemodialysis patients. Clin J Am Soc Nephrol 1: 281–287, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Dinarello CA: Interleukin-1beta and the autoinflammatory diseases. N Engl J Med 360: 2467–2470, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Stampfer MJ, Rimm EB: Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med 351: 2599–2610, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Abbate A, Van Tassell BW, Seropian IM, Toldo S, Robati R, Varma A, Salloum FN, Smithson L, Dinarello CA: Interleukin-1beta modulation using a genetically engineered antibody prevents adverse cardiac remodelling following acute myocardial infarction in the mouse. Eur J Heart Fail 12: 319–322, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Acharyya S, Ladner KJ, Nelsen LL, Damrauer J, Reiser PJ, Swoap S, Guttridge DC: Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest, 114: 370–378, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, Franch H, Guarnieri G, Ikizler TA, Kaysen G, Lindholm B, Massy Z, Mitch W, Pineda E, Stenvinkel P, Trevino-Becerra A, Wanner C: A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int 73: 391–398, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Frison L, Pocock SJ: Repeated measures in clinical trials: Analysis using mean summary statistics and its implications for design. Stat Med 11: 1685–1704, 1992 [DOI] [PubMed] [Google Scholar]
- 38. Senn S: Statistical Issues in Drug Development, Chichester, England, John Wiley & Sons, 2007 [Google Scholar]
- 39. Vickers AJ: Parametric versus non-parametric statistics in the analysis of randomized trials with non-normally distributed data. BMC Med Res Methodol 5: 35, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]