Abstract
WNK kinases stimulate endocytosis of ROMK channels to regulate renal K+ handling. Phosphatidylinositol 3-kinase (PI3K)-activating hormones, such as insulin and IGF 1, phosphorylate WNK1, but how this affects the regulation of ROMK abundance is unknown. Here, serum starvation of ROMK-transfected HEK cells led to an increase of ROMK current density; subsequent addition of insulin or IGF1 inhibited ROMK currents in a PI3K-dependent manner. Serum and insulin also increased phosphorylation of the downstream kinases Akt1 and SGK1 as well as WNK1. A biotinylation assay suggested that insulin and IGF1 inhibit ROMK by enhancing its endocytosis, a process that WNK1 may mediate. Knockdown of WNK1 with siRNA or expression of a phospho-deficient WNK1 mutant (T58A) both prevented insulin-induced inhibition of ROMK currents, suggesting that phosphorylation at Threonine-58 of WNK1 is important to mediate the inhibition of ROMK by PI3K-activating hormones or growth factors. In vitro and in vivo kinase assays supported the notion that Akt1 and SGK1 can phosphorylate WNK1 at this site, and we established that Akt1 and SGK1 synergistically inhibit ROMK through WNK1. We used dominant-negative intersectin and dynamin constructs to show that SGK1-mediated phosphorylation of WNK1 inhibits ROMK by promoting its endocytosis. Taken together, these results suggest that PI3K-activating hormones inhibit ROMK by enhancing its endocytosis via a mechanism that involves phosphorylation of WNK1 by Akt1 and SGK1.
The concentration in the blood of the potassium ion (K+), an important determinant of cell membrane potential, is tightly regulated within a narrow range. The excretion of K+ occurs mainly in the kidney through processes involving glomerular filtration, tubular reabsorption, and secretion. The transepithelial K+ secretion in the kidney takes place predominantly in the aldosterone-sensitive distal nephron and involves K+ uptake into cells by the basolateral sodium-potassium pump and exit into lumen through apical K+ channels, which include the Ca2+-activated maxi-K channel and the renal outer medullary potassium channel, ROMK (also known as Kir1.1).1,2
ROMK channel undergoes constitutive clathrin-dependent endocytosis, which regulates the density of channel at the cell surface, thus controlling renal K+ secretion.3 Recently, WNK (with-no-lysine [K]) kinases have been identified as important regulators of the cell surface abundance of ROMK. WNKs are serine-threonine protein kinases with an unusual position of the catalytic lysine in subdomain I instead of subdomain II.4 Mammalian WNK family includes four members, WNK1-4, which share 85 to 90% sequence identity in their kinase domain.4–6 Mutations in WNK1 and WNK4 in humans cause an autosomal-dominant disease called pseudohypoaldosteronism type 2 (PHA2), featuring hypertension and hyperkalemia.5 Studies have shown that WNK1 and WNK4 regulate renal Na+ and K+ transporters, and dysregulation of these transporters contributes to hypertension and hyperkalemia in PHA2.
WNKs regulate renal Na+ transport through both catalytic and noncatalytic mechanisms. With respect to the catalytic mechanism of regulation, WNK1 and 4 phosphorylate and activate OSR1 (oxidative stress-responsive kinase-1) and its related kinase SPAK (Ste20-related proline-alanine-rich kinase), which in turn phosphorylate and activate the thiazide-sensitive sodium-chloride co-transporter NCC and the bumetanide-sensitive sodium-potassium-2 chloride cotransporter NKCC.7,8 WNK1 and 4 can also regulate ENaC and NCC via noncatalytic mechanisms that involve protein–protein interaction with serum- and glucocorticoid-induced kinase-1 (SGK1) for the regulation of ENaC and with transporter directly for the regulation of NCC.9,10 With respect to K+ transport, WNK1 and 4 stimulate endocytosis of ROMK via a kinase-independent mechanism that involves a direct interaction with an endocytic scaffold protein, intersectin.11
Compared with the downstream effects of WNKs, the physiologic upstream regulators of WNKs are less understood. Vitari et al.12 showed that IGF1 induces phosphorylation of endogenous WNK1 in cultured human embryonic kidney (HEK) cells at threonine-60 (equivalent to threonine-58 of rat WNK1). The effect of IGF1 is through activation of phosphatidylinositol 3-kinase (PI3K), leading to activation of the 3-phosphoinositide–dependent protein kinase-1 (PDK1) and protein kinase B (PKB)/Akt1. Phosphorylation of WNK1 by Akt1 does not affect its kinase activity or subcellular distribution.12 Jiang et al.13 reported that insulin induces a similar phosphorylation of WNK1, which underscores the inhibition of cell proliferation of 3T3-L1 preadipocytes by insulin. Xu et al.14 reported that WNK1 activates SGK1 through direct protein–protein interactions independently of WNK1 kinase activity. Xu et al.9 further showed that phosphorylation of rat WNK1 at threonine-58 by the IGF1–Akt1 pathway enhances the ability of WNK1 to stimulates SGK1 kinase activity, leading to activation of ENaC. Others have also shown that insulin stimulates ENaC via Akt1, although the role of WNK1 was not studied.15 The mechanism for WNK1 regulation of ROMK and ENaC are fundamentally distinct, raising an interesting question as to whether phosphorylation of WNK1 by PI3K-activating hormones, such as insulin and IGF1, affects its regulation of ROMK. We investigate this question in this study.
RESULTS
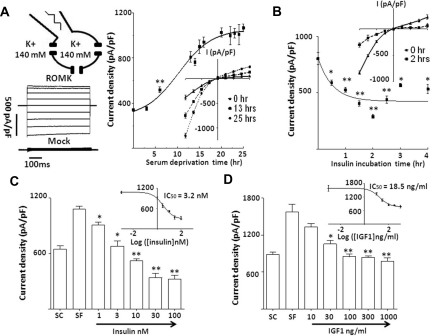
Effects of Serum Deprivation, Insulin, and IGF1 on ROMK
Barium-sensitive K+ currents were measured by ruptured whole-cell recording from HEK cells transfected with ROMK. Under the condition of symmetrical 140 mM [K+], ROMK-mediated K+ currents showed characteristic weak inward rectification (Figure 1A, left). No currents were observed in mock-transfected cells. To allow for testing the effect of insulin and IGF1 on ROMK, we first examined the effect of serum deprivation. After culturing in the serum-containing media for 48 hours to allow maximal expression of the channel, ROMK-transfected cells were deprived of serum for 3 to 25 hours before ruptured whole-cell recording. As shown, ROMK current density (normalized to capacitance, pA/pF) increased progressively with increasing duration of serum deprivation (Figure 1A, right). Six hours after serum deprivation, current density was significantly higher than that before deprivation. ROMK currents continued to increase and reached a plateau at 16 to 20 hours (Figure 1A). Addition of insulin thereafter caused a significant inhibition of ROMK currents in 30 minutes (Figure 1B). ROMK currents reached the nadir at approximately 2-hour incubation with insulin (Figure 1B). The dose–response relationship for inhibition of ROMK was examined by incubating insulin ranging from 1 to 100 nM for 2 hours. Insulin significantly inhibited ROMK at a concentration as low as 1 nM (Figure 1C). The concentration for half-maximal inhibition (IC50) of ROMK for insulin was estimated at 3.2 nM (Figure 1C). For reference, the plasma concentration of insulin in normal individuals is 10 to 150 pM at fasting but may reach 300 to 800 pM after carbohydrate meals.16 IGF and insulin act on similar membrane receptors and elicit overlapping cellular responses.17 Accordingly, we found a similar inhibition of ROMK by IGF1, with an IC50 for IGF1 estimated at 18.5 ng/ml (=2.4 nM; Figure 1D). The normal basal plasma concentration of IGF1 in humans is around 50 to 100 ng/ml.18
Figure 1.
Insulin and IGF1 inhibit ROMK current. (A) Effect of serum deprivation on ROMK current. (Left) Configuration of whole-cell recording, voltage-clamp protocol (from −100 to 100 mV), representative currents from ROMK- and mock-transfected cells are shown. (Right) ROMK current density (pA/pF at −100 mV; normalized to the cell surface area) at different time of serum deprivation are shown (mean ± SEM, n ≥ 6 for each) and analyzed by nonlinear regression curve. Inset shows current-voltage (I-V) relationship curve of ROMK with serum deprivation for 0, 13, and 25 hours. Data in each time point was compared with serum-containing group (0-hour serum deprivation). **P < 0.01. All time points beyond 6 hours are significant compared with the serum-containing group (not indicated by asterisk). All time points between 16 and 25 hours are not significantly different (not indicated). In all experiments throughout this study, ROMK currents shown are after subtracting residual currents in the presence of 5 mM barium. (B) Time course of effect of insulin on serum-deprived ROMK current. Cells were cultured in serum-free medium at least 16 hours before addition of insulin (100 nM) for different time periods. Data points are mean ± SEM (n ≥ 6 for each), compared with serum-deprived (0-hour insulin incubation), and analyzed by nonlinear regression curve. Inset shows I-V curve of ROMK current before and after 2-hour insulin. (C and D) Dose–response curve of insulin and IGF1 on serum-deprived ROMK. ROMK current density (mean ± SE, n ≥ 6) at −100 mV was measured in cells cultured in serum-containing medium (SC), serum-free medium (SF), and 2-hour incubation of different concentration of insulin or IGF1. Data of each insulin or IGF1 treatment group were compared with the SF group. Dose–response curve and IC50 of insulin or IGF1 on ROMK resulted from nonlinear regression analysis. *P < 0.05 versus designated group by unpaired two-tailed t test. **P < 0.01.
Effect of Insulin and IGF1 Is Dependent on PI3K and WNK1-T58 Phosphorylation
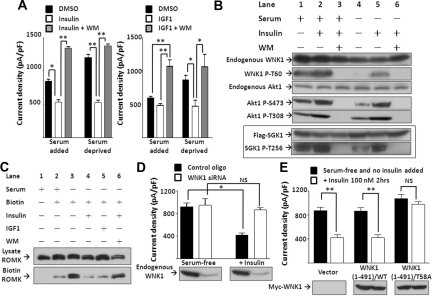
To study whether the inhibition of ROMK by insulin and IGF1 requires PI3K, ROMK-transfected cells were incubated with insulin or IGF1 with or without a specific PI3K inhibitor, wortmannin. In these experiments, we also compared the effects of insulin and IGF1 on ROMK with or without serum. We found that 100 nM of insulin caused a significant inhibition of ROMK even in the presence of serum (Figure 2A), indicating that the receptors are not maximally occupied by insulin present in the serum. For comparison, IGF1 at 100 ng/ml did not cause further inhibition of ROMK in the presence of serum. As before, serum deprivation increased ROMK currents, and application of insulin or IGF1 thereafter inhibited the currents. Co-application of wortmannin (WM, 100 nM) completely abolished the effect of insulin and IGF1 on ROMK (Figure 2A), indicating that the effect of insulin and IGF1 depends on PI3K.
Figure 2.
Insulin and IGF1 inhibit ROMK through PI3K and WNK1–T58 phosphorylation. (A) Effect of insulin and IGF1 on ROMK is blocked by wortmannin. Cells cultured with or without serum were treated by DMSO, insulin 100 nM, or insulin 100 nM plus wortmannin (WM, 100 nM), respectively, for 2 hours (mean ± SEM, n ≥ 6). (B) Effect of serum, insulin, and wortmannin on phosphorylation of endogenous WNK1, Akt1, and overexpressed SGK1. Phosphorylation on specific residues was determined by specific anti-phospho antibodies. Shown is representative of three separate experiments of similar results. (C) Effect of insulin and IGF1 on surface abundance of ROMK. Cells were serum-deprived for 16 hours and treated with insulin (100 nM), IGF1 (100 ng/ml), and/or WM (100 nM) for 2 hours as indicated before biotinylation (Biotin). ROMK in total cell lysates (Lysate ROMK) and elute from avidin beads (Biotin ROMK) were detected by Western blot. (D) Insulin inhibits ROMK through WNK1. Cells transfected with control oligonucleotide or WNK1 siRNA (200 nM each) were deprived of serum for 16 hours (serum-free group). Insulin (100 nM) was added for 2 hours (+Insulin group). Successful knockdown of endogenous WNK1 by siRNA is evident by Western blot analysis. Mean ± SEM (n ≥ 6 each). (E) Insulin inhibits ROMK through phosphorylation on T58 of WNK1. Cells transfected with empty vector, wild-type (WT), or T58A mutant WNK1(1-491) were cultured in serum-free medium for 16 hours and received insulin (100 nM) or not for 2 hours. Equal amount of WT or T58A WNK1(1-491) expression is evident by Western blot analysis. Mean ± SEM (n ≥ 6 each). In A, D, and E, *P < 0.05 between indicated groups by unpaired two-tailed t test. **P < 0.01. NS, not statistically significant.
Stimulation of PI3K by hormones or growth factors produces 3-phosphoinositides in the plasma membrane and leads to activation of downstream AGC kinases, Akt1 and SGK1, via multiple concerted actions.19 First, it stimulates the mammalian target of rapamycin complex-2 (mTORC2) to phosphorylate Akt1 or SGK1 at the hydrophobic motif. Phosphorylation of Akt1 or SGK1 by mTORC2 enhances its binding with PDK1, which phosphorylates Akt1 or SGK1 at the T-loop to activate its catalytic activity. Finally, the recruitment and binding of PDK1 to Akt1 is also facilitated by the production of 3-phosphoinositides in the plasma membrane. To understand the role of PI3K in WNK1 regulation of ROMK, we examined the phosphorylation status of endogenous Akt1 and WNK1 in HEK cells using respective residue-specific phospho-antibodies. In the absence of serum, there was a basal level of phosphorylation of Akt1 at threonine-308 (T308) of the T-loop and serine-473 (S473) of the hydrophobic motif, respectively (Figure 2B, lane 4). Serum and/or insulin increased phosphorylation of Akt1 at both residues (lanes 1, 2, and 5). Wortmannin abrogated basal and serum or insulin-stimulated phosphorylation of Akt1 (lanes 3 and 6). Phosphorylation of WNK1 at threonine-60 (T60 = T58 of rat WNK1) was not detectable in the absence of serum (lane 4) but was enhanced by serum and/or insulin in a wortmannin-sensitive manner (lanes 1, 2, and 5). Serum and insulin also stimulated wortmannin-sensitive phosphorylation of overexpressed SGK1 at the T-loop (threonine-256) (Figure 2B, bottom two gels highlighted in box). Overexpressed SGK1 was used because endogenous SGK1 was below detection by our antibody.
Cell surface abundance of ROMK was examined using a biotinylation assay. Serum deprivation increased ROMK surface abundance (Figure 2C, lane 2 versus 3). Insulin and IGF1 decreased ROMK surface abundance in a wortmannin-sensitive manner (lanes 4 to 6). These results support the notion that insulin and IGF1 inhibit ROMK by enhancing its endocytosis, in which WNK1 may play a role.
To confirm the role of WNK1 in the PI3K-mediated regulation of ROMK, we knocked down endogenous WNK1 using small interference RNA (siRNA). Efficacy of WNK1 siRNA was validated by blotting endogenous WNK1 in HEK cells (Figure 2D). Cells transfected with WNK1 siRNA (“WNK1 siRNA”, white bar) or control oligonucleotides (“Control oligo”, black bar) had similar ROMK current in the absence of serum (“serum-free”). Addition of insulin inhibited ROMK currents in cells transfected with control oligonucleotides but not in cells whose endogenous WNK1 was knocked down by siRNA.
Next, we used a phospho-deficient T58A mutant to examine the role of T58 phosphorylation on WNK1 (Figure 2E). Insulin inhibited ROMK in cells expressing endogenous (“vector”) or exogenous WNK1 [“WNK1(1-491)/WT”] but not in cells expressing exogenous T58A mutant of WNK1 [“WNK1(1-491)/T58A”]. Overexpression of WNK1-T58A exerted a dominant-negative effect on endogenous WNK1 (data not shown). These results strongly support the notion that phosphorylation on T58 of WNK1 is important for inhibition of ROMK by hormones or growth factors that activate PI3K.
Akt1 and SGK1 Phosphorylate WNK1 at Threonine-58 In Vitro and In Vivo
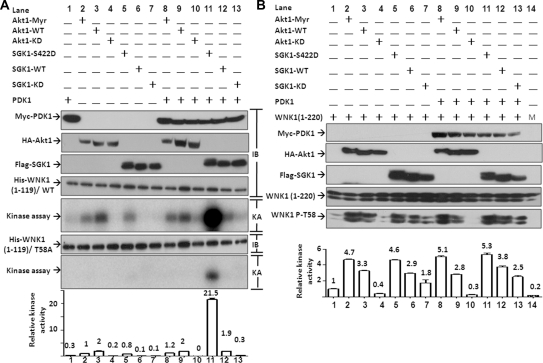
Although WNK1 has been reported as a substrate for Akt1 and SGK1, the efficacy of WNK1 phosphorylation by different status of Akt1 and SGK1 has not been clearly clarified yet. In this study, we decided to examine kinase activity of Akt1 and SGK1 systematically with the goal to guide our physiologic studies. We first examined the ability of exogenously expressed Akt1 and SGK1 to phosphorylate WNK1 in vitro with or without coexpressed PDK1. Expressed epitope-tagged Myc-PDK1, HA-Akt1, or Flag-SGK1 was immunoprecipitated from HEK cell lysates by respective antibodies. Purified bacterial His-tagged fragment of WNK1 consisting of amino acids 1 to 119 was used as the substrate. T58A mutant WNK1 was used as the control for specific phosphorylation at T58. As shown, PDK1 by itself did not phosphorylate WNK1 (Figure 3A, lane 1). For Akt1, both wild-type Akt1 and myristoylated Akt1, but not their kinase dead mutant, phosphorylated WNK1 (lane 2 to 4). Although myristoylated Akt1 is reported catalytically more active than wild type toward certain substrates, we did not find this using WNK1 as a substrate. Co-expression of PDK1 did not further enhance the in vitro kinase activity of Akt1 (lane 8 to 10). For SGK1, only S422D (not wild-type or kinase-dead mutant) phosphorylated WNK1 in the absence of PDK1 (lanes 5 to 7). The SGK1-S422D mutant with serine-422 in the hydrophobic motif substituted by aspartate is constitutively active because it does not require phosphorylation by mTOR for activation by PDK1.20 Co-expression of PDK1 enhanced the kinase activity of WT and SGK1-S422D (lanes 11 to 13). The increase in the kinase activity on the S422D mutant was so much that phosphorylation on WNK1 also occurred at residue(s) other than T58 [see lane 11, on WNK1(1-119)/T58A mutant]. The averaged relative kinase activity of Akt1 and SGK1 (normalized to “Akt1-Myr” in lane 2, which is given as 1), representing specific phosphorylation on WNK1-T58 [i.e., after subtracting signal on T58A mutant from signal on wild type of WNK1(1-119)] from three independent experiments is shown in bar graph in the bottom.
Figure 3.
Akt1 and SGK1 phosphorylate WNK1 at threonine-58. (A) In vitro kinase assay of PDK1, Akt1, and SGK1. Epitope-tagged (Myc-, HA-, or Flag-) wild-type SGK1 (SGK1-WT), kinase-dead SGK1 (SGK1-KD), constitutively active mutant SGK1 (SGK1–S422D), WT Akt1 (Akt1-WT), myristoylated-Akt1 (Akt1-Myr), and kinase-dead Akt1 (Akt1-KD) were expressed in HEK cells with or without wild-type PDK1. Kinase activity of immunoprecipitated PDK1, Akt1, and SGK1 was assayed using wild-type or T58A WNK1(1-119) as a substrate. Immunoblots of precipitated proteins by respective epitope antibody (labeled “IB” on the right) and autoradiograph of kinase assay analyzed by phosphoimager (labeled “KA” on the right) are shown. Bar graph in the bottom is the relative kinase activity (normalized to lane 2, “Akt1-Myr”) specific for phosphorylation at T58 [i.e., subtracting non-T58 phosphorylation signal on WNK1(1-119)/T58A mutant from signal on wild-type WNK1(1-119)]. Mean ± SEM from three separate experiments is shown on top of each bar. (B) In vivo phosphorylation on T58 of WNK1 by Akt1 and SGK1. Cells were transfected with epitope-tagged Akt1, SGK1, or PDK1 with wild-type WNK1(1-220) (lanes 1 to 13) or with WNK1(1-220)/T58A mutant (lane 14 labeled “M”). Protein expressions were blotted by specific antibodies. Phosphorylation on T58 of WNK1(1-220) was detected by anti-phospho-T58 WNK1 antibody. Doublet bands detected by anti-WNK1 and anti-phospho-T58 WNK1 antibodies were always found in WNK1(1-119) and WNK1(1-220) but not WNK1(1-491). The doublets probably represent different conformational forms of eukaryotic WNK1 proteins because they are not observed for purified His-tagged WNK1(1-119) proteins produced in the bacteria (see A). The abundance of each band in the gel reflecting kinase activity of Akt1 or SGK1 was measured by densitometry by the Image J program available at the NIH website. Basal level of T58 phosphorylation on WNK1(1-220) without exogenous Akt1 or SGK1 (lane 1) was defined as 1 for relative kinase activity measurement. Mean ± SEM from three separate experiments is shown on top of each bar.
We further examined the activity of these kinases acting in vivo (i.e., in intact cells). WNK1(1-220) (which lacks kinase domain thus avoiding autophosphorylation) was co-expressed with epitope-tagged Akt1, SGK1, and/or PDK1 as indicated. Phosphorylation at T58 was probed by anti-WNK1-T58 phospho-antibody. T58A mutant of WNK1(1-220) was used as a negative control (Figure 3B, lane 14, labeled as “M” for mutant). As shown, there was a low basal level of phosphorylation on WNK1(1-220) (lane 1), presumably from the endogenous Akt1. Myristoylated and wild-type Akt1, but not kinase-dead mutant, caused phosphorylation of WNK1 above the basal level (lanes 2 to 4). Coexpression with PDK1 slightly enhanced kinase activity of myristoylated Akt1 but not wild-type Akt1 (lanes 8 to 10). The increase in the kinase activity of myristoylated Akt1 by PDK1 in vivo, but not in vitro, may be caused by preferential targeting of the myristoylated Akt1 to the cell membrane, thus increasing co-localization with PDK1. As in vitro studies, S422D and wild-type SGK1 phosphorylated WNK1 in vivo (lanes 5 and 6). PDK1 slightly enhanced SGK1 kinase activity but not as much as the effect found in the in vitro experiments (lanes 11 and 12). Differences in the ratio of substrate relative to kinase and/or the efficiency of kinase may account for the discrepancy. To our surprise, expression of kinase-dead SGK1 caused some phosphorylation of WNK1 above the basal level, although the activity was less compared with wild-type or S422D mutant (lanes 7 and 13). At the moment, we do not have a good explanation for the finding except to speculate that it may be caused by altered activity of endogenous Akt1. Overall, these results from in vitro and in vivo kinase assays support the idea that Akt1 and SGK1 can phosphorylate WNK1 at T58.
Akt1 Inhibits ROMK through WNK1
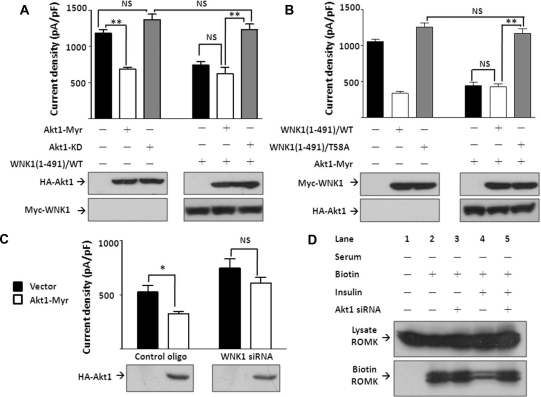
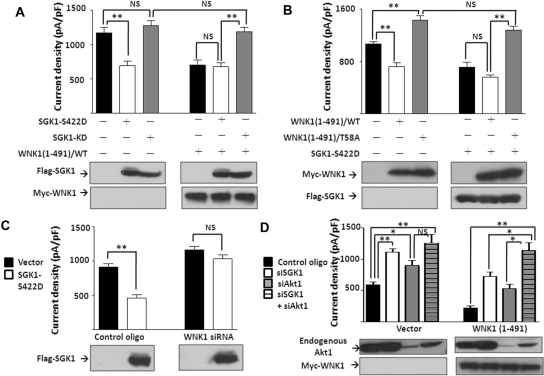
The finding that a supraphysiologic concentration of insulin can further inhibit ROMK in the presence of serum (Figure 2A) suggests that normally the endogenous Akt1 and WNK1 are not maximally activated. Consistent with the idea, exogenous WNK1 inhibits ROMK (Figure 2E; see also ref. 21). We thus asked whether overexpression of Akt1 may inhibit ROMK by enhancing WNK1-T58 phosphorylation. We used myristoylated Akt1 because it can be enhanced by PDK1 in intact cells (Figure 3B). Myristoylated Akt1 (“Akt1-Myr”) inhibited ROMK but did not cause additional effect when WNK1(1-491) was co-transfected (Figure 4A). Kinase dead mutant of Akt1 (Akt1-KD) did not cause inhibition of ROMK but reversed WNK1(1-491)–mediated inhibition. Thus, Akt1 and WNK1 act on the same pathway.
Figure 4.
Akt1 inhibits ROMK in a WNK1–T58 phosphorylation-dependent manner. (A) Effect of Akt1 on ROMK. Cells were transfected with ROMK and with myristoylated Akt1 (Akt1-Myr), kinase-dead Akt1 (Akt1-KD), and/or WNK1(1-491) as indicated. (B) Effect of myristoylated Akt1 (Akt1-Myr) on ROMK in the presence of wild-type (WT) or T58A mutant of WNK1(1-491). (C) Effect of Akt1 on ROMK with or without endogenous WNK1. Cells were transfected with control oligonucleotide or WNK1 siRNA (200 nM each) and with empty vector or myristoylated Akt1 (Akt1-Myr). In each panel, ROMK current density (pA/pF at −100 mV) was represented as mean ± SEM (n ≥ 6). *P < 0.05 between indicated groups by unpaired two-tailed t test. **P < 0.01. NS, not statistically significant. Equal protein expression was confirmed by Western blot. (D) Effect of insulin on membrane abundance of ROMK with or without endogenous Akt1. Cells were transfected with ROMK and control oligonucleotide or Akt1 siRNA (200 nM each) and deprived of serum for 16 hours.
To confirm the role of Akt1 is from phosphorylation on T58 of WNK1, we showed that WNK1(1-481)/T58A had no effect on ROMK but reversed myristoylated Akt1-mediated inhibition of ROMK (Figure 4B). Knocking down endogenous WNK1 by siRNA prevented the inhibition of ROMK by myristoylated Akt1 (Figure 4C). Knocking down Akt1 blunted the effect of insulin to decrease cell surface abundance of ROMK in serum-free conditions (Figure 4D), linking the effect of Akt1-WNK1 on ROMK to insulin.
SGK1 Inhibits ROMK through WNK1 and Works Together with Akt1
We next examined the potential role of SGK1, another member of the AGC kinase family that can also mediate downstream effect of PI3K, in regulating WNK1 inhibition of ROMK. Similar to Akt1, constitutively active SGK1 mutant, S422D, inhibited ROMK but did not cause an additional effect when WNK1(1-491) was co-expressed. Kinase-dead SGK1 did not inhibit ROMK but reversed the inhibition caused by WNK1 (Figure 5A). In the presence of the T58A mutant of WNK1(1-491), even the constitutively active form of SGK1 did not inhibit ROMK (Figure 5B). Knocking down endogenous WNK1 by siRNA also abrogated the effect of SGK1 on ROMK (Figure 5C). Thus, exogenous Akt1 and SGK1 showed a similar inhibitory effect on ROMK, and both effects depend on WNK1-T58 phosphorylation. Next, we examined potential synergistic effects of Akt1 and SGK1 on ROMK by silencing endogenous Akt1 and/or SGK1 using siRNA in the absence (“vector”) or presence of exogenous WNK1 [“WNK1(1-491)”]. Knocking down Akt1 or SGK1 individually increased ROMK current significantly with or without exogenous WNK1 (Figure 5D). The effect of knocking down both Akt1 and SGK1 is greater than knocking down each individually in the “WNK1(1-491)”–transfected but not in the “vector”-transfected group. Differences in the abundance of WNK1 substrate likely account for the different results. In summary, both Akt1 and SGK1 phosphorylate WNK1 and contribute to its regulation of ROMK. The importance of Akt1 versus SGK1 in vivo will depend on the relative abundance of each respective kinase and WNK1 in the setting.
Figure 5.
SGK1 inhibits ROMK through the same pathway as Akt1. (A) Effect of SGK1 on ROMK. Cells were co-transfected with ROMK and constitutively active SGK1 (SGK1–S422D) or kinase-dead SGK1 (SGK1-KD) with or without WNK1(1-491). (B) Effect of SGK1 on ROMK in the presence of wild-type or T58A mutant of WNK1(1-491). SGK1–S422D was co-transfected with wild-type or T58A mutant WNK1(1-491). (C) Effect of SGK1 on ROMK with or without endogenous WNK1. Cells were transfected with control oligonucleotide or WNK1 siRNA (200 nM each) and with empty vector or SGK1–S422D. (D) Effect of siRNA of Akt1 and/or SGK1 on ROMK. Cells were transfected with siRNA for Akt1 (siAkt1) and/or SGK1 (siSGK1, 200 nM each) and with vector or WNK1(1-491). In each panel, ROMK current density was measured and presented as mean ± SEM (n ≥ 6 for each group). *P < 0.05 between indicated groups by unpaired two-tailed t test. **P < 0.01. NS, not statistically significant. Efficacy of Akt1 siRNA and equal expression of protein were confirmed by Western blot.
Inhibition of ROMK by SGK1 via Enhanced Endocytosis and Not by Phosphorylation of ROMK
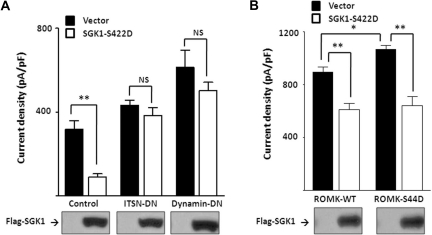
WNK1 inhibits ROMK by enhancing endocytosis through a dynamin-dependent, clathrin-mediated pathway.3,21,22 This effect of WNK1 requires an interaction with intersectin.11 It has also been reported that SGK1 can directly phosphorylate on ROMK1 at serine-44, although this effect is believed to result in an increase of the cell surface abundance of ROMK.23 We found that co-expression of a dominant-negative (“DN”) intersectin (“ITSN”) or dynamin prevented inhibition of ROMK by SGK1-S422D (Figure 6A). These results, together with our previous reports that these experimental maneuvers abolish inhibition of ROMK by WNK1, support that phosphorylation of WNK1-T58 by SGK1 leads to inhibition of ROMK by increasing endocytosis of ROMK. In further support of this idea, we found that SGK1-S422D inhibited ROMK bearing a mutation of serine-44 (S44D) and wild-type ROMK (Figure 6B).
Figure 6.
Effect of SGK1 on ROMK is dynamin and intersectin (ITSN)-dependent, but not dependent on phosphorylation of ROMK at S44. (A) Effect of SGK1 on ROMK in the presence of dominant-negative intersectin (ITSN-DN) or dynamin (dynamin-DN). Cells were transfected with SGK1–S422D or without (“vector”) and with ITSN-DN or dynamin-DN or without (“control”). (B) Effect of SGK1 on wild-type or S44D mutant ROMK. Cells were co-transfected with SGK1–S422D and with wild-type ROMK (ROMK-WT) or S44D mutant of ROMK (ROMK-S44D). In each panel, ROMK current density was measured and presented as mean ± SEM (n ≥ 6 for each group). *P < 0.05 between indicated groups by unpaired two-tailed t test. **P < 0.01. NS, not statistically significant. Equal protein expression was confirmed by Western blot.
Kidney-Specific WNK1 Blocks SGK1 Effect on ROMK without Interfering with Phosphorylation on WNK1
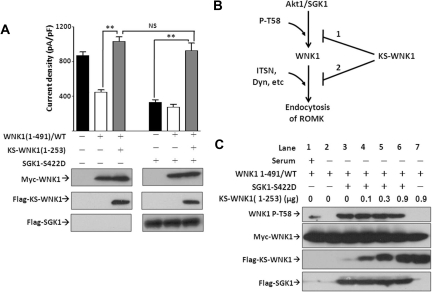
WNK1 has multiple alternatively spliced isoforms, including the full-length WNK1 (also known as long WNK1) and a kidney-specific WNK1 (KS-WNK1) that lacks most of the kinase domain and preceding amino acids in the N terminus.24 Long WNK1 contains T58, the target of Akt1/SGK1. In contrast, KS-WNK1 lacks T58. We showed that KS-WNK1 binds and antagonizes long WNK1–induced inhibition of ROMK.21 Here, we asked whether KS-WNK1 antagonizes the effect of long WNK1 in the presence of Akt1/SGK1. As reported previously, KS-WNK1 reversed WNK1(1-491)–induced inhibition of ROMK (Figure 7A, left three bars). The ability of KS-WNK1 to antagonize WNK1(1-491)–induced inhibition of ROMK was unaltered in the presence of exogenous constitutively active SGK1 (SGK1-S422D) (Figure 7A, last bar on the right). KS-WNK1 may antagonize long WNK1 inhibition of ROMK by interfering with its phosphorylation by Akt1/SGK1 (Figure 7B, mechanism “1”) or interfering with its interaction with downstream effectors of endocytosis, such as intersectin (mechanism “2”). To distinguish between these two possibilities, we examined the effect of KS-WNK1 on serum-induced phosphorylation of WNK1(1-491) in HEK cells coexpressed with SGK1-S422D. Serum deprivation decreased T58 phosphorylation on WNK1, which was enhanced by SGK1-S422D (lanes 1 to 3; Figure 7C). Coexpression of KS-WNK1 did not affect phosphorylation of WNK1 at T58 (lanes 4 to 6). KS-WNK1 alone had no effect on WNK1 phosphorylation (lane 7). Thus, KS-WNK1 likely affects WNK1 interaction with downstream effectors.
Figure 7.
Effect of SGK1 on ROMK is reversed by kidney-specific WNK1 (KS-WNK1). (A) Effect of SGK1 on ROMK in the presence of KS-WNK1. Cells were transfected with SGK1–S422D, WNK1(1-491), and/or KS-WNK1(1-253) as indicated. KS-WNK1(1-253) consists of amino acids 1 to 253 of KS-WNK1. ROMK current density was measured and presented as mean ± SEM (n ≥ 6 for each group). **P < 0.01 between indicated groups by unpaired two-tailed t test. NS, not statistically significant. Equal protein expression was confirmed by Western blot. (B) Possible mechanisms for KS-WNK1 to block SGK1 effect on ROMK. Mechanism 1: Interfering with WNK1 phosphorylation by SGK1. Mechanism 2: Interfering with WNK1 interaction with downstream effectors, such as intersectin (“ITSN”) and dynamin (“Dyn”). (C) Effect of KS-WNK1 on WNK1–T58 phosphorylation by SGK1. Cells were all transfected with WNK1(1-491), KS-WNK1(1-253) (at DNA amount from 0 to 0.9 μg), and/or SGK1–S422D and incubated with or without serum as indicated. Basal level of WNK1(1-491) phosphorylation is shown in lane 1. For experiments shown in lanes 2 to 7, cells were incubated in serum-free media for 16 hours. Protein expression was detected by specific antibodies. Phosphorylation on WNK1–T58 was determined using anti-phospho-T58 WNK1 antibody.
DISCUSSION
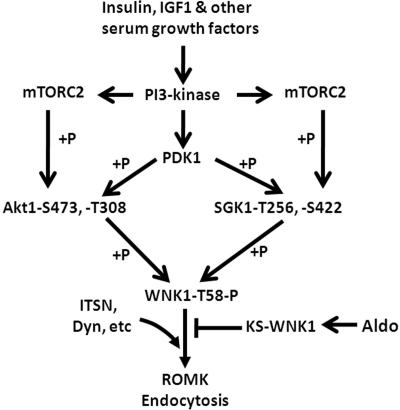
ROMK undergoes constitutive clathrin-mediated endocytosis.3 WNK kinases including WNK1 and WNK4 inhibit ROMK by increasing endocytosis.21,22 We previously reported that WNK1 and 4 interact with intersectin, an endocytic scaffold protein that binds dynamin and other endocytic accessory proteins.11 The interaction with intersectin leads to stimulation of endocytosis of ROMK, probably by enhancing the recruitment and assembly of endocytic machinery. In this study, we showed that activation of PI3K by insulin, IGF1, and likely by other serum growth factors enhances endocytosis of ROMK through phosphorylation of T58 on WNK1. This effect on ROMK via WNK1 depends on two members of AGC kinases: Akt1 and SGK1. As summarized in Figure 8, activation of PI3K stimulates mTORC2 to phosphorylate Akt1 and SGK1 at S473 and S422 in the hydrophobic motif, respectively. Phosphorylation by mTORC2 allows binding of PDK1, which phosphorylates Akt1 and SGK1 at T308 and T256 in the T-loop, respectively, to activate their catalytic activity. Activated Akt1 and SGK1 phosphorylate WNK1 at T58, leading to enhanced endocytosis of ROMK via an intersectin-dependent mechanism.
Figure 8.
A working model for regulation of ROMK by PI3K-activating hormones via Akt1/SGK1 and WNK1 and by aldosterone. See texts for details and for abbreviations.
This mechanism of regulation of ROMK by Akt1 and/or SGK1 via WNK1 has several potential physiologic or pathophysiologic relevancies. One of these is the maintenance of K+ homeostasis during chronic (approximately 1 week) K+ deficiency. IGF1 is produced in the kidney, and production is upregulated by chronic dietary K+ restriction.25 Upregulation of IGF1 is believed to play a role in the K+ deficiency–induced renal hypertrophy. An increase in the level of IGF1, nonetheless, can decrease renal K+ secretion via ROMK, contributing to K+ conservation during K+ deficiency. In support of the idea that IGF1 inhibits renal K+ secretion in vivo, intravenous administration of IGF1 in humans decreases renal K+ excretion without significant changes in the filtered load of K+.26 Conversely, mice with liver-specific deletion of IGF1 have approximately 80% reduction in the circulating IGF1 and increased renal K+ excretion despite a normal filtered load of K+.27 Finally, inhibition of PI3K increases the density of native ROMK channels in mouse CCD,28 providing further support for the physiologic role of PI3K in the inhibition of ROMK and renal K+ secretion.
Insulin also activates PI3K and thus may be another upstream signal that uses the Akt1/SGK1-WNK1 pathway to inhibit renal K+ excretion. A physiologic role of insulin in decreasing renal K+ excretion in vivo, however, is not universally accepted. Although it is known that insulin decreases urinary K+ excretion, some suggested that the effect is entirely from the decrease in the plasma K+ caused by the intracellular shift.29 In contrast, others showed that the decrease in urinary K+ excretion caused by insulin infusion in humans is more than the decrease in the filtered load, supporting that insulin inhibits renal K+ excretion in vivo.30,31 In support of this idea, application of insulin to the basolateral bath of isolated perfused rabbit CCD inhibits the net transepithelial K+ secretion.32 The IC50 for inhibition of K+ secretion in the CCD is 500 pM, which is higher than the normal fasting level of insulin (10 to 150 pM) but within the normal postprandial level (300 to 800 pM).16 The minimal concentration of insulin required for inhibition of ROMK in our studies is 1 nM. This value is not far from the effective concentration of insulin in the CCD and the postprandial levels in vivo, considering that different experimental systems are compared. The inhibition of ROMK-mediated renal K+ secretion by insulin, if it occurs in vivo, will help to maintain the plasma K+ level in the postprandial state during which a very active intracellular K+ shift occurs.
Our finding that SGK1 inhibits ROMK is different from other reports that SGK1 stimulates ROMK.23,33 The in vivo importance of our finding is supported by several animal studies. The abundance of ROMK in the apical membrane of distal nephron in mice homozygous for Sgk1 deletion is higher than that in the wild-type littermates.34 The increase in the ROMK abundance in Sgk1 knockout mice was suggested to be caused by a compensatory response to hyperkalemia caused by reduced Na+ reabsorption and thus reduced electrical driving force for K+ secretion. However, in mice with double knockout of Sgk1 and Sgk3 (in which Na+ wasting is evident in normal Na+ diets), the fractional urinary K+ excretion is higher than that in wild-type littermates, despite a normal blood K+ level and an impairment in Na+ reabsorption in the double knockout mice.35 These results support that SGK1 (perhaps together with SGK3 or isoforms) inhibits ROMK in vivo. It is possible that SGK1 can also exert a stimulatory effect on ROMK under different physiologic contexts or presence of certain co-regulators. Our present cell-based study, nonetheless, provides strong evidence to support the hypothesis that Akt1/SGK1-mediated phosphorylation of WNK1 can mediate inhibition of ROMK and renal K+ secretion by PI3K-activating hormones. Future experiments will examine the effect of insulin and IGF1 on K+ homeostasis in mice with WNK1, SGK1, and/or Akt1 deletions.
Aldosterone is a positive regulator of SGK1.36 Our finding that SGK1 inhibits ROMK may seem to be counterintuitive to the fact that aldosterone stimulates renal K+ secretion. However, besides SGK1, aldosterone also stimulates KS-WNK1,37 which antagonizes the effect of SGK1 phosphorylation of (long) WNK1 on ROMK (see Figures 7 and 8). Thus, whereas insulin and IGF1 activate the Akt1/SGK1-WNK1 signaling cascade to inhibit ROMK, aldosterone activates two opposing effects on the signaling cascade (the negative and positive effect via SGK1 and KS-WNK1, respectively) and therefore may not have a net effect on ROMK. The principal mechanism for aldosterone stimulation of K+ secretion is likely the increase in the electrical driving force for K+ secretion secondary to enhanced Na+ reabsorption, rather than via the SGK1-WNK1 cascade.
CONCISE METHODS
DNA Constructs and Reagents
pEGFP-ROMK1, pCMV5-Myc-WNK1, pIRES-Flag-KS-WNK1, and dominant-negative intersectin and dynamin have been described previously.21 The plasmids encoding N-terminal 60 amino acids truncated and Flag-tagged wild-type, S422D mutant and kinase-dead mutant SGK1 (pCMV7–3xFlag-▵SGK1), HA-tagged wild-type, myristoylated and kinase-dead mutant Akt1 (pCMV-HA-Akt1), and myc-tagged PDK1 (pcDNA3-Myc-PDK1) were generous gifts from M. Cobb (UT Southwestern Medical Center at Dallas).38 Point mutations were generated by site-directed mutagenesis (QuickChange kit; Stratagene) and confirmed by sequencing. Sense and anti-sense oligonucleotides (Dharmacon RNA Technology) for human WNK1 siRNA were 5′-UGUCUAACGAUGGCCGCUUdTdT-3′ and 5′-AAGCGGCCAUCGUUAGACAdTdT-3′. Sense and anti-sense oligonucleotides for human SGK1 were 5′-GUCCUUCUCAGCAAAUCAAUU-3′ and 5′-UUGAUUUGCUGAGAAGGACUU-3′. Sense and anti-sense oligonucleotides for human Akt1 were 5′-GACCGCCUCUGCUUUGUCAdTdT-3′ and 5′-UGACAAAGCAGAGGCGGUCdTdT-3′. Insulin from bovine pancreas and IGF1 were purchased from Sigma. The following antibodies were used: anti-WNK1 antibody (Q256) (1:5000 dilution; described previously),21 anti-Flag antibody (M2) (1:5000 dilution; Sigma), anti-c-Myc (1:5000 dilution; Sigma), anti-HA antibody (12CA5) (1:5000 dilution; Berkeley Antibody), anti-WNK1 phospho-Thr-58 (1:1000 dilution; Abcam), anti-Akt1 (AW24; 1:1000 dilution; Millipore), anti-Akt1 phospho-Thr-308 (C31E5E; 1:1000 dilution; Cell Signaling), anti-Akt1 phospho-Ser-473 (D9E; 1:1000 dilution; Cell Signaling), anti-SGK1 phospho-Thr-256 (1:1000 dilution; Santa Cruz), and anti-GFP horseradish peroxidase conjugate (1:1000 dilution; Invitrogen).
Cell Culture, Transfection, Preparation of Cell Lysates, Immunoblotting, and Kinase Assays
HEK-293 cells were co-transfected with cDNA (0.3 μg per six wells) for GFP-ROMK1 plus other indicated cDNAs using Fugene HD (Roche) as described.11 In each experiment, the total amount of DNA for transfection was balanced by using empty vectors. Transfected cells were identified by green fluorescence. Approximately 36 to 48 hours after transfection, cells were dissociated and placed in a chamber for ruptured whole-cell recordings. For knockdown by siRNA, oligonucleotides (200 nM each) were mixed with cDNAs for ROMK1 and other indicated constructs for co-transfection by PolyFect (Quagen). For serum deprivation studies, cells were washed with PBS two times and cultured in serum-free DMEM for different time periods as indicated.
Cultured cells were incubated with lysis buffer (50 mM HEPES, pH 7.6, 150 mM sodium chloride, 0.5% Triton X-100, 10% glycerol, protease inhibitor cocktail [Mini EDTA-free, Roche], and phosphatase inhibitor cocktail [PhosSTOP, Roche]). After shaking for 30 minutes on a rotator at 4°C, extracts were cleared by centrifugation. Protein concentrations of supernatant were measured by the Bradford assay using BSA as a standard. Equal amounts of lysates were boiled in Laemmli sample buffer and separated by SDS-PAGE under reducing conditions. For Western blotting, proteins were transferred to nitrocellulose membranes, blocked using 5% nonfat milk, incubated with the appropriate antibodies, and detected using enhanced chemiluminescence detection reagent (Pierce).
For kinase assays, epitope-tagged PDK1, Akt1, and/or SGK1 were co-transfected in HEK cells as indicated. Lysates (500 μg) were incubated with respective antibodies (anti-rabbit anti-Myc for PDK1, anti-mouse anti-HA for Akt1, and anti-mouse anti-Flag for SGK1 each at 1:300 dilution) and 30 μl of 50% slurry of protein A (for immunoprecipitating PDK1) or protein G (for immunoprecipitating Akt1 and SGK1) at 4°C overnight. Then beads were washed three times with 1 ml wash buffer (0.25 M Tris, pH 7.4, 1 M NaCl, 0.1% Triton X-100, proteinase, and phosphatase inhibitor cocktails) for 10 minutes and followed by one wash with 1 ml kinase wash buffer (10 mM HEPES, pH 7.6, and 10 mM MgCl2). After removing the kinase wash buffer, beads were incubated with 2 μg WNK1(1-119) wild-type or T58A mutant proteins purified from Escherichia coli in 30 μl of kinase buffer (20 mM HEPES, pH 7.6, 5 μM ATP [5 μCi of γ-32P ATP], 10 mM MgCl2, and 10 mM β-glycerol phosphate) at 30°C for 45 minutes. The samples were boiled with 7.5-μl fivefold sampling buffer at 90°C for 5 minutes. Supernatants were separated by SDS-PAGE for Western blot and phosphoimager analysis. The radioisotope intensity of the bands was determined by phosphoImager, Storm 860 (GE Healthcare, Piscataway, NJ), and ImageQuant 5.2 software (GE Healthcare, Little Chalfont, Buckinghamshire, UK).
Whole-Cell Patch-Clamp Recording of ROMK Channels
After 48-hour transfection, cells were trypsinized and plated on poly-l-lysine–coated coverslips. Whole-cell ROMK currents were recorded using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA) as described previously.11 The pipette resistance was around 1.5 to 3 MΩ. Green fluorescence of GFP-ROMK in transfected cells was identified by epifluorescent microscopy. The pipette solution contained 140 mM KCl and 10 mM HEPES (pH 7.2); the bath solution contained 140 mM KCl, 1 mM MgCl2, 1 mM CaCl2, and 10 mM HEPES (pH 7.4). The cell membrane capacitance and series resistance were monitored and compensated (>75%) electronically. The voltage protocol consisted of 0-mV holding potential and 500-ms steps from −100 to 100 mV in 25-mV increments. ClampX 9.2 software (Axon Instruments) was used for data acquisition. Current density was calculated by dividing current at −100 mV (pA; measured at 25°C) by capacitance (pF). Results were shown as mean ± SEM (n = 6–10). Each experiment (i.e., set of results shown in each panel of a figure) was repeated two to four times.
Surface Biotinylation Assay
For biotinylation of cell surface ROMK, cells were washed with ice-cold PBS and incubated with 0.75 ml PBS containing 1.5 mg/ml EZ-link-NHS-SS-biotin (Thermo Scientific) for 1 hour at 4°C. After quenching with glycine (100 mM), cell were lysed in a RIPA buffer (150 mM NaCl, 50 mM Tris-HCl, 5 mM EDTA, 1% Triton X-100, 0.5% DOC, and 0.1% SDS) containing protease inhibitor cocktail. Biotinylated proteins were precipitated by streptavidin-agarose beads (Thermo Scientific). Beads were subsequently washed four times with PBS containing 1% Triton X-100. Biotin-labeled proteins were eluted in sample buffer, separated by SDS-PAGE, and transferred to nitrocellulose membranes for Western blotting. ROMK proteins on the membrane were detected using anti-GFP horseradish peroxidase conjugate antibody. The biotinylation experiment was performed three times with similar results.
Statistical Analysis
Data analysis and curve fitting were performed with Prism (v5.03) software (GraphPad Software, San Diego, CA). Data are presented as mean ± SEM. Statistical comparisons between two groups of data were made using the two-tailed unpaired t test. Multiple comparisons were determined using one-way ANOVA. Time course and dose–response curves were fitted by nonlinear regression analysis. Statistical significance was defined as P < 0.05 for single comparison and P < 0.01 for multiple comparisons.
DISCLOSURES
These experiments were performed by C.-J.C. in partial fulfillment of the requirements of the PhD degree at the University of Texas Southwestern Medical Center at Dallas.
Acknowledgments
We thank Dr. Melanie Cobb and Aileen Klein at UT Southwestern at Dallas for reagents and for comments and advice on the in vitro kinase assay; our colleague Dr. Seung-Kuy Cha for advice on patch-clamp experiments; and Drs. Yuh-Feng Lin, Shih-Hua Lin, and Pauling Chu at Tri-Service General Hospital, Taipei, Taiwan for support and encouragement. This study is supported by National Institutes of Health Grants RO1DK-59530 and P30DK079328. C.L.H. holds the Jacob Lemann Professorship in Calcium Transport of University of Texas Southwestern Medical Center. C.-J.C. is supported by a scholarship grant from the Ministry of Defense, Taiwan.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. Ho K, Nichols CG, Lederer WJ, Lytton J, Vassilev PM, Kanazirska MV, Hebert SC: Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature 362: 31–38, 1993 [DOI] [PubMed] [Google Scholar]
- 2. Rodan AR, Huang CL: Distal potassium handling based on flow modulation of maxi-K channel activity. Curr Opin Nephrol Hypertens 18: 350–355, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zeng WZ, Babich V, Ortega B, Quigley R, White SJ, Welling PA, Huang CL: Evidence for endocytosis of ROMK potassium channel via clathrin-coated vesicles. Am J Physiol Renal Physiol 283: F630–F639, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Xu B, English JM, Wilsbacher JL, Stippec S, Goldsmith EJ, Cobb MH: WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J Biol Chem 275: 16795–16801, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Wilson FH, Disse-Nicode‘me S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP: Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Verissimo F, Jordan P: WNK kinases, a novel protein kinase subfamily in multi-cellular organisms. Oncogene 20: 5562–5569, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Vitari AC, Deak M, Morrice NA, Alessi DR: The WNK1 and WNK4 protein kinases that are mutated in Gordon's hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem J 391: 17–24, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moriguchi T, Urushiyama S, Hisamoto N, Iemura S, Uchida S, Natsume T, Matsumoto K, Shibuya H: WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem 280: 42685–42693, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Xu BE, Stippec S, Chu PY, Lazrak A, Li XJ, Lee BH, English JM, Ortega B, Huang CL, Cobb MH: WNK1 activates SGK1 to regulate the epithelial sodium channel. Proc Natl Acad Sci USA 102: 10315–10320, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang CL, Angell J, Mitchell R, Ellison DH: WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest 111: 1039–1045, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. He G, Wang HR, Huang SK, Huang CL: Intersectin links WNK kinases to endocytosis of ROMK1. J Clin Invest 117: 1078–1087, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vitari AC, Deak M, Collins BJ, Morrice N, Prescott AR, Phelan A, Humphreys S, Alessi DR: WNK1, the kinase mutated in an inherited high-blood-pressure syndrome, is a novel PKB (protein kinase B)/Akt substrate. Biochem J 378: 257–268, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jiang ZY, Zhou QL, Holik J, Patel S, Leszyk J, Coleman K, Chouinard M, Czech MP: Identification of WNK1 as a substrate of Akt/protein kinase B and a negative regulator of insulin-stimulated mitogenesis in 3T3–L1 cells. J Biol Chem 280: 21622–21628, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Xu BE, Stippec S, Lazrak A, Huang CL, Cobb MH: WNK1 activates SGK1 by a phosphatidylinositol 3-kinase-dependent and non-catalytic mechanism. J Biol Chem 280: 34218–34223, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Lee IH, Dinudom A, Sanchez-Perez A, Kumar S, Cook DI: Akt mediates the effect of insulin on epithelial sodium channels by inhibiting Nedd4–2. J Biol Chem 282: 29866–29873, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Ahmed M, Gannon MC, Nuttall FQ: Postprandial plasma glucose, insulin, glucagon and triglyceride responses to a standard diet in normal subjects. Diabetologia 12: 61–67, 1976 [DOI] [PubMed] [Google Scholar]
- 17. Rechler MM, Nissley SP: The nature and regulation of the receptors for insulin-like growth factors. Annu Rev Physiol 47: 425–442, 1985 [DOI] [PubMed] [Google Scholar]
- 18. Zapf J, Walter H, Froesch ER: Radioimmunological determination of insulinlike growth factors I and II in normal subjects and in patients with growth disorders and extrapancreatic tumor hypoglycemia. J Clin Invest 68: 1321–1330, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pearce LR, Komander D, Alessi DR: The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol 11: 9–22, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Kobayashi T, Cohen P: Activation of serum- and glucocorticoid-regulated protein kinase by agonists that activate phosphatidylinositide 3-kinase is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2. Biochem J 15: 319–328, 1999 [PMC free article] [PubMed] [Google Scholar]
- 21. Lazrak A, Liu Z, Huang CL: Antagonistic regulation of ROMK by long and kidney-specific WNK1 isoforms. Proc Natl Acad Sci USA 103: 1615–1620, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kahle KT, Wilson FH, Leng Q, Lalioti MD, O'Connell AD, Dong K, Rapson AK, MacGregor GG, Giebisch G, Hebert SC, Lifton RP: WNK4 regulates the balance between renal NaCl reabsorption and K+ secretion. Nat Genet 35: 372–376, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Yoo D, Kim BY, Campo C, Nance L, King A, Maouyo D, Welling PA: Cell surface expression of the ROMK (Kir 1.1) channel is regulated by the aldosterone-induced kinase, SGK-1, and protein kinase A. J Biol Chem 278: 23066–23075, 2003 [DOI] [PubMed] [Google Scholar]
- 24. O'Reilly M, Marshall E, Speirs HJ, Brown RW: WNK1, a gene within a novel blood pressure control pathway, tissue-specifically generates radically different isoforms with and without a kinase domain. J Am Soc Nephrol 14: 2447–2456, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Flyvbjerg A, Marshall SM, Frystyk J, Rasch R, Bornfeldt KE, Arnqvist H, Jensen PK, Pallesen G, Orskov H: Insulin-like growth factor I in initial renal hypertrophy in potassium-depleted rats. Am J Physiol 262: F1023–F1031, 1992 [DOI] [PubMed] [Google Scholar]
- 26. Giordano M, DeFronzo RA: Acute effect of human recombinant insulin-like growth factor-1 on renal function in humans. Nephron 71: 10–15, 1995 [DOI] [PubMed] [Google Scholar]
- 27. Svensson J, Tivesten A, Sjögren K, Isaksson O, Bergström G, Mohan S, Mölne J, Isgaard J, Ohlsson C: Liver-derived IGF-I regulates kidney size, sodium reabsorption, and renal IGF-II expression. J Endocrinol 193: 359–366, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Li D, Wei Y, Babilonia E, Wang Z, Wang WH: Inhibition of phosphatidylinositol 3-kinase stimulates activity of the small-conductance K channel in the CCD. Am J Physiol Renal Physiol 290: F806–F812, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen P, Guzman JP, Leong PK, Yang LE, Perianayagam A, Babilonia E, Ho JS, Youn JH, Wang WH, McDonough AA: Modest dietary K+ restriction provokes insulin resistance of cellular K+ uptake and phosphorylation of renal outer medulla K+ channel without fall in plasma K+ concentration. Am J Physiol Cell Physiol 290: C1355–C1363, 2006 [DOI] [PubMed] [Google Scholar]
- 30. DeFronzo RA, Cooke CR, Andres R, Faloona GR, Davis PJ: The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest 55: 845–855, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. DeFronzo RA, Goldberg M, Agus ZS: The effects of glucose and insulin on renal electrolyte transport. J Clin Invest 58: 83–90, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Furuya H, Tabei K, Muto S, Asano Y: Effect of insulin on potassium secretion in rabbit cortical collecting ducts. Am J Physiol 262: F30–F35, 1992 [DOI] [PubMed] [Google Scholar]
- 33. Ring AM, Leng Q, Rinehart J, Wilson FH, Kahle KT, Hebert SC, Lifton RP: An SGK1 site in WNK4 regulates Na+ channel and K+ channel activity and has implications for aldosterone signaling and K+ homeostasis. Proc Natl Acad Sci USA 104: 4025–4029, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang DY, Wulff P, Völkl H, Loffing J, Richter K, Kuhl D, Lang F, Vallon V: Impaired regulation of renal K+ elimination in the sgk1-knockout mouse. J Am Soc Nephrol 15: 885–891, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Grahammer F, Artunc F, Sandulache D, Rexhepaj R, Friedrich B, Risler T, McCormick JA, Dawson K, Wang J, Pearce D, Wulff P, Kuhl D, Lang F: Renal function of gene-targeted mice lacking both SGK1 and SGK3. Am J Physiol Regul Integr Comp Physiol 290: R945–R950, 2006 [DOI] [PubMed] [Google Scholar]
- 36. McCormick JA, Bhalla V, Pao AC, Pearce D: SGK1: A rapid aldosterone-induced regulator of renal sodium reabsorption. Physiology 20: 134–139, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Náray-Fejes-Tóth A, Snyder PM, Fejes-Tóth G: The kidney-specific WNK1 isoform is induced by aldosterone and stimulates epithelial sodium channel-mediated Na+ transport. Proc Natl Acad Sci USA 101: 17434–17439, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen W, Chen Y, Xu BE, Juang YC, Stippec S, Zhao Y, Cobb MH: Regulation of a third conserved phosphorylation site in SGK1. J Biol Chem 284: 3453–3460, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]