Abstract
Although Th17 responses may contribute to the pathogenesis of glomerulonephritis, whether the key transcription factor in Th17 cell development, RORγt, also promotes glomerulonephritis is unknown. Here, we induced crescentic glomerulonephritis in wild-type and RORγt-deficient (RORγt−/−) mice. RORγt−/− mice were protected from disease, with reduced histologic and functional injury and decreased leukocyte infiltration. Because RORγt−/− mice lack lymph nodes, which may influence the development of nephritis, we performed cell-transfer studies. We reconstituted Rag1−/− mice, which lack adaptive immunity but otherwise have normal architecture of the lymphatic system, with splenocytes from naïve wild-type or RORγt−/− mice. Mice receiving wild-type splenocytes exhibited high mortality from renal failure after induction of nephritis whereas mice receiving RORγt−/− cells were protected. To determine the effect of RORγt deficiency specifically in T helper cells, we isolated naïve CD4+ T cells from wild-type and RORγt−/− mice and transferred them into Rag1−/− animals. Recipients of wild-type CD4+ T cells developed severe glomerulonephritis whereas recipients of RORγt−/− cells developed less severe disease. To exclude effects of altered regulatory T cell (Treg) development caused by RORγt deficiency, we transferred naïve CD4+ T cells depleted of Tregs into Rag1−/− mice. Recipients of wild-type, Treg-depleted, CD4+ T cells developed severe glomerulonephritis whereas recipients of RORγt−/−, Treg-depleted CD4+ T cells did not. Taken together, this study demonstrates that RORγt promotes the development of crescentic glomerulonephritis by directing nephritogenic Th17 responses.
The mechanisms underlying the etiology and pathogenesis of glomerulonephritis are incompletely understood. However, previous studies have identified CD4+ T helper cells as important players in proliferative forms of glomerulonephritis.1 In particular, Th1-type infiltrating effector CD4+ cells can initiate and perpetuate renal tissue damage in crescentic and necrotizing forms of glomerulonephritis.2 After the identification of an IL-17A-secreting CD4+ T cell subset (Th17),3 it is now known that Th17 cells are involved in diseases previously thought to be Th1 mediated such as rheumatoid arthritis,4,5 multiple sclerosis,6,7 and colitis.8 We and others have implicated Th17 responses in glomerulonephritis. Deficiency of IL-17A or of IL-23, which is important in the maintenance of Th17 responses, conferred protection from the development of experimental autoimmune glomerulonephritis,9 anti-myeloperoxidase-directed glomerulonephritis,10 and anti-glomerular basement membrane (aGBM) disease.11 Furthermore, using cell transfer studies we have shown that antigen-specific Th17 cells alone can induce proliferative glomerulonephritis in mice.12
However, whether the Th17 axis and especially IL-17A plays a key pathogenic role in autoinflammatory diseases remains uncertain. In contrast to earlier studies, recent work suggested that neither IL-17A deficiency nor T cell-specific overexpression of IL-17A had significant effects on experimental autoimmune encephalitis (EAE).13 Moreover, IL-17A deficiency and antibody neutralization aggravated experimental colitis in two separate studies.14,15 These divergent results are potentially explained by the fact that IL-17A is not the sole cytokine produced by Th17 cells, which also produce IL-17F, IL-21, IL-22,3,16 and IL-9.17 Therefore, combined neutralization of several Th17 cytokines may be needed to limit Th17 effector responses. Supporting this concept, neutralization of IL-17A and IL-17F resulted in improved outcomes in EAE13 and limited injury in an adoptive transfer model of colitis.18
Targeting lineage-specific transcription factors offers the opportunity to simultaneously affect multiple Th cell effector cytokines. Tbet is responsible for programming of Th1 cells,19 GATA3 drives Th2 differentiation,20 and the retinoid orphan receptors (RORs) RORα and RORγt orchestrate Th17 development.21–23 RORγt (more potent and better characterized than RORα) is crucial for Th17 effector cytokine secretion, the expression of IL-23R, the receptor for the Th17 lineage-expanding cytokine IL-23,22,24 and surface expression of the Th17 chemokine receptor CCR6.23 Therefore, inhibiting RORγt may not only influence Th17 effector cytokines but also the Th17 subset's trafficking and expansion and has been proposed as a therapeutic target for Th17-mediated disease.25 The potential benefits of interference with RORγt were demonstrated in EAE, in which absence of RORγt in CD4+ T cells protected mice from development of encephalitis.21 Likewise, the most potent protection in the aforementioned model of colitis (exceeding neutralization of IL-17A and IL-17F) was achieved when RORγt-deficient T cells were transferred.18
To date, the functional role of RORγt in inflammatory renal disease is unknown. We therefore studied immune responses and glomerulonephritis in RORγt-deficient (RORγt−/−) mice using a standard model (accelerated autologous-phase “aGBM” glomerulonephritis, in which injury is dependent on immune responses to sheep globulin localized to the GBM). Because RORγt−/− mice have abnormal lymph node architecture and because RORγt expression is not exclusively confined to Th17 cells, we performed adoptive transfer studies using different RORγt−/− leukocyte subsets to assess effects of leukocyte-specific and CD4+ T cell-specific deficiency.
RESULTS
Glomerulonephritis is Ameliorated in RORγt−/− Mice
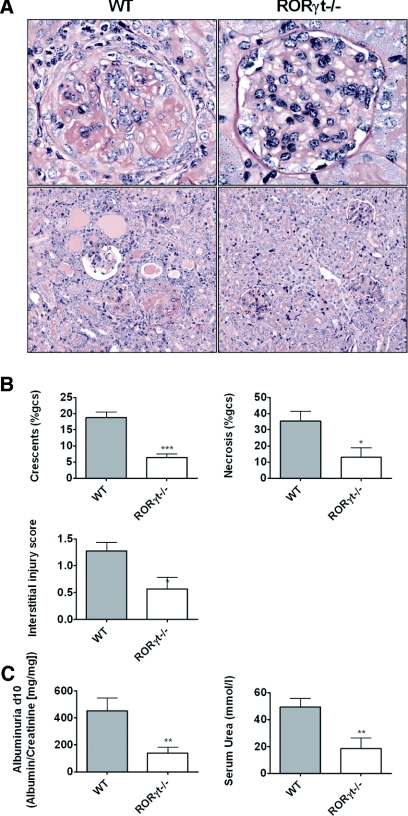
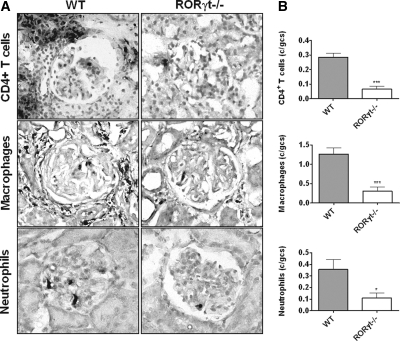
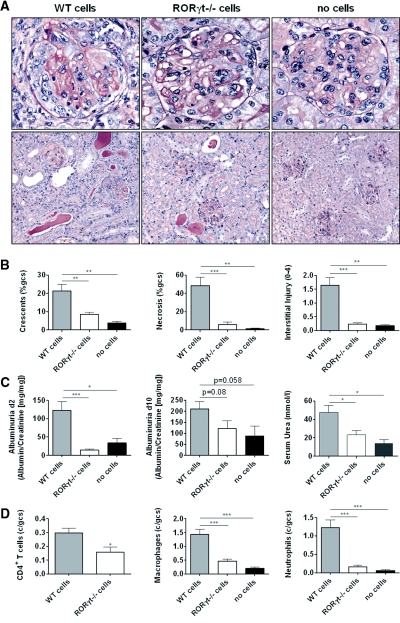
Induction of accelerated autologous-phase aGBM disease in wild-type (WT) mice resulted in development of severe nephritis 10 days after injecting aGBM globulin into sensitized mice. RORγt−/− mice were protected from histologic and functional renal injury with fewer glomerular crescents and less necrosis, less interstitial injury (Figure 1, A and B), and lower albuminuria and serum urea (Figure 1C). Consistent with these findings, RORγt−/− mice demonstrated reduced numbers of glomerular CD4+ T cells, macrophages, and neutrophils compared with WT mice with glomerulonephritis (Figure 2, A and B). Numbers of all three leukocyte populations in the renal interstitium were also reduced in RORγt−/− mice (Supplementary Table 1).
Figure 1.
Histologic and functional renal injury is reduced in RORγt−/− mice with glomerulonephritis. (A) WT mice developed severe glomerulonephritis, whereas RORγt−/− mice were protected. (PAS stain; top row 400×, bottom row 200× [original magnifications]). (B) Quantification of histologic injury showed protection in RORγt−/− mice. (C) Functional injury was reduced as demonstrated by less albuminuria and lower serum urea levels in RORγt−/− mice (n = 10, each group). *P < 0.05, **P < 0.01, ***P < 0.0001.
Figure 2.
Renal leukocyte infiltration is reduced in RORγt−/− mice with glomerulonephritis. (A) Sections from WT and RORγt−/− mice stained for CD4+ T cells, macrophages, and neutrophils are shown (leukocytes in black, original magnification 400×). (B) Quantifying glomerular CD4+ T cells, macrophages, and neutrophils demonstrated fewer leukocytes in kidneys of RORγt−/− mice (n = 10, each group). *P < 0.05, ***P < 0.0001.
Immune Responses in RORγt−/− Mice
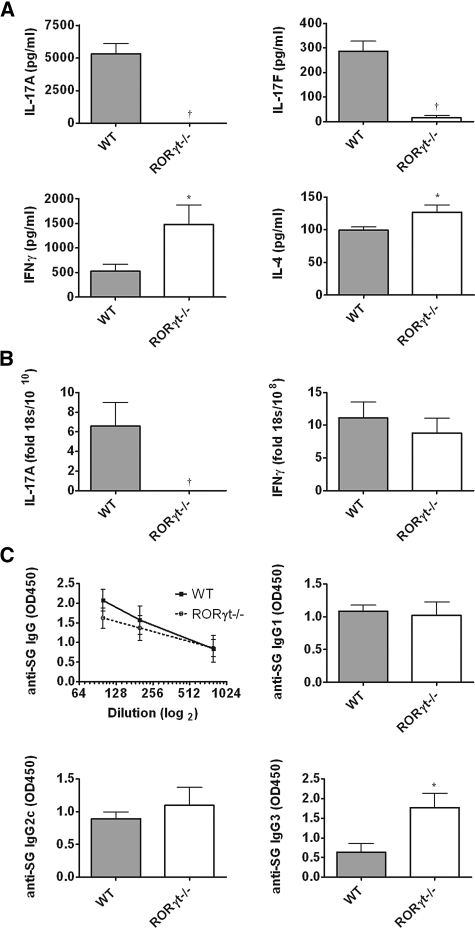
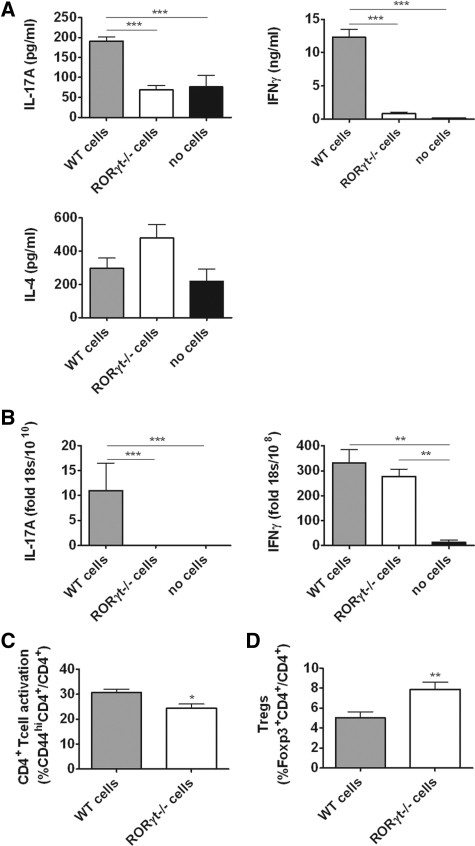
Assessment of splenocyte cytokine production showed expression of IL-17A and IL-17F only in WT animals. In contrast, increased IFNγ and IL-4 were produced by RORγt−/− splenocytes (Figure 3A). Quantification of intrarenal cytokine expression showed no measurable IL-17A mRNA in RORγt−/− mice, and levels of IFNγ were similar in both strains (Figure 3B). Similarly, splenic mRNA expression of the Th1 transcription factor Tbet was increased in RORγt−/− mice, and there was a trend toward increased expression of the Th2 transcription factor GATA3, whereas renal levels were similar. Splenic expression of the Th1-associated cytokine IL-2 was similar in both groups, and although intrarenal IL-2 mRNA expression was low, it was increased in RORγt−/− mice (Supplementary Figure S1). Renal and systemic expression of mRNA for cytokines involved in Th17 differentiation is presented in Supplementary Table 2. Analyses of humoral immune responses revealed no change in serum sheep globulin (SG)-specific IgG titers and similar production of SG-specific IgG1 and IgG2c, but IgG3 titers were enhanced in RORγt−/− mice (Figure 3C).
Figure 3.
Th17 responses are reduced in RORγt−/− mice with glomerulonephritis. (A) IL-17A and IL-17F were produced by splenocytes from WT mice but were not detectable in RORγt−/− mice. IFNγ and IL-4 production were higher in RORγt−/− mice than WT mice. (B) Intrarenal IL-17A mRNA was detectable only in WT animals, whereas IFNγ levels were similar in kidneys of both strains. (C) Serum IgG titers specific for the nephritogenic antigen (SG) were not significantly altered in RORγt−/− mice. Analyses of antigen-specific IgG subtypes showed no differences in IgG1 or IgG2c, whereas IgG3 production was enhanced in RORγt−/− mice (n = 10, each group). *P < 0.05, †P < 0.001.
To ensure that RORγt−/− mice were not suffering from a generalized immunoparesis, additional groups of mice were immunized with SG and immune responses were analyzed after 6 days. Analysis of splenic CD4+ T cell proliferation by in vivo bromodeoxyuridine (BrdU) incorporation showed higher proliferative activity in RORγt−/− animals and CD4+ T cell activation, measured by expression of CD69 and CD44, was enhanced in RORγt−/− mice. Percentages of FoxP3+ regulatory T cells (Tregs) were similar in both strains (Table 1).
Table 1.
CD4+ T cell activation in WT and RORγt−/− mice 6 days after immunization with SG
| WT | RORγt−/− | |
|---|---|---|
| CD4+ T cell proliferation (%BrdU+CD4+/CD4+) | 8.4 ± 0.7 | 13.9 ± 0.8b |
| CD4+ T cell activation (%CD69+CD4+/CD4+) | 11.5 ± 0.6 | 20.7 ± 0.9b |
| CD4+ T cell activation (%CD44hiCD4+/CD4+) | 6.0 ± 0.5 | 9.2 ± 0.9a |
| Tregs (%FoxP3+CD4+/CD4+) | 25.0 ± 1.5 | 24.6 ± 0.8 |
Splenic CD4+ T cell proliferation (BrdU incorporation) was enhanced in RORγt−/− mice. Similarly, CD4 T cell activation as assessed by expression of early (CD69) and late (CD44) activation markers was augmented in RORγt−/− mice. Proportions of Tregs were similar in the two strains of mice (n = 8 WT, n = 7 RORγt−/− mice).
aP < 0.01,
bP < 0.0001.
Splenocyte RORγt Deficiency Results in Attenuated aGBM Disease in a Rag1−/− Transfer Model
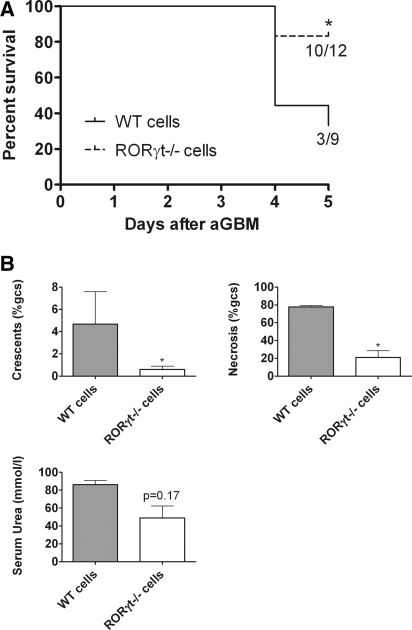
RORγt−/− mice lack Peyer patches and lymph nodes26–28 and show alterations of the splenic microenvironment.29 Therefore, splenocytes from WT and RORγt−/− mice were isolated and transferred into Rag1−/− mice. Induction of aGBM glomerulonephritis resulted in development of severe nephritis in mice that had received WT cells, with death from renal failure in most recipients within 5 days (Figure 4A). Rag1−/− mice injected with RORγt−/− splenocytes were less affected and had improved survival, and when compared with the remaining WT splenocyte recipients they developed less histologic injury and functional impairment (Figure 4B).
Figure 4.
Glomerulonephritis is ameliorated in Rag1−/− mice injected with whole splenocytes from RORγt−/− mice. (A) Survival was prolonged in Rag1−/− mice reconstituted with RORγt−/− splenocytes, whereas most mice injected with WT cells died. (B) Histologic and functional renal injury was reduced in recipients of RORγt−/− cells (n = 3 WT cells, n = 10 RORγt−/− cells). *P < 0.05.
RORγt in CD4+ T Helper Cells Is Crucial for the Development of Nephritis
Spleens from RORγ−/− mice contain lower proportions of CD4+ T cells,29 a finding that we confirmed in RORγt−/− mice by flow cytometric analyses of splenocytes (data not shown). Furthermore, RORγt expression is not only confined to Th17 cells. Therefore, Rag1−/− mice were reconstituted with CD4+ T cells isolated from WT or RORγt−/− mice. A group of control mice did not receive any cells. All mice survived until the end of the experiment (10 days after the induction of nephritis). However, the induction of aGBM glomerulonephritis caused severe renal disease in recipients of WT cells, whereas recipients of RORγt−/− CD4+ T cells were protected and had less glomerular and interstitial injury and lower albuminuria and serum urea. Mice did not develop severe nephritis in the absence of cell transfer (Figure 5, A through C). In line with these findings, glomerular (Figure 5D) and interstitial (Supplementary Table 1) CD4+ T cell, macrophage, and neutrophil infiltrates were decreased in RORγt−/− cell recipients.
Figure 5.
Renal injury and leukocyte infiltration are reduced in Rag1−/− mice reconstituted with CD4+ T cells from RORγt−/− mice. (A and B) Recipients of cells from WT mice showed severe glomerulonephritis, whereas mice receiving RORγt−/− T cells were protected. Only mild histologic injury was seen in control Rag1−/− mice not receiving cells (PAS stain; top row 400×, bottom row 200× [original magnifications]). (C) Albuminuria was lower in mice receiving RORγt−/− T cells at day 2 of nephritis (with a trend toward a decrease at day 10), whereas serum urea was lower in RORγt−/− cell recipients (and similar to control mice) compared with the WT cell recipients. (D) Quantification of glomerular CD4+ T cells, macrophages, and neutrophils showed fewer leukocytes in the glomeruli of RORγt−/− cell recipients and non-cell-injected mice (n = 10 WT cells, n = 9 RORγt−/− cells, n = 4 no cells). *P < 0.05, **P < 0.01, ***P < 0.0001.
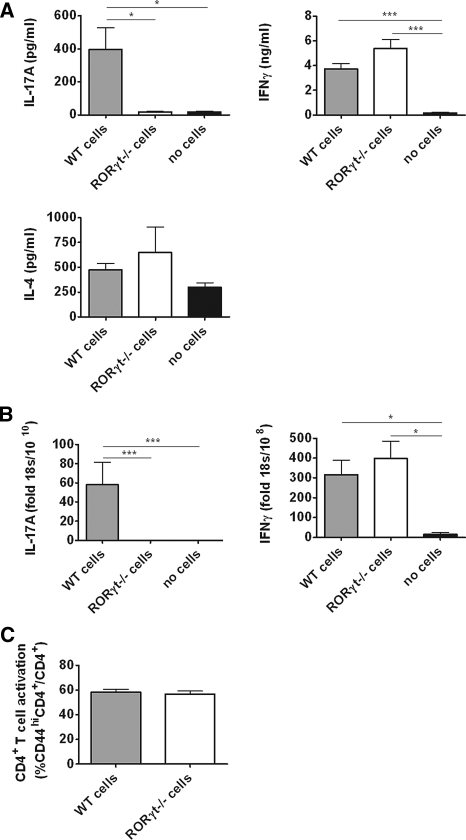
Recipients of RORγt-Deficient CD4+ T Cells Have Suppressed Th17 and Th1 Immunity
SG-stimulated splenocyte IL-17A production was high in recipients of WT cells and low in the RORγt−/− and control groups. Production of IL-17F was below the level of detection (<15.6 pg/ml) in all groups. IFNγ production was suppressed in the RORγt−/− group, suggesting effects on Th1 responses. IL-4 levels were unchanged (Figure 6A). Renal cytokine mRNA expression showed no detectable IL-17A in RORγt-deficient cell recipients and control mice. However, intrarenal IFNγ expression was not reduced in recipients of RORγt−/− cells (Figure 6B). Renal and systemic mRNA levels of lineage-specific transcription factors and cytokines involved in Th17 differentiation were analyzed (Supplementary Figure S2 and Supplementary Table 2). Flow cytometric analyses of splenocytes showed lower CD44 expression in RORγt−/− CD4+ T cells (Figure 6C). Furthermore, higher proportions of splenic FoxP3+ Tregs were found in recipients of RORγt−/− CD4+ T cells (Figure 6D).
Figure 6.
Rag1−/− mice injected with RORγt−/− CD4+ T cells show altered immune responses and higher proportions of Tregs. (A) Splenic IL-17A production was upregulated in WT cell recipients and reduced in RORγt−/− cell recipients, IFNγ production was downregulated in RORγt−/− cell recipients and absent in control mice not injected with cells, and IL-4 was similar in the three groups. (B) Intrarenal IL-17A mRNA was detectable only in WT cell recipients. Renal IFNγ mRNA expression was similar in recipients of WT and RORγt-deficient cells. (C) CD4+ T cell activation was reduced in splenocytes of RORγt−/− mice (% of CD4+ cells also CD44hi). (D) The proportion of Tregs in spleens of RORγt−/− cell recipients was higher (% of CD4+ cells also FoxP3+) (n = 10 WT cells, n = 9 RORγt−/− cells, n = 4 no cells). *P < 0.05, **P < 0.01, ***P < 0.0001.
To investigate the induction of SG-specific immune responses, Rag1−/− mice were reconstituted with WT or RORγt−/− CD4+ T cells, immunized with SG, and analyzed 6 days later. Flow cytometric analysis showed similar numbers of CD4+ T cells in spleens of both groups. In addition, analyses of spleens and lymph nodes showed similar proportions of CD4+ T cells in both groups, suggesting similar homing behavior and effective expansion of transferred cells. There were no significant differences in CD4+ T cell proliferation in spleens and draining lymph nodes, but CD4+ T cell activation was somewhat reduced, with lower CD44 expression in the draining lymph node. Changes in the proportion of Tregs in lymph nodes and spleens in recipients of RORγt-deficient T cells did not reach statistical significance at this time point (Table 2).
Table 2.
CD4+ T cell activation in Rag1−/− mice reconstituted with CD4+ T cells from WT or RORγt−/− mice 6 days after immunization with SG
| WT CD4+ Cell Recipients | RORγt−/− CD4+ Cell Recipients | |
|---|---|---|
| CD4+ T cell proportion (%CD4+/lymphocytes) | 15.4 ± 1.6 LN | 18.0 ± 2.3 LN |
| 4.3 ± 0.4 spleen | 4.50 ± 0.2 spleen | |
| CD4+ T cell number (CD4+ cells/106) | NA LN | NA LN |
| 3.3 ± 0.3 spleen | 3.3 ± 0.4 spleen | |
| CD4+ T cell proliferation (%BrdU+CD4+/CD4+) | 38.4 ± 4.0 LN | 33.1 ± 3.4 LN |
| 39.2 ± 3.9 spleen | 39.3 ± 4.6 spleen | |
| CD4+ T cell activation (%CD69+CD4+/CD4+) | 60.3 ± 0.6 LN | 53.8 ± 2.2 LN |
| 38.8 ± 1.3 spleen | 36.6 ± 2.2 spleen | |
| CD4+ T cell activation (%CD44hiCD4+/CD4+) | 11.4 ± 0.6 LN | 8.6 ± 1.0 LNa |
| 9.2 ± 0.8 spleen | 10.3 ± 1.0 spleen | |
| Tregs (%FoxP3+CD4+/CD4+) | 15.3 ± 0.9 LN | 16.9 ± 1.0 LN (P = 0.26) |
| 11.3 ± 1.1 spleen | 13.5 ± 1.0 spleen (P = 0.18) |
CD4+ T cell proliferation (flow cytometric analysis of CD4 percentages in lymph nodes [LN] and spleens as well as BrdU incorporation) was not different between recipients of RORγt-deficient and WT cells. Splenic CD4+ T cell numbers were also almost identical between the groups. CD4+ T cell activation (CD69 expression) was similar in both groups. Expression of the activation marker CD44 was significantly lower in the LN of mice receiving RORγt-deficient cells. Changes in the proportion of CD4+ cells that were Tregs did not reach statistical significance (n = 7 WT cell recipients, n = 6 RORγt−/− cell recipients). NA, not assessed.
aP < 0.05.
RORγt Deficiency Ameliorates Nephritis Independently of Tregs
Given the potential for altered Treg homeostasis in the absence of RORγt to confound results, Treg-depleted CD4+ T cell populations were prepared from splenocytes of WT and RORγt−/− mice (absence was confirmed by flow cytometric analyses of FoxP3 expression). Cells were transferred into Rag1−/− mice and aGBM nephritis was induced. Although Rag1−/− mice reconstituted with naïve WT Treg-depleted CD4+ cells developed severe glomerulonephritis with prominent glomerular necrosis (one mouse died from renal failure on day 8), mice injected with Treg-depleted CD4+ T cells from RORγt−/− mice showed less histologic and functional renal injury (Figure 7, A through C). Glomerular macrophage and neutrophil numbers were lower in recipients of RORγt−/− Treg-depleted CD4+ T cells and there was a trend toward fewer glomerular CD4+ T cells (Figure 7D), with similar findings in the interstitium (Supplementary Table 1). Rag1−/− mice not reconstituted with CD4+ T cells in which glomerulonephritis was induced did not show significant renal injury and only sparse leukocyte recruitment. Flow cytometric analyses of spleens from all experimental animals showed almost complete absence of FoxP3+ cells (%FoxP3+CD4+/CD4+ cells: 0.21 ± 0.06% WT versus 0.23 ± 0.02% RORγt−/−).
Figure 7.
Renal injury and leukocyte infiltration are reduced in Rag1−/− mice injected with Treg-depleted CD4+ T cells from naïve RORγt−/− mice. (A and B) Recipients of cells from WT mice showed severe necrotizing glomerulonephritis, whereas mice receiving RORγt−/− cells were protected. Only mild histologic injury was seen in control Rag1−/− mice not receiving cells (PAS stain; top row 400×, bottom row 200× [original magnifications]). (C) Albuminuria was reduced in mice receiving RORγt−/− cells at days 2 and 9 after the induction of nephritis to levels comparable with non-cell-injected control mice, and serum urea was lower in mice receiving RORγt-deficient cells. (D) RORγt-deficient cell recipients had a trend toward fewer CD4+ cells in glomeruli, whereas macrophages and neutrophils were significantly reduced (n = 9 WT cells, n = 6 RORγt−/− cells, n = 5 no cells). *P < 0.05, **P < 0.01, ***P < 0.0001.
Depletion of Tregs before Transfer Restores Systemic Th1 Responses and CD4+ T Cell Activation in Recipients of RORγt-Deficient Cells
To investigate the effects of RORγt deficiency on immune responses in the absence of any alterations in Treg homeostasis, cytokine production was assessed. As expected, splenocyte IL-17A production and intrarenal IL-17A mRNA were higher in the WT cell recipients, whereas only low levels were detected in mice injected with RORγt−/− cells. IL-17F was not detected. Importantly, splenocyte IFNγ production and renal IFNγ mRNA expression were not reduced in mice injected with RORγt−/− Treg-depleted CD4+ cells. Splenocyte IL-4 production was not different between the three groups (Figure 8, A and B), and CD4+ T cell activation (CD44 expression) was similar in the RORγt−/− and WT group (Figure 8C). Renal and systemic levels of further cytokines involved in Th17 differentiation were determined and the results are presented in Supplementary Table 2.
Figure 8.
CD4+ T cell activation and Th1 responses are restored in Rag1−/− mice injected with Treg-depleted CD4+ T cells from naïve RORγt−/− mice. (A) Splenic IL-17A production was upregulated in WT cell recipients and reduced in mice injected with RORγt−/− cells. IFNγ production was similar in both groups reconstituted with T cells, and IL-4 was similar in all three groups. (B) Renal IL-17A mRNA expression was detectable only in Rag1−/− mice receiving WT cells. Expression of IFNγ mRNA was similar in both groups of mice that received cells, whereas only low levels were detected in the control group. (C) CD4+ T cell activation was similar in WT and RORγt−/− mice (% of CD4+ cells also CD44hi) (n = 9 WT cells, n = 6 RORγt−/− cells, n = 5 no cells). *P < 0.05, ***P < 0.0001.
DISCUSSION
Several studies imply a pathogenic role for the Th17 subset in experimental inflammatory diseases,4–8 including glomerulonephritis,9–12,30–33 with most focusing on the Th17 effector cytokine IL-17A or the Th17 lineage-expanding cytokine IL-23. However, IL-17A is secreted not only by Th17 cells but also by other cell types, including natural killer (NK) T cells,34,35 γδ T cells,36 noncytotoxic CD8+ T cells,37 neutrophils,38 and mast cells.39 The role of Th17 cell-derived IL-17A was not defined in most previous studies. IL-23, although important in Th17 responses, is produced by and also affects innate immune cells, including macrophages and neutrophils.40,41 Relatively few studies have investigated the effects of RORγt, the key transcription factor orchestrating development of Th17 cells,3,21 on immune mediated injury. Although studies in immune disease centered on IL-17A have shown variable outcomes,13–15 those involving deficiency of RORγt have so far shown only protective effects.18,21
Because the functional role of RORγt in nephritis is unknown, we studied this key Th17 transcription factor in a T cell-dependent model of aGBM disease. Past studies18,21 have used mice with a genetic deletion of the Rorc gene resulting in absence of RORγ, which is expressed in multiple non-T cell tissues, as well as the thymic isoform RORγt, which is expressed only in leukocytes, particularly Th17 cells. The study presented here uses mice with disruption only of the Rorc(γt) element of the Rorc gene; therefore, these mice have intact RORγ expression and are selectively deficient in RORγt. As hypothesized, our results show attenuated glomerulonephritis in mice lacking RORγt. Analyses of immune responses showed intrarenal IL-17A mRNA and systemic IL-17A and IL-17F production in WT mice but undetectable levels in RORγt−/− mice, confirming that these cytokines are RORγt dependent. Cellular systemic immunity in RORγt−/− mice was Th17 deficient with modest increases in Th1 and Th2 cytokine production and largely unaltered humoral responses (aside from the Th1-associated IgG3 subclass). There was no decrease in antigen-stimulated proliferation and activation of CD4+ T cells in RORγt−/− mice, suggesting that deficient Th17 responses (rather than a more generalized immunoparesis) explain the attenuated disease in RORγt−/− mice.
However, RORγt is not only responsible for Th17 programming but also for the development of lymphoid tissue inducer cells, a lack of which results in an absence of lymph nodes and Peyer's patches.26–28 Furthermore, although trafficking properties of RORγt−/− leukocytes are intact, their egress from spleens may be impaired because of an altered splenic microenvironment.29 This trapping of activated leukocytes is likely to have contributed to the elevated Th1 and Th2 splenic cytokine production and increased CD4+ T cell proliferation found in RORγt−/− mouse spleens. To bypass these possible confounders, splenocytes from naïve WT and RORγt−/− animals were transferred into Rag1−/− mice, which lack T and B cells but display normal architecture of secondary lymphoid tissues. Induction of aGBM glomerulonephritis resulted in development of severe nephritis causing death from renal failure in most recipients of WT cells whereas mice that received RORγt−/− cells were protected.
RORγt is not only expressed in CD4+ Th17 cells but may also be found in IL-17A-secreting γδ T cells,36 CD4− NK T cell subsets,34 and RORγt-expressing NKp46+ NK-like cells.42,43 These cell types could contribute to our observation of reduced aGBM antibody-mediated heterologous-phase glomerular neutrophil infiltration in IL-17A−/− mice.10 Furthermore, spleens of RORγ−/− mice contain lower percentages of CD4+ T cells,29 which we found also to be the case in RORγt−/− mice. To exclude possible effects of lower CD4+ T cell numbers or differences in other RORγt-dependent leukocyte subsets, CD4+ T cells were isolated from the spleens of both strains of mice. After transfer of equal numbers into Rag1−/− recipients, nephritis was still attenuated in mice reconstituted with RORγt−/− cells. Control Rag1−/− mice not injected with cells did not develop severe renal disease. These CD4+ cell transfer studies are of additional interest because they provide further direct evidence for the central role for effector CD4+ T cells in this aGBM model of glomerulonephritis, consistent with previous studies.44,45 Unsurprisingly, renal and systemic IL-17A was only detectable in the WT group, but systemic IFNγ production was reduced in RORγt−/− mice, consistent with a recent report.18 In addition, although CD4+ cell proliferation was unaffected, activation was lower in recipients of RORγt-deficient cells. Because the generation of Th17 cells and Tregs is linked,46,47 RORγt deficiency could affect Treg development. Recent studies have suggested that effector T cell lineage-specific Treg subsets exist that depend on the same transcription factors as their proinflammatory counterparts.48–50 Therefore, RORγt deficiency could result in skewing of Treg subtypes and this in turn could explain the deficient T cell activation and IFNγ cytokine secretion in the face of intact T cell proliferation.51 Although we found no differences in the proportions of Tregs after immunizing RORγt−/− mice, these observations might have been influenced by the reported alterations in splenic leukocyte egress that probably leads to preferential accumulation of effector CD4+ T cells. Indeed, analyses of Tregs in reconstituted Rag1−/− mice 6 days after immunization showed a trend toward higher proportions in mice receiving RORγt−/− CD4+ T cells. Differences became statistically significant after aGBM induction, 16 days after CD4+ T cell transfer.
To exclude any possible influences of altered Treg homeostasis from effects mediated by lack of Th17 cells, Treg-depleted CD4+ T cells were isolated and transferred into Rag1−/− mice. Although the absence of Tregs caused severe glomerulonephritis in recipients of WT cells, mice receiving RORγt−/− cells still did not develop severe histologic and functional damage. Analyses of splenocytes showed almost complete absence of Tregs in both groups at the end of experiments, showing that Tregs were not generated de novo from the transferred cells. Furthermore, CD4+ T cell activation and IFNγ production by Treg-depleted RORγt−/− cell-transferred mice were similar to that found in mice reconstituted with WT cells. Therefore, amelioration of disease in Rag1−/− mice reconstituted with RORγt−/− CD4+ T cells is attributable neither to a defective Th1 response nor to altered Treg function, but it is highly likely to be due to impaired Th17 immunity. One recent study has suggested that a new RORγt-dependent Th17 subset (“natural” Th17 cells)52 rapidly produces IL-17A in a transgenic mouse model of airway inflammation without prior antigen priming. Whether these cells comprise an independent subset of Th17 cells remains to be determined. RORγt-expressing NKp46+ NK-like cells are not likely to be contributing to the reductions in disease observed in our transfer studies. These cells are not found in the spleen because they are selectively present in the gastrointestinal tract42 and have therefore not been transferred. Furthermore, these cells do not produce IL-17A42 and they are present in Rag-knockout mice,43 which were used as recipients in our studies.
Renal injury in the absence of T cell RORγt was attenuated but not abrogated. This is not surprising because nephritogenic Th1 responses can also mediate glomerulonephritis.53 In our final, most definitive study, glomerular necrosis was a prominent feature and was markedly reduced in recipients of RORγt−/− CD4+ cells depleted of Tregs, suggesting an important role for the Th17 subset in elements of glomerular injury involving necrosis. Our recent study, showing that Th1 and Th17 cells can independently induce glomerular injury with a different course and pattern, underlines the importance of Th1 and Th17 nephritogenic immune responses in the development of disease.12
In summary, the studies presented here define an important role for RORγt, the key Th17 transcription factor, in severe experimental glomerulonephritis. Not only global but also selective deficiency of RORγt in CD4+ T cells ameliorates disease. These effects were not due to altered T cell activation or proliferation per se, and they were independent of Tregs. RORγt may therefore be a potential therapeutic target in immunologically mediated renal disease.
CONCISE METHODS
Animals, Induction of Accelerated aGBM Glomerulonephritis, and Experimental Design
RORγt−/− mice on the C57BL/6 background were a kind donation from Professor D. Littman (Howard Hughes Medical Institute, New York University School of Medicine, New York)54 and were intercrossed for one further generation with the C57BL/6 strain at Monash Medical Centre (Melbourne, Australia). The RORγt−/− genotype was confirmed by PCR analysis in each animal as described.54 Age-matched C57BL/6 WT mice were used as controls. Both strains were bred at Monash Medical Centre. Rag1−/− mice were purchased from The Walter and Eliza Hall Institute (Melbourne, Australia). The Monash Medical Centre Animal Ethics Committee B approved all experiments.
WT and RORγt−/− mice (n = 10, each group) were sensitized by subcutaneous injection of 0.5 mg of SG in Freund's complete adjuvant (FCA) on day −5. For nephritis induction, SG-immunized mice were injected intraperitoneally with sheep anti-mouse GBM globulin on day 0 at a dose of 30 mg/mouse. Renal injury and systemic immunity were assessed on day 10. For assessment of antigen-specific immune responses, separate groups of mice (n = 8 WT, n = 7 RORγt−/− mice) were injected subcutaneously with SG in FCA on day −6 and culled on day 0.
For cell transfer studies, WT or RORγt−/− mice were used as donors. Whole splenocytes (1 × 107 cells/mouse; WT recipients n = 9, RORγt recipients n = 12), CD4+ T cells (4 × 106 cells/mouse; WT recipients n = 10, RORγt−/− recipients n = 9, no cells n = 4), or Treg-depleted CD4+ T cells (2 × 106 cells/mouse; WT recipients n = 9, RORγt−/− recipients n = 6, no cells n = 5) from WT or RORγt−/− mice were injected intravenously into Rag1−/− mice on day −6. Mice were immunized by subcutaneous injection of 0.5 mg SG in FCA on day −5. Nephritis was induced in SG-immunized Rag1−/− mice by intraperitoneal injection of sheep anti-mouse GBM globulin on day 0 at a dose of 30 mg/mouse in whole splenocyte transfer and 25 mg/mouse in CD4+ T cell- and Treg-depleted CD4+ T cell transfer studies. In Rag1−/− mice receiving splenocytes, the experiment was terminated on day 6 and survival and renal injury were assessed. Renal injury and systemic immunity were assessed on day 10 in mice receiving unfractionated CD4+ T cells and on day 9 in mice receiving Treg-depleted CD4+ T cells. For assessment of antigen-specific immune responses, further groups of Rag1−/− mice were intravenously injected with unfractionated CD4+ T cells on day −7 (WT recipients n = 7, RORγt−/− recipients n = 6), followed by subcutaneous immunization with SG in FCA on day −6. Immune responses were studied on day 0.
Assessment of Renal Injury
Glomerular abnormalities were assessed on periodic acid–Schiff (PAS)-stained, Bouin-fixed, 3-μm-thick, paraffin-embedded sections using coded slides. Abnormalities recorded included crescent formation, (two or more layers of cells visible in Bowman's space55) and severe necrosis (>50% of the glomerular tuft affected). A minimum of 50 glomeruli was analyzed per animal to determine the percentage of crescentic and severely necrotic glomeruli. Semiquantitative analysis of tubulointerstitial damage was performed in each mouse as previously published53 using ten randomly selected cortical areas (×200). Injury was defined as tubular dilation, tubular atrophy, sloughing of tubular epithelial cells, or thickening of the basement membrane.56 Each cortical field was scored (0 to 4) according to the amount of injury: 0, no interstitial damage; 1, <25% of the tubulointerstitium damaged; 2, 25% to 50% damaged; 3, 50% to 75% damaged; and 4, >75% of the tubulointerstitium damaged. For urine collection, mice were housed for 24 hours in metabolic cages at the time points indicated with free access to tap water, whereas serum was collected after mice were humanely culled. Albuminuria was analyzed by ELISA according to the manufacturers' instructions (Mice-Albumin Kit, Bethyl, Montgomery, TX). Serum urea was measured using standard laboratory methods at Monash Medical Centre.
To evaluate renal leukocyte infiltration, kidney sections were fixed in periodate lysine paraformaldehyde for 4 hours, washed with 20% sucrose solution, and then frozen. Tissue sections were cut and a three-layer immunoperoxidase technique was used to stain for T cells, neutrophils, and macrophages.35,55 The primary antibodies used were GK1.5 (anti-mouse CD4; American Type Culture Collection [ATCC], Manassas, VA), RB6–8C5 (anti-Gr-1; DNAX, Palo Alto, CA) for neutrophils, and FA/11 for macrophages (anti-mouse CD68; provided by Dr. G. Koch, MRC Laboratory of Molecular Biology, Cambridge, United Kingdom). The secondary antibody used was rabbit anti-rat biotin (BD Biosciences, San Diego, CA). At least 25 consecutively viewed glomeruli were assessed per animal and results were expressed as cells per glomerular cross section. For assessment of interstitial leukocyte infiltration, 20 randomly selected cortical areas (×400) were analyzed.
Real-Time RT-PCR Analysis
For real-time PCR analyses, 500 ng of RNA was treated with 1 unit of amplification-grade DNase I (Invitrogen, Melbourne, Australia), primed with random primers (Applied Biosystems, Foster City, CA), and reverse transcribed using a high-capacity cDNA reverse transcription kit (Applied Biosystems). Real-time PCR was performed on a Rotor Gene RG-3000 (Corbett Research, Mortlake, Australia) using FastStart DNA master Sybr Green I (Roche, Melbourne, Australia) in the case of IFNγ, IL-6, TGFβ, Tbet, GATA3, and FoxP3. For detection of IL-17A, IL-2, and IL-21, TaqMan predesigned real-time PCR assays were used (Applied Biosystems). Results are expressed as fold change relative to the housekeeping gene 18s. Primer sequences are available on request.
Systemic Cellular and Humoral Immune Responses
Spleens were removed and a single cell suspension was obtained. Splenocytes (4 × 106 cells/ml) were cultured in RPMI/10% FCS with protein G-purified normal sheep IgG (10 μg/ml) at 37°C for 72 hours. IFNγ, IL-4, IL-17A, and IL-17F concentrations were measured by ELISA as described previously.53 The following antibodies were used: rat anti-mouse IFNγ (R4–6A2; BD Biosciences), biotinylated rat anti-mouse IFNγ (XMG1.2; BD Biosciences), rat anti-mouse IL-4 (11B11; ATCC), and biotinylated rat anti-mouse IL-4 (BVD6; DNAX). For IL-17A and IL-17F, ELISA kits (DuoSet, R&D systems, Minneapolis, MN) were used. For assessment of proliferation, spleens and/or lymph nodes were obtained 6 days after immunization of experimental mice with SG in FCA. For measurements of proliferation, mice were administered an intraperitoneal injection of 1 mg of BrdU (Sigma-Aldrich, St. Louis, MO) 48, 36, 24, and 12 hours before organ harvesting. Proliferation of CD4+ T cells was assessed by flow cytometric analysis of intracellular BrdU incorporation.
ELISA was used to detect circulating serum SG-specific IgG titers as described previously.57 Horseradish-peroxidase-conjugated sheep anti-mouse IgG (1:100, 1:200, and 1:800 dilutions, Amersham Biosciences, Rydalmere, Australia), horseradish-peroxidase-conjugated-conjugated goat anti-mouse IgG2c (dilution 1:200, Quantum Scientific, Murrarie, Australia [C57BL/6 mice express IgG2c instead of IgG2a]), biotinylated goat anti-mouse IgG1 (1:100, Silenius, Boronia, Australia), and rat anti-mouse IgG3 (1:10, BD Biosciences) antibodies were used. Results are expressed as OD450.
Isolation of Splenocyte Population for In Vivo Transfer
CD4+ T cells from splenocytes were isolated by magnetic sorting (MACS, Miltenyi, Bergisch Gladbach, Germany) using the L3T4 microbead antibody (Miltenyi). For isolation of Treg-depleted CD4+ T cells, splenocytes were first depleted of CD25+ cells by negative selection using an LD column (CD25 MicroBead kit and LD columns; both Miltenyi). Afterward, CD4+ T cells were isolated using the L3T4 antibody. Purity of CD4+ T cell preparations were assessed by flow cytometric analysis and showed 90.2 ± 3.1% CD4+ T cells. Absence of Tregs was validated using the FoxP3 staining kit (<0.25% FoxP3+ CD4+ T cells before transfer and at the end of experiments in both groups).
Flow Cytometry
Antibodies used for multicolor flow cytometric analysis were as follows: CD4-FITC, CD4-PE, CD4-APC/Cy7, CD25-FITC, CD69-PE, CD44-APC, and anti-BrdU-FITC (all BD Bioscience). For evaluation of FoxP3, a commercially available FoxP3 kit (eBiosciences, San Diego, CA) was used. Flow cytometric analyses were performed on a Mo-Flo flow cytometer (Dako, Botany, New South Wales, Australia). Data were analyzed with the Summit version 4.3 software. To assess absolute splenic CD4+ T cell numbers, percentages were multiplied by results from splenocyte cell counts (derived using a standard hemocytometer).
Statistical Analysis
Analyses were performed using the GraphPad Prism software. Differences between two groups were calculated using a t test; for three experimental groups, ANOVA analysis was performed with subsequent post hoc analysis according to Tukey. For analysis of survival, a log rank test was used. P values <0.05 were considered statistically significant.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
The authors thank Professor D. Littman (Howard Hughes Medical Institute, New York University School of Medicine, New York) for the RORγt−/− mice and Ms A. Wright and Ms K. O'Sullivan for excellent technical assistance.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Constructing an Immune System for Glomerulonephritis Studies, ” on pages 398–399.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Tipping PG, Holdsworth SR: T cells in crescentic glomerulonephritis. J Am Soc Nephrol 17: 1253–1263, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Kitching AR, Holdsworth SR, Tipping PG: Crescentic glomerulonephritis—A manifestation of a nephritogenic Th1 response? Histol Histopathol 15: 993–1003, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Korn T, Bettelli E, Oukka M, Kuchroo VK: IL-17 and Th17 cells. Annu Rev Immunol 27: 485–517, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Nakae S, Nambu A, Sudo K, Iwakura Y: Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol 171: 6173–6177, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Bush KA, Farmer KM, Walker JS, Kirkham BW: Reduction of joint inflammation and bone erosion in rat adjuvant arthritis by treatment with interleukin-17 receptor IgG1 Fc fusion protein. Arthritis Rheum 46: 802–805, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Chen Y, Langrish CL, McKenzie B, Joyce-Shaikh B, Stumhofer JS, McClanahan T, Blumenschein W, Churakovsa T, Low J, Presta L, Hunter CA, Kastelein RA, Cua DJ: Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest 116: 1317–1326, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hofstetter HH, Ibrahim SM, Koczan D, Kruse N, Weishaupt A, Toyka KV, Gold R: Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol 237: 123–130, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D: IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest 116: 1310–1316, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ooi JD, Phoon RK, Holdsworth SR, Kitching AR: IL-23, not IL-12, directs autoimmunity to the Goodpasture antigen. J Am Soc Nephrol 20: 980–989, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gan PY, Steinmetz OM, Tan DS, O'Sullivan KM, Ooi JD, Iwakura Y, Kitching AR, Holdsworth SR: Th17 cells promote autoimmune anti-myeloperoxidase glomerulonephritis. J Am Soc Nephrol 21: 925–931, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paust HJ, Turner JE, Steinmetz OM, Peters A, Heymann F, Holscher C, Wolf G, Kurts C, Mittrucker HW, Stahl RA, Panzer U: The IL-23/Th17 axis contributes to renal injury in experimental glomerulonephritis. J Am Soc Nephrol 20: 969–979, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Summers SA, Steinmetz OM, Li M, Kausman JY, Semple T, Edgtton KL, Borza DB, Braley H, Holdsworth SR, Kitching AR: Th1 and Th17 cells induce proliferative glomerulonephritis. J Am Soc Nephrol 20: 2518–2524, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haak S, Croxford AL, Kreymborg K, Heppner FL, Pouly S, Becher B, Waisman A: IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. J Clin Invest 119: 61–69, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y: Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin Immunol 110: 55–62, 2004 [DOI] [PubMed] [Google Scholar]
- 15. O'Connor W, Jr, Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, Kolls JK, Flavell RA: A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol 10: 603–609, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bettelli E, Korn T, Oukka M, Kuchroo VK: Induction and effector functions of T(H)17 cells. Nature 453: 1051–1057, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nowak EC, Weaver CT, Turner H, Begum-Haque S, Becher B, Schreiner B, Coyle AJ, Kasper LH, Noelle RJ: IL-9 as a mediator of Th17-driven inflammatory disease. J Exp Med 206: 1653–1660, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leppkes M, Becker C, Ivanov II, Hirth S, Wirtz S, Neufert C, Pouly S, Murphy AJ, Valenzuela DM, Yancopoulos GD, Becher B, Littman DR, Neurath MF: RORgamma-expressing Th17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology 136: 257–267, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH: A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100: 655–669, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Zheng W, Flavell RA: The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 89: 587–596, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR: The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126: 1121–1133, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C: T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity 28: 29–39, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Manel N, Unutmaz D, Littman DR: The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol 9: 641–649, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhu J, Yamane H, Paul WE: Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol 28: 445–489, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang Z, Xie H, Wang R, Sun Z: Retinoid-related orphan receptor gamma t is a potential therapeutic target for controlling inflammatory autoimmunity. Expert Opin Ther Targets 11: 737–743, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR: Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science 288: 2369–2373, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Eberl G, Littman DR: The role of the nuclear hormone receptor RORgammat in the development of lymph nodes and Peyer's patches. Immunol Rev 195: 81–90, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Kurebayashi S, Ueda E, Sakaue M, Patel DD, Medvedev A, Zhang F, Jetten AM: Retinoid-related orphan receptor gamma (RORgamma) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis. Proc Natl Acad Sci U S A 97: 10132–10137, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang N, Guo J, He YW: Lymphocyte accumulation in the spleen of retinoic acid receptor-related orphan receptor gamma-deficient mice. J Immunol 171: 1667–1675, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Steinmetz OM, Turner JE, Paust HJ, Lindner M, Peters A, Heiss K, Velden J, Hopfer H, Fehr S, Krieger T, Meyer-Schwesinger C, Meyer TN, Helmchen U, Mittrucker HW, Stahl RA, Panzer U: CXCR3 mediates renal Th1 and Th17 immune response in murine lupus nephritis. J Immunol 183: 4693–4704, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Turner JE, Paust HJ, Steinmetz OM, Panzer U: The Th17 immune response in renal inflammation. Kidney Int 77: 1070–1075, 2010 [DOI] [PubMed] [Google Scholar]
- 32. Zhang Z, Kyttaris VC, Tsokos GC: The role of IL-23/IL-17 axis in lupus nephritis. J Immunol 183: 3160–3169, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kyttaris VC, Zhang Z, Kuchroo VK, Oukka M, Tsokos GC: Cutting edge: IL-23 receptor deficiency prevents the development of lupus nephritis in C57BL/6-lpr/lpr mice. J Immunol 184: 4605–4609, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Coquet JM, Chakravarti S, Kyparissoudis K, McNab FW, Pitt LA, McKenzie BS, Berzins SP, Smyth MJ, Godfrey DI: Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc Natl Acad Sci U S A 105: 11287–11292, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rachitskaya AV, Hansen AM, Horai R, Li Z, Villasmil R, Luger D, Nussenblatt RB, Caspi RR: Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol 180: 5167–5171, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roark CL, Simonian PL, Fontenot AP, Born WK, O'Brien RL: Gammadelta T cells: An important source of IL-17. Curr Opin Immunol 20: 353–357, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu SJ, Tsai JP, Shen CR, Sher YP, Hsieh CL, Yeh YC, Chou AH, Chang SR, Hsiao KN, Yu FW, Chen HW: Induction of a distinct CD8 Tnc17 subset by transforming growth factor-beta and interleukin-6. J Leukoc Biol 82: 354–360, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Li L, Huang L, Vergis AL, Ye H, Bajwa A, Narayan V, Strieter RM, Rosin DL, Okusa MD: IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest 120: 331–342, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hueber AJ, Asquith DL, Miller AM, Reilly J, Kerr S, Leipe J, Melendez AJ, McInnes IB: Mast cells express IL-17A in rheumatoid arthritis synovium. J Immunol 184: 3336–3340, 2010 [DOI] [PubMed] [Google Scholar]
- 40. Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, Powrie F, Maloy KJ: Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med 203: 2473–2483, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Awasthi A, Riol-Blanco L, Jager A, Korn T, Pot C, Galileos G, Bettelli E, Kuchroo VK, Oukka M: Cutting edge: IL-23 receptor gfp reporter mice reveal distinct populations of IL-17-producing cells. J Immunol 182: 5904–5908, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, Diefenbach A: RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol 10: 83–91, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Luci C, Reynders A, Ivanov II, Cognet C, Chiche L, Chasson L, Hardwigsen J, Anguiano E, Banchereau J, Chaussabel D, Dalod M, Littman DR, Vivier E, Tomasello E: Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat Immunol 10: 75–82, 2009 [DOI] [PubMed] [Google Scholar]
- 44. Huang XR, Tipping PG, Shuo L, Holdsworth SR: Th1 responsiveness to nephritogenic antigens determines susceptibility to crescentic glomerulonephritis in mice. Kidney Int 51: 94–103, 1997 [DOI] [PubMed] [Google Scholar]
- 45. Li S, Holdsworth SR, Tipping PG: Antibody independent crescentic glomerulonephritis in mu chain deficient mice. Kidney Int 51: 672–678, 1997 [DOI] [PubMed] [Google Scholar]
- 46. Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK: Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441: 235–238, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Littman DR, Rudensky AY: Th17 and regulatory T cells in mediating and restraining inflammation. Cell 140: 845–858, 2010 [DOI] [PubMed] [Google Scholar]
- 48. Feuerer M, Hill JA, Mathis D, Benoist C: Foxp3+ regulatory T cells: Differentiation, specification, subphenotypes. Nat Immunol 10: 689–695, 2009 [DOI] [PubMed] [Google Scholar]
- 49. Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY: CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science 326: 986–991, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Steinmetz OM, Turner JE, Panzer U: Staying on top of things right from the start: The obsessive-compulsive disorder of regulatory T cells. J Am Soc Nephrol 21: 6–7, 2010 [DOI] [PubMed] [Google Scholar]
- 51. Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ: CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol 8: 1353–1362, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Tanaka S, Yoshimoto T, Naka T, Nakae S, Iwakura Y, Cua D, Kubo M: Natural occurring IL-17 producing T cells regulate the initial phase of neutrophil mediated airway responses. J Immunol 183: 7523–7530, 2009 [DOI] [PubMed] [Google Scholar]
- 53. Phoon RK, Kitching AR, Odobasic D, Jones LK, Semple TJ, Holdsworth SR: T-bet deficiency attenuates renal injury in experimental crescentic glomerulonephritis. J Am Soc Nephrol 19: 477–485, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR: An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol 5: 64–73, 2004 [DOI] [PubMed] [Google Scholar]
- 55. Kitching AR, Tipping PG, Timoshanko JR, Holdsworth SR: Endogenous interleukin-10 regulates Th1 responses that induce crescentic glomerulonephritis. Kidney Int 57: 518–525, 2000 [DOI] [PubMed] [Google Scholar]
- 56. Rangan GK, Pippin JW, Coombes JD, Couser WG: C5b-9 does not mediate chronic tubulointerstitial disease in the absence of proteinuria. Kidney Int 67: 492–503, 2005 [DOI] [PubMed] [Google Scholar]
- 57. Tipping PG, Kitching AR, Huang XR, Mutch DA, Holdsworth SR: Immune modulation with interleukin-4 and interleukin-10 prevents crescent formation and glomerular injury in experimental glomerulonephritis. Eur J Immunol 27: 530–537, 1997 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.