Abstract
The thiazide-sensitive NaCl cotransporter (NCC) plays a key role in renal salt reabsorption and the determination of systemic BP, but the molecular mechanisms governing the regulation of NCC are not completely understood. Here, through pull-down experiments coupled to mass spectrometry, we found that γ-adducin interacts with the NCC transporter. γ-Adducin colocalized with NCC to the distal convoluted tubule. 22Na+ uptake experiments in the Xenopus laevis oocyte showed that γ-adducin stimulated NCC activity in a dose-dependent manner, an effect that occurred upstream from With No Lysine (WNK) 4 kinase. The binding site of γ-adducin mapped to the N terminus of NCC and encompassed three previously reported phosphorylation sites. Supporting this site of interaction, competition with the N-terminal domain of NCC abolished the stimulatory effect of γ-adducin on the transporter. γ-Adducin failed to increase NCC activity when these phosphorylation sites were constitutively inactive or active. In addition, γ-adducin bound only to the dephosphorylated N terminus of NCC. Taken together, our observations suggest that γ-adducin dynamically regulates NCC, likely by amending the phosphorylation state, and consequently the activity, of the transporter. These data suggest that γ-adducin may influence BP homeostasis by modulating renal NaCl transport.
Hypertension is a highly prevalent clinical condition and has been estimated to affect as many as 1.56 billion individuals in the year 2025.1 Primary hypertension is a key contributor to cardiovascular complications. As such, hypertension has been estimated to account for a large fraction of strokes and ischemic heart disease on a global scale.2 The National Vital Statistics Report lists heart disease as the primary cause of death, affecting more than 20 million people in the United States alone.3 In addition, elevated BP is a leading cause in the development of chronic renal failure.4 According to the Guytonian model, the kidney plays an essential role in chronic BP maintenance by adjusting blood volume in response to changes in systemic pressure.5 Such modifications are achieved in part by amending the urinary excretion of NaCl.
The Joint National Committee for the detection, evaluation, and treatment of high BP recommends thiazides as the first line treatment of uncomplicated stage I hypertension.6 Thiazides act by blocking NaCl reabsorption in the distal convoluted tubule (DCT), which reclaims 5 to 10% of Na+ from the renal ultrafiltrate.7 It is therefore not surprising that disorders disturbing transport processes in this segment affect the renal reabsorptive capacity for NaCl and thereby influence systemic BP. The thiazide-sensitive NaCl transporter (NCC) is responsible for the majority of inward Na+ transport in the DCT. In line with this, renal NaCl transport and systemic BP can be changed in Gitelman patients8,9 and patients with pseudohypoaldosteronism type II.10,11
A better understanding of the regulation of NaCl transport by NCC may ultimately increase our understanding of how BP is maintained and the etiology of underlying primary hypertension. During the last decade, research within this field has greatly expanded our knowledge about how NaCl transport via NCC is controlled. The cotransporter contains several phosphorylation sites in its N-terminal domain, a feature that is well conserved among several members of the SLC12 family.12–15 Phosphorylation of these residues is critically important for the activation of NCC in response to chloride (Cl−) depletion.12 Serine/threonine kinases of the STE20 family (such as the Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1)) as well as the With No Lysine (WNK) kinase family have been shown to play an important role in modulating the phosphorylation state of NCC.16–19 Genetically modified mice strains largely support these observations and solidify the important role of NCC regulation in BP maintenance.20–25
The aim of this study was to identify novel interactors of NCC that could be involved in modulating its function. Because the N-terminal domain has been shown to play an important role in activation of the transporter, pull-down experiments were performed with this domain of NCC in mouse kidney lysates and subsequently coupled with mass spectrometry. This study describes the identification of γ-adducin as a novel auxiliary factor interacting with NCC. Adducins were originally identified as heteromeric cytoskeletal proteins implicated in the binding of spectrin to actin.26 γ-Adducin was selected as an interesting candidate on the basis of the previous involvement of this protein family in primary hypertension in both humans and rats.27–29 Here, we delineated the molecular basis by which γ-adducin stimulates the activity of NCC.
RESULTS
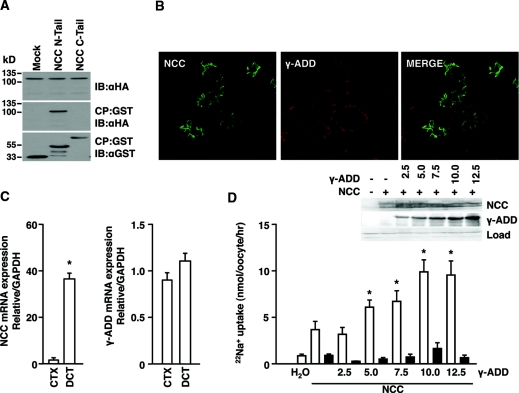
To investigate potential binding partners of the N-terminal domain of NCC, pull-down experiments were performed to screen mouse kidney lysates. The glutathione S-transferase (GST)-coupled N-terminal domain of NCC was used as bait, and the associated proteins were identified by mass spectrometry. The selection of the proteins of interest was based on localization to the distal nephron within the kidney and whether they had previously been involved in hypertension. γ-Adducin was an interesting candidate that was chosen for further evaluation. The interaction was confirmed by pull-down experiments in human embryonic kidney (HEK) 293 cells transiently transfected with GST-conjugated terminal domains of NCC and hemagglutinin (HA)-tagged γ-adducin. The resulting cell lysates were incubated with glutathione-coupled Sepharose beads to precipitate GST-bound complexes. Binding was seen by γ-adducin toward the N-terminal domain of NCC (Figure 1A).
Figure 1.
γ-Adducin binds the N terminus of NCC and stimulates the activity of the cotransporter. (A) GST pull-down assay in HEK293 cells transiently transfected with either the GST-tagged N or C terminus of NCC or GST alone, all in combination with γ-adducin, was used to evaluate binding of γ-adducin to NCC. CP denotes coprecipitate, and IB represents the protein immunoblotted against. (B) γ-Adducin localizes with NCC to the DCT. Representative immunohistochemical images are shown as obtained by confocal laser microscopy. The sections were incubated with specific antibodies against NCC and γ-adducin and stained using Alexa-conjugated secondary antibodies. (C) γ-Adducin mRNA is expressed in the DCT as well as in other nephron segments. Semiquantitative real-time PCR was used to determine the abundance of NCC and γ-adducin mRNA extracted from fluorescently sorted DCT tubules and the kidney cortex (CTX). (D) Effect of γ-adducin upon NCC function. γ-Adducin stimulated NCC activity in a dose-dependent manner. 22Na+ uptakes in X. laevis oocytes microinjected with water, 5 ng of HA-tagged human NCC cRNA alone, or in combination with increasing amounts of HA-tagged γ-adducin cRNA (n = 8). The experiment was performed in the absence (open bars) or presence (closed bars) of 0.1 mM thiazide. The data are presented as the means ± SEM. *P < 0.05 is statistically significant as compared with NCC-injected oocytes. Western blots were used to quantify the expression of NCC and γ-adducin in groups of oocytes injected as described above. The data are presented as the means ± SEM.
γ-Adducin has previously been localized to the renal distal tubules,30 whereas actual colocalization with NCC in the DCT was never reported. Immunofluorescence labeling showed that γ-adducin localizes to the NCC-expressing DCT tubules (Figure 1B). Here, γ-adducin localized to cytoplasmic and (baso)-lateral domains of the cell. γ-Adducin was not restricted to the DCT, because expression was also found in the proximal tubule and thick ascending limb as reported previously.31
To evaluate whether the abundance of γ-adducin is enriched in the DCT in comparison with other tubules in the renal cortex, DCT fragments were isolated from mice expressing enhanced green fluorescence protein (eGFP) after the parvalbumin promoter.32 The restricted renal expression of this protein to the DCT allowed fluorescence-based sorting of the segment using a complex object parametric analyzer and sorter (COPAS).33 In line with the more ubiquitous expression pattern of the protein as observed by immunohistochemistry, the relative enrichment of γ-adducin mRNA in the DCT remained unaltered, whereas that for NCC was markedly enriched (Figure 1C).
To estimate the functional effect of γ-adducin on the transport capacity of NCC, 22Na+ uptake studies were performed using the Xenopus laevis oocyte system. Oocytes injected with cRNA encoding NCC showed a significant thiazide-sensitive 22Na+ uptake compared with water-injected oocytes. Coinjection with increasing amounts of γ-adducin cRNA stimulated NCC activity in a dose-dependent manner (Figure 1D). In addition, no difference in the expression level of HA-NCC could be detected between oocytes injected without or with increasing concentrations of HA-tagged γ-adducin in the presence of HA-NCC. A dose-dependent increase was observed in the abundance of the HA-tagged γ-adducin.
To evaluate whether γ-adducin plays a role in maintaining basal NCC-dependent 22Na+ transport in the oocyte, small interference RNA (siRNA) against endogenous X. laevis γ-adducin was injected in oocytes preinjected with NCC. When siRNA against γ-adducin was injected, a significant decrease in thiazide-sensitive 22Na+ uptake was observed (Supplemental Figure 1A). Because the related family member α-adducin does not affect NCC-dependent transport (Supplemental Figure 1B), the oocytes were injected with siRNA against α-adducin as a negative control. Injection of α-adducin siRNA did not significantly affect the activity of NCC.
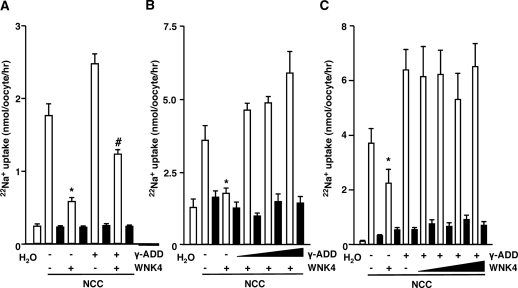
To investigate whether γ-adducin affects NCC activity via the same signaling cascade as WNK4, the effect of WNK4 on γ-adducin-stimulated Na+ transport was evaluated. WNK4 has been shown to reduce NCC activity by directing the protein to the lysosomal compartment.34,35 In line with this, WNK4 was able to inhibit NCC-dependent 22Na+ uptake (Figure 2A). Importantly, the stimulatory effect of γ-adducin was also observed in the presence of WNK4 (Figure 2A). Moreover, injections of increasing concentrations of γ-adducin reverted the inhibitory effect of WNK4 on NCC-mediated 22Na+ uptake (Figure 2B). Furthermore, the effect of γ-adducin abrogated the WNK4-dependent reduction in NCC activity, because the stimulatory effect of γ-adducin was still present when increasing amounts of WNK4 cRNA were injected (Figure 2C).
Figure 2.
γ-Adducin reverts the inhibitory effect of WNK4. (A) WNK4 did not inhibit γ-adducin-stimulated NCC activity. X. laevis oocytes were injected with 5 ng of NCC cRNA, in the presence or absence of 10 ng of γ-adducin as well as 10 ng of WNK4 cRNA (n = 2). (B) Increasing amounts of γ-adducin reverted the inhibitory effect of WNK4 on NCC. 22Na+ uptakes in oocytes coinjected with 5 ng of NCC cRNA, 10 ng of WNK4 cRNA, and increasing amounts of γ-adducin cRNA (from 20 to 40 ng) (n = 3). (C) WNK4 did not revert the stimulatory effect of γ-adducin. 22Na+ uptakes in oocytes coinjected with 5 ng of NCC cRNA, 10 ng of γ-adducin cRNA, and increasing amounts of WNK4 cRNA (from 20 to 60 ng) (n = 3). The closed bars denote the presence of 0.1 mM thiazide. *P < 0.05 is statistically significant from NCC-injected oocytes. #P < 0.05 is statistically significant from the group coinjected with WNK4 and NCC.
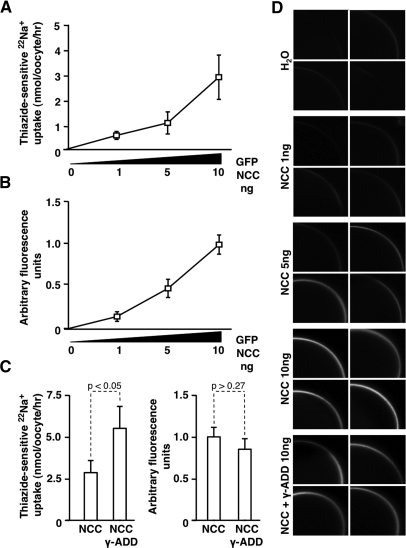
The stimulatory effect of γ-adducin on NCC may be explained by increased trafficking of the transporter to the plasma membrane or by changes in the intrinsic activity of the transporter. To evaluate whether the membrane abundance of NCC increases in the presence of γ-adducin, cell surface expression of eGFP-NCC was determined by confocal laser scanning microscopy. The intensity of the eGFP signal at the plasma membrane as well as the 22Na+ uptake rates correlated dose-dependently with the amount of injected eGFP-NCC cRNA (Figure 3, A and B). Thus, this method was used to determine semiquantitatively the presence of NCC at the plasma membrane. Coinjection of γ-adducin significantly increased eGFP-NCC-dependent 22Na+ uptake, whereas the auxiliary protein γ-adducin did not significantly affect the plasma membrane expression of eGFP-NCC (Figure 3, C and D).
Figure 3.
The effect of γ-adducin occurs independent of NCC trafficking to the oocyte membrane. (A) 22Na+ uptakes in X. laevis oocytes injected with 1 to 10 ng of GFP-NCC. cRNA (n = 4). (B) Corresponding GFP fluorescence at the membrane of oocytes injected with 1 to 10 ng of GFP-NCC cRNA (n = 4). (C) Effect of thiazide-sensitive 22Na+ uptake (n = 4) (left panel) and quantification of the GFP fluorescence in the membrane (n = 4) (right panel) of X. laevis oocytes injected with 10 ng of GFP-NCC cRNA in the presence or absence of 10 ng of γ-adducin cRNA. (D) Representative images of GFP fluorescence at the oocyte membrane after injection with increasing amounts of GFP-NCC cRNA as well as the group coinjected with 10 ng of γ-adducin cRNA using confocal laser scanning microscopy. *P < 0.05 is statistically significant.
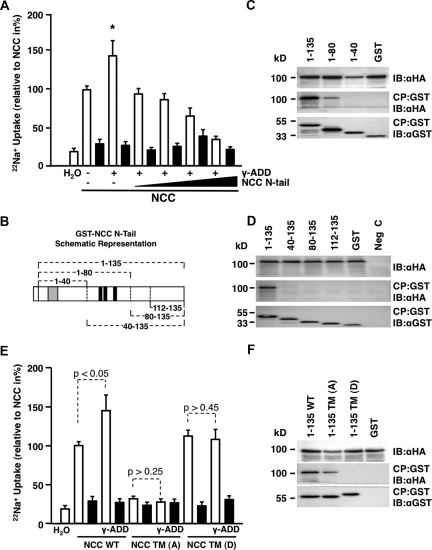
To delineate whether γ-adducin affects NCC function by its interaction with the N terminus of NCC, competition assays were performed in which NCC and γ-adducin were coinjected together with increasing amounts of the N terminus of NCC. During these conditions, injection of the NCC N terminus abolished the stimulatory effect of γ-adducin on NCC activity (Figure 4A).
Figure 4.
Phosphorylation of the N terminus of NCC is essential for the stimulatory effect of γ-adducin. (A) The ability of the NCC N-terminal domain to inhibit γ-adducin-stimulated transport of the cotransporter as evaluated by 22Na+ uptakes. The oocytes were coinjected with 5 ng of NCC cRNA, 10 ng of γ-adducin cRNA, and increasing amounts of the N-terminal domain of NCC (from 2.5 to 10 ng) (n = 3). *P < 0.05 is statistically significant from all other groups. (B) Schematic drawing representing the GST fusion proteins containing different portions of the N-terminal tail of NCC. These were generated to map the binding site of γ-adducin to NCC. Black domains represent the N-terminal phosphorylation sites, whereas gray denotes the SPAK binding site. (C) GST pull-down assay in HEK293 cells. Deletion fragments of the NCC N-terminal domain (amino acids 1 to 135, 1 to 80, and 1 to 40) coupled to GST were generated to determine the HA-γ-adducin-binding site within the cotransporter. (D) GST pull-down assay repeated, but the N terminus was truncated from the opposite end (amino acids 1 to 135, 40 to 135, 80 to 135, and 112 to 135). Note that γ-adducin binds only in the presence of the full-length terminus or the 1 to 80 fragment. (E) The effect of γ-adducin requires phosphorylatable NCC sites. 22Na+ uptakes were performed in oocytes injected with 5 ng of NCC cRNA, 5 ng of constitutively inactive (NCC TM(A); T55A, T60A, S73A), or 5 ng of the constitutively active (NCC TM(D); T55D, T60D, S73D) NCC phospho-mutants, in the presence or absence of 10 ng of γ-adducin cRNA (n = 2). (F) GST pull-down assay in HEK293 cells. Coprecipitation of HA-tagged γ-adducin with the native GST-N terminus and the GST-N terminus containing constitutively inactive and active phosphorylation sites. CP denotes coprecipitate, IB indicates the protein that was immunoblotted, and NCC WT indicates wild-type NCC. The 22Na+ uptake experiments were performed in the presence (closed bars) or absence (open bars) of 0.1 mM thiazide. *P < 0.05 is statistically significant from NCC-injected oocytes.
The binding site of γ-adducin in the N terminus of NCC was subsequently mapped by pull-down analysis. For determination of the binding site, a series of fragments from the N-terminal domain of NCC were generated as depicted in Figure 4B, with residues 1 to 135 representing the full-length N-tail. All of the N-terminal fragments were fused to GST to allow precipitation using glutathione-coupled Sepharose beads. N-terminal deletion fragments of NCC were expressed transiently in HEK293 cells together with γ-adducin. GST pull-down experiments revealed that γ-adducin only binds the N terminus when the 1 to 80 fragment is present, albeit at a lower affinity than to the full length N-tail (Figure 4, C and D).
Because the part of the N-terminal domain of NCC that binds γ-adducin encompasses the three phosphorylation sites of NCC (Thr55, Thr60, and Ser73 in human NCC), which previously have been shown necessary for activation of the protein in response to Cl− depletion,12 the role of γ-adducin on the phosphorylation of NCC was evaluated. γ-Adducin was coinjected with triple phosphorylation site NCC mutants (converted to alanines (A) or aspartates (D) to mimic either the constitutively inactive or active sites, respectively), and 22Na+ uptakes were performed. The stimulatory effect of γ-adducin on NCC-mediated Na+ transport was lost when the three phosphorylation sites were converted into constitutively active sites (D) (Figure 4E), suggesting an important role of γ-adducin in modulating the phosphorylation of NCC. Similarly, when the N-terminal phosphorylation sites were converted to inactive phosphorylation sites (A), NCC-dependent 22Na+ transport dropped markedly in line with previously published data (Figure 4E).12 Also in this experimental setting, when the phosphorylation sites were made constitutively inactive, γ-adducin had no effect on NCC-mediated 22Na+ transport.
These data suggest that the stimulatory effect of γ-adducin is critically dependent on the phosphorylation sites in the N-terminal domain of NCC. To further elucidate the relationship between γ-adducin and NCC, pull-down experiments were performed to determine whether binding of γ-adducin to the N-tail of NCC was dependent on the phosphorylation status of this domain. Thus, GST pull-downs were performed using the N terminus of NCC as well as the N-terminal constitutively inactive or active phosphorylation forms (Figure 4F). Binding of γ-adducin was found to the N terminus of wild-type NCC and the constitutively inactive form, whereas no binding was observed to the constitutive active phosphorylation form of NCC.
DISCUSSION
NCC is of crucial importance for the reabsorption of NaCl by the kidney, thus influencing arterial pressure. Here, we report the identification of γ-adducin, a novel auxiliary protein interacting with the N-terminal domain of NCC. Our study is the first to delineate the stimulatory action of γ-adducin on the thiazide-sensitive NaCl cotransporter and highlights that the effect of γ-adducin is critically dependent on the phosphorylation status of NCC. These observations are based on the following data: (1) the identified protein γ-adducin binds strongly to the N-terminal domain of NCC and markedly stimulates the activity of the transporter; (2) the stimulatory actions of γ-adducin occur in the regulatory cascade before the WNK4-dependent lysosomal shuttling of NCC; (3) competition with increasing amounts of the N-terminal part of NCC completely reverts the stimulatory action of γ-adducin on thiazide-sensitive 22Na+ transport; (4) the γ-adducin-binding site is mapped to the part of the N-terminal domain of NCC that encompasses three phosphorylation sites that previously have been shown to affect the activity of the cotransporter; (5) NCC forms lacking phosphorylatable sites in the N terminus do not exhibit increased 22Na+ transport rates when coinjected with γ-adducin; and (6) γ-adducin dissociates from NCC when the phospho-residues are converted to aspartates, mimicking a constitutively active phosphorylated state.
γ-Adducin was originally cloned from rat kidney.36 The protein exhibits homology with the previously identified α- and β-adducin family members. Adducins are heteromeric membrane skeletal proteins originally implicated in spectrin-actin binding.26 The adducins form heteromeric proteins composed of either α- and β-subunits or α- and γ-subunits. Because the β-subunit has a restricted expression and is primarily found in brain and hemopeotic tissues, it has been suggested that the α- and γ-subunits function as heteromers in tissues where the β-subunit is absent.36 It is interesting to note that although γ-adducin localizes to the DCT with NCC, α-adducin, which is also expressed in the kidney, seems absent from the distal tubules, although expression of both reappears in the collecting duct.31 This may suggest that γ-adducin has a unique function in DCT, where it could function as a homomer. In line with this is our observation that α-adducin fails to stimulate the activity of NCC in the oocyte system. The adducin gene family has previously been implicated in arterial hypertension. A G460W polymorphism in the α-adducin gene showed linkage to primary hypertension in certain patient groups.27 One intronic single nucleotide polymorphism has currently been described in the γ-adducin gene (A/G; rs3731566), which correlates with peripheral and central pulse pressures (because of increases in systolic pressure), changes in the urinary Na+/K+ ratio, and urinary aldosterone excretion, but only in individuals that also harbor the G460W polymorphism in α-adducin.37 These observations suggest that γ-adducin can affect BP; however, to be visible clinically, arterial pressure needs to be perturbed by the G460W polymorphism in α-adducin.
γ-Adducin is able to revert the inhibitory effect of WNK4 on NCC. Consequently, our observation implies that γ-adducin protects against the inhibitory actions of WNK4, either by affecting processes occurring before lysosomal removal of the protein or by directly blocking the WNK4-dependent inhibitory action on NCC. Because no changes in the membrane localization of NCC were observed after coinjection of γ-adducin, one may conclude that γ-adducin controls processes preceding lysosomal shuttling of NCC. The functional effect of phosphorylation of the thiazide-sensitive NCC transporter has not been fully delineated. Previous studies have reported that when the N-terminal phosphorylation sites in NCC are converted to alanines mimicking inactive phosphorylation sites, the activity of the transporter is markedly decreased. This occurs even although the membrane abundance remains unaffected, suggesting that the intrinsic activity is inhibited.12 Similarly, when the transporter is incubated at hypotonic low Cl− conditions, which noticeably increases its phosphorylation level, NCC increases its activity, again independent of trafficking to the oocyte membrane.12 In line with this finding is the observation that the Thr96- and Thr101-phosphorylated form of the related family member, the rat furosemide-sensitive Na+, K+, 2Cl− cotransporter (NKCC2) (corresponding to Thr55 and Thr60 phospho-form of human NCC), is found exclusively at the membrane as evaluated by electron microscopy.38 Similarly to NCC in oocytes, when the phosphorylation level of NKCC2 is increased by growth hormone, no change in trafficking of the transporter can be observed.12,38 Consequently, the γ-adducin-stimulated NCC activity needs to be explained by an intrinsic change in transporter activity, because the amount of transporters in the membrane remains unchanged. Such changes could ensue, for instance, if affinity constants for Na+ or Cl− shift, to such a degree that the maximal velocity is altered. Thus, the data obtained in our study as well as the previous literature strongly imply a link between the phosphorylation state and the intrinsic activity of the transporter.
We found that the effect of γ-adducin is entirely dependent on the phosphorylation sites of NCC. The sequence of γ-adducin does not suggest that the protein is a kinase. Furthermore, there is no experimental evidence showing that γ-adducin or other members of the family possess kinase activity. The most likely explanation is that γ-adducin associates with kinases involved in the phosphorylation of the transporter, thereby anchoring them to the dephosphorylated N terminus of the transporter. The STE20 family of kinases, comprising members such as SPAK and OSR1, has been shown to increase the phosphorylation state of NCC in vitro and in vivo.16,24 In addition, WNK1, via SPAK and OSR1, has previously been shown to be responsible for increasing the transport activity and phosphorylation level of NCC during hypotonic Cl−-depleted conditions.16 Moreover, WNK4 plays an important role in stimulating the NCC phosphorylation via SPAK and OSR1 in response to angiotensin II-mediated receptor activation.39 The phosphorylation state of NCC is also markedly enhanced by dietary NaCl restriction, an effect that appears aldosterone-dependent and is lost in transgenic animals with a pseudohypoaldosteronism type II mutation in WNK4.40 Consequently, phosphorylation of the N-terminal domain in NCC seems to be a common final pathway by which several stimuli converge to regulate the activity of the transporter. Here, we report that γ-adducin may function as an important component in the phosphorylation cascade of NCC.
As can be observed from the immunohistochemical stainings, the γ-adducin protein colocalizes with the NCC cotransporter to the DCT. γ-Adducin is confined primarily to cytoplasmic domains oriented near the basolateral region of the cell, whereas NCC is found in apical domains of the cell. However, the adducins have previously been shown to play multiple roles within the cell, such as promoting cytoskeletal spectrin-actin binding,26 and larger fractions of the γ-adducin protein may be associated with those kind of structures, thus explaining the localization of the majority of protein. It is also unclear how the cytoskeleton is organized in the DCT after periodatelysine paraformaldehyde (PLP) fixation and freezing. Therefore, what is observed in the kidney section may not exactly mirror the subcellular localization of this protein in the native DCT cell. In addition, γ-adducin is a cytoplasmic protein that may show a gradient in its distribution, where the large majority of the protein is localized to cytoskeletal structures, compatible with its multiple functions within the cell. Thus, only a minor fraction could be present at the apical plasma membrane. In primary renal proximal tubule epithelial cells, γ-adducin is found in basolateral domains of the cell, whereas protein kinase C-dependent phosphorylation of the protein induces its redistribution toward the cytoplasm.30 The potential regulation of γ-adducin in the DCT as well as its subcellular distribution deserves further scrutiny, particularly after activation of intracellular signaling cascades that has been shown to affect the phosphorylation of NCC.
On the basis of the data generated in our study, we postulate that γ-adducin stimulates NCC activity by anchoring a kinase, likely SPAK or OSR1 to the dephosphorylated transporter. Subsequently, the kinase increases the phosphorylation level of NCC, thereby stimulating the activity of the transporter. After the kinase-mediated phosphorylation event, γ-adducin dissociates from NCC and may also facilitate the release of the associated kinase. Dephosphorylation of the transporter reduces the activity of NCC to its basal state. However, this event also allows for the binding of γ-adducin to the N terminus again, and the cycle can be repeated. Thus, these speculations infer a dynamic model in which γ-adducin binding and dissociation to NCC is directly correlated with the dephosphorylation and phosphorylation of the transporter, respectively.
In this study, the influence of γ-adducin on NCC-dependent NaCl transport was evaluated using the X. laevis oocyte expression system. This in vitro system provides a powerful approach to directly study NCC function at the cellular and molecular level. In general, the results obtained in the oocyte expression system pertaining to the function and regulation of NCC mirror quite accurately those reported in vivo.21,24,41 Despite the substantial success of the oocyte expression system, there are several limitations that should be noted. Obviously, the oocyte system represents a simplification of the in vivo situation and does not adequately reflect the complexity of the DCT cell, much less the kidney. In addition, several cofactors that may be necessary for the appropriate regulation of NCC could be absent from the oocyte. Thus, by recognizing these limitations in relation to the data obtained in this study, we can only extrapolate that the effect of γ-adducin on NCC function is likely to occur in vivo. However, further studies in appropriate animal models are needed to definitively support this conclusion.
In summary, we identified γ-adducin as a regulator of NCC in oocytes and showed that the stimulatory action of γ-adducin is intimately linked with the N-terminal phosphorylation sites in the cotransporter. On the basis of our data, γ-adducin may contribute importantly to the regulation of NCC and hence BP maintenance. Our findings will aid in the understanding of the complex cascade regulating NCC activity. Importantly, the observations made in our study may help elucidate the molecular events underlying the formation of primary hypertension.
CONCISE METHODS
Constructs
HA-tagged human NCC in pT7Ts has been previously described.42 The human γ-adducin clone was obtained from Imclone Systems (New York, NY), subcloned into a pCI-NEO-IRES-GFP vector, and further subcloned into pT7Ts. GST-conjugated N- and C-terminal fragments of NCC were subcloned by PCR to pGEX-6p-2 for Escherichia Coli protein production and further subcloned into pEBG vectors for expression of GST-fused proteins in HEK293 cells. GST-fused proteins containing different portions of the N-terminal domain of NCC were constructed according to the schematic drawing in Figure 4B. These constructs inserted in pEBG vectors were generated either by PCR subcloning or truncated by stop codons using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). In addition, the N-terminal domain of NCC (amino acids 1 to 135) for the competition assay was generated by insertion of a stop codon by site-directed mutagenesis in the full-length NCC in pT7Ts. The triple phospho sites (Thr55, Thr60, and Ser73) in NCC mimicking constitutively inactive (converted to alanines) or active (converted to aspartates) phospho-proteins were generated by site-directed mutagenesis in pT7Ts vectors. Similarly, the N termini with modified phosphorylation sites were further subcloned by PCR into pEBG for GST pull-down analysis. A Myc tag was inserted in front of WNK4 by subcloning, and the complete insert was placed into a pT7Ts vector using the gateway system (Invitrogen, Breda, The Netherlands). For generation of eGFP-NCC in pT7Ts, human NCC was subcloned by PCR from human NCC pT7Ts and constructed behind eGFP in a pCB7 vector. The complete insert was thereafter subcloned into pT7Ts. Human α-adducin (Imclone Systems) was subcloned into a pT7Ts vector. All of the inserts were verified by direct sequencing.
GST Pull-down and Mass Spectrometry
The intracellular N- and C-terminal domains of NCC coupled to GST were produced in E. Coli BL21 cells. The bacteria were lysed, and the GST-NCC terminal fusion domains were purified on glutathione-Sepharose 4B beads (Amersham Pharmacia Biotech). C57BL/6 mice were killed under 1.5% vol/vol isofluorane anesthesia (Nicholas Piramal Limited, London, UK), and the kidneys were homogenized in lysis buffer (20 mM Tris/HCl, 140 mM NaCl, 1 mM CaCl2,0.2% (vol/vol) Triton X-100, 0.2% (vol/vol) NP-40, pH 7.4) containing protease inhibitors (0.10 mg/ml leupeptin, 0.05 mg/ml pepstatin-A, 1 mM phenylmethylsulfonyl fluoride, and 5 mg/ml aprotinin). The precipitated GST-terminal proteins were incubated overnight at 4°C with mouse kidney lysates. After extensive washing, the precipitates were placed on a SDS-PAGE gel and analyzed by the Nijmegen Proteomics Facility (Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands, http://www.proteomicsnijmegen.nl/). Proteins were in-gel digested with trypsin. After extraction from the gel, the peptides were analyzed using nano-flow liquid chromatography coupled to a linear ion trap Fourier-transform mass spectrometer (LTQ FT; Thermo Fisher Scientific). Peptide and protein identifications were extracted from the data by means of the search program Mascot using the NCBI database 20080103 containing Mus musculus taxonomy with the addition of known contaminant proteins such as trypsin and human keratins. Precipitation of GST-coupled fusion domains of NCC with HA-tagged γ-adducin in HEK293 cells was done as described in detail previously.43
COPAS Sorting and Semiquantitative Real-Time PCR Analysis
Transgene mice expressing eGFP after the parvalbumin promoter have been described previously.32 The animals were anesthetized by an intraperitoneal injection of Hellabrunn mixture (ketamine, 0.05 mg/g of body weight; and xylazine, 0.02 mg/g of body weight) and perfused transcardially with digestion solution (1 mg/ml collagenase A [#1088793; Roche Diagnostics, Mannheim, Germany] and 1 mg/ml hyaluronidase [#H3884; Sigma Aldrich, Zwijndrecht, The Netherlands] in KREBS [145 mM NaCl, 10 mM HEPES, 5 mM KCl, 1 mM NaH2PO4, 2.5 mM CaCl2, 1.8 mM MgSO4, 5 mM glucose, pH 7.3]). The animal ethics board of Radboud University Nijmegen approved all of the experimental procedures. The kidney cortex was finely minced and incubated in digestion solution for 22 minutes at 37°C, subsequently sieved through a series of meshes, and finally collected on a 40-μm filter. Fluorescently labeled DCT tubules were isolated from transgenic animals, using a COPAS sorter (Union Biometrica, Somerville, MA) as described in detail previously.33 The GFP-positive fraction consists of DCT fragments. The remainder was saved and denoted whole kidney tubules. Tubule RNA was extracted using TRIzol Total RNA Isolation Reagent (Life Technologies BRL, Breda, The Netherlands) and processed into cDNA. The cDNA was mixed with Power SYBR® green PCR Mastermix (Applied Biosystems, Foster City, CA) and exon overlapping primers against γ-adducin (5′-CACATCCACACCCTTGCCAC-3′; 5′-CCTGGTAGTCATAGTAGGCGAC-3′), NCC (5′-CTTCGGCCACTGGCATTCTG-3′; 5′-GATGGCAAGGTAGGAGATGG-3′), and the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (5′- AACATCAAATGGGGTGA-3′; 5′-GGTTCACACCCATCACAA-3′).
In Vitro cRNA Translation
pT7Ts plasmid vectors were linearized by restriction digestion at the 3′-end of the insert. The plasmids were transcribed in vitro using the mMESSAGE mMACHINE® T7 Kit (Ambion, Austin, TX) to generate cRNA. The integrity of the product was confirmed on 1% (wt/vol) agarose, 37% (vol/vol) formaldehyde gels. RNA concentrations were determined spectrophotometrically at 260 nm. The cRNA aliquots were stored at −80°C.
Evaluation of NCC Function
All of the animal experiments described below received the approval from the animal ethics board of Radboud University Nijmegen. Isolation and 22Na+ uptakes in oocytes were done as described previously,44,45 with minor modifications. Briefly, X. laevis oocytes were surgically collected after decapitation, manually separated, and incubated in collagenase A (1 mg/ml). Incubations were done in Ca2+-free ND96 solution (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, and 5 mM HEPES/NaOH, pH 7.4, 50 mg/L gentamicin). The following day, stage V and VI oocytes were selected and injected with the appropriate cRNAs in a final volume of 50 nl/oocyte. The oocytes were subsequently incubated in Ca2+-containing ND96 including 1.8 mM CaCl2 for 2 to 3 days at 16°C. 16 hours before the uptake, the oocytes were placed in Cl−-depleted medium (96 mM sodium isethionate, 2 mM potassium gluconate, 1.8 mM calcium gluconate, 1 mM Mg(NO3−)2, 5 mM HEPES/NaOH, pH 7.4, 50 mg/dl gentamicin, approximately 200 mOsm). Before the uptake, the oocytes were preincubated in 1 mM ouabain, 0.1 mM amiloride, and 0.1 mM bumetanide in the presence or absence of 0.1 mM thiazide in Cl−-depleted medium. 22Na+ uptake (1 μCi 22Na+/ml; Perkin Elmer) was done in K+-free isotonic uptake buffer (40 mM NaCl, 56 mM N-methyl-d-glucamine (NMDG)-Cl, 1.8 mM CaCl2, 1 mM MgCl2, 5 mM HEPES/NaOH, pH 7.4) for 1 hour. The oocytes were washed five times at the end of the 22Na+ incubation with ice-cold uptake medium, transferred to scintillation vials, and lysed in 10% (w/v) sodium dodecyl sulfate. Radioactivity was counted in a liquid scintillation counter.
Evaluation of Total Expression of NCC and γ-Adducin in the Oocyte
HA-NCC was detected in total membrane isolates, whereas HA-γ-adducin was detected in the remaining cytosolic fraction. As described in detail previously,42 membrane preparations and total lysates were prepared, blotted onto membranes, and detected using an enhanced chemiluminescence system (Pierce, Rockford, IL).
Quantification of eGFP-NCC in the Oocyte Membrane
The oocytes were injected with 1, 5, or 10 ng of eGFP-NCC cRNA as well as 10 ng of eGFP-NCC cRNA in the presence of 10 ng of γ-adducin. cRNA. Images were acquired on an Olympus FV1000 laser-scanning microscope (Center Valley, PA, USA) using a 20× objective. Background subtraction and semiquantitative determination of eGFP-NCC abundance at the plasma membrane was done using Image J (National Institutes of Health, Bethesda, MD).
siRNA against X. laevis γ-Adducin
To confirm endogenous expression of γ-adducin in X. laevis oocytes, primers designed against γ-adducin in Xenopus tropicalis were used to amplify a fragment by RT-PCR (TaKaRa TaqTM, Shiga, Japan) from oocyte cDNA. The fragment was extracted from the gel and sequenced. Sequence similarity was identical to the previously published mRNA of hypothetical protein LOC432146 from X. laevis. siRNA was designed using double-stranded oligo-probes against the following sequence of endogenous X. laevis γ-adducin, 5′-AATGACCCCGGCTACATCCGC-3′. Double-stranded probes were also designed against X. laevis α-adducin 5′-AATGGTGATTCGAGTGTAG-3′, on the basis of the previously published sequence (NM_001087641). Oocytes were injected with 5 ng of HA-NCC siRNA as described above. siRNAs were injected 24 hours before the uptake.
Immunohistochemistry
Kidneys from C57BL/6 mice were immersion fixated in 1% (w/v) PLP fixative. 10-μm cryosections were prepared and costained with anti-γ-adducin antibodies (1:25, rabbit, H-60; Santa Cruz Biotechnology, Santa Cruz, CA) and NCC (1:100, guinea pig; generously provided by Jan Loffing, Switzerland). The images were acquired on an Olympus FV1000 laser-scanning microscope (Center Valley, PA).
Statistical Analysis
All of the results obtained in oocytes were the averages of two to eight independent experiments, each containing a minimum of ten oocytes per group. Overall statistics between groups was determined by one-way ANOVA. In case of significance, multiple comparisons between groups were performed by Bonferroni post hoc tests. The values are presented as the means ± SEM. P < 0.05 is considered statistically significant.
DISCLOSURES
None.
Acknowledgments
The authors are grateful to our colleagues Jan Janssen, Henk Arnts, Femke van Zeeland, Jolein Gloerich, AnneMiete van der Kemp, Huib Croes, and Ron Engels for technical assistance and helpful suggestions. We greatly appreciate the support of Dr. Hannah Monyer, Universitätsklinikum Heidelberg, Germany, for kindly donating the transgenic mouse line B-Pv-E. This study was supported financially by the Dutch Kidney Foundation (C05.4106), The Netherlands Organization for Scientific Research (ZonMw 9120.6110; ALW 700.55.302), and a EURYI award from the European Science Foundation to J.H.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J: Global burden of hypertension: Analysis of worldwide data. Lancet 365: 217–223, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Lawes CM, Vander Hoorn S, Law MR, Elliott P, MacMahon S, Rodgers A: Blood pressure and the global burden of disease 2000. Part II: Estimates of attributable burden. J Hypertens 24: 423–430, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Minino AM, Arias E, Kochanek KD, Murphy SL, Smith BL: Deaths: Final data for 2000. Natl Vital Stat Rep 50: 1–119, 2002 [PubMed] [Google Scholar]
- 4. Bakris GL, Ritz E: The message for World Kidney Day 2009: Hypertension and kidney disease: A marriage that should be prevented. J Hypertens 27: 666–669, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Guyton AC: Blood pressure control: Special role of the kidneys and body fluids. Science 252: 1813–1816, 1991 [DOI] [PubMed] [Google Scholar]
- 6. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ: The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA 289: 2560–2572, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Reilly RF, Ellison DH: Mammalian Distal Tubule: Physiology, Pathophysiology, and Molecular Anatomy. Physiol Rev 80: 277–313, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Gitelman HJ, Graham JB, Welt LG: A new familial disorder characterized by hypokalemia and hypomagnesemia. Trans Assoc Am Physicians 79: 221–235, 1966 [PubMed] [Google Scholar]
- 9. Simon DB, Nelson-Williams C, Bia MJ, Ellison D, Karet FE, Molina AM, Vaara I, Iwata F, Cushner HM, Koolen M, Gainza FJ, Gitleman HJ, Lifton RP: Gitelman's variant of Bartter's syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet 12: 24–30, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Gordon RD: The syndrome of hypertension and hyperkalemia with normal glomerular filtration rate: Gordon's syndrome. Aust N Z J Med 16: 183–184, 1986 [DOI] [PubMed] [Google Scholar]
- 11. Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson- Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP: Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Pacheco-Alvarez D, Cristobal PS, Meade P, Moreno E, Vazquez N, Munoz E, Diaz A, Juarez ME, Gimenez I, Gamba G: The Na+:Cl− cotransporter is activated and phosphorylated at the amino-terminal domain upon intracellular chloride depletion. J Biol Chem 281: 28755–28763, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Darman RB, Forbush B: A regulatory locus of phosphorylation in the N terminus of the Na-K-Cl cotransporter, NKCC1. J Biol Chem 277: 37542–37550, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Gimenez I, Forbush B: Short-term stimulation of the renal Na-K-Cl cotransporter (NKCC2) by vasopressin involves phosphorylation and membrane translocation of the protein. J Biol Chem 278: 26946–26951, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Gimenez I, Forbush B: Regulatory phosphorylation sites in the NH2 terminus of the renal Na-K-Cl cotransporter (NKCC2). Am J Physiol Renal Physiol 289: F1341–F1345, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Richardson C, Rafiqi FH, Karlsson HK, Moleleki N, Vandewalle A, Campbell DG, Morrice NA, Alessi DR: Activation of the thiazide-sensitive Na+-Cl- cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci 121: 675–684, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Vitari AC, Deak M, Morrice NA, Alessi DR: The WNK1 and WNK4 protein kinases that are mutated in Gordon's hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem J 391: 17–24, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gagnon KB, England R, Delpire E: Volume sensitivity of cation-Cl- cotransporters is modulated by the interaction of two kinases: Ste20-related proline-alanine-rich kinase and WNK4. Am J Physiol Cell Physiol 290: C134–C142, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Rozansky DJ, Cornwall T, Subramanya AR, Rogers S, Yang YF, David LL, Zhu X, Yang CL, Ellison DH: Aldosterone mediates activation of the thiazide-sensitive Na-Cl cotransporter through an SGK1 and WNK4 signaling pathway. J Clin Invest 119: 2601–2612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ohta A, Rai T, Yui N, Chiga M, Yang SS, Lin SH, Sohara E, Sasaki S, Uchida S: Targeted disruption of the Wnk4 gene decreases phosphorylation of Na-Cl cotransporter, increases Na excretion and lowers blood pressure. Hum Mol Genet 18: 3978–3986, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Yang SS, Morimoto T, Rai T, Chiga M, Sohara E, Ohno M, Uchida K, Lin SH, Moriguchi T, Shibuya H, Kondo Y, Sasaki S, Uchida S: Molecular pathogenesis of pseudohypoaldosteronism type II: Generation and analysis of a Wnk4(D561A/+) knockin mouse model. Cell Metab 5: 331–344, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Zambrowicz BP, Abuin A, Ramirez-Solis R, Richter LJ, Piggott J, BeltrandelRio H, Buxton EC, Edwards J, Finch RA, Friddle CJ, Gupta A, Hansen G, Hu Y, Huang W, Jaing C, Key BW, Jr., Kipp P, Kohlhauff B, Ma ZQ, Markesich D, Payne R, Potter DG, Qian N, Shaw J, Schrick J, Shi ZZ, Sparks MJ, Van Sligtenhorst I, Vogel P, Walke W, Xu N, Zhu Q, Person C, Sands AT: Wnk1 kinase deficiency lowers blood pressure in mice: A gene-trap screen to identify potential targets for therapeutic intervention. Proc Natl Acad Sci U.S.A. 100: 14109–14114, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kahle KT, Wilson FH, Leng Q, Lalioti MD, O'Connell AD, Dong K, Rapson AK, MacGregor GG, Giebisch G, Hebert SC, Lifton RP: WNK4 regulates the balance between renal NaCl reabsorption and K+ secretion. Nat Genet 35: 372–376, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Rafiqi FH, Zuber AM, Glover M, Richardson C, Fleming S, Jovanovic S, Jovanovic A, O'Shaughnessy KM, Alessi DR: Role of the WNK-activated SPAK kinase in regulating blood pressure. EMBO Mol Med 2: 63–75, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vallon V, Schroth J, Lang F, Kuhl D, Uchida S: Expression and phosphorylation of the Na+-Cl- cotransporter NCC in vivo is regulated by dietary salt, potassium, and SGK1. Am J Physiol Renal Physiol 297: F704–F712, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gardner K, Bennett V: Modulation of spectrin-actin assembly by erythrocyte adducin. Nature 328: 359–362, 1987 [DOI] [PubMed] [Google Scholar]
- 27. Cusi D, Barlassina C, Azzani T, Casari G, Citterio L, Devoto M, Glorioso N, Lanzani C, Manunta P, Righetti M, Rivera R, Stella P, Troffa C, Zagato L, Bianchi G: Polymorphisms of alpha-adducin and salt sensitivity in patients with essential hypertension. Lancet 349: 1353–1357, 1997 [DOI] [PubMed] [Google Scholar]
- 28. Bianchi G, Tripodi G, Casari G, Salardi S, Barber BR, Garcia R, Leoni P, Torielli L, Cusi D, Ferrandi M, et al. : Two point mutations within the adducin genes are involved in blood pressure variation. Proc Natl Acad Sci U.S.A. 91: 3999–4003, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zagato L, Modica R, Florio M, Torielli L, Bihoreau MT, Bianchi G, Tripodi G: Genetic mapping of blood pressure quantitative trait loci in Milan hypertensive rats. Hypertension 36: 734–739, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Fowler L, Dong L, Bowes RC, 3rd, van de Water B, Stevens JL, Jaken S: Transformation-sensitive changes in expression, localization, and phosphorylation of adducins in renal proximal tubule epithelial cells. Cell Growth Differ 9: 177–184, 1998 [PubMed] [Google Scholar]
- 31. Fowler L, Everitt J, Stevens JL, Jaken S: Redistribution and enhanced protein kinase C-mediated phosphorylation of alpha- and gamma-adducin during renal tumor progression. Cell Growth Differ 9: 405–413, 1998 [PubMed] [Google Scholar]
- 32. Meyer AH, Katona I, Blatow M, Rozov A, Monyer H: In vivo labeling of parvalbumin-positive interneurons and analysis of electrical coupling in identified neurons. J Neurosci 22: 7055–7064, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miller RL, Zhang P, Chen T, Rohrwasser A, Nelson RD: Automated method for the isolation of collecting ducts. Am J Physiol Renal Physiol 291: F236–F245, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Yang CL, Angell J, Mitchell R, Ellison DH: WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest 111: 1039–1045, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhou B, Zhuang J, Gu D, Wang H, Cebotaru L, Guggino WB, Cai H: WNK4 enhances the degradation of NCC through a sortilin-mediated lysosomal pathway. J Am Soc Nephrol 21: 7–9, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dong L, Chapline C, Mousseau B, Fowler L, Ramsay K, Stevens JL, Jaken S: 35H, a sequence isolated as a protein kinase C binding protein, is a novel member of the adducin family. J Biol Chem 270: 25534–25540, 1995 [DOI] [PubMed] [Google Scholar]
- 37. Cwynar M, Staessen JA, Ticha M, Nawrot T, Citterio L, Kuznetsova T, Wojciechowska W, Stolarz K, Filipovsky J, Kawecka-Jaszcz K, Grodzicki T, Struijker-Boudier HA, Thijs L, Van Bortel LM, Bianchi G: Epistatic interaction between alpha- and gamma-adducin influences peripheral and central pulse pressures in white Europeans. J Hypertens 23: 961–969, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Dimke H, Flyvbjerg A, Bourgeois S, Thomsen K, Frokiaer J, Houillier P, Nielsen S, Frische S: Acute growth hormone administration induces antidiuretic and antinatriuretic effects and increases phosphorylation of NKCC2. Am J Physiol Renal Physiol 292: F723–F735, 2007 [DOI] [PubMed] [Google Scholar]
- 39. San-Cristobal P, Pacheco-Alvarez D, Richardson C, Ring AM, Vazquez N, Rafiqi FH, Chari D, Kahle KT, Leng Q, Bobadilla NA, Hebert SC, Alessi DR, Lifton RP, Gamba G: Angiotensin II signaling increases activity of the renal Na-Cl cotransporter through a WNK4-SPAK-dependent pathway. Proc Natl Acad Sci U.S.A. 106: 4384–4389, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chiga M, Rai T, Yang SS, Ohta A, Takizawa T, Sasaki S, Uchida S: Dietary salt regulates the phosphorylation of OSR1/SPAK kinases and the sodium chloride cotransporter through aldosterone. Kidney Int 74: 1403–1409, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Lalioti MD, Zhang J, Volkman HM, Kahle KT, Hoffmann KE, Toka HR, Nelson-Williams C, Ellison DH, Flavell R, Booth CJ, Lu Y, Geller DS, Lifton RP: Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet 38: 1124–1132, 2006 [DOI] [PubMed] [Google Scholar]
- 42. de Jong JC, Willems PH, Mooren FJ, van den Heuvel LP, Knoers NV, Bindels RJ: The structural unit of the thiazide-sensitive NaCl cotransporter is a homodimer. J Biol Chem 278: 24302–24307, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Schoeber JP, Topala CN, Lee KP, Lambers TT, Ricard G, van der Kemp AW, Huynen MA, Hoenderop JG, Bindels RJ: Identification of Nipsnap1 as a novel auxiliary protein inhibiting TRPV6 activity. Pflugers Arch 457: 91–101, 2008 [DOI] [PubMed] [Google Scholar]
- 44. De Jong JC, Van Der Vliet WA, Van Den Heuvel LP, Willems PH, Knoers NV, Bindels RJ: Functional expression of mutations in the human NaCl cotransporter: Evidence for impaired routing mechanisms in Gitelman's syndrome. J Am Soc Nephrol 13: 1442–1448, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Gamba G, Saltzberg SN, Lombardi M, Miyanoshita A, Lytton J, Hediger MA, Brenner BM, Hebert SC: Primary structure and functional expression of a cDNA encoding the thiazide-sensitive, electroneutral sodium-chloride cotransporter. Proc Natl Acad Sci U.S.A. 90: 2749–2753, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]