Abstract
Rf-3, a quantitative trait locus (QTL) on rat chromosome 3, affects the development of CKD in Fawn-Hooded Hypertensive (FHH) rats. This QTL spans 110 Mb and approximately 1400 genes; therefore, narrowing the position of this locus is necessary to elucidate potential candidate genes. Here, we used congenic models and comparative genomics to refine the Rf-3 candidate region. We generated congenic lines carrying smaller intervals (subcongenics) of the Rf-3 region and used these lines to reduce the Rf-3 candidate region by 94% (to 7.1 Mb). We used comparative genomics to identify QTL for both nephropathy and albuminuria in the syntenic region of this interval for both human and mouse. We also used the overlapping homologous regions to reduce the number of likely positional candidate genes to 13 known or predicted genes. By combining congenic models and cross-species studies, we narrowed the list of candidate genes to a level that we could sequence the whole interval to further identify the causative gene in future studies.
Chronic kidney disease (CKD) is a growing health risk in the United States and worldwide, with the incidence continuing to rise at an alarming rate.1 Epidemiologic studies have shown that familial and ethnic components contribute to an individual's risk of developing renal complications as a result of hypertension and/or diabetes.1–5 Human association and linkage studies have identified specific regions of the genome that significantly contribute to renal disease susceptibility6–12; however, the degree of genetic heritability accounted for by these genes is only a small percentage of the total heritability.13 Consequently, there is a need to pursue other strategies for identifying genes and their associated pathways that are driving CKD. The rat model offers numerous advantages, such as an abundance of physiologic data on many well-characterized disease models and available consomic and congenic strains that can be used to study the genetic basis of kidney disease.14,15
As in humans, genes play a role in the development of CKD in rats. The Fawn-Hooded Hypertensive (FHH) rat is a well-established model for hypertension-associated kidney disease.16–20 This particular strain spontaneously develops systolic and glomerular hypertension, and consequently, renal complications, as indicated by proteinuria, albuminuria, and glomerular sclerosis.16,18–23 Because of its robust phenotype, we crossed this strain with the normotensive, renal failure–resistant August Copenhagen Irish (ACI) rat and performed F2 linkage analyses to identify regions of the rat genome that cause kidney disease susceptibility in the FHH rat.20,21 These linkage analyses showed the presence of five renal failure quantitative trait loci (QTLs) called Renal failure 1 through 5 (Rf-1 through Rf-5). Subsequent phenotypic analysis of single and double congenic animals has shown a synergistic relationship between the various Rf QTLs. Specifically, an interaction was identified between Rf-1 and Rf-3, and Rf-1 and Rf-4, whereas, the Rf-3 and Rf-4 loci had little to no apparent effect on renal function alone.24,25
To elucidate the specific gene variant(s) causing the observed phenotype, it is necessary to narrow the candidate region to a manageable number of genes, because the Rf-3 region is 110 Mbp in size and contains >1400 genes. Our group has previously used this strategy to narrow the Rf-2 QTL region and identify Rab38 as a candidate gene.22 Because gene–gene interactions have been identified between the various Rf QTLs and because of the polygenic nature of renal disease, we received triple congenic animals from Dr. Abraham Provoost (Erasmus MC, Rotterdam, The Netherlands) that have an ACI disease-resistant background and FHH-sensitive locus introgressed onto Rf-1 (D1Mit18-D1Rat90), Rf-3 (D3Rat84-D3Rat59), and Rf-4 (D14Mit11-D14Rat33/D14Rat65-D14Rat90), called Rf-1 + 3+4, because the presence of FHH alleles on the Rf-1 locus, and possibly the Rf-4 locus, is necessary for Rf-3 to have a measurable effect on renal disease.24,25 In preliminary studies, we observed that, after unilateral nephrectomy (UNX), albumin excretion (UAV) of Rf-1 + 3+4 is almost three times higher than that of the Rf-1 + 4 double congenics (ACI.FHH [D1Mit18-D1Rat90]/[D14Mit11-D14Rat33/D14Rat65-D14Rat90]), showing the utility of the triple congenic model for mapping disease-causing variant(s) in Rf-3 on a resistant genome background. In this study, we crossed Rf-1 + 3+4 animals to Rf-1 + 4 animals to generate subcongenic lines targeting the Rf-3 QTL. Phenotypic analysis of these subcongenic lines showed a 7.1-Mb region of rat chromosome 3 that significantly contributes to the early development of renal disease. Interestingly, three separate linkage analysis studies in human and mouse have mapped kidney disease loci concordant to this 7.1-Mb candidate region,12,26,27 suggesting that the same genetic elements may play a role in renal disease susceptibility across species. By comparing the breakpoints of our candidate region to the boundaries of mouse and human renal function QTLs, we were able to further narrow down the list of candidate genes from 1400 to just 13 known and predicted genes. In addition to cross-species analysis, we also sequenced the entire 7.1-Mb region to identify variants between ACI and FHH potentially responsible for the Rf-3 renal phenotype.
RESULTS
Assessment of Albumin Excretion and BP in Rf-1 + 3+4 Congenic Strains
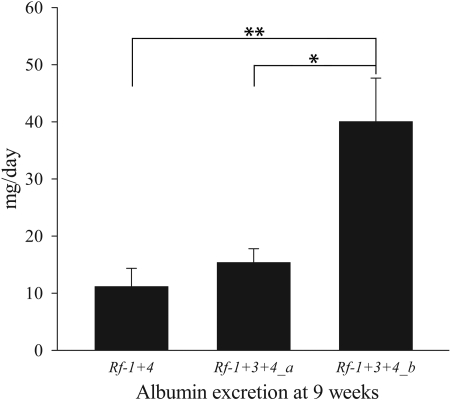
To physically narrow the candidate region of the Rf-3 QTL, we generated and phenotyped a panel of subcongenic lines targeting the Rf-3 QTL for UAV at 9 weeks of age after UNX. We found that congenic lines containing the FHH genotype between genetic markers D3Got102 and D3Got121 had higher UAV compared with other subcongenic lines (data not shown). To further study the contribution of this region to renal impairment, we selected two subcongenic lines—Rf-1 + 3+4_a (ACI.FHH [D1Mit18-D1Rat90]/[D3Rat6-D3Got149]/[D14Mit11-D14Rat33/D14Rat65-D14Rat90]) and Rf-1 + 3+4_b (ACI.FHH [D1Mit18-D1Rat90]/[D3Got102-D3Got149]/[D14Mit11-D14Rat33/D14Rat65-D14Rat90])—for phenotypic analysis. These overlapping congenic lines are genetically identical to Rf-1 + 4 except for the Rf-3 region, where Rf-1 + 3+4_a is FHH from D3Rat6 to D3Got149 and Rf-1 + 3+4_b is FHH from D3Got102 to D3Got149 (Figure 1). At 9 weeks of age, Rf-1 + 3+4_b had significantly higher levels of UAV than Rf-1 + 3+4_a (40.06 ± 7.60 versus 15.40 ± 2.40 mg/d, respectively, P = 0.024) and also compared with Rf-1 + 4 (11.18 ± 3.18 mg/d, P = 0.002; Figure 2), indicating that gene(s) in the 7.1-Mb region differentiating these lines (D3Got102-D3Got121 [called Rf-3_b]) are causing increased UAV at an early age.
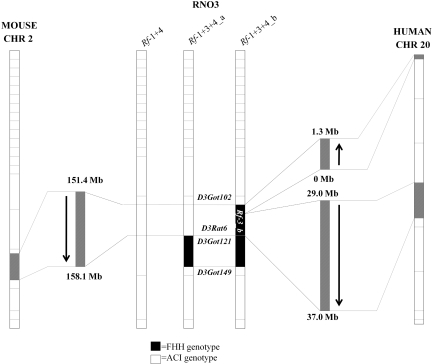
Figure 1.
Schematic representation of the flanking markers on rat chromosome 3 for Rf-1 + 4, Rf-1 + 3+4_a, and Rf-1 + 3+4_b congenic lines and the homologous regions in human and mouse. The flanking markers for the FHH rat chromosome 3 (RNO3) are D3Got102-D3Got149 for Rf-1 + 3+4_b and for Rf-1 + 3+4_a are D3Rat6-D3Got149. The region differentiating lines a and b (Rf-3_b), from D3Got102 to D3Got121, is homologous to two regions on human chromosome 20, from the p end of the chromosome to approximately 1.3 Mb and from 29 to 37 Mb. Rf-3_b is homologous to mouse chromosome 2 from approximately 151.4 to 158.1 Mb.
Figure 2.
Rf-1+3+4_b animals excrete higher levels of albumin than Rf-1+4 and Rf-1+3+4_a animals at 9 weeks of age. Number of animals was 9, 13, and 16 for Rf-1 + 4, Rf-1 + 3+4_a, and Rf-1 + 3+4_b, respectively. *P < 0.05; **P < 0.01. Datasets were not normally distributed, so each data point was log transformed before statistical analysis. Statistical comparison was done using a one-way ANOVA followed by a Holm-Sidak post hoc test.
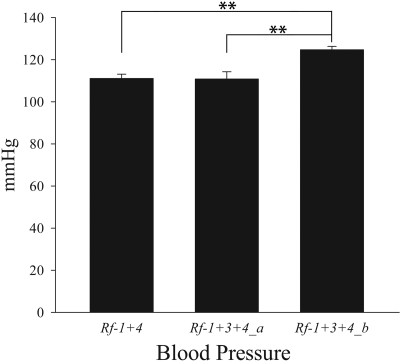
To assess whether differences in protein excretion between congenic strains is an independent event or whether it is secondary to differences in BP that can accelerate renal damage in a susceptible background, we also measured mean arterial pressure (MAP) in the congenic strains. Rf-1 + 3+4_b showed a slight but significantly higher MAP (124.71 ± 1.62 mmHg) compared with Rf-1 + 3+4_a (110.82 ± 3.45 mmHg, P = 0.002) and Rf-1 + 4 strains (111.08 ± 2.04 mmHg, P = 0.003; Figure 3).
Figure 3.
Mean arterial pressure (MAP) is elevated in Rf-1+3+4_b animals. Number of animals was 6, 7, and 6 for Rf-1 + 4, Rf-1 + 3+4_a, and Rf-1 + 3+4_b, respectively. **P < 0.01. Statistical comparison was done using a one-way ANOVA followed by a Holm-Sidak post hoc test.
Histologic Analysis
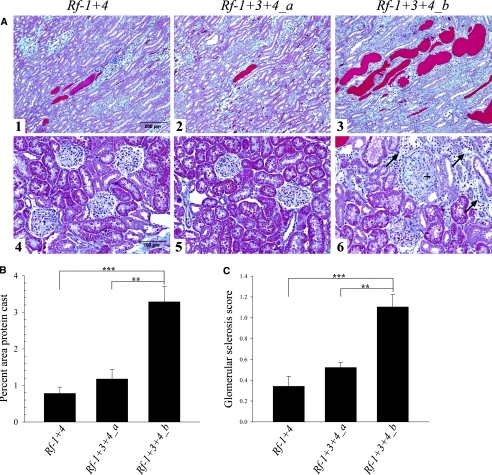
Histologic analysis showed significant differences in kidney morphology between strains. Protein casts observed in stained kidney sections were larger and more abundant in the outer medulla and cortex of Rf-1 + 3+4_b compared with Rf-1 + 3+4_a and Rf-1 + 4 animals (Figure 4A). Quantification of the observed difference was obtained using a color threshold analytical method. Rf-1 + 3+4_b had higher percent area of protein cast (3.29 ± 0.43) in the outer stripe of the medulla and cortex compared with Rf-1 + 4 (0.78 ± 0.17, P < 0.001) and Rf-1 + 3+4_a (1.18 ± 0.26, P = 0.002) kidneys (Figure 4B).
Figure 4.
Rf-1+3+4_b kidneys have increased protein casting and glomerular sclerosis compared with Rf-1+4 and Rf-1+3+4_a kidneys. (A) Histologic sections (10×) of (1) Rf-1 + 4,(2) Rf-1 + 3+4_a, and (3) Rf-1 + 3+4_b medulla and histologic sections (20×) of (4) Rf-1 + 4,(5) Rf-1 + 3+4_a, and (6) Rf-1 + 3+4_b cortex. All kidney sections were stained using Gomori's one-step trichrome stain. Rf-1 + 3+4_b kidneys showed larger and more abundant protein casts (stained red) compared with Rf-1 + 4 and Rf-1 + 3+4_a kidneys (panels 1 to 3). Panel 6 shows glomerular sclerosis (+) and interstitial fibrosis (→) in Rf-1 + 3+4_b cortex. (B) Quantification of percent area of protein casting in the outer stripe of the medulla and cortex for Rf-1 + 4 (n = 4), Rf-1 + 3+4_a (n = 3), and Rf-1 + 3+4_b (n = 4). (C) Average sclerosis score for Rf-1 + 4 (n = 4), Rf-1 + 3+4_a (n = 3), and Rf-1 + 3+4_b (n = 4) glomeruli. **P < 0.01; ***P < 0.001. Statistical comparisons were done using a one-way ANOVA followed by a Holm-Sidak post hoc test.
Furthermore, Rf-1 + 3+4_b kidney sections showed an increased presence of focal segmental glomerular sclerosis, as well as focal tubular interstitial fibrosis and tubular atrophy compared with Rf-1 + 4 and Rf-1 + 3+4_a kidneys (Figure 4A). Average glomerular sclerosis score was significantly higher in Rf-1 + 3+4_b (1.10 ± 0.12) glomeruli compared with Rf-1 + 4 (0.34 ± 0.10, P < 0.001) and Rf-1 + 3+4_a (0.52 ± 0.05, P = 0.004) glomeruli (Figure 4C).
Comparative Genomics
The Rf-3_b region is comprised of 7.1 Mb containing 181 known and predicted genes (Table S1) and is syntenic to two regions on human chromosome 20 (Figure 1). One megabase (Mb) region toward the p terminal of Rf-3_b (141.9 to 142.9 Mb) is homologous to the p end of human chromosome 20 and extends approximately 1.3 Mb in the reverse orientation (p to q) compared with the rat. The human homolog to Rf-3_b from 143 to 149 Mb is also located on human chromosome 20 spanning from approximately 29 to 37 Mb in the same orientation (p to q) as the rat. Rf-3_b is syntenic to a 6.7-Mb contiguous region on mouse chromosome 2 (151.4 to 158.1 Mb) in the same orientation (p to q) as the rat.
Two mouse26,27 and one human12 renal function QTLs spanning the Rf-3_b syntenic region have been previously mapped. Sheehan et al.26 performed an F2 linkage analysis by crossing the C57BL/6J with the DBA/2J mouse strains and identified an albuminuria QTL (Albq5) located on mouse chromosome 2. The boundaries of Albq5 were compared with another mouse renal function QTL also located on chromosome 2.27 The concordant region of a human diabetic nephropathy QTL identified in the Pima Indian population12 was compared with the mouse region, further narrowing the candidate genes of the Albq5 region (137 to 152.4 Mb on mouse chromosome 2) to just 133 genes. A small piece of the Rf-3_b region, D3Got102 (141.9 Mb) to approximately 143.2 Mb, overlaps the Albq5 candidate region defined by the mouse and human. Forty known and predicted genes are located within this interval, and of these, 25 genes map to the p end of human chromosome 20 that does not overlap with the human diabetic nephropathy QTL, and two genes map to other regions of the human genome. The remaining 13 genes, shown in Table 1, map to chromosome 20 from 29 to 31 Mb, which is concordant with all three QTLs for renal function in rat, mouse, and human.
Table 1.
List of candidate genes in the Rf-3_b region that overlap the syntenic mouse and human renal function QTLs
| Symbol | Name | Start | Stop |
|---|---|---|---|
| LOC690064 | Similar to 40S ribosomal protein S10 | 142,853,462 | 142,853,996 |
| Defb29 | Defensin β 29 | 142,860,674 | 142,865,955 |
| Defb21 | Defensin β 21 | 142,910,151 | 142,911,465 |
| Defb24 | Defensin β 24 | 142,913,877 | 142,920,010 |
| Defb27 | Defensin β 27 | 142,932,437 | 142,937,373 |
| Defb36 | Defensin β 36 | 142,946,483 | 142,959,997 |
| Defb25 | Defensin β 25 | 142,972,019 | 142,972,859 |
| Rem1 | RAS-like GTP binding 1 | 142,976,928 | 142,985,368 |
| H13 | Histocompatability 13 | 143,020,612 | 143,056,203 |
| Id1 | Inhibitor of DNA binding 1 | 143,086,162 | 143,087,289 |
| LOC499921 | Similar to high mobility group protein 1 | 143,095,429 | 143,096,094 |
| Cox4i2 | Cytochrome c oxidase subunit IV isoform 2 | 143,103,348 | 143,114,236 |
| Bcl2l1 | Bcl2-like1 | 143,129,087 | 143,180,199 |
Start and stop are base positions on rat chromosome 3.
Candidate Region Sequence Analysis
Using the sequence capture array followed by GS-FLX 454 sequencing, we were able to obtain a sequence covering 96.98 and 96.87% of the 7.1-Mb target region for FHH and ACI, respectively. We identified a total of 9556 sequence variants between the two strains. Thirty-three variants resulted in nonsynonymous amino acid changes in a total of 22 genes within the Rf-3_b region (Tables S1 and S2). Within the candidate region deduced by comparative mapping, we found exon variants in 2 of the 13 candidate genes and found 21 intron or highly conserved intergenic variants potentially affecting transcription factor binding (Table S3).
DISCUSSION
Van Dijk et al.24 reported that the Rf-3 region does not cause severe renal damage in the rat without the presence of Rf-1, yet the combination of Rf-1 and Rf-3 causes a remarkable augmentation in proteinuria. The original interacting Rf-3 congenic region spanned about 110 Mb and contained >1400 genes. In this study, we generated subcongenic lines targeting the Rf-3 95% confidence interval of the QTL to physically narrow the candidate region. By comparing renal damage susceptibility of the Rf-1 + 3+4_b to that of the Rf-1 + 3+4_a, we successfully reduced the interval by 94% to a 7.1-Mb region of rat chromosome 3 that makes a significant contribution to renal function in the FHH rat.
The Rf-1 region carries genes that cause impaired autoregulation in the FHH rat,18,28 likely leading to an increase in glomerular capillary pressure (PGC). This insult is necessary to amplify the affects of Rf-3, which does not influence renal autoregulation.24 These data suggest that the Rf-3_b gene (or genes) directly confers susceptibility to dysfunction in the nephron, affecting glomerular permeability or tubular reabsorption of proteins, and in the absence of a stressor such as increased PGC (Rf-1), the dysfunction does not manifest. The presence of Rf-1 is also required to amplify the effects of Rf-4,25 whereas the interaction between Rf-3 and Rf-4 has never been directly examined. We have combined the Rf-1, Rf-3, and Rf-4 QTLs to generate a triple congenic model to study the effects of genes in the Rf-3 region on renal function. The UAV fold change of Rf-1 + 3+4 versus Rf-1 + 4 is similar to the observed change in Rf-1 + 3 animals compared with Rf-1 animals,24 suggesting there is no profound epistasis between the Rf-3 and Rf-4 regions. The triple congenic model exhibited significantly higher levels of UAV than that of the Rf-1 + 4 animals, making this a useful model for fine mapping of the Rf-3 gene(s). As the Rf-3 congenic region alone does not confer susceptibility to measureable renal disease, it was necessary to incorporate the Rf-1 and/or Rf-4 regions in our congenic model to obtain a robust phenotype for dissecting the Rf-3 locus. This triple congenic model shows the significant role of gene–gene interactions in the development and severity of complex disease.
We found that Rf-1 + 3+4_b exhibited slightly elevated (10 mmHg) MAP compared with Rf-1 + 4 and Rf-1 + 3+4_a congenic animals. Compared with Rf-1 and Rf-3 single congenic animals, Van Dijk et al.24 also reported a slight but significant increase in systolic BP in the Rf-1 + 3 animals at 18 weeks after UNX. Because Rf-1 has been shown to affect renal autoregulation resulting in an increased PGC, it is conceivable that a slight increase in BP coupled with impaired autoregulation could cause the observed renal impairment in the Rf-1 + 3+4_b animals, and we cannot formally exclude this hypothesis. However, Van Dijk et al.24 administered Nω-Nitro-l-arginine methyl ester to raise BP to an average of 180 mmHg in combination with UNX on the Rf-1 + 4 genetic background, resulting in only a twofold increase in UAV. That our animals show a threefold increase in UAV at a young age with slight (approximately 10 mmHg) increase in BP suggests that the Rf-3 gene(s) is directly causing the observed kidney damage. Also, the original F2 analysis significantly linked Rf-3 with UAV and FGS but not with BP,21 and it has been previously reported that, unlike the FHH rat, Rf-1, Rf-3, and Rf-1 + 3 two kidney animals do not show elevated BP,24 further supporting the hypothesis that Rf-3 does not directly increase BP. Further studies are needed to determine whether Rf-3_b directly affects kidney function resulting in a secondary increase in BP or whether the initial insult of elevated BP causes the observed renal damage.
With the region reduced to just 7.1 Mb, we were able to use pyrosequencing technology to identify potentially damaging sequence variants within the candidate region. Genetic variants underlying QTLs can be caused by alterations in regulatory elements, such as transcription factor binding sites or polymorphisms that result in protein sequence changes. We analyzed the protein coding region of all annotated genes in this 7.1-Mb region and identified 22 genes with nonsynonomous amino acid changes between ACI and FHH. These genes can be considered promising candidates for future studies, and these sequence data provide an important resource for identifying genetic variants responsible for the Rf-1 + 3+4_b phenotype.
Genetic studies of complex disease in rodent models have resulted in identification of causative gene variants that have been shown to affect the corresponding disease in humans, suggesting that genetic elements share function across species.29–31 We used comparative genomics to both validate the utility of cloning this gene by position and further reduce the interval. This strategy has been used by several groups to reduce complex disease QTL intervals in the mouse and human for atherosclerosis,32–35 hypertension,32,36 and renal disease.26 Sheehan et al.26 compared mouse and human homologous renal function QTL to narrow the candidate region, Albq5. The original Rf-3 boundaries could not be used to further narrow their Albq5 candidate region because the Rf-3 breakpoints were outside of the Albq5 interval. In this study, we used congenic approaches to narrow the Rf-3 region and further minimize the candidate region that Sheehan et al. had identified. By comparing the endpoint of Albq5 with our current Rf-3_b interval breakpoints, we were able to preliminarily narrow the list of candidate genes to 13 known and predicted genes. We cannot formally exclude the possibility that any of the 181 genes in the Rf-3_b region may contribute to renal insufficiency in the FHH rat, but at this point, we can use this synteny to reduce the predicted interval further, without the need to generate additional congenics.
One of these 13 candidate genes, Bcl2l1, belongs to the Bcl-2 family, which is known to consist of anti-apoptotic genes expressed in renal tubular cells.37–40 Transgenic mice deficient in Bcl-2 have renal hypoplasia and distal tubular damage, whereas other regions of the nephron such as glomeruli and proximal tubules seem unaffected in these mice.41 Bcl2l1 is an interesting candidate gene in our model because the Rf-1 + 3+4_b line shows severe tubular dilation and protein casting at an early age. Another gene in this region, Rem1, is functionally related to the previously identified Rf-2 gene, Rab38, because both are GTPase members of the Ras superfamily,42 making this an interesting candidate as well. However, we did not find any potentially damaging sequence variants in these two genes, reducing the likelihood that they are functionally responsible for renal insufficiency in the FHH rat. In the 13 gene interval, we did identify highly conserved intergenic variants potentially affecting transcription factor binding, and we also found highly conserved exon variants in the predicted genes LOC690064 and LOC100363271. The latter does not map to human or mouse homologous renal function QTL, but it is located in the intron of Bcl2l1. In future studies, we plan to investigate whether these, or any of the 246 variants within this 13 gene interval, are causal. If not, we will return to the larger gene set.
In summary, we physically reduced the Rf-3 QTL to a 7.1-Mb region of rat chromosome 3 that significantly contributes to renal impairment in the FHH rat. We compared our candidate region to the concordant mouse and human renal function QTLs to narrow the list of 181 candidate genes in this region down to 13 known and predicted genes. Additional studies are needed to elucidate the specific variant(s) causing the observed phenotype and to determine how these candidate genes affect kidney function in the FHH rat. In the interim, these genes can be tested in subsets of genome wide association studies and other species or strains.
CONCISE METHODS
Generation of Triple Congenic Overlapping Subcongenic Strains
Rats were housed in the Biomedical Resource Center of the Medical College of Wisconsin, an American Association for the Accreditation of Laboratory Animal Care–approved facility. All protocols used in these studies were approved by the local Animal Care and Use Committee. We performed an initial cross between congenic strains ACI.FHH (D1Mit18-D1Rat90)/(D14Mit11-D14Rat33/D14Rat65-D14Rat90), referred to as Rf-1 + 4,25 and ACI.FHH (D1Mit18-D1Rat90)/(D3Rat84-D3Rat59)/(D14Mit11-D14Rat33/D14Rat65-D14Rat90), referred to as Rf-1 + 3+4, to generate an F1 generation. We intercrossed F1 littermates, and the F2 generation was genotyped with 19 microsatellite markers across 110 Mbp of the Rf-3 region by fluorescence genotyping, as described previously.43 Recombinant animals were selected for breeding to generate a panel of subcongenic lines targeting the Rf-3 95% confidence interval of the QTL.
In Vivo Renal Damage Assessment
Male rats between 5 and 6 weeks of age were anesthetized with ketamine (30 mg/kg) plus xylazine (2.5 mg/kg) and acepromazine (0.6mg/kg), the right kidney was exposed by a flank incision, the renal artery and vein were ligated, and the kidney was removed. After surgery, the animal was placed on a purified AIN-76A rodent diet containing 0.4% NaCl (Dyets, Bethlehem, PA) and allowed to recover for 4 weeks. After recovery, the animals were placed in metabolic cages (Nalgene, Rochester, NY) and allowed to adapt to the cages for 2 days, followed by urine collection for two consecutive 24-hour periods. Albumin concentration was determined using Albumin Blue 580 assay (Molecular Probes, Eugene, OR).
BP Measurement
After the metabolic cage experiment, MAP was measured by radiotelemetry (Data Sciences, St. Paul, MN) in 10- to 11-week-old rats. Telemetry transmitters (TA11PA-C40) were implanted subcutaneously (under isoflurane anesthesia), and the catheter was inserted into the abdominal aorta via the femoral artery. Animals were allowed 4 days for recovery after surgery, and BP was recorded in conscious, freely moving animals for 3 consecutive days at 500 Hz. Ten-second intervals were continuously recorded every 2 minutes, and these data were averaged over a 3-hour period each day to estimate MAP.
Histologic Examination
Three-month-old uniphrectomized males were killed by isoflurane overdose, and the left kidney was removed and immediately placed in 10% buffered formalin (Sigma-Aldrich) for fixation. Fixed kidneys were sectioned and stained using Gomori's one-step trichrome stain for histologic analysis. Percent area of protein casting was quantified in the outer stripe of outer medulla and cortex region. To quantify percent area of protein casting, stained kidney sections were scanned at 4000 dpi using a Nikon Coolscan V ED (Nikon Instruments) and analyzed using MetaMorph version 7.1.3 (Molecular Devices). The outer stripe of the outer medulla and cortex region was encircled on the scanned images, and a color threshold was set to selectively detect the deep red color that matched the hue of protein casts. The percent protein casting was calculated by (Protein Cast Area/Total Encircled Area) × 100. Glomeruli were scored based on percent sclerosis on a scale from 0 (approximately 0%) to 4 (approximately 100%), and scores for 30 glomeruli were averaged for each animal.
Capture, Sequencing, and Analysis
ACI and FHH genomic DNA for the 7.1-Mb candidate region was captured using a custom tiling 385K array (coordinates: rat chr3:141,890,119 to 149,038,368) designed and manufactured by Roche-Nimblegen. Genomic DNA was sheared using nebulization, and adaptors were ligated to the resulting fragments. Linker mediated PCR was performed to amplify the library, and the amplified library was hybridized to the custom array according to the manufacture protocol (Roche Applied Science/Nimblegen). Unbound DNA was washed away, and hybridized DNA was eluded off of the chip. One microgram of target-enriched DNA was sequenced on the Roche GS-FLX sequencer running 454 sequencing technology (Roche Applied Science). Sequence reads were assembled, and variants were detected using the gsMapper software (Roche Applied Science). Highly conserved variants were identified using the VISTA genome browser (http://pipeline.lbl.gov/cgi-bin/gateway2), and transcription factor binding sites were found using the TFSearch website algorithm (http://molsun1.cbrc.aist.go.jp/research/db/TFSEARCH.html).
Statistical Analysis
Data are presented as mean ± SEM. We analyzed data by t test or a one-way ANOVA followed by the Holm-Sidak multiple comparison test using Sigma Plot 11.0 software. Because albumin data failed the equal variance test, we transformed the data by taking the logarithm of each value and performed a one-way ANOVA followed by the Holm-Sidak multiple comparison test.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This study was performed with financial support from the National Heart, Lung, and Blood Institute (NHLBI-5R01HL069321) to H.J.J. The authors thank Mike Tschannen and Jaime Wendt Andrae for excellent technical assistance.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Lindner TH, Monks D, Wanner C, Berger M: Genetic aspects of diabetic nephropathy. Kidney Int 63: S186–S191, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Freedman BI, Spray BJ, Tuttle AB, Buckalew VM: The familial risk of end-stage renal disease in African Americans. Am J Kidney Dis 21: 387–393, 1993 [DOI] [PubMed] [Google Scholar]
- 4. Marin R, Gorostidi M, Fernandez-Vega F, Alvarez-Navascues R: Systemic and glomerular hypertension and progression of chronic renal disease: The dilemma of nephrosclerosis. Kidney Int 68: S52–S56, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Freedman BI, Iskandar SS, Appel RG: The link between hypertension and nephrosclerosis. Am J Kidney Dis 25: 207–221, 1995 [DOI] [PubMed] [Google Scholar]
- 6. Pattaro C, Aulchenko YS, Isaacs A, Vitart V, Hayward C, Franklin CS, Polasek O, Kolcic I, Biloglav Z, Campbell S, Hastie N, Lauc G, Meitinger T, Oostra BA, Gyllensten U, Wilson JF, Pichler I, Hicks AA, Campbell H, Wright AF, Rudan I, van Duijn CM, Riegler P, Marroni F, Pramstaller PP; EUROSPAN Consortium: Genome-wide linkage analysis of serum creatinine in three isolated European populations. Kidney Int 76: 297–306, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Fox CS, Yang Q, Cupples LA, Guo C-Y, Larson MG, Leip EP, Wilson PWF, Levy D: Genomewide linkage analysis to serum creatinine GFR, and creatinine clearance in a community-based population: The Framingham Heart Study. J Am Soc Nephrol 15: 2457–2461, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Kottgen A, Glazer NL, Dehghan A, Hwang SJ, Katz R, Li M, Yang Q, Gudnason V, Launer LJ, Harris TB, Smith AV, Arking DE, Astor BC, Boerwinkle E, Ehret GB, Ruczinski I, Scharpf RB, Chen YD, de Boer IH, Haritunians T, Lumley T, Sarnak M, Siscovick D, Benjamin EJ, Levy D, Upadhyay A, Aulchenko YS, Hofman A, Rivadeneira F, Uitterlinden AG, van Duijn CM, Chasman DI, Paré G, Ridker PM, Kao WH, Witteman JC, Coresh J, Shlipak MG, Fox CS: Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet 41: 712–717, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schelling JR, Abboud HE, Nicholas SB, Pahl MV, Sedor JR, Adler SG, Arar NH, Bowden DW, Elston RC, Freedman BI, Goddard KA, Guo X, Hanson RL, Ipp E, Iyengar SK, Jun G, Kao WH, Kasinath BS, Kimmel PL, Klag MJ, Knowler WC, Nelson RG, Parekh RS, Quade SR, Rich SS, Saad MF, Scavini M, Smith MW, Taylor K, Winkler CA, Zager PG, Shah VO; Family Investigation of Nephropathy and Diabetes Research Group: Genome-wide scan for estimated glomerular filtration rate in multi-ethnic diabetic populations. Diabetes 57: 235–243, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Mottl AK, Vupputuri S, Cole SA, Almasy L, Göring HH, Diego VP, Laston S, Franceschini N, Shara NM, Lee ET, Best LG, Fabsitz RR, MacCluer JW, Umans JG, North KE: Linkage analysis of glomerular filtration rate in American Indians. Kidney Int 74: 1185–1191, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rogus JJ, Poznik GD, Pezzolesi MG, Smiles AM, Dunn J, Walker W, Wanic K, Moczulski D, Canani L, Araki S, Makita Y, Warram JH, Krolewski AS: High-density single nucleotide polymorphism genome-wide linkage scan for susceptibility genes for diabetic nephropathy in type 1 diabetes. Diabetes 57: 2519–2526, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Imperatore G, Hanson RL, Pettitt DJ, Kobes S, Bennett PH, Knowler WC: Sib-pair linkage analysis for susceptibility genes for microvascular complications among Pima Indians with type 2 diabetes. Pima Diabetes Genes Group. Diabetes 47: 821–830, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Altshuler D, Daly MJ, Lander ES: Genetic mapping in human disease. Science 322: 881–888, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mullins L, Mullins J: Insights from the rat genome sequence. Genome Biol 5: 221, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mattson DL, Dwinell MR, Greene AS, Kwitek AE, Roman RJ, Cowley AW, Jacob HJ: Chromosomal mapping of the genetic basis of hypertension and renal disease in FHH rats. Am J Physiol Renal Physiol 293: F1905–F1914, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Kriz W, Hosser H, Hahnel B, Simons JL, Provoost AP: Development of vascular pole-associated glomerulosclerosis in the Fawn-hooded rat. J Am Soc Nephrol 9: 381–396, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Simons JL, Provoost AP, De Keijzer MH, Anderson S, Rennke HG, Brenner BM: Pathogenesis of glomerular injury in the fawn-hooded rat: Effect of unilateral nephrectomy. J Am Soc Nephrol 4: 1362–1370, 1993 [DOI] [PubMed] [Google Scholar]
- 18. Van Dokkum RPE, Alonso-Galicia M, Provoost AP, Jacob HJ, Roman RJ: Impaired autoregulation of renal blood flow in the fawn-hooded rat. Am J Physiol Regul Integr Comp Physiol 276: R189–R196, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Van Dokkum RPE, Sun C-W, Provoost AP, Jacob HJ, Roman RJ: Altered renal hemodynamics and impaired myogenic responses in the fawn-hooded rat. Am J Physiol Regul Integr Comp Physiol 276: R855–R863, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Brown DM, Provoost AP, Daly MJ, Lander ES, Jacob HJ: Renal disease susceptibility and hypertension are under independent genetic control in the fawn-hooded rat. Nat Genet 12: 44–51, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Shiozawa M, Provoost AP, Van Dokkum RPE, Majewski RR, Jacob HJ: Evidence of gene–gene interactions in the genetic susceptibility to renal impairment after unilateral nephrectomy. J Am Soc Nephrol 11: 2068–2078, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Rangel-Filho A, Sharma M, Datta YH, Moreno C, Roman RJ, Iwamoto Y, Provoost AP, Lazar J, Jacob HJ: Rf-2 gene modulates proteinuria and albuminuria independently of changes in glomerular permeability in the Fawn-Hooded Hypertensive rat. J Am Soc Nephrol 16: 852–856, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Brown DM, Van Dokkum RPE, Korte MR, McLauglin MG, Shiozawa M, Jacob HJ, Provoost AP: Genetic control of susceptiblity for renal damage in Hypertensive Fawn-Hooded rats. Renal Failure 20: 407–411, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Van Dijk SJ, Specht PAC, Lazar J, Jacob HJ, Provoost AP: Synergistic QTL interactions between Rf-1 and Rf-3 increase renal damage susceptibility in double congenic rats. Kidney Int 69: 1369–1376, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Van Dijk SJ, Specht PAC, Lutz MM, Lazar J, Jacob HJ, Provoost AP: Interaction between Rf-1 and Rf-4 quantitative trait loci increases susceptibility to renal damage in double congenic rats. Kidney Int 68: 2462–2472, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Sheehan S, Tsaih S-W, King BL, Stanton C, Churchill GA, Paigen B, DiPetrillo K: Genetic analysis of albuminuria in a cross between C57BL/6J and DBA/2J mice. Am J Physiol Renal Physiol 293: F1649–F1656, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Shike T, Gohda T, Tanimoto M, Kobayashi M, Makita Y, Funabiki K, Horikoshi S, Hirose S, Shirai T, Tomino Y: Chromosomal mapping of a quantitative trait locus for the development of albuminuria in diabetic KK/Ta mice. Nephrol Dial Transplant 20: 879–885, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Lopez B, Ryan RP, Moreno C, Sarkis A, Lazar J, Provoost AP, Jacob HJ, Roman RJ: Identification of a QTL on chromosome 1 for impaired autoregulation of RBF in fawn-hooded hypertensive rats. Am J Physiol Renal Physiol 290: F1213–F1221, 2006 [DOI] [PubMed] [Google Scholar]
- 29. O'Brien SJ, Menotti-Raymond M, Murphy WJ, Nash WG, Wienberg J, Stanyon R, Copeland NG, Jenkins NA, Womack JE, Marshall Graves JA: The promise of comparative genomics in mammals. Science 286: 458–481, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Bottger A, van Lith HA, Kren V, Krenová D, Bílá V, Vorlícek J, Zídek V, Musilová A, Zdobinská M, Wang JM, van Zutphen BF, Kurtz TW, Pravenec M: Quantitative trait loci influencing cholesterol and phospholipid phenotypes map to chromosomes that contain genes regulating blood pressure in the spontaneously hypertensive rat. J Clin Invest 98: 856–862, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rapp JP, Wang SM, Dene H: A genetic polymorphism in the renin gene of Dahl rats cosegregates with blood pressure. Science 243: 542–544, 1989 [DOI] [PubMed] [Google Scholar]
- 32. Chang Y-PC, Liu X, Kim JD, Ikeda MA, Layton MR, Weder AB, Cooper RS, Kardia SL, Rao DC, Hunt SC, Luke A, Boerwinkle E, Chakravarti A: Multiple genes for essential-hypertension susceptibility on chromosome 1q. Am J Hum Genet 80: 253–264, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang X, Ishimori N, Korstanje R, Rollins J, Paigen B: Identifying novel genes for atherosclerosis through mouse-human comparative genetics. Am J Hum Genet 77: 1–15, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Burgess-Herbert SL, Cox A, Tsaih S-W, Paigen B: Practical applications of the bioinformatics toolbox for narrowing quantitative trait loci. Genetics 180: 2227–2235, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yu X, Teng H, Marques A, Ashgari F, Ibrahim SM: High resolution mapping of Cia3: A common arthritis quantitative trait loci in different species. J Immunol 182: 3016–3023, 2009 [DOI] [PubMed] [Google Scholar]
- 36. DiPetrillo K, Tsaih S-W, Sheehan S, Johns C, Kelmenson P, Gavras H, Churchill GA, Paigen B: Genetic analysis of blood pressure in C3H/HeJ and SWR/J mice. Physiol Genomics 17: 215–220, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Gobe G, Zhang XJ, Willgoss DA, Schoch E, Hogg NA, Endre ZH: Relationship between expression of Bcl-2 genes and growth factors in ischemic acute renal failure in the rat. J Am Soc Nephrol 11: 454–467, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Basile DP, Liapis H, Hammerman MR: Expression of bcl-2 and bax in regenerating rat renal tubules following ischemic injury. Am J Physiol Renal Physiol 272: F640–F647, 1997 [DOI] [PubMed] [Google Scholar]
- 39. Valdés F, Pásaro E, Díaz I, Centeno A, López E, García-Doval S, González-Roces S, Alba A, Laffon B: Segmental heterogeneity in Bcl-2, Bcl-xL and Bax expression in rat tubular epithelium after ischemia-reperfusion. Nephrology 13: 294–301, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Takase O, Minto AW, Puri TS, Cunningham PN, Jacob A, Hayashi M, Quigg RJ: Inhibition of NF-[kappa]B-dependent Bcl-xL expression by clusterin promotes albumin-induced tubular cell apoptosis. Kidney Int 73: 567–577, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Sorenson CM, Padanilam BJ, Hammerman MR: Abnormal postpartum renal development and cystogenesis in the bcl-2(-/-) mouse. Am J Physiol Renal Physiol 271: F184–F193, 1996 [DOI] [PubMed] [Google Scholar]
- 42. Pochynyuk O, Stockand JD, Staruschenko A: Ion channel regulation by Ras, Rho, and Rab small GTPases. Exp Biol Med 232: 1258–1265, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Moreno C, Kennedy K, Andrae JW, Jacob HJ: Genome-wide scanning with SSLPs in the rat. Methods Mol Med 108: 131–138, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.