Abstract
Steroid-resistant focal segmental glomerulosclerosis (FSGS) often recurs after renal transplantation. In this international survey, we sought to identify genotype–phenotype correlations of recurrent FSGS. We surveyed 83 patients with childhood-onset primary FSGS who received at least one renal allograft and analyzed 53 of these patients for NPHS2 mutations. The mean age at diagnosis was 6.7 years, and the mean age at first renal transplantation was 13 years. FSGS recurred in 30 patients (36%) after a median of 13 days (range, 1.5 to 152 days). Twenty-three patients received a second kidney transplant, and FSGS recurred in 11 (48%) after a median of 16 days (range, 2.7 to 66 days). None of the 11 patients with homozygous or compound heterozygous NPHS2 mutations developed recurrent FSGS compared with 45% of patients without mutations. These data suggest that genetic testing for pathogenic mutations may be important for prognosis and treatment of FSGS both before and after transplantation.
Primary focal segmental glomerulosclerosis (FSGS) is a “malignant” renal disease, accounting for nearly 10 to 15% of all pediatric and adult patients requiring chronic dialysis or transplantation, and its incidence seems to be rising.1 Additionally, the etiology of FSGS remains obscure, and there is no proven effective therapy. The risk of recurrence is approximately 30%; therefore, kidney transplantation in patients with FSGS is a major challenge.2–4 Recurrence is associated with a significantly higher risk of early graft loss.5 Several risk factors for recurrence have been described: age <6 years at disease onset, rapid progression to ESRD, and recurrence in a previous transplant are reported to be associated with a higher risk of disease recurrence after renal transplantation.6
Mutations in the NPHS2 gene were first described in patients with familial autosomal recessive FSGS7 and thereafter also in sporadic steroid-resistant nephrotic syndrome.8 Positional cloning in the 1q25-q31 region was used to identify the causative gene in steroid-resistant nephrotic syndrome.7 The NPHS2 gene (OMIM 604766) encodes for an integral podocyte membrane protein named podocin. Recurrence after renal transplantation seems to also be influenced by genetic background.9,10
To further define the clinical course and prognosis of patients with FSGS after renal transplantation, we retrospectively analyzed 83 patients of the European FSGS Transplantation Study group with complete clinical data and 53 patients in whom genetic testing was available.
RESULTS
Epidemiology
Of the 83 included patients 75 were white, 6 were Arabs, and 2 were Asians. Forty-eight patients were men and 35 were women. Seventy-eight patients had biopsy-proven FSGS in the native kidney, and the remaining 5 patients were either familial cases (3) or had a documented homozygous or compound heterozygous NPHS2 mutation (2). Twenty-two of the 78 showed minimal change disease in their first biopsy result but were reclassified as FSGS in the second biopsy of the native kidney.
Diagnosis of nephrotic syndrome was made at a mean age of 6.7 ± 0.7 (SEM) years (range, 1 to 34 years). Patients progressed to ESRD in 4.9 ± 0.8 years on average. Patients >6 years of age proceeded faster to ESRD, with a mean time of 3.5 ± 0.8 years, compared with the patients under 6 years of age, with a mean of 5.9 ± 0.8 years (P < 0.05, t test). Waiting time to first transplantation was 17.6 ± 1.5 months for deceased donors (DD) only. Mean age at first transplantation was 12.9 ± 0.8 years (range, 7.4 to 15.6 years).
All patients were transplanted once, 23 were transplanted twice, 6 patients received a third graft, and 1 patient received a fourth graft. Regarding the first transplantation, 20 grafts (24%) were living-related donations (LRD), and 63 grafts (76%) were from a DD. The second transplantation was performed with LRD in 5 patients (22%) and a DD in 18 patients (78%); all third or fourth Renal transplantation (RTx) were performed with a DD. Mean age at time of entry into the study was 23.5 ± 1.0 (SEM) years. Mean observation period was 12.7 ± 0.9 years.
Patient characteristics are listed in Table 1.
Table 1.
Demographic data on all 83 patients compared with the subset of 53 patients subjected to NPHS2 genetic screening
| n = 83 | n = 53 | P | |
|---|---|---|---|
| Male /female | 48/35 | 27/26 | NSa |
| White/Arab/Asian | 75/6/2 | 49/2/2 | NSa |
| LRD/DD at first RTx | 20/63 | 16/37 | NSa |
| LRD/DD at second RTx | 5/18 | 5/14 | NSa |
| Number of RTx (1x/2x/3x/4x) | 83/23/6/1 | 53/19/5/1 | NSa |
| Age at diagnosis (years, mean ± SEM) | 6.8 ± 0.7 | 7.4 ± 1.0 | NSb |
| Time to ESRD (years, mean ± SEM) | 5.0 ± 0.8 | 5.7 ± 0.8 | NSb |
| Time of dialysis for LRD (months, mean ± SEM) | 10.2 ± 1.9 | 9.1 ± 1.8 | NSb |
| Time of dialysis for DD (months, mean ± SEM) | 17.6 ± 1.5 | 17.1 ± 1.9 | NSb |
| Age at first RTx (years, mean ± SEM) | 12.9 ± 0.8 | 14.1 ± 1.1 | NSb |
| Observation period (years ± SEM) | 12.7 ± 0.9 | 13.8 ± 1.3 | NSb |
NS, not significant.
aChi-Quadrate test (Pearson).
bt test.
Genetic Analysis
Fifty-three of 83 patients were screened for mutations by direct sequencing of the eight exons comprising the sequence of the NPHS2 gene on chromosome 1q25–q31 encoding the podocyte protein podocin. Demographic data are listed separately in Table 1.
Eleven of the 53 investigated individuals (20.8%) showed either homozygous (n = 8) or compound heterozygous (n = 3) NPHS2 mutations in an autosomal recessive disease. Two of the compound heterozygous mutations caused a frame shift, leading to a premature stop codon. The most frequent mutation was homozygous at location R138Q (n = 6, Table 2). Four of the 11 patients with homozygous or compound heterozygous NPHS2 mutations had a history of familial predisposition: they had a brother or sister with an identical mutation. Age at time of diagnosis in patients with homozygous or compound heterozygous mutations was 4.2 ± 1.2 years compared with 8.2 ± 1.2 years in patients without a mutation or with heterozygous mutations (P = 0.04, Mann-Whitney U test). Progression to ESRD took 6.2 ± 1.4 years compared with 5.6 ± 0.9 years, respectively (not significant). Four patients (7.5%) showed a heterozygous NPHS2 mutation, they were diagnosed with nephrotic syndrome at an average age of 2.6 ± 1.2 years, and proceeded to ESRD in 3.7 ± 1.7 years.
Table 2.
Eleven of 53 patients genetically analyzed with two pathogenic NPHS2 mutations and 4 patients with one NPHS2 sequence variant
| Position | Mutation Status |
|---|---|
| DelAA, 855 + 856 | Homozygous |
| DelG, 417 + R138Q | Compound heterozygous |
| DelG, 417 + R138Q | Compound heterozygous |
| C851T/A284V | Compound heterozygous |
| R138Q | Homozygous |
| R138Q | Homozygous |
| R138Q | Homozygous |
| R138Q | Homozygous |
| DelAA, 855 + 856 | Homozygous |
| R138Q | Homozygous |
| R138Q | Homozygous |
| R229Q | Heterozygous |
| R229Q | Heterozygous |
| Not available | Heterozygous |
| L207I | Heterozygous |
A small subset of patients were screened for other mutations. NPHS1 was found as a heterozygous mutation in one patient, who also had a heterozygous NPHS2 mutation. Thirteen other patients screened for NPHS1 were negative. In the seven patients screened for TRPC6, no mutations were identified. Mean age at diagnosis in these seven patients was 15.5 ± 4.9 years, and one patient had a familial predisposition.
Recurrence
Thirty of 83 (36.1%) patients had a recurrence after the first RTx, 25 of those within 6 months after transplantation. Eleven of 23 patients (47.8%) had a recurrence after the second RTx and 100% after the third and fourth RTx. One hundred percent of patients who had lost their first graft because of recurrence and were retransplanted had another recurrence in the second graft. The time period after first RTx to recurrence of proteinuria was 13 days (median; range, 1.5 to 152 days) or 152 ± 55 (mean ± SEM) days, after the second RTx, median duration to recurrence was 16 days (range, 2.7 to 66.0 days) or 97 ± 72 days, and after the third RTx 45 days (range, 3 to 125 days) or 58 ± 33 days, respectively.
The 53 patients subjected to NPHS2 screening did not differ significantly with respect to demographic features (Table 1) and recurrence rate compared with the entire study group. In this genetically analyzed subgroup, the overall recurrence rate was 17/53 (32.1%). Regarding NPHS2 mutations, none of the 11 patients with both NPHS2 alleles having mutations had a recurrence after the first RTx, nor did the 4 patients with a solitary heterozygous mutation. Of the remaining 38 tested individuals, 17 showed recurrence of proteinuria after the first RTx (44.7%; Figure 1A). The difference in recurrence rate between patients with homozygous or compound heterozygous NPHS2 mutations and patients without or with a heterozygous mutation was significant (Kaplan-Meier, P < 0.05). Given that patients with heterozygous mutations might have another yet unidentified mutation leading to a nonimmunologic FSGS, they were also evaluated as part of the patient group with both NPHS2 alleles having mutations. The difference was even more significant (P < 0.005). Thus, homozygous and compound heterozygous NPHS2 mutations, and in this population also solitary heterozygous mutations, were not associated with recurrent disease after transplant (Fisher exact test, P < 0.01). Patients with unknown mutation status (n = 30) showed a recurrence rate of 43.3% (n = 13), similar to the recurrence rate of patients without any mutation (44.7%).
Figure 1.
No disease recurrence in patients with NPHS2 mutation compared to 45% recurrence in patients without NPHS2 mutation. (B) No significant difference between recurrence rates of LRD versus DD by Kaplan-Meier analysis. The four individuals with a heterozygous status had an overall grafts survival rate of 50%, one graft was lost to acute rejection after a time of 25 days, one to chronic allograft nephropathy after 23 years.
When analyzing the entire group (n = 83), recurrence rate proved to be independent of gender, donor source (Figure 1B), ethnic origin, and time of progression to ESRD. Younger age at time of first diagnosis correlated with a reduced recurrence rate. Mean age at first diagnosis of patients with recurrence was 9 ± 1.3 years; mean age of those that did not have a recurrence was 5.5 ± 0.8 years (P = 0.008).
Patient and Graft Survival
Patient survival was 94% (n = 78/83) after a mean observation period of 12.5 ± 0.9 years. Reasons for death were sepsis, breast carcinoma, Epstein-Barr virus disease, thrombotic complications with cardiogenic shock, and an unknown reason in one patient. Mean age at time of death was 29.7 ± 6.2 years.
Overall graft loss after the first RTx was 36.1% (n = 30) and occurred after a mean time of 5.1 ± 1.2 years. The percentage of graft loss caused by recurrence was 40% (12 of 30 patients), caused by chronic allograft nephropathy was 50% (15 of 30), and caused by other reasons was 10%. Graft loss caused by recurrence occurred at a mean of 1.7 ± 0.4 years compared with 8.1 ± 2 years when caused by chronic allograft nephropathy (P < 0.01) and 4 years when caused by other reasons. Graft loss after the second RTx was 52.2% (12 of 23) after 3.4 ± 1.3 years and was caused by recurrence in 67% (8 of 12), by chronic allograft nephropathy in 17%, and by other reasons in 16%. Time to graft loss was 1.2 ± 0.4 years in recurring patients compared with 7.4 ± 6.2 years in nonrecurring patients (P < 0.05). Six of eight patients (75%) who lost their second graft because of recurrence had lost their first graft for the same reason. The two remaining patients who lost their first graft because of recurrence and were retransplanted also recurred after 48 and 3 days, respectively, and kept their graft for 5 and 10 months at the time the evaluation was performed. Graft loss after the third RTx occurred in three of six patients (50%) after a mean time of 2.0 ± 1.5 years. All three cases had a history of graft loss caused by recurrence of disease and again lost their transplant by recurrence. One patient with a third graft died with a functioning graft, the two remaining patients also had recurrences after 2 and 137 days, respectively, yet still had a functioning third graft after 9 months and 3 years, respectively.
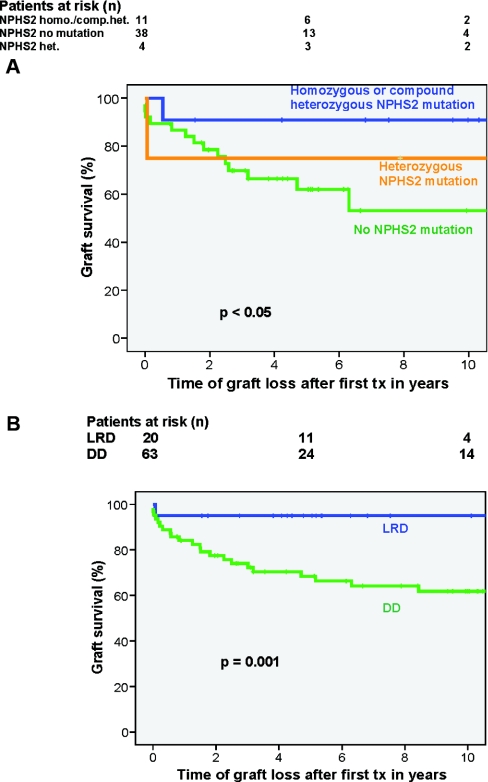
Referring to the entire study population and different observation periods of patients, graft loss according to NPHS2 mutations was as low as 27% (n = 3) of the 11 patients with homozygous or compound heterozygous NPHS2 mutations after a mean observation period of 11.4 ± 5.9 years versus 45% (n = 17) of the 38 patients without NPHS2 mutations (log rank, P < 0.05; Figure 2A). The four individuals with a heterozygous status had an overall graft survival rate of 50%, one graft was lost to acute rejection, one to chronic allograft nephropathy after a time of 25 days after 23 years, respectively.
Figure 2.
(A) Better graft survival in patients with NPHS2 mutation (homozygous or compound heterozygous) compared with patients without NPHS2 mutations after 10 years. (B) Better graft survival in LRD- versus DD-associated transplantation.
Influence of LRD/DD and Preemptive Transplantation
Overall recurrence rate was 25% in LRD versus 39.7% in DD (not significant; Figure 1B), and graft survival was 95% in LRD versus 54% in DD (P = 0.001; Figure 2B). In association with genetic groups, the distribution of LRD versus DD was 3 versus 8 in patients with homozygous or compound heterozygous NPHS2 mutations and 12 versus 26 in patients without a mutation. Recurrence rate in subjects with both NPHS2 alleles having mutations was overall 0%; therefore, no difference between LRD and DD was found. In patients without mutations, recurrence occurred in 3 of 12 LRD patients (25%) and 14 of 26 DD patients (54%; log rank, P = 0.07). Despite the three cases of recurring disease, no graft loss occurred in the LRD group of patients without NPHS2 mutations, whereas 17 of 26 DD patients (65%) lost their graft within the observation period (P < 0.005). Among patients with homozygous or compound heterozygous NPHS2 mutations, graft survival was 100% (zero graft losses in three patients) for LRD patients versus 62.5% (three graft losses in eight patients) in DD patients (not significant).
Preemptive transplantation was performed in four patients with three DD. These four patients showed no recurrence compared with 30 recurrences in the 79 not preemptively transplanted patients. However, the number of preemptive transplants was insufficient for separate analysis.
DISCUSSION
This is the largest clinical/genetic series of patients diagnosed with FSGS who have been examined after renal transplantation for FSGS-associated podocin mutations. No patients with homozygous or compound heterozygous mutations in podocin had recurrent FSGS after transplant. In our series, patients with one mutation did not have recurrence either. These results highlight how genetic testing in patients with FSGS, other than familial forms, may be helpful to assess the risk of recurrence after RTx. In contrast to the slow progress of knowledge about immunologic forms of FSGS, significant advances have been made with regard to the genetic forms.
Genetic Analysis
Mutations in several genes have been identified as a cause of hereditary forms of FSGS, including WT1, ACTN4, CD2AP, NPHS1, NPHS2, and TRPC6. Mutations in podocin (NPHS2) were also found responsible for sporadic forms of steroid-resistant nephrotic syndrome (SRNS).8,11 We identified 28.3% of screened individuals with either a homozygous, compound heterozygous, or heterozygous mutation in the NPHS2 gene; 20.8% had either a homozygous or compound heterozygous mutation. Excluding patients with a positive family history, we found an NPHS2 mutation rate in spontaneous forms of SRNS of 13%, which agrees with previously published data of European and North American groups.9,11,12 All nonfamilial mutations were missense mutations; the most frequent one in our study group was R138Q, which was homozygous in six patients and compound heterozygous in another two patients and is most often detected in Northern Europe patients with spontaneous forms of FSGS.9 Only two of the patients with a familial history showed a frame shift mutation at 855/856delAA. However, all homozygous and compound heterozygous mutations were located in the highly conserved region of the stomatin family.9
Patients with a solitary heterozygous sequence variant remain unclear. The aspect that they behave clinically (no recurrence) like patients with homozygous or compound heterozygous mutations underscores our hypothesis that another unidentified mutation may be present. Two publications dealing with the polymorphism R229Q, traceable in at least 3.6% of a control population, showed that those patients are more susceptible to renal injury in the compound heterozygous state of the R229Q polymorphism and to microalbuminuria.13,14 Caridi et al.11 showed a higher incidence of R229Q in nephrotic siblings compared with healthy subjects. In FSGS patients, a slightly higher number of patients heterozygous for R229Q were found than in control groups.13 Furthermore, in vitro experiments found decreased nephrin binding to R229Q podocin.13 Two of the patients analyzed in this study and identified with a heterozygous mutation showed the R229Q polymorphism (3.7%), one with a variant that is not yet known (L207I).
Recurrence and Graft Survival
In our cohort, recurrence rate was 36% in the entire study group (and 32% in the subgroup subjected to genetic screening) after the first transplant, which is in the upper range of findings described in the literature.10,15,16 Graft loss caused by recurrence occurred in 12 of 30 patients with recurrence and in 30 patients with graft loss. Three additional patients with recurrence lost their graft because of an episode of rejection. The latter may confirm the observation of Pinto et al.17 regarding the possibility of acute rejections triggered by an episode of recurrence. Thus, 50% of documented graft losses occurred in patients with at least one episode of recurrence. This underscores the tremendous influence of recurrence on the outcome, especially because time to graft loss is significantly shorter (1.7 years) compared with graft losses caused by reasons other than recurrence (8 years). Those who did not lose their transplant after recurrence, i.e., by successful treatment (n = 15), showed similar renal function compared with individuals who did not recur. However, 10-year graft survival of patients with an episode of recurrence remains significantly worse compared with patients who did not have recurrence.
Controversial risk factors for recurrence identified in the literature are also questionable in this cohort of individuals. An absolute risk factor for recurrence of disease in a second graft is recurrence in the first one, even when Stephanian et al.18 distinguished between patients who kept their primary graft for a substantial time and those who lost the organ quickly after transplantation. In this cohort, 100% of retransplanted patients who lost their first graft because of recurrence also experienced recurrence in the second graft and lost it earlier compared with patients without recurrence. Additionally, other risk factors are less evident without the knowledge of their genetic background. Namely, a younger age at diagnosis was not a risk factor in our cohort; this might be because of a high percentage of patients with NPHS2 mutations, who did not have recurrence, in this younger age group. Furthermore, progression to ESRD was equally long in patients with homozygous or compound heterozygous NPHS2 mutations compared with those without NPHS2 mutations or patients with a heterozygous mutation in this cohort, with a significantly higher recurrence rate in patients without NPHS2 mutations after RTx.
With regard to graft loss based on NPHS2 mutations, patients with homozygous or compound heterozygous mutations did not recur and therefore showed no graft losses caused by recurrence. In this analysis, the four patients with a solitary heterozygous mutation did not recur either and showed no recurrence-associated graft loss. Thus, according to most published data, all recurrences occurred in patients without NPHS2 mutations.9,19 In contrast to this finding, Bertelli et al.10 found an equal recurrence rate in genetic versus nongenetic etiologies for FSGS after first transplantation.10 However, they included heterozygous mutations in the group of genetic FSGS.
Influence of LRD/DD
Interestingly, LRD was distributed equally among patients with (27% LRD) versus those without (31% LRD) NPHS2 mutations, despite the significantly different risk of recurrence. However, on examining patients without NPHS2 mutations, we found a trend toward a reduced recurrence rate in LRD versus DD (P = 0.07) and a significant advantage regarding graft survival in LRD transplantations (P < 0.005). Because of the small number of patients with NPHS2 mutations, only a trend toward better graft survival in LRD transplants could be detected. Thus, according to our study, LRD is still the better option, regardless of the etiology of FSGS, even if overall graft outcome of children with FSGS is worse compared with patients with other primary diseases.20
Genetic Testing of LRDs
In most LRDs, genetic testing for NPHS2 was not performed. Furthermore, information on the long-term course of these donors was not available. Therefore, a recommendation cannot be given in general.
However, our personal experience can be given as follows: we would screen the donor for mutations if the recipient had a mutation. Because the disease is recessive in homozygous patients and to our knowledge does not occur in heterozygous patients, we would accept a heterozygous donor. With the sequence variant R229Q, the discussion is not final. At present, the two thoughts are that R229Q is only a polymorphism (one could accept the person as donor) or it is a dominant-negative effect (there would be a risk for the donor). As long as this issue is not completely solved, we would not accept such a donor.
CONCISE METHODS
In this international multicenter study, 83 patients with childhood onset of biopsy-proven primary FSGS and at least one RTx from seven countries (Austria, United States, Czech Republic, France, Germany, Norway, and Switzerland) were included and evaluated by reviewing their clinical records and completing a standardized questionnaire. ESRD was defined as persistent need for dialysis or transplantation.
The Duke University Medical Center (Durham, NC) Institutional Review Board and the Medical University, Innsbruck, Austria Ethics Committee approved this project, and all participants provided signed informed consent before data and DNA collection.
Genetics
Evaluation of genetic background included the familial history of nephrotic syndrome and mutational analyses of NPHS1 (n = 14), NPHS2 (n = 53), ACTN4 (n = 7), TRPC6 (n = 7), and WT1 (n = 1). Because of the multicenter and retrospective design and limited availability, not all patients could be analyzed for NPHS2. Screening for NPHS1, ACTN4, TRPC6, and WT1 were additional investigations of certain centers.
NPHS2 mutational screening was performed in two laboratories (see Acknowledgments) through direct sequence analysis of exons 1 to 8 on chromosome 1q25-q31. The analysis for TRPC6 was done in the laboratory of Dr. Michelle P. Winn, Department of Medicine, Duke University Medical Center, Durham, NC.21 Data for the seven TRPC6-analyzed patients are not shown here because all of them were negative for a mutation. This study is ongoing.
The laboratories of F. Hildebrandt (Ann Arbor, MI) and C. Antignac (INSERM U574, Néphropathies Héréditaires et rein en Développement, Hôpital Necker, Paris, France) performed the genetic testing of NPHS1, ACTN4, and WT1.
Definitions
Recurrence was defined according to previous publications as proteinuria (not necessarily with the development of full nephrotic syndrome) >960 mg/m2 per day in childhood or 3.5g/1.73 m2 per day in adults in the absence of signs of rejection.10 Biopsy confirmation was recommended and performed in 60% of recurrences; 40% were diagnosed on clinical grounds. Information on how many recurrence biopsies were studied ultrastructurally was not available. Rejection was defined using the most recent Banff criteria.22
Complete remission was achieved if proteinuria was reduced to <150 mg/m2 per day; partial remission was defined as proteinuria <960 mg/m2 per day.
Statistical Analysis
Values are reported using descriptive statistical methods. Statistical comparisons were performed using the nonparametric Mann-Whitney U test. Survival analysis was performed using Kaplan-Meier estimates. Survival distributions were tested for statistical significance by log rank testing. P < 0.05 was considered significant. SPSS version 14.0 was used.
DISCLOSURES
None.
Acknowledgments
This study was supported by the Gesellschaft pädiatrische Nephrologie (GPN), the European Society of Pediatric Nephrology (ESPN), Duke University Medical Center, Durham, NC, and the Medical University Innsbruck, Austria. Parts of this study have been presented before and appeared in abstract form (WTC 2006, IPTA 2007, IPNA 2007, ASN 2007). This abstract was selected as one of the eight best abstracts at the Annual Meeting of the ASN in San Francisco 2007 (JASN, SU-FC121). One part of the genetic analyses was done in the laboratory of Professor F. Hildebrandt, University of Michigan, and was published before; another part was performed at the laboratory of Professor F. Schaefer, University Children Hospital, Heidelberg, Germany; and the third part by the group of Professor Winn, Duke University Medical Center, Durham, NC. We thank Dr. Oberaigner, Institute for Epidemiology and Statistics, TILAK, Innsbruck, Austria, for statistical assistance. We thank the following centers and colleagues for their important contribution to this study: Hacettepe, Üniversitesi, Ankara, Turkey, Prof. Söylemezoglu, Dr. Bilginer; University Childrens Hospital Erlangen, Germany, Prof. Dötsch, Dr. Plank; Landeskrankenhaus Feldkirch, Austria, Dr. Neyer; University Childrens Hospital Freiburg, Germany, Dr. Pohl; Medical University Innsbruck, Austria, Prof. Mayer, Dr. Rudnicki; Medical University Innsbruck, Austria, Pediatrics I, Dr. Clara, cand.med. Celik; Childrens Hospital München-Schwabing, Germany, Prof. Montoya; Rikshospitalet University Hospital, Oslo, Norway, Dr. Bjerre, Dr. Kyte; Dialysezentrum Villingen-Schwenningen, Germany, Dr. Schulz.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. Swaminathan S, Leung N, Lager DJ, Melton LJ, III, Bergstralh EJ, Rohlinger A, Fervenza FC: Changing incidence of glomerula disease in Olmsted County, Minnesota: A 30-year renal biopsy study. Clin J Am Soc Nephrol 1: 483–487, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Tejani A, Stablein DH: Recurrence of focal segmental glomerulosclerosis posttransplantation: A special report of the North American Pediatric Renal Transplant Cooperative Study. J Am Soc Nephrol 2: 258–263, 1992 [DOI] [PubMed] [Google Scholar]
- 3. Cochat P, Kassir A, Colon S, Glastre C, Tourniare B, Parchoux B, Martin X, David L: Recurrent nephritic syndrome after transplantation: Early treatment with plasmaphaeresis and cyclophosphamide. Pediatr Nephrol 7: 50–54, 1993 [DOI] [PubMed] [Google Scholar]
- 4. Jungraithmayr TC, Bulla M, Dippell J, Greiner C, Griebel M, Leichter HE, Plank C, Tönshoff B, Weber LT, Zimmerhackl LB; German MMF Study Group: Primary focal segmental glomerulosclerosis: Long-term outcome after pediatric renal transplantation. Pediatr Transplant 9: 226–231, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Hariharan S, Adams MB, Brennan DC, Davis CL, First MR, Johnson CP, Ouseph R, Peddi VR, Pelz CJ, Roza AM, Vincenti F, George V: Recurrent and de novo glomerular disease after renal transplantation: A report from Renal Allograft Disease Registry (RADR). Transplantation 68: 635–641, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Fine RN: Recurrence of nephrotic syndrome/focal segmental glomerulosclerosis following renal transplantation in children. Pediatr Nephrol 22: 496–502, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C: NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24: 349–354, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Karle SM, Uetz B, Ronner V, Glaeser L, Hildebrandt F, Fuchshuber A: Novel mutations in NPHS2 detected in both familial and sporadic steroid-resistant nephrotic syndrome. J Am Soc Nephrol 13: 388–393, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Weber S, Gribouval O, Esquivel EL, Morinière V, Tête MJ, Legendre C, Niaudet P, Antignac C: NPHS2 mutation analysis shows genetic heterogeneity of steroid-resistant nephrotic syndrome and low post-transplant recurrence. Kidney Int 66: 571–579, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Bertelli R, Ginevri F, Caridi G, Dagnino M, Sandrini S, Di Duca M, Emma F, Sanna-Cherchi S, Scolari F, Neri TM, Murer L, Massella L, Basile G, Rizzoni G, Perfumo F, Ghiggeri GM: Recurrence of focal segmental glomerulosclerosis after renal transplantation in patients with mutations of podocin. Am J Kidney Dis 41: 1314–1321, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Caridi G, Bertelli R, Di Duca M, Dagnino M, Emma F, Onetti Muda A, Scolari F, Miglietti N, Mazzucco G, Murer L, Carrea A, Massella L, Rizzoni G, Perfumo F, Ghiggeri GM: Broadening the spectrum of diseases related to podocin mutations. J Am Soc Nephrol 14: 1278–1286, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Caridi G, Perfumo F, Ghiggeri GM: NPHS2 (Podocin) mutations in nephrotic syndrome. Clinical spectrum and fine mechanisms. Pediatr Res 57: 54R–61R, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Tsukaguchi H, Sudhakar A, Le TC, Nguyen T, Yao J, Schwimmer JA, Schachter AD, Poch E, Abreu PF, Appel GB, Pereira AB, Kalluri R, Pollak MR: NPHS2 mutations in late-onset focal segmental glomerulosclerosis: R229Q is a common disease-associated allele. J Clin Invest 110: 1659–1666, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pereira AC, Pereira AB, Mota GF, Cunha RS, Herkenhoff FL, Pollak MR, Mill JG, Krieger JE: NPHS2 R229Q functional variant is associated with microalbuminuria in the general population. Kidney Int 65: 1026–1030, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Newstead CG: Recurrent disease in renal transplants. Nephrol Dial Transplant 18[Suppl 6]: 68–74, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Pardon A, Audard V, Caillard S, Moulin B, Desvaux D, Bentaarit B, Remy P, Sahali D, Roudot-Thoraval F, Lang P, Grimbert P: Risk factors and outcome of focal and segmental glomerulosclerosis recurrence in adult renal transplant recipients. Nephrol Dial Transplant 21: 1053–1059, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Pinto J, Lacerda G, Cameron JS, Turner DR, Bewick M, Ogg CS: Recurrence of focal segmental glomerulosclerosis in renal allografts. Transplantation 32: 83–89, 1981 [DOI] [PubMed] [Google Scholar]
- 18. Stephanian E, Matas AJ, Mauer SM, Chavers B, Nevins T, Kashtan C, Sutherland DE, Gores P, Najarian JS: Recurrence of disease in patients retransplanted for focal segmental glomerulosclerosis. Transplantation 53: 755–757, 1992 [DOI] [PubMed] [Google Scholar]
- 19. Ruf RG, Lichtenberger A, Karle SM, Haas JP, Anacleto FE, Schultheiss M, Zalewski I, Imm A, Ruf EM, Mucha B, Bagga A, Neuhaus T, Fuchshuber A, Bakkaloglu A, Hildebrandt F; Arbeitsgemeinschaft Für Pädiatrische Nephrologie Study Group: Patients with mutations in NPHS2 (podocin) do not respond to standard steroid treatment of nephrotic syndrome. J Am Soc Nephrol 15: 722–732, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Baum MA, Stablein DM, Panzarino VM, Tejani A, Harmon WE, Alexander SR: Loss of living donor renal allograft survival advantage in children with focal segmental glomerulosclerosis. Kidney Int 59: 328–333, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S, Burchette JL, Pericak-Vance MA, Howell DN, Vance JM, Rosenberg PB: A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308: 1801–1804, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Yamaguchi Y, et al. : The Banff 97 working classification of renal allograft pathology. Kidney Int 55: 713–723, 1999 [DOI] [PubMed] [Google Scholar]