Abstract
The cytidine deaminase APOBEC3G, which is incorporated into nascent virus particles, possesses potent antiviral activity and restricts Vif-deficient HIV-1 replication at the reverse transcription step through deamination-dependent and -independent effects. HIV-1 Vif counteracts the antiviral activity of APOBEC3G by inducing APOBEC3G polyubiquitination and its subsequent proteasomal degradation. In this study, we show that overexpression of heat shock protein 70 (HSP70) blocked the degradation of APOBEC3G in the ubiquitin-proteasome pathway by HIV-1 Vif, rendering the viral particles non-infectious. In addition, siRNA targeted knock-down of HSP70 expression enhanced the Vif-mediated degradation of APOBEC3G. A co-immunoprecipitation study revealed that overexpression of HSP70 inhibited APOBEC3G binding to HIV-1 Vif. Thus, we provide evidence for a host protein-mediated suppression of HIV-1 replication in an APOBEC3G-dependent manner.
Keywords: Antiviral Agents, Heat Shock Protein, HIV, Ubiquitination, Viral Replication, APOBEC3G, Vif
Introduction
Human immunodeficiency virus type-1 (HIV-1),3 the retrovirus that causes AIDS, efficiently replicates within human CD4+ T cells. However, Vif-deficient virions produced by non-permissive cells, including CD4+ T cells and immortalized lines, such as Hut78 or CEM, are non-infections, whereas virions produced in permissive cells, such as SupT1 or CEM-SS, are infectious (1, 2). Previous studies have demonstrated that HIV-1 Vif counteracts the innate antiviral activity of APOBEC3G, a member of the APOBEC family of cytidine deaminase-editing enzymes (3). In the absence of Vif, APOBEC3G induces the deamination of cytidine (C) and its conversion to uridine (U) (4, 5), which can be packaged into budding retroviruses through a direct interaction with the Gag polyprotein (6–11). The C to U conversion in the HIV-1 minus strand leads to a G to A hypermutation, preferentially at CCCA sequences. This motif corresponds to TGGG in the plus-strand sequence, thereby mutating the TGG tryptophan codon to a TAG stop codon and affecting subsequent stages of the viral life cycle (12). Vif predominant mechanism for overcoming the antiviral activity of APOBEC3G is to form an E3 ubiquitin ligase with cullin 5 (Cul5), elongin B (EloB), and elongin C (EloC) and target these proteins for degradation by the ubiquitin-proteasome pathway (13–16). Vif may also inhibit APOBEC3G activity through mechanisms independent of proteasomal degradation (17–19).
Heat shock proteins play critical roles in the life cycle of a variety of RNA and DNA viruses (20–23). For example, heat shock protein 70 (HSP70) is specifically incorporated into HIV-1 virions (24). However, the formation of the P-TEFb/Tat/TAR complex is required to stabilize the CDK9/cyclinT1 heterodimer by HSP70 and HSP90 (25).
To better develop potential novel therapeutic strategies to exploit the APOBEC3G antiviral function, we investigated the role of HSP70 in APOBEC3G function. We found that siRNA against HSP70 significantly reduced the level of APOBEC3G in the presence of HIV-1 Vif, but not in the absence of Vif. In addition, overexpression of HSP70 in 293T cells reduced the Vif-mediated degradation of APOBEC3G by inhibiting APOBEC3G polyubiquitination. This effect is attributed to the impairment of APOBEC3G-Vif binding. Furthermore, overexpression of HSP70 in the presence, but not in the absence, of APOBEC3G clearly suppressed the infectivity of virions in a dose-dependent manner. These results suggest that HSP70 acts as a potential antiviral host factor through interaction with APOBEC3G and may form the basis for new anti-HIV-1 therapies.
EXPERIMENTAL PROCEDURES
Immunoprecipitation
293T cells (5 × 105) were transfected with 1.0 μg of each Vif expression plasmid using Lipofectamine2000 (Invitrogen). At 48 h post-transfection, cells were suspended in a lysis buffer (50 mm Tris-HCl, pH 7.0, 150 mm NaCl, 1% Nonidet P-40, and 10% glycerol) and incubated with 5 μl of anti-HSP70 antibody (Santa Cruz Biotechnology) and 30 μl of Dynabeads-protein G (Invitrogen). The beads were washed with PBS containing 0.02% Tween 20. The immunocomplex was eluted by boiling with 20 μl of 5× sample buffer and analyzed by SDS-PAGE and Western blot.
Protein Stability Assay
293T cells (5 × 105) were co-transfected with 1.0 μg of pc-Hu-APOBEC3G-HA and 0.5 μg of a GFP expression plasmid (CS-CDF-CG-PRE), or 1.0 μg of pc-Hu-APOBEC3G-HA, 0.5 μg of CS-CDF-CG-PRE and 0.5 μg of pcDNA-Vif along with either 2.0 μg of pFLAG-HSP70 or an empty plasmid. At 24 h post-transfection, cells were treated with 100 μg/ml of cycloheximide. Cells were harvested, and cell lysates were analyzed by Western blotting with horseradish peroxidase-conjugated anti-HA antibody (Roche Diagnostics) and anti-GFP antibody (Medical & Biological Laboratories). The blots were semi-quantified using ImageJ 1.43u software.
Polyubiquitination Assay
293T cells (3 × 106) were co-transfected with 2.0 μg of pc-Hu-APOBEC3G-HA, 2.0 μg of pVif-V5, 4.0 μg of pFLAG-HSP70, and 2.0 μg of pCMV-Myc-Ubi (26). At 24 h post-transfection, cells were treated with 5 μm MG-132 for 24 h. Cells were suspended in a lysis buffer. Cell lysates were immunoprecipitated using anti-Myc antibody (Cell Signaling) followed by Western blotting with horseradish peroxidase-conjugated anti-HA antibody.
MAGI Assay
MAGI cells were plated in 96-well plates at 1 × 104 cells per well in Dulbecco's modified Eagle's medium with 10% fetal bovine serum. The next day, cells were infected with dilutions of the virus in a total volume of 50 μl in the presence of 20 μg/ml DEAE-dextran for 2 h. At 2 days post-infection, cells were fixed with 100 μl of fix solution (1% formaldehyde/0.2% glutaraldehyde in PBS) at room temperature for 5 min and then washed twice with PBS. Cells were incubated with 100 μl of staining solution (4 mm potassium ferrocyanide, 4 mm potassium ferricyanide, 2 mm MgCl2, and 0.4 mg/ml X-Gal) for 50 min at 37 °C. The reaction was stopped by removing the staining solution, and blue cells were counted under a microscope.
Construction of Plasmids
To generate pcDNA-Vif, HIV-1 Vif fragments were amplified from pNL4-3 by PCR with the following primers: forward 5′-GAT ATC ATG GAA AAC AGA TGG CAG GTG ATG-3′ and reverse 5′-CTC GAG CTA GTG TCC ATT CAT TGT ATG CT-3′. The PCR products were inserted into pcDNA3.1 (Invitrogen).
To construct pFLAG-HSP70, whole RNA was isolated from 293T cells with TRIzol (Invitrogen) and amplified by RT-PCR with the following primers: forward 5′-GTT GAA TTC CGC CAA AGC CGC GGC GAT-3′ and reverse 5′-CGC GGA TCC CTA ATC TAC CTC CTC AAT-3′. The products were inserted into pFLAG-CMV2 (Sigma).
To generate pVif-V5, HIV-1 Vif fragments were amplified from pNL4-3. The PCR products were inserted into pENTR using TOPO Cloning kits (Invitrogen) and transferred into pLenti6/V5-DEST (Invitrogen) by LR recombination. This construct contains the β-globin intron sequence of pMDL-g/p-RRE downstream of the CMV promoter. A plasmid construct encoding human APOBEC3G tagged with the influenza hemagglutinin (HA) sequence was a kind gift from Darlene Chen (The Salk Institute for Biological Studies). Vif-defective variants of NL4-3 have been described previously (27).
To generate pCS-U6, the U6 promoter was amplified by PCR. The resulting products were inserted into pCS-CDF-CG-PRE. pCS-U6-shControl or pCS-U6-shHSP70 was constructed by ligating the annealed product of sense oligonucleotide 5′-GAT CCT TCT CCG AAC GTG TCA CGT TTC AAG AGA ACG TGA CAC GTT CGG AGA ATT T-3′ and antisense oligonucleotide 5′-CTA GAA ATT CTC CGA ACG TGT CAC GTT CTC TTG AAA CGT GAC ACG TTC GGA GAA G-3′ or sense oligonucleotide 5′-GAT CCC ACG GCA AGG TGG AGA TCA TTC AAG AGA TGA TCT CCA CCT TGC CGT GTT T-3′ and antisense oligonucleotide 5′-CTA GAA ACA CGG CAA GGT GGA GAT CAT CTC TTG AAT GAT CTC CAC CTT GCC GTG G-3′, respectively, with the BamHI-XbaI fragment from pCS-U6. These plasmids contain a transcriptional termination signal sequence downstream of the shControl and shHSP70 sequences.
Transfection of siRNA
293T cells (3 × 106) were transfected with siRNAs (100 nm) using Lipofectamine2000. Control siRNA (5′-UUC UCC GAA CGU GUC ACG UdTdT-3′) and HSP70-siRNA (5′-CAC GGC AAG GUG GAG AUC AdTdT-3′) were purchased from B-Bridge International.
Preparation of Lentiviral Vectors
293T cells (5 × 105) were cotransfected with the lentiviral vector (1.6 μg), vesicular stomatitis virus G expression vector pMD.G (0.4 μg), rev expression vector pRSV-Rev (0.8 μg), and gag-pol expression vector pMDLg/pRRE (1.2 μg) using Lipofectamine2000. At 48 h after transfection, culture supernatants were harvested and filtered through 0.45-μm filters. In all experiments, H9 cells (3 × 105) were transduced with equal amounts of the lentivirus vector.
Statistical Analysis
The results are shown as means ± S.D., and statistical analysis was performed using the paired Student's t test. A p value of <0.05 was considered significant. At least three replicates were performed for each experiment.
RESULTS
HSP70 Leads to Stabilization of APOBEC3G
HSPs are induced by a variety of stress-related stimuli, including heat, UV radiation, and microbial/viral infections (28). HSPs are involved in the folding and translocation of cellular proteins under normal conditions, whereas under stressful conditions, HSPs bind to proteins and inhibit their misfolding or irreversible aggregation (29). Recent studies revealed that the binding of HSPs to HIV-1 proteins enhances antiviral immunity (30). HSP70 is selectively expressed soon after HIV-1 infection, suggesting that these proteins might be involved in the innate cellular antiviral immune response (31). However, the specific targets of HSPs and their role in the response to HIV infection remain unclear.
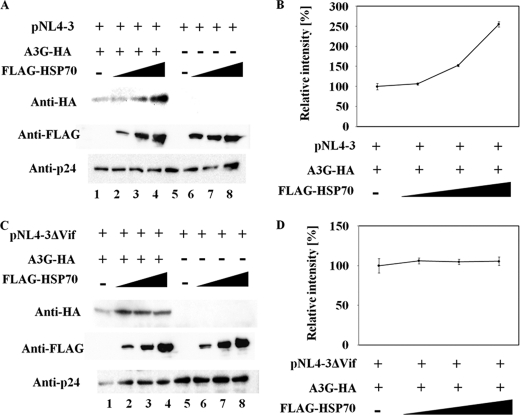
HIV-1 Vif targets APOBEC3G for ubiquitination by forming an Skp1-cullin-F-box (SCF)-like complex, which subsequently leads to the degradation of APOBEC3G. To evaluate how HSP70 affects Vif-dependent ubiquitination and degradation, we examined the steady-state level of APOBEC3G in 293T cells co-transfected with FLAG-tagged HSP70 (FLAG-HSP70) and pNL4-3. The expression of HSP70 in 293T cells significantly increased the amount of APOBEC3G in a dose-dependent manner but not the amount of the HIV-1 Gag p24 antigen (Fig. 1A, lanes 1–4 and Fig. 1B). Importantly, HSP70 had no effect on the expression level of APOBEC3G in 293T cells transfected with Vif-deleted HIV-1 proviral plasmid (Fig. 1, C, lanes 1–4 and D). Our results suggest that HSP70 may inhibit the degradation of APOBEC3G by HIV-1 Vif.
FIGURE 1.
Expression of FLAG-tagged HSP70 blocks APOBEC3G degradation in cells transfected with pNL4-3, but not those transfected with pNL4-3-delta-Vif. 293T cells (5 × 105) were co-transfected with 1.0 μg of pc-Hu-APOBEC3G-HA and increasing amounts of pFLAG-HSP70 (0, 0.5, 1.0, or 2.0 μg), adjusted with an empty vector to 2.0 μg of total, along with either 0.1 μg of pNL4-3 (A) or 0.1 μg of pNL4-3-delta-Vif (C). At 48 h post-transfection, cell lysates were analyzed by Western blotting. The relative intensity of APOBEC3G-HA bands was determined by densitometry (B and D). Results are representative of three independent experiments, and error bars show the standard deviations of the means.
HSP70 Blocks HIV-1 Vif-mediated Degradation of APOBEC3G
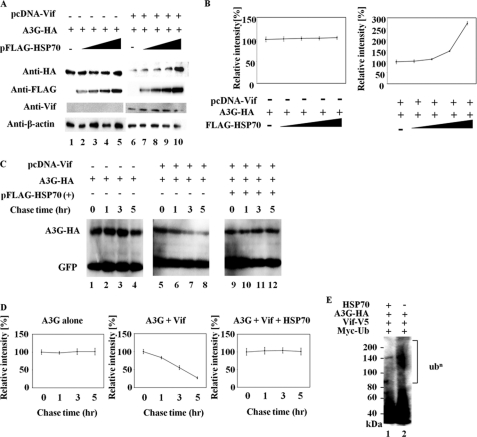
Next, we investigated whether expression of HSP70 directly blocks APOBEC3G degradation by HIV-1 Vif. 293T cells were co-transfected with pc-Hu-APOBEC3G-HA and pFLAG-HSP70 in the absence or presence of pcDNA-Vif. We found that the steady-state levels of APOBEC3G in the presence of pcDNA-Vif were increased by the expression of HSP70 in a dose-dependent manner (Fig. 2, A, lanes 6–10 and B, right panel). By contrast, HSP70 did not significantly affect the amount of APOBEC3G expression in the absence of pcDNA-Vif (Fig. 2, A, lanes 1–5 and B, left panel). These data indicate that the effects of HSP70 on APOBEC3G expression depend on HIV-1 Vif.
FIGURE 2.
HSP70 expression inhibits Vif-mediated APOBEC3G ubiquitination and degradation. A, 293T cells (5 × 105) were co-transfected with 1.0 μg of pc-Hu-APOBEC3G-HA and increasing amounts of pFLAG-HSP70 (0, 0.5, 1.0, or 2.0 μg), adjusted to 2.0 μg of total DNA with 0.5 μg of an empty plasmid (pcDNA3.1) or pcDNA-Vif. At 48 h post-transfection, cell lysates were subjected to Western blotting and were then analyzed with the indicated antibody. β-Actin was used as a control for protein levels. B, relative intensity of APOBEC3G-HA bands in A was determined by densitometry. C, 293T cells (5 × 105) were transfected with 1.0 μg of pc-Hu-APOBEC3G-HA alone (lanes 1–4); 0.5 μg of pcDNA-Vif and 1.0 μg of pc-Hu-APOBEC3G-HA (lanes 5–8); and 0.5 μg of pcDNA-Vif, 1.0 μg of pc-Hu-APOBEC3G-HA and 2.0 μg of pFLAG-HSP70 (lanes 9–12). The transfected cells were treated with cycloheximide to block de novo protein synthesis. The level of APOBEC3G was detected by immunoblotting after cycloheximide treatment lasting 1, 3, or 5 h. CS-CDF-CG-PRE (0.5 μg), which expresses the green fluorescent protein (GFP), was co-transfected with each plasmid into 293T cells as a control plasmid. D, relative intensity of APOBEC3G-HA bands in C was determined by densitometry. E, 293T cells (3 × 106) were co-transfected with 2.0 μg of pCMV-Myc-Ubi, 2.0 μg of pVif-V5, and 2.0 μg of pc-Hu-APOBEC3G-HA along with 4.0 μg of an empty plasmid or pFLAG-HSP70. At 24 h post-transfection, cells were treated with 5 μm MG132. After 24 h, cell lysates were immunoprecipitated with anti-Myc antibody, followed by immunoblotting analysis with horseradish peroxidase-conjugated anti-HA antibody. Results are representative of three independent experiments, and error bars show the standard deviations of the means.
Previous studies have reported that microbial HSP70 up-regulates APOBEC3G mRNA (32, 33). To rule out this possibility, pulse-chase experiments were performed using 293T cells that were co-transfected with pc-Hu-APOBEC3G-HA, pVif-V5, pFLAG-HSP70, and a GFP expression plasmid (CS-CDF-CG-PRE). Cycloheximide was used to block protein synthesis. When 293T cells were transfected with pc-Hu-APOBEC3G-HA alone, there was no change in the level of APOBEC3G (Fig. 2, C, lanes 1–4 and D, left panel). Consistent with previous reports, degradation of APOBEC3G-HA was induced in the presence of HIV-1 Vif (Fig. 2, C, lanes 5–8 and D, middle panel). In contrast, HSP70 expression significantly suppressed the degradation of APOBEC3G by HIV-1 Vif (Fig. 2, C, lanes 9–12 and D, right panel). To further evaluate whether HSP70 expression inhibits the ubiquitination of APOBEC3G by HIV-1 Vif, we performed ubiquitination assays. Lysates of cells co-expressing pVif-V5, Myc-tagged ubiquitin (Myc-Ub), pc-Hu-APOBEC3G-HA and either empty plasmid or pFLAG-HSP70 were analyzed for the polyubiquitination of APOBEC3G. We detected the ubiquitination of APOBEC3G as a ladder band (Fig. 2E, lane 2). The expression of HSP70 resulted in a significant reduction in polyubiquitinated APOBEC3G (Fig. 2E, lane 1). Thus, the expression of HSP70 causes an increase in the steady-state levels of APOBEC3G by blocking the Vif-mediated ubiquitination and degradation of APOBEC3G.
HSP70 Interacts with Both APOBEC3G and HIV-1 Vif
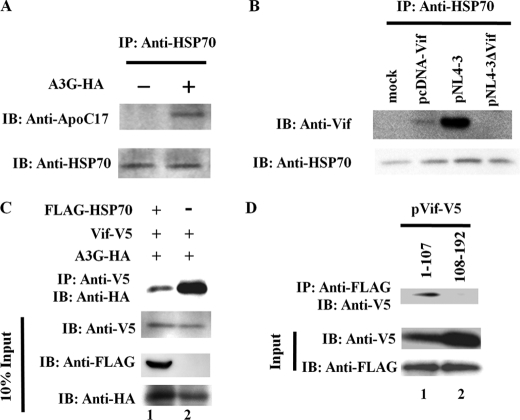
We performed an immunoprecipitation assay to evaluate the binding between HSP70 and APOBEC3G or HIV-1 Vif (Fig. 3). 293T cells were transfected with pc-Hu-APOBEC3G-HA, pcDNA-Vif, pNL4-3, or pNL4-3-delta-Vif. Cell lysates were precipitated with anti-HSP70 antibody, followed by immunoblotting with anti-ApoC17 or anti-Vif antibody. HSP70 interacted with both APOBEC3G (Fig. 3A) and HIV-1 Vif (Fig. 3B). These interactions and the intracellular localization of HSP70 and HA-tagged APOBEC3G were confirmed by immunostaining assays (data not shown). To further investigate the role of HSP70 in APOBEC3G-Vif interactions, 293T cells were co-transfected with pc-Hu-APOBEC3G-HA and pVif-V5 along with either an empty plasmid or pFLAG-HSP70 in the presence of a proteasome inhibitor (MG-132). Consistent with previous studies, HIV-1 Vif was bound to APOBEC3G (Fig. 3C, lane 2). Strikingly, the expression of HSP70 in 293T cells led to the inhibition of APOBEC3G-Vif binding. (Fig. 3C, lane 1). Because a previous study reported that APOBEC3G binds the N-terminal region of HIV-1 Vif (34), we tested the hypothesis that HSP70 competes with APOBEC3G for binding to the N-terminal region of HIV-1 Vif. We found that FLAG-HSP70 efficiently co-immunoprecipitated with the N-terminal region of Vif (amino acids 1–107) (Fig. 3D, lane 1). However, the C-terminal region of Vif (amino acids 108–192) exhibited no detectable interaction with FLAG-HSP70 (Fig. 3D, lane 2). These results suggest that APOBEC3G-Vif binding is reduced by HSP70 through an interaction with the N-terminal region of Vif, resulting in the inhibition of the Vif-mediated ubiquitination and the degradation of APOBEC3G.
FIGURE 3.
HSP70 interacts with APOBEC3G and HIV-1 Vif. A, 293T cells (5 × 105) were transfected with 1.0 μg of pc-Hu-APOBEC3G-HA. After 48 h, cell lysates were immunoprecipitated with anti-HSP70 antibody, followed by immunoblotting analysis with anti-ApoC17 antibody. B, 293T cells (5 × 105) were transfected with 1.0 μg of the indicated plasmids. At 48 h post-transfection, cell lysates were subjected to immunoprecipitation using anti-HSP70 antibody, followed by immunoblotting analysis with anti-Vif antibody. C, 293T cells (5 × 105) were co-transfected with 1.0 μg of pc-Hu-APOBEC3G-HA and 1.0 μg of pVif-V5 together with 2.0 μg of either an empty plasmid or pFLAG-HSP70. At 24 h post-transfection, cells were treated with 5 μm MG132. At 24 h post-treatment, cell lysates were immunoprecipitated with anti-V5 antibody, followed by immunoblotting analysis with horseradish peroxidase-conjugated anti-HA antibody. D, 293T cells (5 × 105) were co-transfected with 1.0 μg of pFLAG-HSP70 and either 1.0 μg of pVif-1–107-V5 or pVif-108–192-V5. At 48 h post-transfection, cell lysates were immunoprecipitated with anti-FLAG antibody, followed by immunoblotting analysis with anti-V5 antibody. Results are representative of three independent experiments.
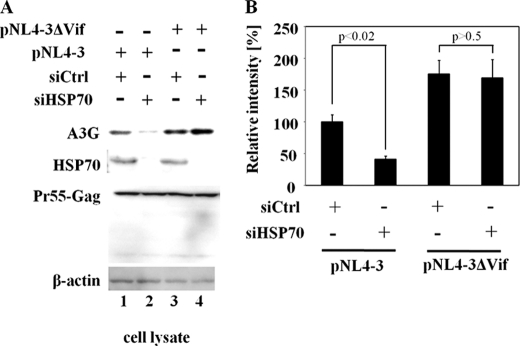
Knock-down of HSP70 in 293T Cells Enhances APOBEC3G Degradation by HIV-1 Vif
To further investigate the effect of endogenous HSP70 on the stability of APOBEC3G, we silenced endogenous HSP70 expression by RNA interference. 293T cells were transfected with control siRNA (siCtrl) or HSP70-specific siRNA (siHSP70) for 4 h prior to transfection along with pc-Hu-APOBEC3G-HA and either pNL4-3 or pNL4-3-delta-Vif. At 48 h post-transfection, cells were harvested and subjected to Western blotting. As expected, the level of APOBEC3G in pNL4-3-transfected cells was less stable than that in the pNL4-3-delta-Vif transfected cells (Fig. 4A, compare lane 1 to lane 3). Quantification of the relative intensities revealed that transfection with pNL4-3 induced APOBEC3G degradation with a potency ∼1.8 times higher than that of pNL4-3-delta-Vif (Fig. 4B). Moreover, in the case of transfection with pNL4-3, the level of APOBEC3G, but not the level of HIV-1 Gag, in the siHSP70-transduced cells were lower than in the siCtrl-transduced cells (Fig. 4A, compare lane 1 to lane 2). The amount of APOBEC3G in HSP70 knock-down cells decreased to half the amount in the control cells (Fig. 4B). However, in terms of transfection with pNL4-3-delta-Vif, treatment with siHSP70 had no effect on the stability of APOBEC3G (Fig. 4A, compare lane 3 to lane 4). These data indicate that depletion of HSP70 facilitates Vif-mediated degradation of APOBEC3G.
FIGURE 4.
Depletion of HSP70 in 293T cells impairs the stability of APOBEC3G. A, 293T cells (3 × 106) were treated with 100 nm HSP70-siRNA (siHSP70) or 100 nm control-siRNA (siCtrl) for 4 h, prior to co-transfection with 1.0 μg of pc-Hu-APOBEC3G-HA with either 1.0 μg of pNL4-3 or pNL4-3-delta-Vif. At 48 h post-transfection, cell lysates were analyzed by Western blotting using the indicated antibodies. B, relative intensity of APOBEC3G bands in A was determined by densitometry. Results are representative of three independent experiments, and error bars show the standard deviations of the means.
HSP70 Suppresses HIV-1 Vif-mediated Degradation of Endogenous APOBEC3G in Non-permissive Cells
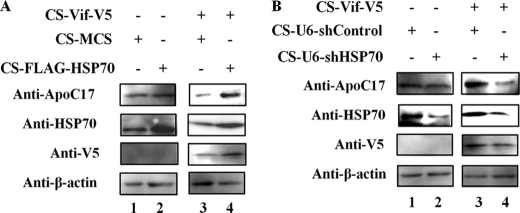
Most experiments in this study used permissive cells. To investigate whether our findings have physiologic relevance in non-permissive cells, we used a lentiviral vector encoding FLAG-HSP70 or HIV-1 Vif-V5. In the absence of Vif-V5, there was no significant effect of FLAG-HSP70 on the level of endogenous APOBEC3G in H9 cells (Fig. 5A, compare lane 1 to lane 2). When Vif-V5 was expressed in H9 cells, expression of FLAG-HSP70 increased the amount of endogenous APOBEC3G (Fig. 5A, compare lane 3 to lane 4). Next, we suppressed the expression of HSP70 using a lentiviral vector to express shHSP70 under the control of the human U6 promoter in H9 cells. APOBEC3G expression in shHSP70-transduced H9 cells was similar to that in shControl-transduced H9 cells (Fig. 5B, compare lane 1 to lane 2). The level of endogenous APOBEC3G was lower in H9 cells transduced with shHSP70 than in H9 cells transduced with shControl by expression of Vif-V5 (Fig. 5B, compare lane 3 to lane 4). Therefore, HSP70 suppresses Vif-mediated degradation of endogenous APOBEC3G in non-permissive cells.
FIGURE 5.
HSP70 affects the level of endogenous APOBEC3G expression in non-permissive T cells expressing HIV-1 Vif. A, H9 cells (3 × 105) were infected with a lentiviral vector encoding an artificial multiple cloning site (MCS) or FLAG-HSP70 in the presence of 8 μg/ml of polybrene. At 48 h after infection, cells were suspended with lysis buffer (left panel) or transduced with HIV-1 Vif using a lentivirus vector system (right panel). At 48 h post-transduction, cell lysates were analyzed by Western blotting using the indicated antibodies. B, H9 cells (3 × 105) were infected with lentivirus-based vectors to express shControl or shHSP70 under the control of the human U6 promoter in the presence of 8 μg/ml of polybrene. At 48 h post-infection, cells were treated as in A. Data are representative of three independent experiments.
Expression of HSP70 in the Presence of APOBEC3G Augments APOBEC3G Restriction of HIV-1
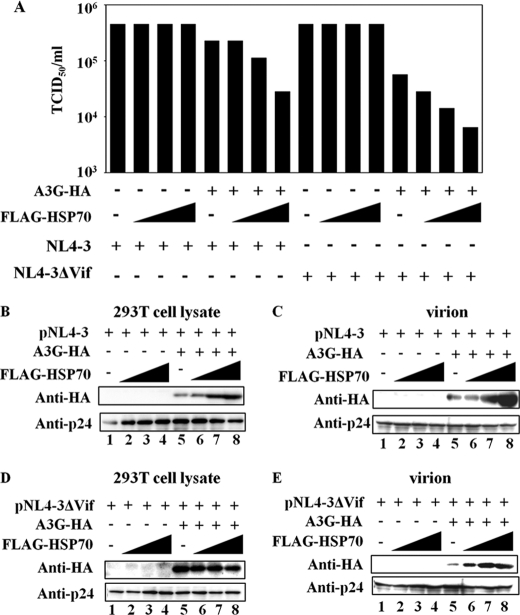
To examine whether HSP70 expression influences the function of APOBEC3G, pNL4-3, or pNL4-3-delta-Vif was transfected into 293T cells along with either pFLAG-HSP70 alone or pFLAG-HSP70 and pc-Hu-APOBEC3G-HA. The viral infectivity was measured by MAGI assay. As shown in Fig. 6A, expression of FLAG-HSP70 clearly suppressed the infectivity of wild-type HIV-1 in the presence of APOBEC3G in a dose-dependent manner. In the absence of APOBEC3G, FLAG-HSP70 did not affect the infectivity of the wild-type HIV-1. Unexpectedly, HSP70 expression in APOBEC3G-HA-transfected 293T cells led to a dose-dependent inhibition of the infectivity of Vif-deficient HIV-1 particles (Fig. 6A). Moreover, no effect of HSP70 expression on the infectivity of the Vif-deficient HIV-1 particles produced by mock-transfected 293T cells was observed. To further demonstrate whether expression of HSP70 affects virion packaging of APOBEC3G, viral particles produced by 293T cells expressing HSP70 were analyzed for APOBEC3G expression by Western blotting. We found that expression of HSP70 significantly increased the amount of intracellular and wild type virion-associated APOBEC3G (Fig. 6, B and C). Interestingly, HSP70 expression enhanced the level of APOBEC3G packaging in Vif-deficient virions, but had no effect on intracellular APOBEC3G and viral release (Fig. 6, D and E). These results indicate that HSP70 blocks Vif-mediated APOBEC3G degradation and enhances the incorporation of APOBEC3G into both wild type and Vif-deficient virions, which result from inhibition of HIV-1 replication through HSP70 interaction with APOBEC3G.
FIGURE 6.
HSP70 regulates HIV-1 infectivity in an APOBEC3G-dependent manner. A, 293T cells (5 × 105) were co-transfected with 0.1 μg of pNL4-3 or pNL4-3-delta-Vif and 1.0 μg of pc-Hu-APOBEC3G-HA alone, pFLAG-HSP70 (0.5, 1.0, or 2.0 μg) alone or 1.0 μg of pc-Hu-APOBEC3G-HA and pFLAG-HSP70 (0.5, 1.0 or 2.0 μg). At 48 h post-transfection, supernatants were harvested, and the amount of each virus was normalized to the equivalent level of p24. MAGI cells (1 × 104) were infected with serially diluting supernatants of each stock of virus, and infected cells were stained with X-Gal 2 days later. 50% tissue culture infective doses (TCID50) is determined by the last virus dilution that is still capable of infecting the cells. B, each stock of cell lysate or virus in A was subjected to Western blotting and was then analyzed with the indicated antibody. All data are representative of three independent experiments.
DISCUSSION
APOBEC3G, which is incorporated into progeny virus particles, restricts the replication of Vif-deficient HIV-1 through cytidine deamination-dependent and independent mechanisms (3–5, 17, 35–41). This restriction can be overcome by HIV-1 Vif, which induces the polyubiquitination of APOBEC3G through recruitment of a ubiquitin E3 ligase complex composed of cullin 5, elongin B, elongin C, and Ring box-1 and facilitates the proteasomal degradation of APOBEC3G (13, 14, 16, 42–45). Thus, mechanistic insights into the quality control of APOBEC3G protein are important for understanding the molecular basis of APOBEC3G-mediated HIV-1 restriction. In this study, we showed that HSP70 suppressed Vif-mediated APOBEC3G degradation. In contrast to our results for HSP70, Pin1 suppresses the HIV restriction activity of APOBEC3G (46). Overexpression of Pin1 reduces the levels of intracellular APOBEC3G. One possibility is that HSP70 regulates Pin1 function, which results in the stimulation of APOBEC3G function, although further analysis is needed to properly address this question.
Pido-Lopez et al. (32) have reported that microbial HSP70 up-regulates APOBEC3G mRNA and protein expression in human CD4+ T cells. Our data indicate that in 293T cells, overexpression of human HSP70 in the absence of HIV-1 Vif did not affect the amount of APOBEC3G protein. The stabilization of APOBEC3G is attributed to a reduction in the Vif-dependent polyubiquitination of APOBEC3G (Fig. 2E). Whereas we have focused on human HSP70 activity on APOBEC3G stability, it would be interesting to investigate whether human HSP70 can affect the level of endogenous APOBEC3G mRNA.
APOBEC3G associates with ribonucleoprotein (RNP) complexes and is not only dispersed throughout the cytoplasm but is also markedly concentrated in cytoplasmic foci that are identified as mRNA-processing bodies (P bodies) (47). Localization of APOBEC3G in P bodies is not important for its LINE-1 suppression activity (48). However, Y3 and 7SL RNAs, which compose RNP complexes, are required for efficient APOBEC3G packaging (49). Stimulation of cells at 44 °C induces the rapid accumulation of APOBEC3G and many cellular RNA-binding proteins (50). We examined whether HSP70 plays a role in packaging APOBEC3G into virus particles and found that overexpression of HSP70 enhanced APOBEC3G packaging in the absence of Vif (Fig. 6E). It is possible that HSP70 interacts with cytoplasmic APOBEC3G, but it remains unclear whether HSP70 induces the accumulation of APOBEC3G in P bodies and increases the association of APOBEC3G with RNP complexes. Further studies will be required to clarify the details of how, where and when HSP70 and APOBEC3G co-localize within cells.
Recently, Nathans et al. (51) have identified a small molecule, termed RN-18, that degrades HIV-1 Vif only in the presence of APOBEC3G, resulting in enhanced APOBEC3G abundance and virion incorporation, similar to the function of HSP70. The possibility has been raised that HSP70 may be the target of RN-18. However, HSP70 has no significant effect on HIV-1 Vif expression and leads to the increase of APOBEC3G packaging into virions in a Vif-independent manner. Moreover, RN-18 exhibits a strong dependence on APOBEC3G, whereas HSP70 can interact directly with both HIV-1 Vif and APOBEC3G. Thus, RN-18 probably does not target HSP70. Taken together, the results of the present study suggest that stimulation of innate immunity, such as that mediated by APOBEC3G, may aid in the development of antiviral therapies.
Acknowledgments
We thank Dr. Darlene Chen for providing pc-Hu-APOBEC3G-HA, Dr. Miyoshi Hiroyuki for providing pCS-CDF-CG-PRE, and Haruki Naganuma and Hiroshi Koseki for technical assistance.
This work was supported in part by a Grant-in-aid for High Technology Research (No. 09309011) from the Ministry of Education, Science, Sports, and Culture of Japan and by a grant-in-aid for AIDS research from the Ministry of Health, Labor, and Welfare, Japan.
- HIV-1
- human immunodeficiency virus type-1
- RNP
- ribonucleoprotein
- HSP
- heat shock protein
- Ub
- ubiquitin.
REFERENCES
- 1. Gabuzda D. H., Lawrence K., Langhoff E., Terwilliger E., Dorfman T., Haseltine W. A., Sodroski J. (1992) J. Virol. 66, 6489–6495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. von Schwedler U., Song J., Aiken C., Trono D. (1993) J. Virol. 67, 4945–4955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sheehy A. M., Gaddis N. C., Choi J. D., Malim M. H. (2002) Nature 418, 646–650 [DOI] [PubMed] [Google Scholar]
- 4. Zhang H., Yang B., Pomerantz R. J., Zhang C., Arunachalam S. C., Gao L. (2003) Nature 424, 94–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harris R. S., Bishop K. N., Sheehy A. M., Craig H. M., Petersen-Mahrt S. K., Watt I. N., Neuberger M. S., Malim M. H. (2003) Cell 113, 803–809 [DOI] [PubMed] [Google Scholar]
- 6. Cen S., Guo F., Niu M., Saadatmand J., Deflassieux J., Kleiman L. (2004) J. Biol. Chem. 279, 33177–33184 [DOI] [PubMed] [Google Scholar]
- 7. Svarovskaia E. S., Xu H., Mbisa J. L., Barr R., Gorelick R. J., Ono A., Freed E. O., Hu W. S., Pathak V. K. (2004) J. Biol. Chem. 279, 35822–35828 [DOI] [PubMed] [Google Scholar]
- 8. Alce T. M., Popik W. (2004) J. Biol. Chem. 279, 34083–34086 [DOI] [PubMed] [Google Scholar]
- 9. Douaisi M., Dussart S., Courcoul M., Bessou G., Vigne R., Decroly E. (2004) Biochem. Biophys. Res. Commun. 321, 566–573 [DOI] [PubMed] [Google Scholar]
- 10. Schäfer A., Bogerd H. P., Cullen B. R. (2004) Virology 328, 163–168 [DOI] [PubMed] [Google Scholar]
- 11. Kremer M., Bittner A., Schnierle B. S. (2005) Virology 337, 175–182 [DOI] [PubMed] [Google Scholar]
- 12. Yu Q., König R., Pillai S., Chiles K., Kearney M., Palmer S., Richman D., Coffin J. M., Landau N. R. (2004) Nat. Struct. Mol. Biol. 11, 435–442 [DOI] [PubMed] [Google Scholar]
- 13. Yu X., Yu Y., Liu B., Luo K., Kong W., Mao P., Yu X. F. (2003) Science 302, 1056–1060 [DOI] [PubMed] [Google Scholar]
- 14. Mehle A., Goncalves J., Santa-Marta M., McPike M., Gabuzda D. (2004) Genes Dev. 18, 2861–2866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yu Y., Xiao Z., Ehrlich E. S., Yu X., Yu X. F. (2004) Genes Dev. 18, 2867–2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kobayashi M., Takaori-Kondo A., Miyauchi Y., Iwai K., Uchiyama T. (2005) J. Biol. Chem. 280, 18573–18578 [DOI] [PubMed] [Google Scholar]
- 17. Iwatani Y., Chan D. S., Wang F., Maynard K. S., Sugiura W., Gronenborn A. M., Rouzina I., Williams M. C., Musier-Forsyth K., Levin J. G. (2007) Nucleic. Acids Res. 35, 7096–7108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stopak K., de Noronha C., Yonemoto W., Greene W. C. (2003) Mol. Cell 12, 591–601 [DOI] [PubMed] [Google Scholar]
- 19. Mercenne G., Bernacchi S., Richer D., Bec G., Henriet S., Paillart J. C., Marquet R. (2010) Nucleic. Acids Res. 38, 633–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parent R., Qu X., Petit M. A., Beretta L. (2009) Hepatology 49, 1798–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Padwad Y. S., Mishra K. P., Jain M., Chanda S., Karan D., Ganju L. (2009) Immunobiology 214, 422–429 [DOI] [PubMed] [Google Scholar]
- 22. Ujino S., Yamaguchi S., Shimotohno K., Takaku H. (2009) J. Biol. Chem. 284, 6841–6846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chase G., Deng T., Fodor E., Leung B. W., Mayer D., Schwemmle M., Brownlee G. (2008) Virology 377, 431–439 [DOI] [PubMed] [Google Scholar]
- 24. Gurer C., Cimarelli A., Luban J. (2002) J. Virol. 76, 4666–4670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O'Keeffe B., Fong Y., Chen D., Zhou S., Zhou Q. (2000) J. Biol. Chem. 275, 279–287 [DOI] [PubMed] [Google Scholar]
- 26. Ryo A., Suizu F., Yoshida Y., Perrem K., Liou Y. C., Wulf G., Rottapel R., Yamaoka S., Lu K. P. (2003) Mol. Cell 12, 1413–1426 [DOI] [PubMed] [Google Scholar]
- 27. Karczewski M. K., Strebel K. (1996) J. Virol. 70, 494–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neidhardt F. C., VanBogelen R. A., Vaughn V. (1984) Annu. Rev. Genet. 18, 295–329 [DOI] [PubMed] [Google Scholar]
- 29. Bukau B., Weissman J., Horwich A. (2006) Cell 125, 443–451 [DOI] [PubMed] [Google Scholar]
- 30. SenGupta D., Norris P. J., Suscovich T. J., Hassan-Zahraee M., Moffett H. F., Trocha A., Draenert R., Goulder P. J., Binder R. J., Levey D. L., Walker B. D., Srivastava P. K., Brander C. (2004) J. Immunol. 173, 1987–1993 [DOI] [PubMed] [Google Scholar]
- 31. Wainberg Z., Oliveira M., Lerner S., Tao Y., Brenner B. G. (1997) Virology 233, 364–373 [DOI] [PubMed] [Google Scholar]
- 32. Pido-Lopez J., Whittall T., Wang Y., Bergmeier L. A., Babaahmady K., Singh M., Lehner T. (2007) J. Immunol. 178, 1671–1679 [DOI] [PubMed] [Google Scholar]
- 33. Babaahmady K., Oehlmann W., Singh M., Lehner T. (2007) J. Virol. 81, 3354–3360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mehle A., Wilson H., Zhang C., Brazier A. J., McPike M., Pery E., Gabuzda D. (2007) J. Virol. 81, 13235–13241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bishop K. N., Holmes R. K., Malim M. H. (2006) J. Virol. 80, 8450–8458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bishop K. N., Verma M., Kim E. Y., Wolinsky S. M., Malim M. H. (2008) PLoS Pathog. 4, e1000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guo F., Cen S., Niu M., Yang Y., Gorelick R. J., Kleiman L. (2007) J. Virol. 81, 11322–11331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lecossier D., Bouchonnet F., Clavel F., Hance A. J. (2003) Science 300, 1112. [DOI] [PubMed] [Google Scholar]
- 39. Li X. Y., Guo F., Zhang L., Kleiman L., Cen S. (2007) J. Biol. Chem. 282, 32065–32074 [DOI] [PubMed] [Google Scholar]
- 40. Mangeat B., Turelli P., Caron G., Friedli M., Perrin L., Trono D. (2003) Nature 424, 99–103 [DOI] [PubMed] [Google Scholar]
- 41. Suspène R., Sommer P., Henry M., Ferris S., Guétard D., Pochet S., Chester A., Navaratnam N., Wain-Hobson S., Vartanian J. P. (2004) Nucleic. Acids Res. 32, 2421–2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sheehy A. M., Gaddis N. C., Malim M. H. (2003) Nat. Med. 9, 1404–1407 [DOI] [PubMed] [Google Scholar]
- 43. Marin M., Rose K. M., Kozak S. L., Kabat D. (2003) Nat. Med. 9, 1398–1403 [DOI] [PubMed] [Google Scholar]
- 44. Conticello S. G., Harris R. S., Neuberger M. S. (2003) Curr. Biol. 13, 2009–2013 [DOI] [PubMed] [Google Scholar]
- 45. Mehle A., Strack B., Ancuta P., Zhang C., McPike M., Gabuzda D. (2004) J. Biol. Chem. 279, 7792–7798 [DOI] [PubMed] [Google Scholar]
- 46. Watashi K., Khan M., Yedavalli V. R., Yeung M. L., Strebel K., Jeang K. T. (2008) J. Virol. 82, 9928–9936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gallois-Montbrun S., Kramer B., Swanson C. M., Byers H., Lynham S., Ward M., Malim M. H. (2007) J. Virol. 81, 2165–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Niewiadomska A. M., Tian C., Tan L., Wang T., Sarkis P. T., Yu X. F. (2007) J. Virol. 81, 9577–9583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang T., Tian C., Zhang W., Luo K., Sarkis P. T., Yu L., Liu B., Yu Y., Yu X. F. (2007) J. Virol. 81, 13112–13124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gallois-Montbrun S., Holmes R. K., Swanson C. M., Fernández-Ocaña M., Byers H. L., Ward M. A., Malim M. H. (2008) J. Virol. 82, 5636–5642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nathans R., Cao H., Sharova N., Ali A., Sharkey M., Stranska R., Stevenson M., Rana T. M. (2008) Nat. Biotechnol. 26, 1187–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]