Abstract
Saccharomyces cerevisiae cells lacking Mne1 are deficient in intron splicing in the gene encoding the Cox1 subunit of cytochrome oxidase but contain wild-type levels of the bc1 complex. Thus, Mne1 has no role in splicing of COB introns or expression of the COB gene. Northern experiments suggest that splicing of the COX1 aI5β intron is dependent on Mne1 in addition to the previously known Mrs1, Mss116, Pet54, and Suv3 factors. Processing of the aI5β intron is similarly impaired in mne1Δ and mrs1Δ cells and overexpression of Mrs1 partially restores the respiratory function of mne1Δ cells. Mrs1 is known to function in the initial transesterification reaction of splicing. Mne1 is a mitochondrial matrix protein loosely associated with the inner membrane and is found in a high mass ribonucleoprotein complex specifically associated with the COX1 mRNA even within an intronless strain. Mne1 does not appear to have a secondary function in COX1 processing or translation, because disruption of MNE1 in cells containing intronless mtDNA does not lead to a respiratory growth defect. Thus, the primary defect in mne1Δ cells is splicing of the aI5β intron in COX1.
Keywords: Cytochrome Oxidase, Mitochondria, Mitochondrial DNA, Yeast, Yeast Metabolism
Introduction
Cytochrome c oxidase (CcO)3 biogenesis requires the expression and interaction of subunits encoded by mitochondrial and nuclear genomes. A myriad of nuclear-encoded assembly factors mediate CcO biogenesis (1, 2). These factors function in the processing of mitochondrial CcO subunit mRNAs, their translation and insertion into the inner membrane, and formation of metal and heme cofactor centers. Assembly is initiated with the synthesis and maturation of the Cox1 subunit, one of the three catalytic core components (3, 4). COX1 is the one CcO gene that is universally present in the mitochondrial genome. The COX1 mRNA requires processing prior to translation on mitochondrial ribosomes. Cox1 contains three of the redox centers of the enzyme with heme a, a3, and CuB cofactors (5). The last redox center, the binuclear CuA center, exists within the Cox2 subunit.
Mitochondrial genomes of non-metazoan species tend to be larger due to the presence of non-coding regions including introns and additional genes not present in animals (6–8). In Saccharomyces cerevisiae, introns are commonly found in COX1 as well as COB (the cytochrome b subunit of the bc1 complex) and the large ribosomal RNA gene (9). Two types of introns, groups I and II, exist in COX1. Some of these introns contain open reading frames encoding maturases, related to DNA endonucleases for group I introns and reverse transcriptases for group II introns (8). Group I intron-containing COX1 alleles have been generated numerous times during evolution due to the invasive nature of the introns containing a DNA homing endonuclease (10). Most commonly used laboratory yeast strains contain up to seven introns within COX1 and in such multiple-intron strains, exons may be as short as 24–37 bases. No compelling rationale is known why introns are maintained in COX1 and COB.
The generation of mature COX1 mRNA transcript requires processing of introns in addition to cleavage of the polycistronic precursor RNA. Both group I and group II introns catalyze their own splicing, but require different combinations of nuclear-encoded factors as well as intron-encoded maturases to mediate intron excision and ligation of flanking exons (11). Cells unable to properly process mitochondrial introns are deficient or attenuated in translation of the respective mRNAs and present with defects in respiratory growth.
Some nuclear-encoded factors function in the processing of a specific intron, whereas others, such as Mss116, aid in splicing of all mtDNA introns in yeast (12). Group I intron splicing requires that the RNA folds into an active conformation permitting the self-splicing reaction (13). Some of the nuclear factors, e.g. Mss116, have been shown to facilitate the RNA folding reaction or stabilize the RNA tertiary fold (14, 15).
COX1 introns in yeast are annotated as aI1-aI4 and aI5α, -β, and -γ. The aI3, aI4, aI5α, and aI5β introns are group I introns, whereas aI1, aI2, and aI5γ are group II introns. Concerning accessory factors, Cox24 is necessary in the processing of aI2 and aI3 COX1 introns (16) and in addition may have a role in mitochondrial translation (17). Intron aI4 processing requires Nam2, Ccm1, and a maturase encoded by the COB bI4 intron (18, 19). Excision of the aI5β intron of COX1 depends on several accessory factors including Pet54, Mrs1, Mss18, Mss116, and Suv3 (20). The requirement for multiple factors may arise due to the fact that the 3′ splice site of aI5β lies unusually far from the catalytic core of the intron. Mrs1 also functions in the excision of the bI3 of COB (21). Yeast harboring mutations in one of these accessory factors are respiratory deficient due to attenuated levels of mature COX1 transcripts. The growth defect of certain mutants, e.g. cox24Δ cells (16) and mss18Δ cells (22), are partially rescued in intronless strains suggestive of additional functions.
Our present study showing that the mitochondrial protein Mne1 has a role in COX1 intron splicing was initiated by our interest in characterizing mitochondrial proteins with limited functional annotation. Mne1 was reported to be a mitochondrial protein in a large-scale localization study (23). In addition, a high throughput screen of the growth sensitivity of the yeast disruptome to 1144 chemicals allowed the grouping of deletion strains according to growth fitness in the presence of chemical or stress conditions (24). The mne1Δ strain exhibited a drug sensitivity pattern similar to a number of mutants (cox17, cox5b, cox23, mss51, and cox10) impaired in CcO biogenesis suggesting a role for Mne1 in this process. Cells lacking Mne1 were found to have a specific impairment in CcO assembly. Studies presented show that mne1Δ cells are defective in processing of the aI5β COX1 intron and that this defect can be partially suppressed by overexpression of Mrs1. Our results identify Mne1 as a novel component of the splicing apparatus responsible for processing of group I intron aI5β. This conclusion is supported by previous preliminary observations (25).
EXPERIMENTAL PROCEDURES
Yeast Strains, Plasmids, and Media
S. cerevisiae strains used in the study are listed in Table 1. A derivative of W303 containing the long form of COX1 with 7 introns consisting of aI1, aI2, aI3, aI4, aI5α, aI5β, and aI5γ was used (26). An intronless variant was obtained from Dr. Brigitte Meunier. Strain BY4741 contains the same long COX1 genes with the seven COX1 introns (27). Yeast cells were grown in YP (1% yeast extract, 2% bactopeptone) or amino acid-supplemented SC medium, containing 2% glucose, lactate, or glycerol as a carbon source. Cloning procedures were performed in Escherichia coli DH5α as described (28). The MNE1 open reading frame was PCR amplified from the WT genomic DNA with or without addition of a single Myc epitope tag using the primers as listed in Table 2. The resulting constructs were cloned into pRS426 vector under control of the MET25 promoter and CYC1 terminator (29). We also used pRS415-based pMRS1 plasmid containing MRS1 ORF under its own promoter and terminator (30). All constructs were verified by sequencing. A genomically Myc-tagged variant of MNE1 was generated by homologous recombination, inserting the 13xMyc tag 3′ to the ORF using the template plasmid pFA6a-13Myc-HIS3 (31). The lithium acetate protocol (32) was used to transform the yeast cells.
TABLE 1.
Yeast strains used in this work
| Strain | Genotype | Reference |
|---|---|---|
| W303 | MATα ade2–1 his3–1,15 leu2–3,112 trp1–1 ura3–1 | 26 |
| W303-1B | MATα ade2–1 his3–1,15 leu2–3,112 trp1–1 ura3–1 [Δi] | B. Meunier |
| W303 mne1Δ | MATα ade2–1 his3–1,15 leu2–3,112 trp1–1 ura3–1 mne1Δ::CaURA3 | This study |
| W303-1B mne1Δ | MATα ade2–1 his3–1,15 leu2–3,112 trp1–1 ura3–1 [Δi] mne1Δ::HIS3MX6 | This study |
| BY4741 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Invitrogen |
| BY4741 ade2Δ | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 ade2Δ::URA3 | 30 |
| BY4741 ade2Δ mrs1Δ | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 ade2Δ::URA3 mrs1Δ::kanMX4 | 30 |
| BY4741 ade2Δ mss18Δ | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 ade2Δ::URA3 mss18Δ::kanMX4 | 30 |
| BY4741 ade2Δ pet54Δ | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 ade2Δ::URA3 pet54Δ::kanMX4 | 30 |
| BY4741 ade2Δ mne1Δ | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 ade2Δ::URA3 mne1Δ::kanMX4 | This study |
| BY4743 | MATa/α his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 met15Δ0/MET15 ura3Δ0/ura3Δ0 lys2Δ0/LYS2 | Invitrogen |
| BY4743 mne1Δ | MATa/α his3Δ1/hisΔ31 leu2Δ0/leu2Δ0 met15Δ0/MET15 ura3Δ0/ura3Δ0 lys2Δ0/LYS2 mne1Δ::kanMX4/mne1Δ::kanMX4 | Invitrogen |
| NB40–36A | MATα lys2 leu2–3,112 arg8Δ::hisG ura3–52 | 45 |
| cox1Δ::ARG8m | MATa lys2 leu2–3,112 arg8Δ::hisG ura3–52 [cox1Δ::ARG8m] | 45 |
| cox1Δ::ARG8m mne1Δ | MATa lys2 leu2–3,112 arg8Δ::hisG ura3–52 [cox1Δ::ARG8m] mne1Δ::URA3 | This study |
| DTY833 | MATa ade1Δ arg4Δ aro2Δ his7Δ lys5Δ ura2Δ | |
| DY5113 | MATa ade2–1 his3–1,15 leu2–3,112 trp1Δ ura3–1 | |
| MNE1-13Myc | MATa ade2–1 his3–1,15 leu2–3,112 trp1Δ ura3–1 MNE1-13Myc::HIS3MX6 | This study |
| MNE1-13Myc (Δi) | MATa ade2–1 his3–1,15 leu2–3,112 trp1Δ ura3–1 [Δi] MNE1-13Myc::HIS3MX6 | This study |
| MNE1-13Myc rho0 | MATa ade2–1 his3–1,15 leu2–3,112 trp1Δ ura3–1 MNE1-13Myc::HIS3MX6 [rho0] | This study |
| MSS51-13Myc COA1-3HA | MATa ade2–1 his3–1,15 leu2–3,112 trp1 ura3–1 COA1-3HA::TRP1 MSS51-13Myc::HIS3MX6 | 42 |
| MSS51-13Myc mne1Δ | MATa ade2–1 his3–1,15 leu2–3,112 trp1 ura3–1 COA1-3HA::TRP1 MSS51-13Myc::HIS3MX6 mne1Δ::CaURA3 | This study |
| COA1-13Myc | MATa ade2–1 his3–1,15 leu2–3,112 trp1Δ ura3–1 COA1-13Myc::HIS3MX6 | 42 |
| COA1-13Myc mne1Δ | MATa ade2–1 his3–1,15 leu2–3,112 trp1Δ ura3–1 COA1-13Myc::HIS3MX6 mne1Δ::TRP1 | This study |
TABLE 2.
Oligonucleotides used in this work
| Product | Position | Oligonucleotide (5′ to 3′) |
|---|---|---|
| MNE1 | Forward | TATTTAGGATCCATGAAGTTACTTTTTAAAAGATATTCGTCT |
| Reverse | TATTTACTCGAGTTATTTTTGTTGCATTTGCTTAGATCTTAT | |
| MNE1-Myc | Forward | TATTTAGGATCCATGAAGTTACTTTTTAAAAGATATTCGTCT |
| Reverse | TATTTACTCGAGCTACAAGTCCTCTTCAGAAATGAGCTTTTGCTCGAGTTTTTGTTGCATTTGCTTAGA | |
| mne1Δ::HIS3MX6 cassette | Forward | CGGCGAGAAAAAATGATAGTAGTGTGCCAAGAAGAATATGCGGATCCCCGGGTTAATTAA |
| Reverse | CGGCAAATTTTACATATAATCATTATTTTTGTTGCATTTGCGAATTCGAGCTCGTTTAAAC | |
| MNE1-13Myc::HIS3MX6 cassette | Forward | CTATTTTCTTACATAAGATCTAAGCAAATGCAACAAAAACGGATCCCCGGGTTAATTAA |
| Reverse | GAGTCTGATATCACTTTATAGAATGTAAACGGCAAATTTTACGAATTCGAGCTCGTTTAAAC | |
| COX2 exon fragment | Forward | GATTCGTTGTAACAGCTGCTGATG |
| Reverse | GACCTGTCCCACACAACTCAG | |
| COX1 E5β-E5γ | Forward | GCTCTAATCCATGGTGGTTCAATTAG |
| Reverse | GAAAATGTCCCACCACGTAGTAAG | |
| COX1 E5β-aI5β fragment | Forward | GCTCTAATCCATGGTGGTTCAATTAG |
| Reverse | AATAATTATAAGAGTTTCCCCGTTAGC | |
| COX1 aI5β-E5γ fragment | Forward | AAGAGATTATAAATCTGGTGCTACAGC |
| Reverse | GAAAATGTCCCACCACGTAGTAAG | |
| COX1 aI5β-pre-mRNA probe | Forward | AATTAACCCTCACTAAAGGGAAAGCTTAAGAGTAAAATTCTTAAAGTG |
| Reverse | AATACGACTCACTATAGGGCGTCGACAAAATGTCCCACCACGTAGTAAGTC | |
| COX1 E6 probe | GAATAATGATAATAGTGCAATGAATGAACC | |
| 21S rRNA exon probe | CAACATCAACCTGTTCGATCG | |
| COX1 aI1 probe | GTTTCTAATGTTGTACCTGGAG | |
| COX1 aI2 probe | GAATAAACAGAGATATGTTTATC | |
| COX1 aI3 probe | GCTAAATAAGGTCCCAACTTATC | |
| COX1 aI4 probe | CCATCACCATCAATTAATCCAGC | |
| COX1 aI5α probe | TCTATTTGATCTTGGATAAATATC | |
| COX1 aI5β probe | CCATCTCCTTCAAATAATCC | |
| COX1 aI5γ probe | GGTTTATTCTGTTTTATC |
Mitochondria Isolation and Procedures
Mitochondria were isolated as described previously (33). Mitochondrial protein concentrations were quantified by the Bradford assay. CcO and succinate dehydrogenase/succinate cytochrome c reductase (bc1) enzymatic activities were determined as described previously (34). The specific activities were normalized to mitochondrial protein levels and presented as a percentage of wild type.
Localization Studies
Cells were fractionated into cytosolic and mitochondrial fractions as described (35). For selective rupture of the outer membrane, mitochondria were sonicated (3 × 30 s with 50% duty cycle) in hypotonic buffer (50 mm NaCl, 20 mm HEPES, pH 7.4). The resulting mixtures were fractionated at 165,000 × g for 1 h at 2 °C. Alkaline extraction using 0.1 m Na2CO3, pH 11.5, was performed as described (36). Proteinase K treatment of isolated mitochondria was done as described previously (37).
Blue Native Gel Electrophoresis (BN-PAGE)
BN-PAGE was performed as described (38). Clarified mitochondrial lysates were run on a continuous 5–13% gradient gel. For RNase or DNase treatment experiments, lysates were treated with the indicated amounts of RNase A or RNase-free TURBO-DNase (Ambion). Separated protein complexes were transferred onto PVDF membrane (Bio-Rad) and analyzed by standard immunoblotting.
In Vivo Labeling of Mitochondrial Translation Products
The cells were pre-grown overnight in either complete or supplemented SC medium containing 2% raffinose or galactose, reinoculated, and grown to an A600 of 0.8. The labeling, preparation, and separation of the samples by SDS-PAGE were done as described previously (39). Radiolabeled proteins were visualized by autoradiography.
Mitochondrial RNA Isolation and Procedures
Cells were grown overnight in SC medium and harvested by centrifugation at 3,500 × g for 5 min. Collected cells were washed with diethyl pyrocarbonate (DEPC)-treated autoclaved distilled water and resuspended in ice-cold DEPC water-based STE buffer (0.65 m sorbitol, 20 mm Tris-HCl, pH 7.2, 1 mm EDTA) with 1 mm PMSF. Cold sterile glass beads (0.2–0.45 mm) were added to the cell suspension and the cells were broken by extensive vortexing for 5 min. After sedimentation of the glass beads, respective supernatants were placed into new tubes and the beads were washed with an equal volume of ice-cold STE buffer. Upon pooling of the supernatants, intact cells and cell debris were removed by a short centrifugation step at 4,000 × g at 4 °C for 3 min. The cleared extracts were fractionated for 15 min at 20,000 × g (4 °C) to pellet mitochondria, which were subsequently used for RNA isolation. Mitochondria were resuspended in 650 μl of sterile TES buffer (10 mm Tris-HCl, pH 7.5, 10 mm EDTA, pH 8.0, 0.5% SDS, DEPC-treated), mixed with the equal volume of acidic phenol, pH 4.5, and incubated for 60 min at 65 °C with careful vortexing every 20 min. Following the incubation, mixtures were cooled on ice for 2 min and centrifuged for 5 min at 11,750 × g at room temperature. The upper layer of each respective supernatant was mixed with 0.4 ml of chloroform and spun down for 5 min at 11,750 × g at RT. RNA was precipitated from the obtained supernatants upon the addition of 50 μl of 3 m NaAc, pH 5.2 (DEPC-treated), followed by addition of 0.9 ml of molecular biology grade absolute ethanol and centrifugation for 10 min at 16,000 × g at 4 °C. The resulting pellet was washed with 70% ethanol, air-dried, and resuspended in RNase-free water (Ambion). Isolated mitochondrial RNAs were treated with the DNA-free kit (Ambion) as described by the manufacturer. Reverse transcription-polymerase chain reaction (RT-PCR) was performed using the One-step RT-PCR kit from Qiagen, using 350 ng of RNA as a template. Primers used are shown in Table 2.
RNA for Northern analysis was isolated as described (40) from S. cerevisiae W303 WT and mne1Δ strains grown at 30 °C in 50 ml of YP with 2% raffinose to A600 = 0.8–1.0. Northern blot analysis of COX1 introns was performed as previously described (41) with the following exception: normalized RNA samples equivalent to 10 μg were glyoxylated and run on a 1.2% agarose gel with RNA-grade 1× TAE buffer (40 mm Tris acetate, 1 mm EDTA), pH 8, at RT. The 5′-end labeled DNA oligonucleotide probes used for hybridization are listed in Table 2. To assess the aI5β splicing defect, we used an RNase protection assay described previously (30).
Immunoprecipitations and Immunoblot Analysis
Immunoprecipitations of Mne1-Myc were performed essentially as described (42) using anti-Myc agarose-coupled beads (Santa Cruz Biotechnology), except that DEPC-treated, sterile water and RNase inhibitor (RNasin Plus, Promega) were used. For immunoblotting, mitochondrial or cytosolic protein was loaded onto a 12% polyacrylamide gel, separated by SDS-PAGE, and transferred onto a nitrocellulose membrane. Membranes were decorated with the indicated primary antibodies and visualized with ECL reagents (Pierce), following incubation with horseradish peroxidase-conjugated secondary antibodies or with the Odyssey Infrared Imaging System (LI-COR Biosciences) when fluorescent secondary antibodies were used. Anti-Myc antibody was obtained from Roche Diagnostics. Antibodies to CcO subunits Cox1, Cox2, and Cox3 were obtained from Mitosciences, and antisera to the mitochondrial outer membrane porin and cytosolic phosphoglycerol kinase (Pgk1) were from Invitrogen and Molecular Probes, respectively. Anti-Sod2 was a gift from Dr. Val Culotta. Dr. Alex Tzagoloff provided Atp2 (F1) antiserum. Dr. Bernard Trumpower provided anti-Rip1 and anti-Cyt1.
Miscellaneous
The oxygen consumption of cells grown to stationary phase was determined using a 5300A Biological Oxygen Monitor (Yellow Springs Instrument Co.). The rate of oxygen consumption presented as a percentage of wild type was calculated from the linear response (43).
RESULTS
Mne1 Is Required for Normal Respiration
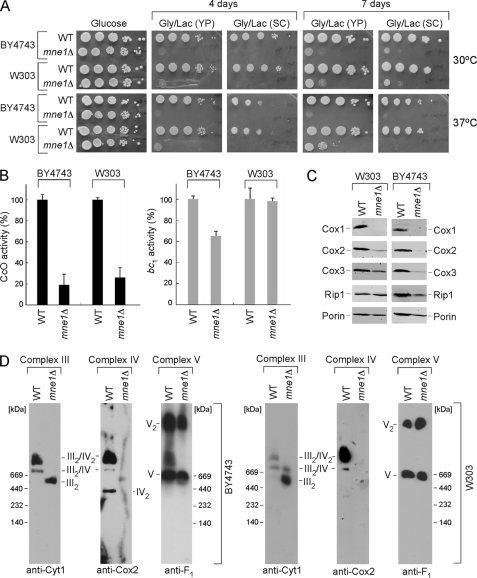
MNE1 was disrupted in two yeast genetic backgrounds, W303 and BY4743. The disruptants were found to propagate normally on glucose-containing growth medium but exhibited a growth impairment on medium containing glycerol and lactate as carbon sources (Fig. 1A). The growth defect was slightly exacerbated at 37 °C. As mentioned, a high throughput drug sensitivity screen revealed a pattern of drug inhibition of mne1Δ cells resembling that of mutants impaired in CcO biogenesis suggesting a role for Mne1 in this process (24). Therefore, we investigated CcO biogenesis in yeast cells lacking Mne1 in both genetic backgrounds and found them to have attenuated CcO activity but bc1 activity (Complex III) was unaffected in W303 cells (Fig. 1B). Consistent with attenuated CcO activity, steady-state levels of the three mitochondrially encoded subunits Cox1–3 were reduced in mne1Δ cells with Cox1 levels being most markedly depleted (Fig. 1C). Rip1 levels reporting on the bc1 complex were slightly attenuated as was the outer membrane protein Por1. BN-PAGE of digitonin-solubilized mitochondria was carried out to visualize respiratory complexes (Fig. 1D). The monomeric and dimeric forms of ATP synthase (Complex V) were unaffected in mne1Δ cells, but CcO supercomplexes were markedly impaired as reported by the Cox2 subunit immunoblot. Cells lacking Mne1 mainly have the dimeric Complex III in both backgrounds. Thus, mne1Δ cells have a specific defect in CcO.
FIGURE 1.
Deletion of Mne1 results in a CcO-specific respiratory defect. A, respiratory growth of mne1Δ strains. Mutant and the isogenic wild-type (WT) cells were pre-grown in complete (YP) or synthetic (SC) liquid medium, serially diluted, and spotted onto the respective plates containing 2% glucose or glycerol/lactate as a carbon source. Pictures of the plates were taken after 4 and 7 days of incubation at 30 or 37 °C. B, CcO and SDH/bc1 activities from the mne1Δ and corresponding WT mitochondria, normalized to total protein. Enzymatic activities are shown as a percentage of wild-type specific activity, error bars indicate S.D. (n = 3). C, steady-state levels of core CcO subunits (Cox1, Cox2, and Cox3) and Rip1 were analyzed by immunoblotting 20 μg of mitochondria isolated from the indicated WT and mne1Δ strains. The mitochondrial outer membrane porin served as a loading control. D, BN-PAGE analysis of the respiratory complexes in WT and mne1Δ mitochondria. Isolated organelles (75 μg) were solubilized in a lysis buffer containing 1% digitonin. Protein complexes were separated on the continuous 5–13% gradient gel under native conditions. Western blotting with antibodies against Cox2, Cyt1, and Atp2 (anti-F1) was used to assess the distribution of respiratory complexes.
Mne1 Is a Mitochondrial Matrix Protein
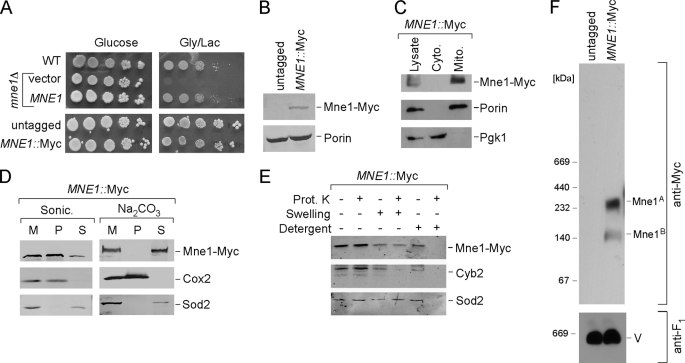
To confirm that Mne1 is a mitochondrial protein, MNE1 was chromosomally tagged with a Myc epitope tag to permit visualization of the Mne1 protein. The Myc-tagged Mne1 was expressed and equally functional to the untagged Mne1 protein (Fig. 2A). Mne1-Myc was found to be associated with gradient-purified yeast mitochondria with no apparent localization in the cytoplasm (Fig. 2C). Sonication of purified mitochondria to separate membranes from the soluble fraction revealed the presence of Mne1-Myc in both fractions with an enrichment in the membrane-associated fraction (Fig. 2D). Incubation of mitochondria with sodium carbonate resulted in solubilization of Mne1 suggesting that Mne1 is only loosely associated with the membrane fraction. To confirm the mitochondrial subcompartmentalization of Mne1, protease protection assays were carried out using proteinase K susceptibility (Fig. 2E). Mne1 was protected against the addition of proteinase K in intact mitochondrial as well as in mitochondria with outer membranes permeabilized by hypotonic lysis. The intermembrane space protein Cyb2 was degraded after hypotonic swelling but not the matrix-localized Sod2 or Mne1-Myc. However, the addition of dodecyl maltoside resulted in digestion of Mne1-Myc by proteinase K. These studies are consistent with Mne1 residing within the matrix compartment and being loosely associated with the inner membrane. The tagged Mne1 is predicted to have a mass of ∼90 kDa, but BN-PAGE analysis of digitonin-solubilized mitochondria revealed that Mne1-Myc fractionates as a large complex in excess of 200 kDa as well as a smaller, less apparent complex of ∼140 kDa (Fig. 2F).
FIGURE 2.
Mne1 is a soluble matrix protein associated with mitochondrial inner membrane. A, respiratory growth of mne1Δ cells complemented with the vector-borne MNE1 and cells with chromosomally tagged Mne1. Cells were grown and tested as in E. B, whole cell lysates of untagged and MNE1::Myc strains were analyzed by SDS-PAGE and Western blot with anti-Myc and anti-porin. C, immunoblot of mitochondria (Mito.) and the post-mitochondrial fraction (Cyto.) purified from the MNE1::Myc strain. Anti-Myc antibodies have been used to detect tagged Mne1. Phosphoglycerol kinase (Pgk1), a cytosolic marker and Porin, a mitochondrial outer membrane protein were used to verify fractionations. D, isolated mitochondria (50 μg) were sonicated in 20 mm HEPES (pH 7.4) and 50 mm NaCl or incubated on ice for 30 min with 0.1 m sodium bicarbonate (pH 11.5). 2 mm phenylmethylsulfonyl fluoride was added in both cases. The soluble and pellet fractions were separated by centrifugation at 165,000 × g for 1 h, and analyzed by Western blot. Soluble matrix protein Sod2 and integral membrane protein Cox2 were detected with the respective antibodies. M, total mitochondria; S, supernatant; P, pellet. E, intact or osmotically swollen mitochondria were incubated with or without 0.1 mg/ml of proteinase K (Prot. K) for 30 min on ice. A combination of detergent and proteinase K treatment was used to exclude the possibility that Mne1-Myc is a proteinase-resistant protein. Following centrifugation, the treated organelles were separated by SDS-PAGE and analyzed by immunoblotting with anti-Myc, anti-Cyb2, and anti-Sod2. F, mitochondria (50 μg) isolated from untagged or MNE1::Myc cells were solubilized with 1% digitonin and analyzed by BN-PAGE as described in the legend to Fig. 1D.
Lack of Mne1 Affects Cox1 Transcript Processing
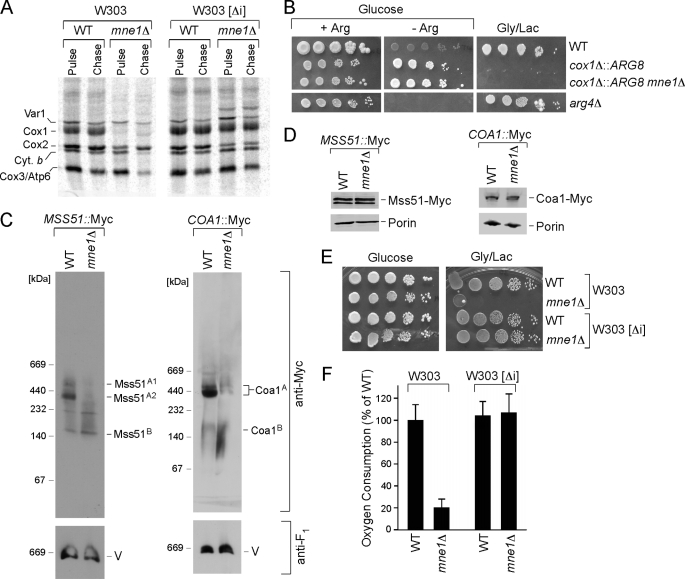
Mne1 is thus found to be a mitochondrial matrix protein that influences CcO biogenesis. Mutations in numerous nuclear-encoded CcO assembly factors lead to attenuated Cox1 synthesis due to sequestration of the COX1 translation activator Mss51 in a stalled assembly intermediate (44). To assess whether mne1Δ cells exhibited a defect in translation of Cox1, a mitochondrial protein translation assay was conducted in which cells were treated with [35S]methionine in the presence of cycloheximide to inhibit protein synthesis in the cytoplasm. Only mitochondrial translation products can be synthesized after cycloheximide treatment. Cells lacking Mne1 showed normal labeling of certain mitochondrial proteins, but marked attenuation in Cox1 labeling in the initial 15-min pulse as well as the subsequent 60-min chase period (Fig. 3A, left panels). During the chase period the labeled Cox2 and Cox3 molecules diminished, as they are unstable in the absence of Cox1 (39).
FIGURE 3.
The absence of Mne1 affects early steps of Cox1 biogenesis in the strains containing mitochondrial introns. A, in vivo labeling of mitochondrial translation products in WT and mne1Δ cells with or without ([Δi]) mitochondrial introns. Cells were pulsed for 15 min with [35S]methionine at 30 °C. The reaction was stopped by addition of cold methionine; following a 60-min chase at 30 °C samples were subjected to SDS-PAGE and analyzed by autoradiography. B, MNE1 was deleted in the arg8Δ strain with ectopic ARG8 replacing the COX1 codons (cox1Δ::ARG8m). The cells were grown in complete synthetic medium containing 2% glucose and 0.2× arginine (4 mg/liter), serially diluted, and spotted on glucose-containing SC with (+Arg) or without (−Arg) 1× arginine (20 mg/liter) and SC containing 2% glycerol/lactate. The plates were incubated at 30 °C for 2 (glucose plates) or 4 days (glycerol plates). The arg4 mutant strain was used as a control. C, BN-PAGE analysis of Cox1 early assembly intermediates. Mitochondria (30 to 50 μg) purified from the WT and mne1Δ strains containing a 13xMyc epitope-tagged version of MSS51 or COA1 were analyzed by native electrophoresis and immunoblot with anti-Myc antibodies. The monomeric form of complex V that served as a loading control was visualized with anti-F1 serum. D, steady-state levels of the indicated proteins in WT and mne1Δ mitochondria assessed by SDS-PAGE. The outer membrane protein porin, visualized by the respective antibody was used as a loading control. E, respiratory growth of mne1Δ strains with and without mitochondrial introns. Cells were handled as described in the legend to Fig. 1A, except that plates were incubated at 30 °C for 2 (glucose plates) or 4 days (glycerol plates). F, oxygen consumption by mne1Δ cells with or without ([Δi]) mitochondrial introns. Cells were grown overnight in liquid complete medium with 1% glucose and oxygen consumption was measured. The results are shown as a percentage of WT oxygen consumption and represent the averages of three independent experiments. Error bars indicate S.D.
The dramatic attenuation in Cox1 labeling in mne1Δ cells may arise from impaired translational initiation. Sequestration of Mss51 within early Cox1 assembly intermediates was ruled out, because overexpression of MSS51 failed to restore Cox1 synthesis in mne1Δ cells (data not shown). To assess the status of expression at the COX1 locus in mne1Δ cells, we used an Arg8 reporter strain constructed by Fox and co-workers (45). Arg8 is encoded by a nuclear gene and is imported to the mitochondrial matrix where it participates in the biosynthesis of arginine. The reporter strain, constructed in an arg8Δ background, contained the ARG8 gene in place of mtDNA COX1 ORF (cox1Δ::ARG8) such that translation of Arg8 is under the control of the translational activators of Cox1 (45). The growth of this strain in medium lacking arginine indicates the mitochondrial translation of ARG8 mRNA. Deletion of MNE1 in this strain did not produce arginine auxotrophy, implying that translation occurred normally at the COX1 locus in the mutant cells (Fig. 3B). As expected, no growth of these strains occurred on glycerol as the Cox1 ORF was replaced with Arg8.
Newly synthesized Cox1 readily associates with the Mss51 translational activator as the initial Cox1 assembly intermediate (45, 46). Formation of the ∼440 kDa Mss51 complex containing Cox1, Cox14, and Ssc1 correlates with Cox1 translation and early assembly (46). The presence of the high mass Mss51 complex was assessed in mne1Δ cells containing a chromosomally Myc-tagged Mss51. This complex was not present in mne1Δ cells, although smaller Mss51 complexes were apparent and these correlate with the translational activator function of Mss51 (46) (Fig. 3C). Similarly, a downstream Cox1 assembly complex involving Coa1 was markedly attenuated in mne1Δ cells.
The lack of the Mss51 complex containing newly synthesized Cox1 and the wild-type translation of ARG8 expressed from the COX1 locus led us to consider COX1 mRNA transcript processing. The involvement of numerous accessory factors in intron splicing reactions motivated us to delete MNE1 in a yeast strain lacking any mitochondrial introns. Previous work with mutants lacking the intron-splicing factors Cox24 or Mss18 showed partial restoration of respiratory function with intronless mtDNA (16, 22). An mne1Δ derivative within an intronless variant of W303 lacked the respiratory growth phenotype characteristic of mne1Δ in the intron-containing W303 strain (Fig. 3E). Labeling mitochondrial proteins in the [35S]-labeled translation assay showed normal Cox1 labeling in the pulse and no diminution during the chase phase of the reaction (Fig. 3A). Consistent with these results, oxygen consumption (Fig. 3F) and CcO activity were at WT levels. Thus, the growth impairment observed in mne1Δ cells is dependent on the presence of introns in the mitochondrial genome.
Mne1 Functions in aI5β Intron Processing
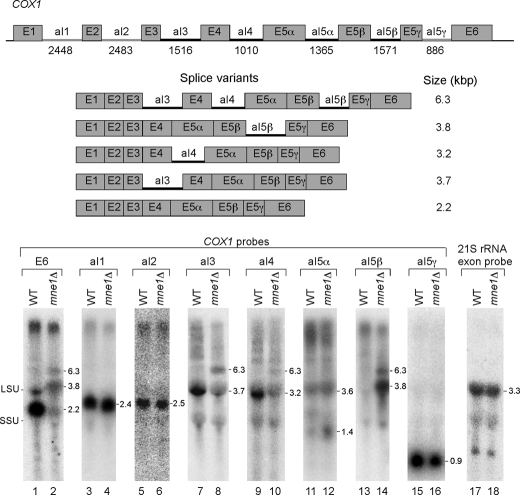
Northern analysis was carried out with total mitochondrial RNA isolated from W303 WT and mne1Δ strains harboring a COX1 with seven confirmed mtDNA introns (Fig. 4). 5′-End labeled probes complementary to COX1 exon 6 or to each intron were used for the analyses. Hybridization with the COX1 exon 6 probe shows a 2.2-kb mature COX1 mRNA in the WT strain (lane 1). This band is greatly diminished in mne1Δ cells where instead two larger bands of ∼3.8 and 6.3 kb are apparent (lane 2, transcript sizes are indicated to the right of each panel in Fig. 4). Intron-specific probes for the three COX1 group II introns show that splicing of the aI1, aI2, and aI5γ introns is not affected in the absence of Mne1 (lanes 4, 6, and 16). The spliced aI3, aI4, and aI5β introns are not visible in the Northern blots and appear to be unstable in mitochondria (1.4 kb signals seen in lanes 7–10, 13, and 14 are most likely cross-hybridization to the 15S rRNA). Strong hybridization signals running just below the large ribosomal subunit band with the aI3 and aI4 intron probes are detected in the WT strain and suggest that these bands represent the splicing intermediates containing the single introns and exons. The same band is also visible with the exon probe in the WT strain in lane 1. These aI3 and aI4 splicing intermediates are greatly reduced in the mne1Δ strain (lanes 8 and 10). However, with both probes we see a signal for a 6.3-kb band also seen with the COX1 exon probe. This suggests that this band contains more than one unspliced intron. The aI5α probe shows little difference between the WT and mne1Δ cells with the exception of a slightly more intense band running below the small ribosomal subunit rRNA, which is possibly the excised aI5α intron (lane 12). The greatest difference in hybridization signals is seen with the aI5β probe. The strongest signal (at 3.8 kb) in the mne1Δ strain comigrates with the large ribosomal subunit band and most likely represents a COX1 RNA that has all introns but aI5β removed. A second signal is detected for a RNA of about 6.3 kb, which comigrates with the signals seen for the aI3 and aI4 intron probes. This suggests that the 6.3-kb band represents multiple RNA species that all contain the aI5β intron and also aI3 and/or aI4.
FIGURE 4.
The absence of Mne1 attenuates splicing of the aI5β intron of COX1. Northern blot analysis of COX1 introns in WT and mne1Δ cells. The blots were hybridized with 32P-labeled probes complementary to COX1 exon 6, COX1 introns aI1, aI2, aI3, aI4, aI5α-γ, and 21S rRNA exon 1. The schematic (top) shows the molecular organization of the COX1 gene from the intron-containing W303 strain and the size (in bp) of each intron is given below its name. Group I introns are indicated by a black line and group II introns by a gray line. Sizes for COX1 mRNA splice variants containing either single or multiple introns aI3, aI4, and/or aI5β are shown below. In the Northern panels we have indicated the position of the large and small ribosomal rRNAs as LSU and SSU, respectively, on the left. The numbers to the right of each Northern panel indicate the approximate size of the indicated transcripts.
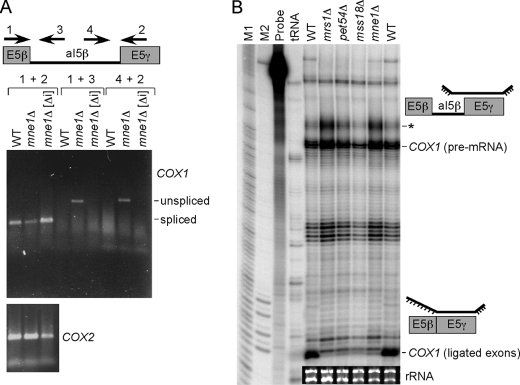
One interpretation of these data is that aI5β intron splicing is defective in mne1Δ cells. To test this prediction, RT-PCR and nuclease protection assays were carried out. Probes generated to the E5β and E5γ exons and two in the aI5β intron were used for RT-PCR (Fig. 5A). RT-PCR with the two exonic probes showed a fragment whose size is consistent with the spliced RNA. The level of this spliced band is attenuated in mne1Δ cells. RT-PCR using one of the two exon primers and an aI5β primer showed a band enriched in mne1Δ cells from cells with mtDNA containing introns but absent from intronless mne1Δ cells. A control experiment verified the absence of contaminating DNA (data not shown). RT-PCR with COX2 primers showed similar levels of product in the RNA from the three strains. An RNase protection assay was performed using a radiolabeled probe containing a complementary sequence to the E5γ exon and a 3′ segment of the aI5β intron as shown in Fig. 5B. Hybridization of this probe to RNA isolated from WT or mne1Δ cells revealed primarily ligated exons in WT cells but enhanced accumulation of the aI5β-containing COX1 pre-mRNA in mne1Δ cells. This accumulation is more apparent in the band representing the incomplete digestion of the pre-mRNA bound probe (marked by an asterisk) consistent with unspliced aI5β. The intensity of this band is a sensitive indicator of a change in the pre-mRNA level compared with the intense band just below that represents the complete digestion of the probe. The combination of Northern, RT-PCR, and RNase protection assays documents an aI5β splicing defect in mne1Δ cells.
FIGURE 5.
Mne1 contributes to the splicing of the aI5β intron. A, RT-PCR analysis of aI5β splicing defect in mne1Δ cells with and without mitochondrial introns. The schematic depicts primers used for RT-PCR. The upper panel shows COX1 mRNA with (unspliced) and without (spliced) aI5β intron. The bottom panel shows amplification of intronless COX2 mRNA that served as a loading control. The PCR products were amplified with 16 cycle reactions. B, RNase protection assay for aI5β splicing defect. Mitochondrial mRNAs were isolated from WT, mrs1Δ, pet54Δ, mss18Δ, and mne1Δ cells. Schematics show the identity of the protected fragments, COX1 pre-mRNA containing aI5β intron and COX1 ligated exon. The asterisk depicts incomplete digestion of the single-stranded portion of the pre-mRNA bound probe. M1 and M2, markers; probe, no RNase control; tRNA, no mitochondrial RNA control. The inset shows rRNA levels that were used as a loading control.
A splicing defect of the aI5β intron is also seen in mrs1Δ, pet54Δ, and mss18Δ cells (Fig. 5B). The abundance of the unspliced intron is most prominent in mrs1Δ and mne1Δ cells.
Mne1 Forms a Riboprotein Complex
As mentioned, Mne1-Myc forms a high mass complex seen on BN-PAGE. With a defined role for Mne1 in splicing of the aI5β intron, we addressed whether the BN-PAGE Mne1 complex contained RNA. Digitonin-solubilized mitochondrial lysates were incubated with RNase A or TURBO DNase prior to BN-PAGE. DNase treatment failed to perturb the complexes, but protease-free RNase treatment markedly attenuated the larger Mne1 complex (Fig. 6A). The Mne1-Myc complexes are not evident in rhoo cells lacking mtDNA (Fig. 6B), although Mne1 is equally abundant by steady-state immunoblotting (Fig. 6C). To assess whether the RNA associated with Mne1-Myc was from COX1, Mne1-Myc was immunoprecipitated from digitonin-solubilized mitochondria. RT-PCR was carried out on the resuspended precipitates. A positive RT-PCR signal was observed in intron-containing Mne1-Myc mitochondria but not in intronless or untagged organelles using probes that amplify the aI5β intron (Fig. 6D). Using COX1 exon probes, a positive RT-PCR signal was observed in the immunoprecipitations from tagged strains regardless of whether COX1 introns were present (Fig. 6D). No RT-PCR signal was observed using primers for COB or COX3 even using two additional PCR cycles (data not shown). Likewise, BN-PAGE showed the same Mne1-Myc complex in strains with and without introns (Fig. 6E). Thus, Mne1 associates with the COX1 mRNA and not exclusively with the aI5β intron.
FIGURE 6.
Mne1 is a component of RNase-sensitive high molecular weight complex containing aI5β intron. A, mitochondria (50 μg) purified from the WT MNE1::Myc strain were lysed in 1% digitonin and clarified lysates were treated with either 4 units of RNase A or Turbo-DNase, or left untreated. Following a 30-min incubation at room temperature, samples were subjected to BN-PAGE and analyzed by Western blot with antibodies against Myc and F1. B, distribution of Mne1-Myc containing complexes in MNE1::Myc strain with (rho+) and without (rhoO) mitochondrial DNA analyzed by native electrophoresis as described above. Anti-porin antibody was used to detect the respective complex that served as a loading control. C, steady-state levels of Mne1-Myc in the strains with and without mitochondrial DNA were assessed by immunoblotting as described in the legend to Fig. 2D. D, clarified lysates obtained from 450 μg of untagged or MNE1::Myc mitochondria with and without introns were immunoprecipitated with goat polyclonal anti-Myc beads under RNA-protecting conditions. Half of the entire fraction of each respective bead eluate was analyzed by immunoblotting with anti-Myc antibodies; the second half served as a template for an RT-PCR analysis with the primers to aI5β intron or ligated COX1 E5β-E6γ exon. The PCR products were amplified with 30 and 24 reaction cycles, respectively. E, BN-PAGE analysis of the Mne1 complexes in mitochondria with and without introns was performed as described above.
Respiratory Growth in mne1Δ Cells Is Partially Restored by Overexpression of Mrs1
Previous work has shown that splicing of the COX1 aI5β intron is dependent on Mrs1, Pet54, Mss116, Mss18, and Suv3 (30). In an in vitro study on splicing of the aI5β intron, Mrs1 was found to promote the first step in splicing, whereas Mss116 acts subsequently in the exon ligation reaction (47). Because Mrs1 functions at an early step in aI5β splicing, we sought to assess the functional relationship of Mrs1 and Mne1. The overexpression of MRS1 in mne1Δ cells partially restores respiratory growth at 30 °C but not 37 °C (Fig. 7). In contrast, overexpression of MNE1 failed to suppress the respiratory growth defect of mrs1Δ cells (data not shown). The lack of an effect of MNE1 overexpression in mrs1Δ cells is an expected result, because Mrs1 is also important in the splicing of the bI3 intron of COB. In contrast, mne1Δ cells show no defect in function of the Cob-containing bc1 complex (Fig. 1B).
FIGURE 7.
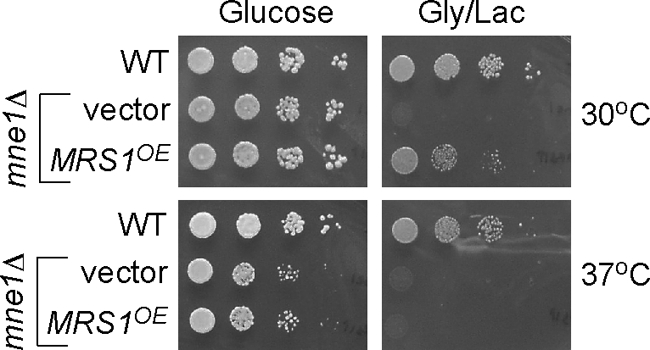
Overexpression of MRS1 suppresses the respiratory growth defect of the cells lacking Mne1. Cells lacking Mne1 (W303 background) transformed with an empty episomal vector or plasmid expressing MRS1 were grown in the supplemented synthetic liquid medium allowing plasmid propagation. Cells were serially diluted and spotted onto the respective plates containing 2% glucose or glycerol/lactate as a carbon source. Pictures of the plates were taken after 2 (glucose plates) and 4 (glycerol plates) days of incubation at 30 or 37 °C.
DISCUSSION
Mne1 is shown for the first time to be an important accessory factor in the splicing of COX1 preRNA through the removal of the aI5β intron. Mne1 does not appear to have a secondary function in CcO biogenesis under standard laboratory growth conditions, because the disruption of MNE1 in cells containing intronless mtDNA does not lead to a respiratory growth defect. Mne1 contrasts therefore from Cox24 and Mss18 that function in splicing of COX1 introns aI2/aI3 and aI5β, respectively, in that deletion strains lacking either Cox24 or Mss18 remain partially compromised without mtDNA introns (16, 22). Mne1 is a mitochondrial inner membrane-associated, matrix-localized protein. Mne1 is a constituent of a dynamic high molecular weight ribonucleoprotein complex specifically containing COX1 RNA. Splicing of aI5β is strongly compromised in mne1Δ and mrs1Δ cells but less so in pet54Δ and mss18Δ strains. The respiratory defect of mne1Δ cells is partially ameliorated by overexpression of Mrs1 that functions in the first step of aI5β intron excision. Mne1 may function with Mrs1 in an early step of aI5β processing. In vitro studies are required to define the precise role of Mne1 in intron splicing.
Splicing of the aI5β intron is known to require at least 5 proteins including Mrs1, Mss18, Mss116, Pet54, and Suv3 (22, 48). Pet54 and Mss116 act downstream of Mrs1 and enhance the efficiency of the exon ligation in the presence of Mrs1 (47). The aI5β intron contains insertions that appear to disrupt the RNA conformation. Thus, accessory factors are likely needed to stabilize the RNA conformer responsible for the splicing reaction. Pet54 may serve this role as it contains an RNA recognition motif and has an additional function as a translational activator of COX3 in yeast (20, 49). Mss116 may also contribute to the folding of the aI5β intron. Mss116 was shown recently to stabilize the tertiary fold of the aI5γ intron (14, 15). Mne1 may also contribute to this stabilization function through its ability to bind COX1 mRNA, although its interaction is not exclusively via the aI5β intron. Mss116 has RNA helicase activity (50) and is required for splicing of all group I and II mitochondrial introns (12). The helicase Suv3 functions in the degradosome to remove spliced introns and in the process release limiting splicing factors, but has an additional role in aI5β processing (51).
The aI5β intron is large (1571 nucleotides) and contains an ORF of 43 kDa. This intron is very inefficient in self-splicing, although the first step occurs in vitro in the presence of high magnesium concentrations. The addition of purified Mrs1 stimulates the first transesterification reaction but the second reaction does not proceed (47). Mrs1 is believed to stabilize the catalytically competent conformation of the RNA. Mrs1 is known to have a stabilizing role for the bI3 COB RNA (52, 53). Mss18 is believed to function in the initial cleavage of the 5′ exon-intron junction of aI5b (22). Exon ligation is stimulated by the addition of Mss116 (47). The role of Mne1 in this splicing reaction is unresolved and will be the subject of future investigations. Mrs1 function is not totally impaired in mne1Δ cells, because Mrs1 has an additional unimpaired role in processing the bI3 intron of COB and the aI5γ of COX1 (21).
Mne1 resembles Mrs1, Mss18, and Pet54 in having only a limited distribution within fungal species. Mne1 and Mrs1 are found in Saccharomyces cerevisiae and Candida glabrata. MRS1 was recently identified as a gene that causes cytonuclear incompatibility in post-zygotic hybrids of Saccharomyces cerevisiae (Sc) and S. bayanus (54). Chromosomal replacement hybrids of these two species revealed that Sc-MRS1 fails to complement the respiratory defect of S. bayanus mrs1Δ cells. COX1 mRNA is not translated in this hybrid likely due to the inability of Sc-Mrs1 to splice a COX1 intron in S. bayanus cells. Mrs1 may have co-evolved with the COX1 introns. A related co-evolution connection was reported previously (55).
Acknowledgments
We thank Stephen Gren for assistance at the initial stages of this project and Dr. A. Lambowitz for insightful comments. We acknowledge Dr. Tom Fox for the generous gift of the ARG8 reporter strain, Dr. Brigitte Meunier for kindly providing of the intronless W303-1B strain, and Dr. Alex Tzagoloff for helpful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grants GM083292 (to D. R. W.) and GM037951 (to Alan M. Lambowitz, University of Texas at Austin), and American Heart Association Grant 10POST4300044 (to O. K.).
- CcO
- cytochrome c oxidase
- COB
- cytochrome b subunit of the bc1 complex
- DEPC
- diethyl pyrocarbonate.
REFERENCES
- 1. Carr H. S., Winge D. R. (2003) Acc. Chem. Res. 36, 309–316 [DOI] [PubMed] [Google Scholar]
- 2. Fontanesi F., Soto I. C., Horn D., Barrientos A. (2006) Am. J. Physiol. Cell Physiol. 291, C1129–C1147 [DOI] [PubMed] [Google Scholar]
- 3. Barrientos A., Gouget K., Horn D., Soto I. C., Fontanesi F. (2009) Biochim. Biophys. Acta 1793, 97–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fontanesi F., Soto I. C., Barrientos A. (2008) IUBMB Life 60, 557–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yoshikawa S., Shinzawa-Itoh K., Tsukihara T. (2000) J. Inorg. Biochem. 82, 1–7 [DOI] [PubMed] [Google Scholar]
- 6. Bullerwell C. E., Leigh J., Forget L., Lang B. F. (2003) Nucleic Acids Res. 31, 759–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Signorovitch A. Y., Buss L. W., Dellaporta S. L. (2007) PLoS Genet. 3, e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lang B. F., Laforest M. J., Burger G. (2007) Trends Genet. 23, 119–125 [DOI] [PubMed] [Google Scholar]
- 9. Bonitz S. G., Coruzzi G., Thalenfeld B. E., Tzagoloff A., Macino G. (1980) J. Biol. Chem. 255, 11927–11941 [PubMed] [Google Scholar]
- 10. Lambowitz A. M., Belfort M. (1993) Annu. Rev. Biochem. 62, 587–622 [DOI] [PubMed] [Google Scholar]
- 11. Lambowitz A. M., C. M. G., Zimmerly S., Perlman P. S. (1999) in The RNA World II (Gesteland R. F., Atkins J. F., Cech T. R. eds) pp. 451–485, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 12. Huang H. R., Rowe C. E., Mohr S., Jiang Y., Lambowitz A. M., Perlman P. S. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 163–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Woodson S. A. (2005) Curr. Opin. Struct. Biol. 15, 324–330 [DOI] [PubMed] [Google Scholar]
- 14. Karunatilaka K. S., Solem A., Pyle A. M., Rueda D. (2010) Nature 467, 935–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Del Campo M., Tijerina P., Bhaskaran H., Mohr S., Yang Q., Jankowsky E., Russell R., Lambowitz A. M. (2007) Mol. Cell 28, 159–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barros M. H., Myers A. M., Van Driesche S., Tzagoloff A. (2006) J. Biol. Chem. 281, 3743–3751 [DOI] [PubMed] [Google Scholar]
- 17. Shin J., Tibbetts A. S., Appling D. R. (2010) FASEB J. 24, 685.2 [Google Scholar]
- 18. Labouesse M. (1990) Mol. Gen. Genet. 224, 209–221 [DOI] [PubMed] [Google Scholar]
- 19. Moreno J. I., Buie K. S., Price R. E., Piva M. A. (2009) Curr. Genet. 55, 475–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaspar B. J., Bifano A. L., Caprara M. G. (2008) Nucleic Acids Res. 36, 2958–2968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bousquet I., Dujardin G., Poyton R. O., Slonimski P. P. (1990) Curr. Genet. 18, 117–124 [DOI] [PubMed] [Google Scholar]
- 22. Séraphin B., Simon M., Faye G. (1988) EMBO J. 7, 1455–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huh W. K., Falvo J. V., Gerke L. C., Carroll A. S., Howson R. W., Weissman J. S., O'Shea E. K. (2003) Nature 425, 686–691 [DOI] [PubMed] [Google Scholar]
- 24. Hillenmeyer M. E., Fung E., Wildenhain J., Pierce S. E., Hoon S., Lee W., Proctor M., St. Onge R. P., Tyers M., Koller D., Altman R. B., Davis R. W., Nislow C., Giaever G. (2008) Science 320, 362–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nyberg T. M. (2006) In Vivo Studies of Yeast Mitochondrial Intron Splicing: Ectopic Branching and a Screen for Nuclear Encoded Splicing Factors, pp. 190–191, Department of Molecular Biology, University of Texas Southwestern Medical Center, Dallas, TX [Google Scholar]
- 26. Kispal G., Csere P., Prohl C., Lill R. (1999) EMBO J. 18, 3981–3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Luban C., Beutel M., Stahl U., Schmidt U. (2005) Gene 354, 72–79 [DOI] [PubMed] [Google Scholar]
- 28. Sambrook J., Fritsch E. F., Mantiatis T. (1989) Molecular Cloning, A Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 29. Mumberg D., Müller R., Funk M. (1994) Nucleic Acids Res. 22, 5767–5768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Turk E. M., Caprara M. G. (2010) J. Biol. Chem. 285, 8585–8594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Longtine M. S., McKenzie A., 3rd, Demarini D. J., Shah N. G., Wach A., Brachat A., Philippsen P., Pringle J. R. (1998) Yeast 14, 953–961 [DOI] [PubMed] [Google Scholar]
- 32. Schiestl R. H., Gietz R. D. (1989) Curr. Genet. 16, 339–346 [DOI] [PubMed] [Google Scholar]
- 33. Daum G., Böhni P. C., Schatz G. (1982) J. Biol. Chem. 257, 13028–13033 [PubMed] [Google Scholar]
- 34. Capaldi R. A., Marusich M. F., Taanman J. W. (1995) Methods Enzymol. 260, 117–132 [DOI] [PubMed] [Google Scholar]
- 35. Diekert K., de Kroon A. I., Kispal G., Lill R. (2001) Methods Cell Biol. 65, 37–51 [DOI] [PubMed] [Google Scholar]
- 36. Fujiki Y., Fowler S., Shio H., Hubbard A. L., Lazarow P. B. (1982) J. Cell Biol. 267, 103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Khalimonchuk O., Ott M., Funes S., Ostermann K., Rödel G., Herrmann J. M. (2006) Eukaryot. Cell 5, 997–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Khalimonchuk O., Bestwick M., Meunier B., Watts T. C., Winge D. R. (2010) Mol. Cell. Biol. 30, 1004–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barrientos A., Korr D., Tzagoloff A. (2002) EMBO J. 21, 43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schmitt M. E., Brown T. A., Trumpower B. L. (1990) Nucleic Acids Res. 18, 3091–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mohr S., Stryker J. M., Lambowitz A. M. (2002) Cell 109, 769–779 [DOI] [PubMed] [Google Scholar]
- 42. Pierrel F., Bestwick M. L., Cobine P. A., Khalimonchuk O., Cricco J. A., Winge D. R. (2007) EMBO J. 26, 4335–4346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Horng Y. C., Leary S. C., Cobine P. A., Young F. B., George G. N., Shoubridge E. A., Winge D. R. (2005) J. Biol. Chem. 280, 34113–34122 [DOI] [PubMed] [Google Scholar]
- 44. Barrientos A., Zambrano A., Tzagoloff A. (2004) EMBO J. 23, 3472–3482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Perez-Martinez X., Broadley S. A., Fox T. D. (2003) EMBO J. 22, 5951–5961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fontanesi F., Soto I. C., Horn D., Barrientos A. (2010) Mol. Cell. Biol. 30, 245–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bifano A. L., Caprara M. G. (2008) J. Mol. Biol. 383, 667–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Valencik M. L., Kloeckener-Gruissem B., Poyton R. O., McEwen J. E. (1989) EMBO J. 8, 3899–3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Costanzo M. C., Seaver E. C., Fox T. D. (1989) Genetics 122, 297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Séraphin B., Simon M., Boulet A., Faye G. (1989) Nature 337, 84–87 [DOI] [PubMed] [Google Scholar]
- 51. Dziembowski A., Piwowarski J., Hoser R., Minczuk M., Dmochowska A., Siep M., van der Spek H., Grivell L., Stepien P. P. (2003) J. Biol. Chem. 278, 1603–1611 [DOI] [PubMed] [Google Scholar]
- 52. Duncan C. D., Weeks K. M. (2010) Biochemistry 49, 5418–5425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Duncan C. D., Weeks K. M. (2010) PLoS ONE 5, e8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chou J. Y., Hung Y. S., Lin K. H., Lee H. Y., Leu J. Y. (2010) PLoS Biol. 8, e1000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Herbert C. J., Macadre C., Bécam A. M., Lazowska J., Slonimski P. P. (1992) Gene Expr. 2, 203–214 [PMC free article] [PubMed] [Google Scholar]