Abstract
The sarcoplasmic reticulum calcium ATPase (SERCA) plays a central role in regulating intracellular Ca2+ homeostasis and myocardial contractility. Several studies show that improving Ca2+ handling in hypertrophied rodent hearts by increasing SERCA activity results in enhanced contractile function. This suggests that SERCA is a potential target for gene therapy in cardiac hypertrophy and failure. However, it raises the issue of increased energy cost resulting from a higher ATPase activity. In this study, we determined whether SERCA overexpression alters the energy cost of increasing myocardial contraction in mouse hearts with pressure-overload hypertrophy using 31P NMR spectroscopy. We isolated and perfused mouse hearts from wild-type (WT) and transgenic (TG) mice overexpressing the cardiac isoform of SERCA (SERCA2a) 8 weeks after ascending aortic constriction (left ventricular hypertrophy (LVH)) or sham operation. We found that overexpressing SERCA2a enhances myocardial contraction and relaxation in normal mouse hearts during inotropic stimulation with isoproterenol. Energy consumption was proportionate to the increase in contractile function. Thus, increasing SERCA2a expression in the normal heart allows an enhanced inotropic response with no compromise in energy supply and demand. However, this advantage was not sustained in LVH hearts in which the energetic status was compromised. Although the overexpression of SERCA2a prevented the down-regulation of SERCA protein in LVH hearts, TG-LVH hearts showed no increase in inotropic response when compared with WT-LVH hearts. Our results suggest that energy supply may be a limiting factor for the benefit of SERCA overexpression in hypertrophied hearts. Thus, strategies combining energetic support with increasing SERCA activity may improve the therapeutic effectiveness for heart failure.
Keywords: Calcium, Calcium ATPase, Cardiac Muscle, Cardiac Hypertrophy, Energetics, NMR, Myocardial Contraction, SERCA2a
Introduction
Recent studies show that improving Ca2+ handling in hypertrophied hearts by increasing expression of the cardiac isoform of the sarcoplasmic reticulum calcium ATPase 2a (SERCA2a) in rodent hearts via either transgenesis or adenoviral transfer results in enhanced contractile function (1–3). Although these findings suggest that SERCA4 gene therapy is a potential treatment strategy for heart failure, they also raise the question of whether an intervention that energetically demanding can be beneficial for the failing heart in the long term. SERCA is a pump that consumes one molecule of ATP to transport two molecules of Ca2+ from the cytosol to sarcoplasmic reticulum, thereby maintaining sarcoplasmic reticulum load and regulating the cytosolic [Ca2+] during both systole and diastole. The pump has a high thermodynamic efficiency and uses 85–90% of the energy released by ATP hydrolysis to maintain the 10,000-fold Ca2+ gradient across the sarcoplasmic reticulum membrane (4–6). Because they are chemically linked in this way, altered intracellular Ca2+ homeostasis and impaired myocardial energetics are two major characteristics of the failing heart (7, 8). Thus, in this study, we determined whether SERCA overexpression alters the energy cost of myocardial contraction in mouse hearts with pressure-overload hypertrophy. Our results show that overexpressing SERCA2a enhances myocardial contraction and relaxation in normal mouse hearts without compromising myocardial energetics. However, this advantage is not sustained in hypertrophied hearts, suggesting that energy supply could be a limiting factor for the benefit of SERCA overexpression in hypertrophied and failing hearts.
EXPERIMENTAL PROCEDURES
Animal Models
Transgenic mice overexpressing rat SERCA2a (TG) were produced as described previously (9). Ascending aortic constriction was performed in TG and age-matched wild-type mice (WT) to induce pressure-overload left ventricular hypertrophy (LVH) (10). Mice were studied at 8 weeks after surgery. Sham-operated mice were used as controls. All experiments were performed as four-way or three-way paired comparisons. All procedures conformed to the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee at the University of California, San Diego and the Harvard Medical School.
31P NMR Spectroscopy of Isolated Perfused Hearts
Mice were euthanized by cervical dislocation, and hearts were removed and perfused in the Langendorff mode with phosphate-free Krebs-Henseleit buffer containing (in mmol/liter) 118 NaCl, 25 NaHCO3, 5.3 KCl, 2.0 CaCl2, 1.2 MgSO4, 0.5 EDTA, 10 glucose, and 0.5 pyruvate as described previously (11). All sham hearts were perfused with a constant perfusion pressure of 75 mm Hg, and the perfusion pressure was increased to 100 mm Hg for LVH hearts. Prior experience showed that this approach would achieve comparable myocardial flow rate per unit of LV weight for the two groups (12). Perfused hearts were placed in a 9.4-tesla superconducting magnet and maintained at 37 °C throughout the protocol. After stabilization, 31P NMR spectra were collected during base line, low dose (0.5 μmol/liter), and high dose (10 μmol/liter) of isoproterenol stimulation. Ventricular function was simultaneously monitored by a water-filled balloon inserted into the LV and connected to a pressure transducer. In this way, the dynamic relationship between myocardial contractile performance and the energetic status can be assessed in a wide range of cardiac workload. All hearts were weighed and snap-frozen at the end of the experiment for biochemical assays.
Biochemical Assays
Total RNA was isolated from left ventricular tissue of unperfused hearts isolated from WT and TG hearts 8 weeks after ascending aortic constriction. This group of hearts had a similar degree of LVH as the group used for perfusion study (data not shown). Northern blotting was performed to assess the SERCA2a expression, and Western blotting was performed to measure the amount of SERCA2a protein in the LVH hearts (9).
Data Analysis and Statistics
The ATP peak area of the base-line NMR spectrum was normalized by the heart weight and set to 10.0 mmol/liter for the control group. Concentrations of phosphocreatine, [PCr], and inorganic phosphate ([Pi]) for both control and LVH hearts, as well as [ATP] for LVH hearts, were calculated using the ratios of their normalized peak areas to that of the base-line ATP area for the controls (13). Intracellular pH (pHi) was determined by comparing the chemical shift of the Pi and PCr resonances in each spectrum with values from a standard curve. Cytosolic free [ADP] was calculated using the creatine kinase equilibrium expression and metabolite values obtained by NMR spectroscopy and biochemical assay as described previously (6). The free energy stored in the terminal high energy phosphate bonds of ATP (ΔG∼P) is released by ATP hydrolysis and is calculated as
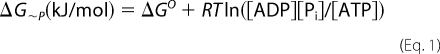 |
where ΔGo (−30.5 kJ/mol) is the value of ΔG∼P under standard conditions of molarity, temperature, pH, and [Mg2+], R is gas constant (8.3 J/mol·K), and T is temperature in Kelvin. Although ΔG∼P is a negative value, the change in free energy state is a positive value; here we describe changes in ΔG∼P in terms of absolute values, |ΔG∼P|.
All results were expressed as means ± S.E. Measurements made before and during isoproterenol stimulation were compared by repeated measures analysis of variance. Comparisons between two groups at the time points of interest were performed by unpaired t test. Wald test statistics were used for comparing the relationship between contractile work and ΔG∼P by pairwise contrasts of mean slopes in a random coefficient mixed model (SAS version 8.2). A value of p < 0.05 was considered significant.
RESULTS
LV Hypertrophy, Contractile Function, and SERCA Expression
The general characteristics of mice used for this study are summarized in Table 1. Ascending aortic constriction caused a similar degree of LV hypertrophy in WT and TG mice that resulted in an 82% increase in heart weight-to-body weight ratio and a 55% increase in heart weight-to-tibia length in both groups (p = not significant between the LVH groups). LV volume, estimated by the balloon volume at base line and normalized to LV weight, was similar for hypertrophied and sham-operated groups, indicating no ventricular dilation. Thus, WT-LVH and TG-LVH are in a compensated hypertrophic state.
TABLE 1.
General characteristics and LV hypertrophy
All data are shown as mean ± S.E. m, males; f, females; HW, heart weight; BW, body weight; TL, tibia length; vol, volume. *, p < 0.05 versus corresponding sham group.
| Number/sex | Body weight | Heart weight | HW/BW | HW/TL | LV vol/LV weight | |
|---|---|---|---|---|---|---|
| g | mg | mg/g | mg/mm | μl/g | ||
| WT-sham | 8 (3 m, 5 f) | 28 ± 1 | 133 ± 8 | 4.8 ± 0.1 | 7.6 ± 0.4 | 145 ± 12 |
| TG-sham | 7 (3 m, 4 f) | 25 ± 2 | 122 ± 6 | 4.7 ± 0.1 | 7.1 ± 0.3 | 131 ± 12 |
| WT-LVH | 6 (3 m, 3 f) | 24 ± 1 | 196 ± 23* | 8.8 ± 1.4* | 11.5 ± 1.4* | 143 ± 15 |
| TG-LVH | 6 (3 m, 3 f) | 24 ± 1 | 191 ± 14* | 8.5 ± 1.1* | 11.3 ± 0.9* | 120 ± 18 |
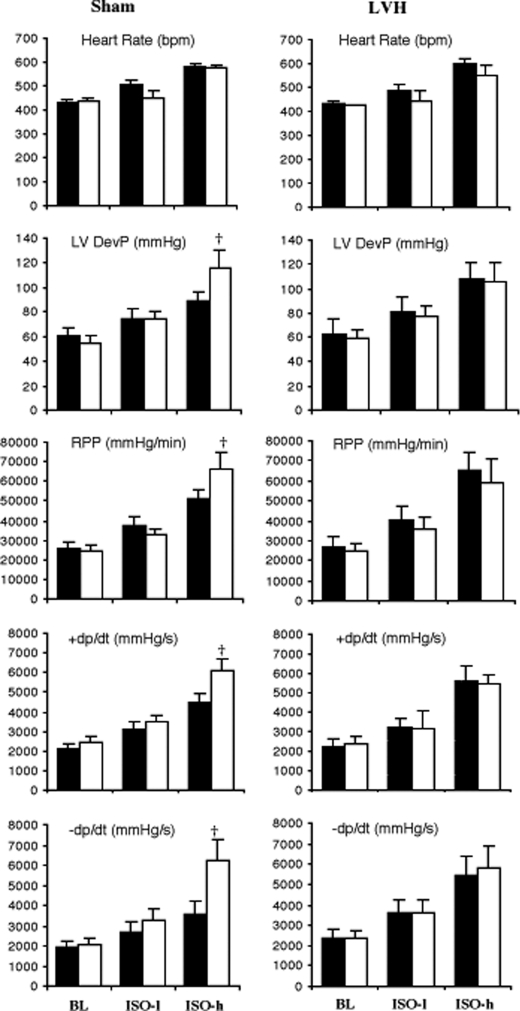
In sham-operated hearts, base-line isovolumic contractile function was similar for WT and TG hearts, but the response to isoproterenol (ISO) was greater in TG hearts (Fig. 1, left panels). At high doses of ISO, the LV-developed pressure and rate pressure product, +dp/dt and −dp/dt, were all significantly higher in TG hearts, whereas the heart rate response was not different from that of the WT, suggesting that SERCA2a overexpression leads to increased contraction and relaxation of the cardiac muscle. In contrast, increased positive inotropic and lusitropic effects were not observed in hypertrophied TG hearts (Fig. 1, right panels). Moreover, the response to ISO was not different in hypertrophied WT and hypertrophied TG hearts. These results suggest that the positive effect of overexpressing SERCA2a on myocardial contractility was not maintained in LVH hearts.
FIGURE 1.
Left ventricular function measured in isolated perfused Langendorff hearts from sham-operated (left panels) and hypertrophied (LVH) hearts (right panels) during base-line (BL), low dose (0. 5 μmol/liter), and high dose (10 μmol/liter) isoproterenol (ISO-l and ISO-h) infusion. Black bars represent WT, and white bars represent TG hearts. Data are shown as mean ± S.E., n = 6–8/group. †, p < 0.05 versus WT. RPP, rate pressure product. DevP, developed pressure; +dp/dt, rate of tension development; −dp/dt, rate of relaxation.
To confirm that increased SERCA2a expression was sustained in hypertrophied TG hearts, mRNA and protein levels of SERCA2a were determined in WT-LVH and TG-LVH hearts. When compared with WT-sham hearts, and as expected, both SERCA2a mRNA and protein levels were decreased by more than 50% in WT-LVH hearts (mRNA, 1 ± 0.1 AU in WT-sham versus 0.42 ± 0.02 AU in WT-LVH; protein levels, 1 ± 0.17 in WT-sham versus 0.48 ± 0.05 AU in WT-LVH; both were normalized to WT-sham levels set to 1). Consistent with our previous findings that SERCA2a overexpression can normalize the marked decrease of SERCA2a in hypertrophied hearts, both SERCA2a mRNA (1.15 ± 0.1 AU) and protein levels (0.89 ± 0.16 AU) in TG-LVH hearts were similar to normal WT hearts (set to 1) (1).
Myocardial Energetics
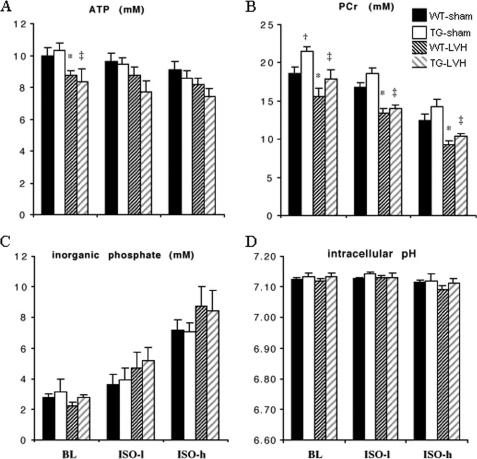
Myocardial ATP concentration ([ATP]) was similar for sham-operated WT and TG hearts at base line, and ISO stimulation resulted in ∼10% decrease in [ATP] in both groups (Fig. 2A). [ATP] was 10–15% lower in LVH hearts when compared with sham-operated hearts during base-line perfusion, and there is no difference between WT-LVH and TG-LVH hearts either at base line or during ISO stimulation (Fig. 2A). [PCr], the high energy reserve compound, was higher in TG-sham hearts at base line (p < 0.05 versus WT-sham), but not during ISO stimulation, as more PCr was used to support the higher energy demand for increased contractile function in TG-sham hearts (Fig. 2B). In LVH hearts, a lower base line [PCr] was observed for both WT and TG groups when compared with their corresponding sham-operated controls, showing a depletion of energy reserve in LVH hearts as reported previously (8, 14). Myocardial content of Pi increased as [PCr] decreased during ISO stimulation (Fig. 2C). pHi was ∼7.1 for all groups (Fig. 2D).
FIGURE 2.
31P NMR measurements of the hearts during base-line (BL), low dose (0.5 μmol/liter), and high dose (10 μmol/liter) isoproterenol (ISO-l and ISO-h) infusion. A, ATP. B, PCr. C, Pi. D, pHi. Data shown were obtained from WT-sham (black), TG-sham (white), WT-LVH (dark hatched), and TG-LVH (gray hatched) hearts. Data are shown as mean ± S.E., n = 6–8/group. *, p < 0.05 WT-LVH versus WT-sham group; ‡, p < 0.05 TG-LVH versus TG-sham; †, p < 0.05 TG-sham versus WT-sham.
Energetic Cost of Increasing Contraction
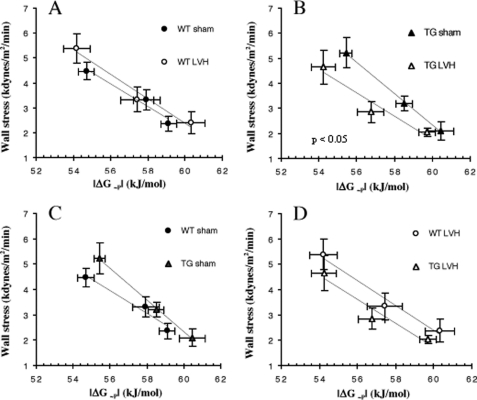
To determine the energetic cost of increasing isovolumic contractile performance, we analyzed the relationship between the contractile force developed during isovolumic contraction and the myocardial energetic status for each group of hearts during ISO stimulation. We used minute wall stress during systole as the measure of force development because this index takes into consideration any geometric changes in LVH hearts. The energetic status of the heart was assessed by the free energy available from ATP hydrolysis (ΔG∼P) because the free energy released from ATP is the driving force for all ATPase reactions. The relationship between force development and ΔG∼P for each group of hearts was linear. The relationships for WT and WT-LVH hearts are indistinguishable, suggesting that the cost of increasing contraction was not altered for moderate hypertrophy in the normal mouse heart with 2-fold differences in SERCA2a protein levels (Fig. 3A). In contrast, the force ΔG∼P relation is shifted to the left and downward in TG-LVH hearts when compared with TG-sham hearts (p = 0.05, Fig. 3B). This was primarily due to a rightward shift of the relationship for TG-sham when compared with WT-sham hearts (p < 0.05, Fig. 3C) as there was no difference between TG-LVH and WT-LVH hearts (Fig. 3D). These results suggest that TG-sham hearts are able to increase contractile function with high energy efficiency, but this advantage is not maintained in TG-LVH hearts.
FIGURE 3.
The relationship between LV force development, estimated by systolic minute wall stress, and myocardial energetic status, estimated by free energy availability (|ΔG∼P|), in the four groups of hearts during inotropic stimulation by isoproterenol infusion. A, WT-sham (wall stress = −0.51|ΔG∼P| + 32) and LVH (wall stress = −0.48|ΔG∼P| + 31). B, TG sham (wall stress = −0.63|ΔG∼P| + 40) and LVH (wall stress = −0.46|ΔG∼P| + 30). C, WT and TG sham. D, WT-LVH and TG-LVH. *, p < 0.05 when the slopes of the two groups are compared.
DISCUSSION
In this study, we found that increasing SERCA2a expression in mouse hearts by transgenesis enhanced myocardial contraction and relaxation during inotropic challenge and that the increased contractile function was achieved without compromising myocardial energetics. However, these favorable effects were not maintained in SERCA2a TG hearts with moderate pressure-overload hypertrophy, suggesting that alterations intrinsic to cardiac hypertrophy limit the efficacy of enhancing myocardial contractility by increasing SERCA2a protein level.
SERCA2a Overexpression and Energetics of Contraction in Normal Hearts
Previous studies have reported that the energy reserve of the heart decreases with an increase of SERCA activity by either phospholamban ablation (15) or adenoviral transfer of SERCA2a (16). However, in contrast, rat hearts overexpressing the skeletal SERCA isoform (SERCA1) are able to maintain normal energetics under base-line conditions (17). Considering that these interventions also result in enhanced contractility and force generation, it becomes critical to analyze the relationship between changes in energetics and contractile function, i.e. the cost of increasing contraction, under these conditions. Such an analysis has been performed in this study, and the results suggest that for the normal heart, the amount of energy spent on operating a moderately increased number of SERCA pumps (by 20% (8)) is “well invested” (Fig. 3C). Increased SERCA activity facilitates Ca2+ removal from the cytosol and increases sarcoplasmic reticulum loading, thus enhancing excitation-contraction coupling and rendering the contractile machinery highly efficient. Our study shows that overexpression of SERCA2a in normal mouse hearts enhanced their inotropic response (Fig. 3C). This is consistent with previous reports demonstrating increased contractile function in rodent hearts overexpressing SERCA2a or in hearts with phospholamban ablation resulting in uninhibited SERCA pump activity (15, 18). A novel and important finding of the present study is that the higher contractile performance achieved by increasing SERCA does not result in additional energy cost. On the contrary, the cost of increasing contraction is less in normal hearts overexpressing SERCA2a. This is unexpected because the presence of a greater number of functioning SERCA pumps should increase energy consumption of the heart.
SERCA2a Overexpression and Energetics of Contraction in Hypertrophied Hearts
Despite the finding of greater efficiency with higher levels of SERCA2a in normal TG hearts, we did not find a beneficial effect in hypertrophied hearts overexpressing SERCA2a. Instead, we observed a leftward and downward shift of the relationship between contractile force and free energy available from ATP hydrolysis in hypertrophied TG hearts when compared with sham TG hearts (Fig. 3B), suggesting a decreased energy efficiency in hypertrophied when compared with non-hypertrophied myocardium overexpressing SERCA2a (compare Fig. 3, B and C). This shift is not caused by down-regulation of SERCA2a protein in hypertrophied TG hearts as we found that SERCA2a protein was >2-fold higher in hypertrophied TG hearts when compared with hypertrophied WT hearts, leading to a complete normalization of SERCA2a level in LVH. However, the increased amount of SERCA2a in hypertrophied hearts was not sufficient to support an increase in contractile function of these hearts above the level achieved by hypertrophied WT hearts. Our results using SERCA2a overexpression in the hypertrophied mouse heart extend the study of O'Donnell et al. (17) investigating SERCA1 overexpression in the hypertrophied rat heart. They observed that the beneficial effect of SERCA1 overexpression on contractile function during adrenergic stimulation could not be maintained despite an increase in heart rate. Taken together, these results strongly suggest that neither overexpression of SERCA1 nor overexpression of SERCA2a in rodent models of myocardial hypertrophy has beneficial effects on contractile reserve.
Energy Cost of Varying SERCA2a Expression
It is important to point out that despite the finding that hypertrophied TG hearts failed to sustain a favorable energy efficiency during inotropic stimulation, the energy cost of contraction for hypertrophied TG hearts is the same as for hypertrophied WT hearts (Fig. 3D). The maximal inotropic responses in both groups of hypertrophied hearts are achieved at the ΔG∼P of −54 ± 1 kJ/mol, a level that is near the minimal free energy requirement to drive the SERCA pump (5). We have previously demonstrated that neither increases in intracellular Ca2+ transient nor increases in contractile force can be achieved beyond the level of ΔG∼P required by the SERCA pumps (−52 to −53 kJ/mol) (6, 19). In the study presented here, the finding that the SERCA pump operates at its apparent thermodynamic limit in both WT-LVH and TG-LVH hearts during ISO stimulation suggests that for these hearts, energetic support outweighs the amount of SERCA pump in determining the maximal contractile performance. Our results suggest that increasing SERCA2a protein in the hypertrophied heart does not incur extra energy expenditure, but its efficacy of enhancing contractile performance is limited by intrinsic changes associated with pressure-overload hypertrophy. Importantly, our results also show that the cost of increasing contraction in WT-LVH hearts with half-normal amounts of SERCA2a was not different from WT hearts (Fig. 3A), suggesting that the relationship between increased work and energy expenditure at the whole heart level is neither decreased nor increased by decreasing the amount of SERCA2a more than 2-fold.
Energetics of Increasing SERCA Activity in Cardiac Hypertrophy and Failure
Impaired SERCA pump activity is a major contributor to the abnormal intracellular Ca2+ homeostasis observed in hypertrophied and failing hearts. A number of studies have shown that increasing SERCA activity either by increasing the amount of SERCA protein or by decreasing the amount of its inhibitor, phospholamban, leads to improved contractile function in animal models of heart failure (2, 3, 20). It is indeed attractive to consider manipulating SERCA function as a novel inotropic therapy for heart failure. However, given the overwhelming clinical evidence indicating an adverse long term outcome of inotropic therapy in heart failure, one needs to carefully evaluate the cost of enhancing contraction in the failing heart. Although the SERCA strategy has the advantage of avoiding the stimulation of the β-adrenergic/adenyl cyclase system, known to be detrimental for heart failure patients in the long term, increased energy consumption associated with SERCA overexpression has been a major concern. Interestingly, del Monte et al. (16) found that gene transfer of SERCA2a to failing rat hearts improved myocardial energetics evidenced by increased concentration of energy reserve compound PCr. In the del Monte study, SERCA2a was delivered to markedly dilated hearts with substantial decreases in contractile function. Increasing SERCA activity in that case increased contractility and reduced ventricular dilatation. It is likely that improved geometry and thus a favorable ventricular efficiency contributed to the improved energetics. In our study, SERCA2a was overexpressed prior to the development of LVH and the hearts were not in failure. Although SERCA2a overexpression in hypertrophied hearts prior to failure did not further compromise myocardial energetics, this strategy showed minimal effect in boosting contractile reserve. We suggest that the SERCA pump function is limited by its energetic support at least in this case. These findings are consistent with literature reports suggesting that the strategy of increasing SERCA activity may not be effective in all models and/or at all stages of cardiac dysfunction (21–23). Because energy reserve is decreased in hypertrophied hearts and the SERCA pump has a high energetic requirement, it will be important to determine whether improving energetic support combined with increasing SERCA activity represents an effective therapeutic strategy at each stage of cardiac dysfunction.
Footnotes
- SERCA
- sarcoplasmic reticulum calcium ATPase
- TG
- transgenic
- LV
- left ventricle
- LVH
- left ventricular hypertrophy
- PCr
- phosphocreatine
- ISO
- isoproterenol
- AU
- arbitrary units.
REFERENCES
- 1. Ito K., Yan X., Feng X., Manning W. J., Dillmann W. H., Lorell B. H. (2001) Circ. Res. 89, 422–429 [DOI] [PubMed] [Google Scholar]
- 2. Miyamoto M. I., del Monte F., Schmidt U., DiSalvo T. S., Kang Z. B., Matsui T., Guerrero J. L., Gwathmey J. K., Rosenzweig A., Hajjar R. J. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 793–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Müller O. J., Lange M., Rattunde H., Lorenzen H. P., Müller M., Frey N., Bittner C., Simonides W., Katus H. A., Franz W. M. (2003) Cardiovasc Res. 59, 380–389 [DOI] [PubMed] [Google Scholar]
- 4. Bers D. M., Perez-Reyes E. (1999) Cardiovasc Res. 42, 339–360 [DOI] [PubMed] [Google Scholar]
- 5. Kammermeier H. (1987) Basic Res. Cardiol. 82, Suppl. 2, 31–36 [DOI] [PubMed] [Google Scholar]
- 6. Tian R., Ingwall J. S. (1996) Am. J. Physiol. 270, H1207–H1216 [DOI] [PubMed] [Google Scholar]
- 7. Houser S. R., Margulies K. B. (2003) Circ. Res. 92, 350–358 [DOI] [PubMed] [Google Scholar]
- 8. Ingwall J. S., Weiss R. G. (2004) Circ. Res. 95, 135–145 [DOI] [PubMed] [Google Scholar]
- 9. He H., Giordano F. J., Hilal-Dandan R., Choi D. J., Rockman H. A., McDonough P. M., Bluhm W. F., Meyer M., Sayen M. R., Swanson E., Dillmann W. H. (1997) J. Clin. Invest. 100, 380–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ito K., Yan X., Tajima M., Su Z., Barry W. H., Lorell B. H. (2000) Circ. Res. 87, 588–595 [DOI] [PubMed] [Google Scholar]
- 11. Pinz I., Ostroy S. E., Hoyer K., Osinska H., Robbins J., Molkentin J. D., Ingwall J. S. (2008) Am. J. Physiol. Heart Circ. Physiol. 294, H1459–H1466 [DOI] [PubMed] [Google Scholar]
- 12. Eberli F. R., Apstein C. S., Ngoy S., Lorell B. H. (1992) Circ. Res. 70, 931–943 [DOI] [PubMed] [Google Scholar]
- 13. Javadpour M. M., Tardiff J. C., Pinz I., Ingwall J. S. (2003) J. Clin. Invest. 112, 768–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tian R., Nascimben L., Ingwall J. S., Lorell B. H. (1997) Circulation 96, 1313–1319 [DOI] [PubMed] [Google Scholar]
- 15. Chu G., Luo W., Slack J. P., Tilgmann C., Sweet W. E., Spindler M., Saupe K. W., Boivin G. P., Moravec C. S., Matlib M. A., Grupp I. L., Ingwall J. S., Kranias E. G. (1996) Circ. Res. 79, 1064–1076 [DOI] [PubMed] [Google Scholar]
- 16. del Monte F., Williams E., Lebeche D., Schmidt U., Rosenzweig A., Gwathmey J. K., Lewandowski E. D., Hajjar R. J. (2001) Circulation 104, 1424–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O'Donnell J. M., Fields A., Xu X., Chowdhury S. A., Geenen D. L., Bi J. (2008) Am. J. Physiol. Heart Circ. Physiol. 295, H2483–H2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baker D. L., Hashimoto K., Grupp I. L., Ji Y., Reed T., Loukianov E., Grupp G., Bhagwhat A., Hoit B., Walsh R., Marban E., Periasamy M. (1998) Circ. Res. 83, 1205–1214 [DOI] [PubMed] [Google Scholar]
- 19. Tian R., Halow J. M., Meyer M., Dillmann W. H., Figueredo V. M., Ingwall J. S., Camacho S. A. (1998) Am. J. Physiol. 275, H2064–H2071 [DOI] [PubMed] [Google Scholar]
- 20. Minamisawa S., Hoshijima M., Chu G., Ward C. A., Frank K., Gu Y., Martone M. E., Wang Y., Ross J., Jr., Kranias E. G., Giles W. R., Chien K. R. (1999) Cell 99, 313–322 [DOI] [PubMed] [Google Scholar]
- 21. Chen Y., Escoubet B., Prunier F., Amour J., Simonides W. S., Vivien B., Lenoir C., Heimburger M., Choqueux C., Gellen B., Riou B., Michel J. B., Franz W. M., Mercadier J. J. (2004) Circulation 109, 1898–1903 [DOI] [PubMed] [Google Scholar]
- 22. del Monte F., Lebeche D., Guerrero J. L., Tsuji T., Doye A. A., Gwathmey J. K., Hajjar R. J. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 5622–5627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dorn G. W., 2nd, Molkentin J. D. (2004) Circulation 109, 150–158 [DOI] [PubMed] [Google Scholar]