Abstract
IL-23, produced by dendritic cells (DCs) and macrophages, plays a critical role in innate immunity against bacterial infection. Our previous studies show that morphine disrupts the IL-23/IL-17 mediated pulmonary mucosal host defense and increases susceptibility to Streptococcus pneumoniae lung infection. To determine the mechanism by which morphine modulates IL-23 production, mouse bone marrow-derived dendritic cells (BMDCs) and macrophages (BMDMs) were treated with morphine, and infected with S. pneumoniae or stimulated with Toll-like receptor (TLR) and Nod2 ligands. We found that a significant increase in IL-23 protein production was observed in S. pneumoniae, TLR2 ligand lipoteichoic acid (LTA), and TLR4 ligand pneumolysin (PLY) stimulated BMDCs and BMDMs. Interestingly, although Nod2 ligand muramyldipeptide (MDP) alone had no effect on IL-23 production, it potentiated LTA induced IL-23 production to the same level as that observed following S. pneumoniae infection, suggesting that S. pneumoniae induced IL-23 production in DCs involves activation of both TLR2 and Nod2 signaling mechanisms. Furthermore, pretreatment of DCs with MyD88 (myeloid differentiation primary response gene 88) and IL-1 receptor-associated kinase (IRAK) 1/4 inhibitors, or TLR2 antibody diminished the S. pneumoniae induced IL-23 and abolished the inhibitory effects of morphine, indicating that S. pneumoniae induced IL-23 production depends on activation of the TLR2-MyD88-IRAK1/4 signaling pathway. Moreover, morphine decreased S. pneumoniae induced phosphorylation of interferon regulatory factor 3 (IRF3) and activating transcription factor 2 in DCs. Taken together, our study shows that morphine impairs S. pneumoniae induced IL-23 production through MyD88-IRAK1/4-dependent TLR2 and Nod2 signaling in DCs.
Keywords: Bacteria, Cytokine, Dendritic Cell, Innate Immunity, Signal Transduction
Introduction
Host innate immune defenses in the airways, in most cases, efficiently clear invading Streptococcus pneumoniae and thereby prevent bacterial entry into the lungs and dissemination into the bloodstream. However, any compromise in the host innate immune function increases the risk of bacterial invasion resulting in pneumococcal disease (1, 2). Our previous studies show that morphine impairs host innate immune response and increases susceptibility to S. pneumoniae lung infection (3, 4). Furthermore, our current study show that chronic morphine disrupts the IL-23/IL-17 mediated pulmonary mucosal host defense against S. pneumoniae lung infection in an in vivo murine pulmonary S. pneumoniae infection model and in an in vitro cell infection model (5). However, the cellular and molecular mechanisms by which morphine modulates S. pneumoniae induced IL-23 production remain to be elucidated.
Resident lung phagocytic cells, primarily dendritic cells and alveolar macrophages, are likely the first immune cells exposed to S. pneumoniae upon inhalation of the organism into the lungs. Dendritic cells and alveolar macrophages express various pattern recognition receptors such as Toll-like receptors (TLRs)4 and Nod (nucleotide-binding oligomerization domain) receptors. These receptors are involved in innate immunity signaling and phagocytosis in response to microbial infections. The TLRs play a crucial role against microbial infections through initiating and activating both innate and adaptive immunity (6). TLR4 recognizes pneumolysin (PLY), a cytolytic toxin produced by most clinical isolates of S. pneumoniae (7). TLR2 binds to a broad range of ligands such as cell wall peptidoglycan and lipoteichoic acid (LTA) (8). Although both TLR2 and TLR4 signaling are triggered by pneumococci, mice deficient in either one of these two receptors only show marginal differences in their susceptibility to invasive pneumococcal disease (9). TLR9 recognizes unmethylated CpG motifs, playing a nonredundant role in host protection during the early stage of pulmonary infections (10). This protection occurs prior to the infiltration of inflammatory cells and is believed to be due to TLR9-MyD88-dependent induction of the phagocytic pathway in resident macrophages (11). Nod1 and Nod2 are known to recognize bacteria and/or their components. Nod1 and Nod2 recognize the bacterial peptidoglycan components γ-d-glutamyl-mesodiaminopimelic acid and muramyl dipeptide (MDP), respectively (12). Nod2 activation seems to play an important role in host cell activation by internalized pneumococci (13). Findings from our lab indicate that IL-23 is produced by DCs and macrophages within a few hours after exposure to S. pneumoniae. Morphine treatment disrupts the IL-23/IL-17 axis, which leads to a diminished host release of antimicrobial proteins S100A8/A9. This results in decreased neutrophil recruitment and more severe infection (5). However, the cellular and molecular mechanisms by which morphine treatment leads to compromised IL-23 production in response to S. pneumoniae has not been delineated.
In this study, bone marrow-derived dendritic cells (BMDCs) and macrophages (BMDMs) were treated with morphine and infected with S. pneumoniae or stimulated with TLR ligands (LTA, PLY, and CpG), and Nod2 ligand (MDP). Using a well established in vitro cell S. pneumoniae infection model, we demonstrate that morphine impairs S. pneumoniae induced IL-23 production through MyD88-IRAK1/4-dependent TLR2 and Nod2 signaling in DCs.
EXPERIMENTAL PROCEDURES
Experimental Animals
Wild type mice (B6129PF1) were obtained from The Jackson Laboratory. μ-Opioid receptor knock-out mice (C57BL/6 × 129Ola male) were produced as described previously (14). Mice were housed in a specific pathogen-free facility under barrier conditions. A maximum of four mice were housed per cage. Food and tap water were available ad libitum. The animal housing facilities were maintained on a 12-h light/dark cycle, with a constant temperature (72 ± 1 °F) and 50% humidity. All animal experiments were done in accordance with the Institutional Animal Care and Use Committee guidelines at the University of Minnesota.
In Vitro Morphine Treatment and Cell Infection with S. pneumoniae or Stimulation with TLRs and Nod2 Ligands
Mouse BMDCs and BMDMs were generated following a method described previously (5).
BMDCs and BMDMs (1 × 106) were treated for 24 h in medium containing vehicle (PBS control) or various concentrations of morphine (1 nm to 1 μm; National Institute on Drug Abuse, Rockville, MD). Then, cells were infected with S. pneumoniae, serotype 3 (m.o.i., 20:1), or stimulated with TLRs (5 μg/ml LTA, 1 μg/ml rPLY, 10 μg/ml CpG) or Nod2 (5 μg/ml MDP) ligands, for various periods of time, depending on the experiment. The rPLY protein was purified from Escherichia coli, which expressed full-length rPLY in our laboratory following the protocol of Dr. Braun (15). The E. coli were kindly provided by Dr. Johann S. Braun (Departments of Neurology, Cell Biology, and Neurobiology, Charité Universitaetsmedizin Berlin, Berlin, Germany). The levels of endotoxin were determined by Limulus assay. Hemolytic activity was verified qualitatively by dropping eluted rPLY fractions on sheep blood agar plates.
To inhibit MyD88-dependent signaling, cells were pretreated with MyD88 homodimerization inhibitory peptide or control peptide (Imgenex) at 100 μm for 24 h before in vitro cell infection with S. pneumoniae. The inhibitory peptide contains a sequence from the MyD88 TIR homodimerization domain, which specifically inhibits the MyD88-dependent pathway (5).
To block IRAK1/4 signaling, DCs were pretreated with IRAK1/4 inhibitor N-(2-morpholinylethyl)-2-(3-nitrobenzoylamido)-benzimidazole (Sigma-Aldrich) at 50 μm for 2 h prior to infection of DCs with S. pneumoniae.
Transfection and Detection of Luciferase Reporter Activity
BMDCs (2 × 107) were transiently transfected with 5 μg of IL-23 promoter firefly luciferase reporter plasmid pGL3B-IL-23p19 (16) and 0.5 μg of control plasmid containing the pRL-TK (Promega, Madison, WI) driving Renilla luciferase as an internal standard, according to the manufacturer's instructions using the Nucleofector (Amaxa). In this experiment, DCs were treated with morphine (1 nm–1 μm) or vehicle for 24 h prior to the infection. Lysates were harvested at 1 h following S. pneumoniae in vitro infection and assayed for firefly and Renilla luciferase activity using a dual luciferase reporter assay system (Promega) with a luminometer (Turner TD20/20). All assays were done in triplicate, and luciferase values were normalized for transfection efficiency against Renilla luciferase activity as described previously (14).
ELISA
The levels of IL-23 in cell culture supernatant of in vitro-infected cells were quantified using cytokine-specific ELISA kits (R&D Systems) according to the manufacturer's instructions.
Real-time RT-PCR
Total RNA from mouse BMDCs was isolated using the RNeasy mini kit (Qiagen). Ten nanograms of total RNA was subjected to two-step RT-PCR. Real-time SYBR Green PCR analysis was performed for IL-23p19 (sense, 5′-CCAGCGGGACATATGAATCT-3′; antisense, 5′-AGGCTCCCCTTTGAAGATGT-3′) and 18 S rRNA. Relative quantification was performed using the ABI Prism 7500 sequence detection system (Applied Biosystems). IL-23p19 and IL-17 transcript levels were normalized to 18 S rRNA transcript levels from the same preparations of cDNA. Our previous study show that morphine treatment alone does not change 18 S rRNA transcript levels (5).
Flow Cytometry Analysis for TLRs
Staining for cell surface expression of TLRs was performed using anti-TLR2, -4, and -9 antibodies (R&D Systems). A FACScan flow cytometer with CellQuest software (BD Biosciences) was used.
Immunofluorescence Staining
Mouse BMDCs for immunofluorescence staining were grown on Lab-Tek II chamber slides (Nalge Nunc Int.) and fixed in 4% paraformaldehyde. Fc receptors were blocked using human Ig (Globuman Berna) at a concentration of 10 μg/ml for 15 min. Slides were incubated for 1 h at 37 °C with primary antibodies (IRF3 and ATF2, Abcam) which were diluted in PBS supplemented with 10% FCS. At the end of each incubation period, slides were washed with PBS containing 0.1% Tween 20. Secondary antibody, DyLight 488 affiniPure donkey anti-rabbit IgG, (Jackson ImmunoResearch Laboratories) was then incubated for 1 h at room temperature in the dark. Nuclei were visualized using Hoechst dye (Sigma). Stained cells were visualized with a Nikon confocal microscope.
Statistical Analysis
Data were collected from three independent experiments and expressed as the means ± S.E. Where appropriate, mean values were compared using a paired Student's t test or two-way analysis of variance. A p value of <0.05 was considered statistically significant.
RESULTS
Dendritic Cells Are a Main Source of IL-23 in Response to S. pneumoniae Infection
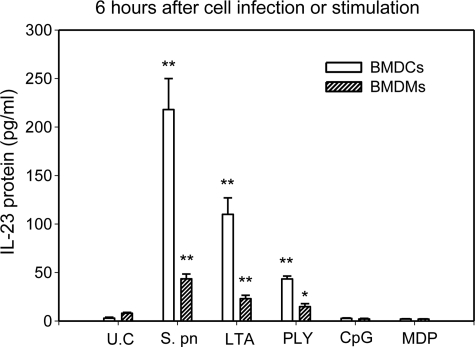
Upon bacterial infection, IL-23 is rapidly produced by activated macrophages and dendritic cells at the site of infection (17). In this study, we first compared the relative ability of BMDCs versus BMDMs to produce IL-23 in response to S. pneumoniae infection or stimulation with TLR ligands (PLY, LTA, and CpG), and Nod2 ligand (MDP). As shown in Fig. 1, BMDCs and BMDMs constitutively released IL-23 at very low levels. Following infection with S. pneumoniae or stimulation with single cognate TLR2 or -4 agonist (LTA or PLY), the levels of IL-23 in the culture supernatants of BMDCs and BMDMs were significantly increased. Interestingly, treatment with cognate TLR9 or Nod2 agonist (CpG or MDP) did not result in a significant induction in IL-23 production in BMDCs and BMDMs. The levels of IL-23 were ∼5-fold higher in culture supernatants of BMDCs compared with those of BMDMs in response to S. pneumoniae infection. This result indicates that DCs are more potent producers of IL-23 in response to S. pneumoniae infection than macrophages.
FIGURE 1.
S. pneumoniae infection and TLR2/4 activation induced IL-23 production in BMDCs and BMDMs. Mouse BMDCs and BMDMs (1 × 106) were infected with S. pneumoniae (m.o.i., 20:1) or stimulated with TLR ligands (5 μg/ml LTA, 1 μg/ml rPLY, 10 μg/ml CpG) or Nod2 ligand (5 μg/ml MDP) for 6 h, and IL-23 concentrations were measured in the supernatant by ELISA. *, p < 0.05; **, p < 0.01, compared with unstimulated controls (U.C.).
Morphine Acts through μ-Opioid Receptor in DCs to Modulate IL-23 Promoter Activity, mRNA Transcription, and Protein Synthesis following Infection with S. pneumoniae
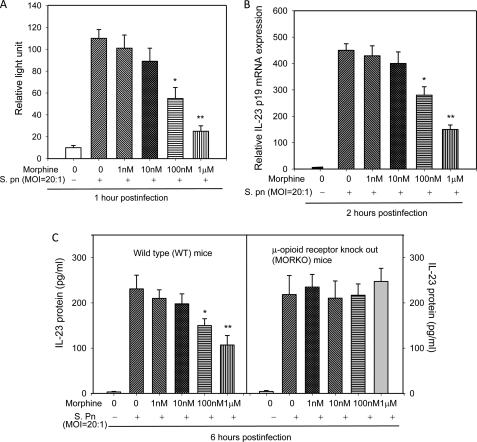
In previous studies, we show that morphine down-regulated S. pneumoniae induced IL-23 protein expression in both in vivo lung infection model and in vitro cell infection model. Although our previous works have established that IRF3, ATF2, and NF-κB were selectively inactivated in morphine-treated and S. pneumoniae-infected DCs, the mechanism for this repression has never been defined (5). In this study, DCs were treated with morphine and then infected with S. pneumoniae. Our results show that higher doses of morphine (100 nm and 1 μm) significantly down-regulated IL-23 in a dose-dependent manner. Furthermore, treatment with high dose of morphine treatment (1 μm) resulted in an ∼65% reduction in IL-23 promoter activity (Fig. 2A) and mRNA levels (Fig. 2B), 50% decline in IL-23 protein production (Fig. 2C) when compared with vehicle control in DCs derived from WT mice. Moreover, the morphine effect on IL-23 protein production was completely abolished in DCs derived from μ-opioid receptor knock-out mice. These results suggest that morphine modulation of IL-23 synthesis is mediated through μ-opioid receptors. As we described previously, micromolar concentrations of morphine are typical drug abuse doses and concentration achieved in patients that are prescribed morphine for moderate to severe pain. Plasma levels that are in the nanomolar range are generally observed in patients who receive morphine for mild and acute pain (18–20). Our results suggest that chronic morphine use and abuse disrupts IL-23 production following S. pneumoniae infection.
FIGURE 2.
Effect of morphine treatment on IL-23 promoter activity, mRNA expression, and protein synthesis in BMDCs infected with S. pneumoniae. A, BMDCs were transfected with 5 μg of the pGL3B-IL-23p19 firefly luciferase reporter plasmid and co-transfected with 0.5 μg of pRL-TK plasmid-driving Renilla luciferase. After 2 h of transfection, cells were treated with vehicle or morphine (1 nm–1 μm) for 24 h prior to infection with S. pneumonia (S. pn). Lysates were harvested after 1 h and assayed for firefly and Renilla luciferase activity using a Dual-Luciferase reporter kit. Data are presented as relative light units after correction for transfection efficiency by normalization with pRL-TK driving Renilla luciferase. B, BMDCs were treated with low and high dose morphine for 24 h and then infected with S. pneumoniae (m.o.i., 20:1) for 2 h. mRNA expression of IL-23 was determined by real-time PCR. C, BMDCs from WT and μ-opioid receptor knock-out (MORKO) mice were treated with low and high dose morphine for 24 h and then infected with S. pneumoniae (m.o.i., 20:1) for 6 h. The protein expression of IL-23 in culture supernatant was measured by enzyme-linked immunosorbent assay. Data shown are the mean ± S.E. of three separate experiments. Statistical differences were determined by a factorial analysis of variance followed by an unpaired t test. *, significance at level p < 0.05; **, significance at level p < 0.01 compared with the control group.
S. pneumoniae Infection Modulate IL-23 Production through Activation of Both TLR2 and Nod2 Signaling Pathway: Morphine Treatment Inhibits Signaling Pathway
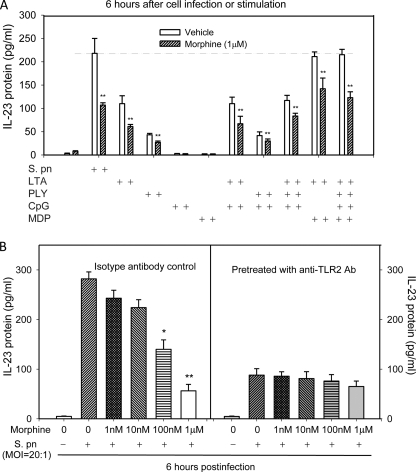
The host defense against S. pneumoniae is primarily localized to the lungs and depends on the immediate recognition of bacteria via TLRs to mount a proper inflammatory response. TLR2, TLR4, TLR9, and Nod2 play important roles in recognizing S. pneumoniae infection (8, 10, 21–23). To determine whether S. pneumoniae induced IL-23 production is mediated through TLRs and Nod2-dependent signaling pathways, DCs were stimulated with either an individual TLR or Nod2 agonist or a combination of TLRs and Nod2 agonists. As shown in Fig. 3A, although the single Nod2 agonist (MDP) did not induce IL-23 production to the same extent as that observed with S. pneumoniae, the combination of Nod2 (MDP) and TLR2 (LTA) agonist treatment resulted in a significant induction in IL-23 production, which was at the same level as S. pneumoniae infection. TLR2 and Nod2 ligands displayed a synergistic effect in the production of IL-23 in DCs, suggesting that S. pneumoniae infection induced IL-23 production may be mediated through both TLR2 and Nod2 signaling pathways. Morphine treatment significantly inhibited both S. pneumoniae and TLR2- and Nod2-induced IL-23 production.
FIGURE 3.
Effect of morphine treatment on IL-23 production by BMDCs in response to Nod2 and various TLR ligands. A, BMDCs were treated with morphine (1 μm) or vehicle for 24 h and then infected with S. pneumoniae (S. pn) or stimulated with rPLY (1 μg/ml), LTA (5 μg/ml), CpG (10 μg/ml), MDP (5 μg/ml), or PBS as a unstimulated control (UC) for 6 h. IL-23 production was determined by ELISA. B, BMDCs were preincubated with anti-TLR2 (10 μg/ml) or isotype control antibody 2 h before S. pneumoniae infection. After 6 h of in vitro cell infection, supernatant of cell culture was collected and used to determine IL-23 production. *, p < 0.05; **, p < 0.01, compared with vehicle controls. Results are representative of three independent experiments.
To further determine whether the TLR2-dependent signaling pathway is essential for IL-23 production following S. pneumoniae infection, DCs were preincubated with anti-TLR2 (10 μg/ml) or isotype control antibody 2 h before S. pneumoniae infection. After 6 h of in vitro cell infection, cell culture supernatant was collected and used to determine IL-23 production. Pretreatment of DCs with anti-TLR2 antibody partially attenuated S. pneumoniae induced IL-23 production, indicating that the IL-23 production induced by S. pneumoniae infection is a downstream event that depends on TLR2 activation. Furthermore, pretreatment of DCs with anti-TLR2 antibody abolished the inhibitory effects of morphine on IL-23 production following infection, indicating that morphine down-regulates TLR2-dependent IL-23 production (Fig. 3B).
Effect of S. pneumoniae Infection and Morphine Treatment on Expressions of TLRs
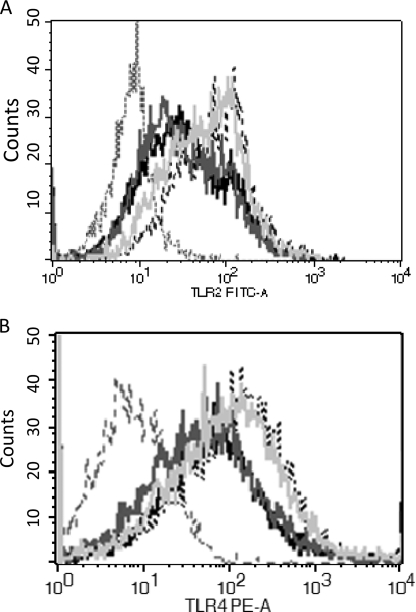
To determine whether morphine treatment alters expressions of TLRs, which leads to compromised TLR-IL-23 signaling, TLR2, -4, and -9 expression levels were measured following morphine treatment and S. pneumoniae in vitro infection using FACS. Upon S. pneumoniae infection, increased number of TLR2 and -4, but not TLR9 (data not shown), positive cells were observed, suggesting that S. pneumoniae infection induced the TLR2 and -4 expressions in DCs. However, morphine treatment did not significantly alter the expressions of TLRs in DCs, suggesting that modulation of TLRs expression is not involved in the actions of morphine following S. pneumoniae infection (Fig. 4).
FIGURE 4.
Effect of morphine treatment and S. pneumoniae infection on TLRs expression in BMDCs. BMDCs were treated with morphine (1 μm) or vehicle for 24 h. Then, cells were infected with S. pneumoniae (m.o.i., 20:1) for 6 h. TLR expression was analyzed by flow cytometry assay. Similar results were obtained in three independent experiments. Gray dashed line, isotype control; gray line, vehicle + unstimulated control; black line, 1 μm morphine; light gray line, S. pneumoniae infection; black dashed line, 1 μm morphine + S. pneumoniae infection.
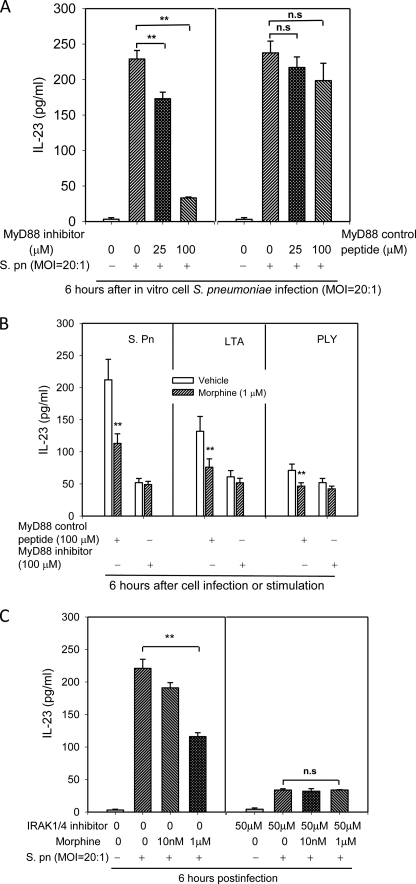
Morphine Treatment Impairs IL-23 Production in a MyD88-IRAK1/4-dependent Manner
To determine whether morphine modulates MyD88-dependent IL-23 production following infection, DCs were preincubated with MyD88 inhibitors or control peptides 24 h before cell infection with S. pneumoniae or stimulation with TLR2/4 ligands. As shown in Fig. 5A, MyD88 inhibitory peptide significantly inhibited S. pneumoniae-induced IL-23 production in a dose-dependent manner; meanwhile, MyD88 control peptide did not show a significant effect on IL-23 production. When DCs were pretreated with MyD88 control peptide, morphine treatment resulted in a decrease in S. pneumoniae, LTA and PLY induced IL-23 production. However, when DCs were pretreated with 100 μm MyD88 inhibitor, no difference in IL-23 production was observed between morphine and vehicle-treated DCs following infection with S. pneumoniae or stimulated with LTA or PLY, suggesting that morphine treatment impairs MyD88-dependent IL-23 production following infection (Fig. 5B).
FIGURE 5.
MyD88-IRAK1/4 signaling pathway participated in the morphine treatment induced inhibitory IL-23 production. A and B, to inhibit MyD88-dependent signaling, cells were pretreated with MyD88 homodimerization inhibitory peptide or control peptide at 24 h before in vitro cell infection with S. pneumoniae (S. pn). C, BMDCs were preincubated with IRAK1/4 inhibitor (50 μm) or control vehicle 2 h before cell infection with S. pneumoniae. IL-23 concentration was measured in the supernatant by ELISA. **, p < 0.01, compared with the vehicle controls, or comparisons indicated by brackets. Results are representative of three independent experiments. ns, not significant.
To further determine whether morphine modulates MyD88-IRAK1/4-dependent IL-23 production following infection, DCs were preincubated with IRAK1/4 inhibitor (50 μm) or control vehicle 2 h before S. pneumoniae cell infection. The IRAK1/4 inhibitor caused a clear reduction in IL-23 production following infection. Pretreatment of DCs with IRAK1/4 inhibitor totally attenuated the inhibitory actions of morphine on IL-23 production following S. pneumoniae infection (Fig. 5C). Taken together these results suggest that morphine treatment impairs S. pneumoniae induced IL-23 production in a MyD88-IRAK1/4-dependent manner.
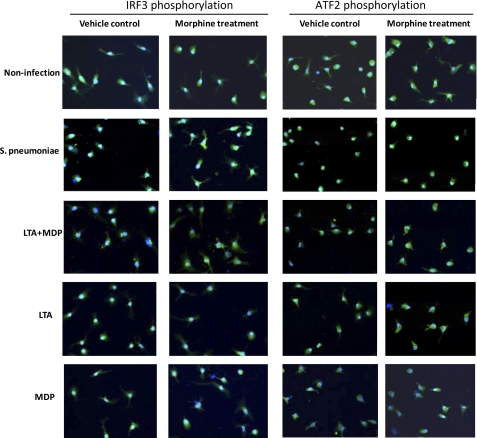
Phosphorylation of ATF2 and IRF3 following Infection with S. pneumoniae or when Stimulated with TLR2 and Nod2 Ligands Is Inhibited by Morphine Treatment
IRF3 and ATF2 are retained in the nucleus only after activation-induced phosphorylation (24, 25). Therefore, to investigate whether morphine modulates the phosphorylation of IRF3 and ATF2, the subcellular localizations of IRF3 and ATF2 in response to S. pneumoniae, and TLRs and Nod2 ligands were determined by immunofluorescence staining. DCs were infected with S. pneumoniae or stimulated with LTA, MDP, or LTA plus MDP for 45 min, then fixed, and stained for the subcellular distribution of IRF3 and ATF2. Consistent with IL-23 production, we only detected nuclear translocation of IRF3 and ATF2 in response to infection with S. pneumoniae, and stimulation with LTA plus MDP, or LTA alone. Morphine treatment significantly decreased the phosphorylation of IRF3 and ATF2 (Fig. 6). These results indicate that morphine modulates IL-23 production through both TLR2/Nod2-IRF3 and TLR2/Nod2-ATF2 signaling pathways.
FIGURE 6.
Effect of morphine treatment on phosphorylation of IRF3 and ATF-2 following infection with S. pneumoniae or stimulation with TLR and Nod2 ligands. Immunofluorescence staining reveals the subcellular distribution of IRF3 and ATF2 after infection with S. pneumoniae and stimulation with TLR or Nod 2 ligands. Of note, IRF3 and ATF2 were stained with green labeled secondary antibody. Original magnification was ×400.
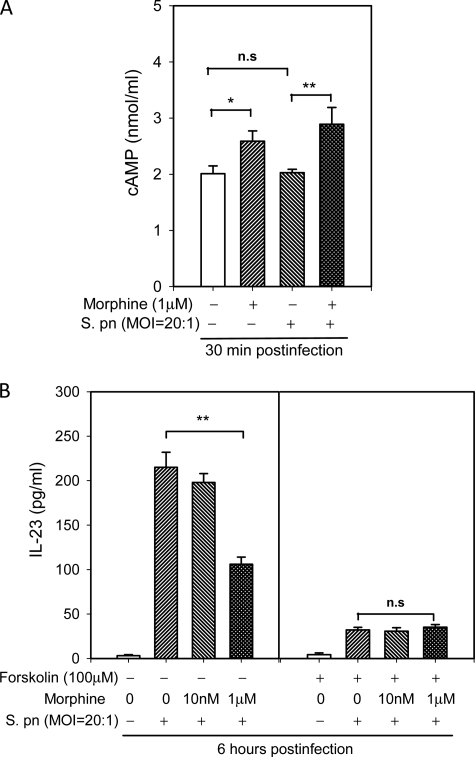
cAMP Is Involved in Inhibitory Effects of Morphine on S. pneumoniae-induced IL-23 Production
Our previous studies have shown that chronic stimulation of the μ-opioid receptor with morphine leads to adenylyl cyclase superactivation, which is completely abolished by pertussis toxin pretreatment. Downstream of adenylyl cyclase, cAMP plays an important role in morphine-mediated IFN-γ inhibition in activated T cells (14). Although the role of adenylyl cyclase superactivation in T cells is well known, the intracellular signal transduction events following morphine treatment that modulate DCs functions remains to be elucidated. In the present study, DCs were treated with morphine and then infected with S. pneumoniae. Intracellular cAMP was then determined by immunoassay. When compared with noninfected DCs, S. pneumoniae infection did not result in an alteration in the levels of intracellular cAMP. Consistent with our previous studies, chronic morphine treatment resulted in a significant increase in intracellular cAMP in DCs.
To determine the potential involvement of an increase in cAMP in the inhibitory effect of morphine on IL-23 production, we used forskolin, an activator of adenylate cyclase, to increase intracellular cAMP in DCs. As shown in Fig. 7, forskolin treatment significantly inhibited IL-23 production in S. pneumoniae-infected DCs, and further exacerbated the inhibitory effect of morphine, indicating that a cAMP-dependent signaling pathway may be responsible for the inhibitory effects of morphine on IL-23 synthesis.
FIGURE 7.
Role of cAMP in the regulation of IL-23 production by morphine in DCs following S. poneumoniae infection. A, morphine treatment resulted in an increase in intracellular cAMP. BMDCs were treated with morphine or vehicle for 24 h and then infected with S. pneumoniae (S. pn) for 30 min. The levels of intracellular cAMP were determined by competitive enzyme immunoassay. B, forskolin inhibited IL-23 synthesis, and the effect of morphine was abolished by pretreatment with forskolin. BMDCs were preincubated with forskolin (100 μm) or vehicle 30 min before cell infection with S. pneumoniae. The data shown are the mean ± S.E. of three experiments. *, p < 0.05; **, p < 0.01 comparisons as indicated by brackets. n.s., not significant.
DISCUSSION
Our previous studies have shown that infection with S. pneumoniae induces IL-23 response in both in vitro models and in vivo murine models. Furthermore, morphine treatment causes a decrease in IL-23 production, leading to delayed and reduced neutrophil migration, which results in severe lung infection and the initiation of systemic infection (5). However, the cellular and molecular mechanisms by which morphine modulates IL-23 production following S. pneumoniae infection have not been clearly delineated. In this report, we demonstrate that IL-23 is produced by DCs and macrophages in response to S. pneumoniae infection and acts as an initiator of the innate immune response. DCs are the main source of IL-23, which is produced through activation of both TLR2 and Nod2 signaling pathways. Morphine treatment modulates the MyD88-IRAK1/4-dependent TLR2 and Nod2 signaling, leading to a diminished IL-23 production in DCs following S. pneumoniae infection.
Previous studies have reported that TLRs are critical receptors for recognizing invading pathogens and triggering protective immune responses. TLR2 was identified as a key pattern recognition receptor in the immune response to cell wall components of S. pneumoniae such as peptidoglycan and LTA (26). There is evidence that TLR4 also has a role in the innate immune response to pneumolysin and intact pneumococci (27). TLR9 plays a nonredundant role in host protection at an early stage of pulmonary S. pneumoniae infections. In addition, pneumococci are recognized intracellularly by cytosolic receptors such as Nod2 (12). In this study, we found that S. pneumoniae induced IL-23 production in DCs through activation of TLR2 and -4 but not through the TLR9-Nod2 signaling pathway. TLR2 activation with LTA induced 5-fold more IL-23 in comparison with TLR4 activation, however, the induction was not as robust as that observed with S. pneumoniae. Although Nod2 activation alone did not cause significant IL-23 production, Nod2 agonist in synergy with TLR2 ligand induced a significant increase in IL-23 production to the same extent as that observed with S. pneumoniae infection. Blockade of TLR2 with anti-TLR2 antibody significantly attenuated S. pneumoniae induced IL-23 production, suggesting that TLR2 is a critical receptor required for the induction of IL-23 following S. pneumoniae infection. Activation of Nod2 signaling amplifies TLR2 signaling response to mimic the response observed with S. pneumoniae. Our previous studies showed that morphine treatment disrupts IL-23 production following S. pneumoniae lung infection, leading to delayed initiation of innate immune responses (5). In this study, we further show that morphine disruption of IL-23 production is mediated through modulation of both TLR2 and Nod2 signaling pathways.
MyD88 is the central signaling adaptor that mediates cellular stimulation downstream to most TLRs, with the exception of TLR3 (28). TLR2 synergizes with both TLR4 and TLR9 to induce the MyD88-dependent splenic cytokine and chemokine response to S. pneumoniae (11). Our previous studies have shown that IL-23 production in response to S. pneumoniae infection requires MyD88 and that morphine treatment impairs MyD88-dependent IL-23 production (5). IRAK1/4 plays an essential role in TLRs-MyD88-mediated signaling. Inherited human IRAK4 deficiency leads to recurrent and invasive pneumococcal disease (29). In the present study, we elucidated a key role for MyD88 downstream mediators such as IRAK1 and -4 following S. pneumoniae infection induced IL-23 production in DCs. For this purpose, DCs were pretreated with IRAK1/4 inhibitor before cell infection with S. pneumoniae. We found that pretreatment of DCs with IRAK1/4 inhibitor reduced IL-23 production and completely attenuated the inhibitory actions of morphine on IL-23 production following infection. Thus, our findings reveal that MyD88-dependent IRAK1/4 pathways are crucial for S. pneumoniae induced IL-23 production, and morphine treatment impairs S. pneumoniae induced IL-23 production through MyD88-IRAK1/4-dependent signaling.
Previous studies have shown that regulatory elements for NK-κB, IRF3, ATF2, and SMAD (Sma- and Mad-related protein)-3 at the IL-23p19 promoter are essential for promoter activity (30). Previously, we have demonstrated that morphine treatment decreases S. pneumoniae-induced phosphorylation of IRF3, ATF2, and NF-κBp65, suggesting that inhibition of IRF3, ATF2, and NF-κB signaling pathways is a critical mechanism by which morphine inhibits IL-23 production in S. pneumoniae-infected DCs (5). Streptococcal cell walls and cellular components such as LTA, pneumolysin, MDP, and CpG oligonucleotide that serve as TLR or Nod ligands can induce inflammatory mediator production (31, 32). In the present study, we used TLR ligands to examine the contribution of signaling by TLRs in the activation of IRF3 and ATF2 in DCs following S. pneumoniae infection. Activations of IRF3 and ATF2 were induced by infection with S. pneumoniae and stimulation with LTA (TLR2 ligand) plus MDP (Nod2 ligand) or LTA alone. Morphine treatment significantly decreased the activation of IRF3 and ATF2. Our results indicate that morphine modulate IL-23 production through both TLR2/Nod2-IRF3 and TLR2/Nod2-ATF2 signaling pathways.
Chronic morphine treatment up-regulates adenynyl cyclase activation both in neuron cells and in macrophages (33, 34). In previous study, we show elevation of intracellular cAMP levels following chronic morphine treatment in both naïve and activated T cells (14). In the present study, we show that treatment of DCs with chronic morphine also results in a significant increase in intracellular cAMP. To investigate the role of cAMP in morphine-induced inhibition of IL-23 production, the effect of forskolin, a cAMP-elevating agent, was investigated in S. pneumoniae-infected DCs. Our results show that pretreatment of DCs with forskolin decreased S. pneumoniae-induced IL-23 production and further inhibited morphine's inhibitory effect on IL-23 production. This suggests that morphine-induced increase in intracellular cAMP may be a potential downstream mechanism following μ-opioid receptor ligation that converges with TLR2/Nod2 signaling to inhibit S. pneumoniae infection induced IL-23 production.
In summary, we demonstrate that DCs are potent producers of IL-23 in response to S. pneumoniae infection. IL-23 production following S. pneumoniae infection is a downstream event that depends on activations of TLR2/Nod2, leading to MyD88 recruitment and IRAK1/4 activation. Morphine treatment impairs S. pneumoniae induced IL-23 production by modulating the MyD88-IRAK1/4 dependent TLR2 and Nod2 signaling in DCs. This study may define specific pathways and effector cells that are involved in the diminished innate immune response following S. pneumoniae infection and identify innate immunity-based therapies and prevention strategies for S. pneumoniae-infected opioid drug abusers.
Acknowledgments
We thank Dr. Youhai H. Chen (University of Pennsylvania) for IL-23 promoter plasmid, Dr. Johann S. Braun (Departments of Neurology, Cell Biology, and Neurobiology, Charité Universitaetsmedizin Berlin, Berlin, Germany) for E. coli which expressed full-length rPLY.
This work was supported, in whole or in part, by National Institutes of Health Grants R03 DA023353 (to J. W.) and R01 DA12104, K02 DA015349, R01 DA022935, and P50 DA11806 (to S. R.). This work was also supported by funds from the Minneapolis Veterans Affairs Medical Center (to R. B.).
- TLR
- Toll-like-receptor
- BMDC
- bone marrow-derived dendritic cell
- BMDM
- bone marrow derived macrophage
- rPLY
- recombinant pneumolysin
- IRAK
- interleukin-1 receptor-associated kinase
- IRF3
- interferon regulatory factor 3
- ATF2
- activating transcription factor 2
- Nod2
- nucleotide-binding oligomerization domain containing 2
- MDP
- muramyldipeptide
- LTA
- lipoteichoic acid
- m.o.i.
- multiplicity of infection.
REFERENCES
- 1. Alifrangis C., Thompson P., Thwaites G., Churchill D. (2006) Int. J. STD. AIDS. 17, 779–780 [DOI] [PubMed] [Google Scholar]
- 2. Garrelts J. C., Herrington A. M. (1996) Pharmacoeconomics 10, 36–58 [DOI] [PubMed] [Google Scholar]
- 3. Wang J., Barke R. A., Charboneau R., Roy S. (2005) J. Immunol. 174, 426–434 [DOI] [PubMed] [Google Scholar]
- 4. Wang J., Barke R. A., Charboneau R., Schwendener R., Roy S. (2008) J. Immunol. 180, 3594–3600 [DOI] [PubMed] [Google Scholar]
- 5. Ma J., Wang J., Wan J., Charboneau R., Chang Y., Barke R. A., Roy S. (2010) Infect. Immun. 78, 830–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sabroe I., Parker L. C., Dower S. K., Whyte M. K. (2008) J. Pathol. 214, 126–135 [DOI] [PubMed] [Google Scholar]
- 7. Srivastava A., Henneke P., Visintin A., Morse S. C., Martin V., Watkins C., Paton J. C., Wessels M. R., Golenbock D. T., Malley R. (2005) Infect. Immun. 73, 6479–6487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dessing M. C., Florquin S., Paton J. C., van der Poll T. (2008) Cell Microbiol. 10, 237–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Klein M., Obermaier B., Angele B., Pfister H. W., Wagner H., Koedel U., Kirschning C. J. (2008) J. Infect. Dis. 198, 1028–1036 [DOI] [PubMed] [Google Scholar]
- 10. Albiger B., Dahlberg S., Sandgren A., Wartha F., Beiter K., Katsuragi H., Akira S., Normark S., Henriques-Normark B. (2007) Cell Microbiol. 9, 633–644 [DOI] [PubMed] [Google Scholar]
- 11. Lee K. S., Scanga C. A., Bachelder E. M., Chen Q., Snapper C. M. (2007) Cell Immunol. 245, 103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Opitz B., Püschel A., Schmeck B., Hocke A. C., Rosseau S., Hammerschmidt S., Schumann R. R., Suttorp N., Hippenstiel S. (2004) J. Biol. Chem. 279, 36426–36432 [DOI] [PubMed] [Google Scholar]
- 13. Zola T. A., Lysenko E. S., Weiser J. N. (2008) J. Immunol. 181, 7909–7916 [DOI] [PubMed] [Google Scholar]
- 14. Wang J., Barke R. A., Charboneau R., Loh H. H., Roy S. (2003) J. Biol. Chem. 278, 37622–37631 [DOI] [PubMed] [Google Scholar]
- 15. Braun J. S., Novak R., Gao G., Murray P. J., Shenep J. L. (1999) Infect. Immun. 67, 3750–3756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carmody R. J., Ruan Q., Liou H. C., Chen Y. H. (2007) J. Immunol. 178, 186–191 [DOI] [PubMed] [Google Scholar]
- 17. Riol-Blanco L., Lazarevic V., Awasthi A., Mitsdoerffer M., Wilson B. S., Croxford A., Waisman A., Kuchroo V. K., Glimcher L. H., Oukka M. (2010) J. Immunol. 184, 1710–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dighe S. V., Madia P. A., Sirohi S., Yoburn B. C. (2009) Pharmacol. Biochem. Behav. 92, 537–542 [DOI] [PubMed] [Google Scholar]
- 19. Osikowicz M., Mika J., Makuch W., Przewlocka B. (2008) Pain 139, 117–126 [DOI] [PubMed] [Google Scholar]
- 20. Tiseo P. J., Thaler H. T., Lapin J., Inturrisi C. E., Portenoy R. K., Foley K. M. (1995) Pain 61, 47–54 [DOI] [PubMed] [Google Scholar]
- 21. Knapp S., Wieland C. W., van 't Veer C., Takeuchi O., Akira S., Florquin S., van der Poll T. (2004) J. Immunol. 172, 3132–3138 [DOI] [PubMed] [Google Scholar]
- 22. Branger J., Knapp S., Weijer S., Leemans J. C., Pater J. M., Speelman P., Florquin S., van der Poll T. (2004) Infect. Immun. 72, 788–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moreira L. O., El Kasmi K. C., Smith A. M., Finkelstein D., Fillon S., Kim Y. G., Núñez G., Tuomanen E., Murray P. J. (2008) Cell Microbiol. 10, 2067–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang H., Lin C. H., Ma G., Orr M., Baffi M. O., Wathelet M. G. (2002) Eur. J. Biochem. 269, 6142–6151 [DOI] [PubMed] [Google Scholar]
- 25. Reimer T., Schweizer M., Jungi T. W. (2007) J. Immunol. 179, 1166–1177 [DOI] [PubMed] [Google Scholar]
- 26. Yoshimura A., Lien E., Ingalls R. R., Tuomanen E., Dziarski R., Golenbock D. (1999) J. Immunol. 163, 1–5 [PubMed] [Google Scholar]
- 27. Malley R., Henneke P., Morse S. C., Cieslewicz M. J., Lipsitch M., Thompson C. M., Kurt-Jones E., Paton J. C., Wessels M. R., Golenbock D. T. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 1966–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yamamoto M., Takeda K., Akira S. (2004) Mol. Immunol. 40, 861–868 [DOI] [PubMed] [Google Scholar]
- 29. Picard C., von Bernuth H., Ku C. L., Yang K., Puel A., Casanova J. L. (2007) Immunol. Res. 38, 347–352 [DOI] [PubMed] [Google Scholar]
- 30. Al-Salleeh F., Petro T. M. (2008) J. Immunol. 181, 4523–4533 [DOI] [PubMed] [Google Scholar]
- 31. Henneke P., Dramsi S., Mancuso G., Chraibi K., Pellegrini E., Theilacker C., Hübner J., Santos-Sierra S., Teti G., Golenbock D. T., Poyart C., Trieu-Cuot P. (2008) J. Immunol. 180, 6149–6158 [DOI] [PubMed] [Google Scholar]
- 32. Li X., Bradford B. U., Dalldorf F., Goyert S. M., Stimpson S. A., Thurman R. G., Makarov S. S. (2004) Arthritis. Res. Ther. 6, R273–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kelschenbach J., Ninkovic J., Wang J., Krishnan A., Charboneau R., Barke R. A., Roy S. (2008) J. Immunol. 180, 3670–3679 [DOI] [PubMed] [Google Scholar]
- 34. Börner C., Warnick B., Smida M., Hartig R., Lindquist J. A., Schraven B., Höllt V., Kraus J. (2009) J. Immunol. 183, 882–889 [DOI] [PubMed] [Google Scholar]