Abstract
Heat shock-binding protein HspBP1 is a member of the Hsp70 co-chaperone family. The interaction between HspBP1 and the ATPase domain of the major heat shock protein Hsp70 up-regulates nucleotide exchange and reduces the affinity between Hsp70 and the peptide in its peptide-binding site. Previously we have shown that Tag7 (also known as peptidoglycan recognition protein PGRP-S), an innate immunity protein, interacts with Hsp70 to form a stable Tag7-Hsp70 complex with cytotoxic activity against some tumor cell lines. This complex can be produced in cytotoxic lymphocytes and released during interaction with tumor cells. Here the effect of HspBP1 on the cytotoxic activity of the Tag7-Hsp70 complex was examined. HspBP1 could bind not only to Hsp70, but also to Tag7. This interaction eliminated the cytotoxic activity of Tag7-Hsp70 complex and decreased the ATP concentration required to dissociate Tag7 from the peptide-binding site of Hsp70. Moreover, HspBP1 inhibited the cytotoxic activity of the Tag7-Hsp70 complex secreted by lymphocytes. HspBP1 was detected in cytotoxic CD8+ lymphocytes. This protein was released simultaneously with Tag7-Hsp70 during interaction of these lymphocytes with tumor cells. The simultaneous secretion of the cytotoxic complex with its inhibitor could be a mechanism protecting normal cells from the cytotoxic effect of this complex.
Keywords: Cell Death, Heat Shock Protein, Immunology, Lymphocyte, Protein-Protein Interactions, HspBP1, Tag7 (PGRP-S)
Introduction
The multifunctional protein heat shock-binding protein 70 (Hsp70)2 is a member of the large stress-induced protein family. The main function of these proteins is to protect cells against damage produced by various stress factors (1). Hsp70 is an ATP-dependent chaperone, which promotes folding of nascent proteins or refolding of the proteins that were partially denatured after exposure of cells in an unfavorable environment (1–3). In addition, Hsp70 is involved in assembly of multiprotein complexes and protein translocation across the plasma membrane (4).
In the chaperone reaction, the Hsp70 peptide-binding domain binds to the misfolded protein. After restoration of folding, the peptide substrate is released from the active site of Hsp70. The N-terminal ATP-binding domain is involved in the control of binding of the protein substrate. The ATP-carrying form of Hsp70 has low affinity for substrate proteins and a high rate of nucleotide exchange. The ATPase activity of the N-terminal domain results in an ADP version of the chaperone with high affinity for the substrate (5).
Research during the last decade has revealed a group of proteins called co-chaperones, which are involved in the control of ATPase activity of Hsp70, thereby modifying its affinity for protein substrates (3, 6, 7). One of these proteins, Hsp70-binding protein I (HspBP1), was initially identified as an Hsp70-binding protein inhibiting its chaperone activity and was shown to bind to the ATP domain of Hps70 and inhibit its ATPase activity (8). HspBP1 can accelerate nucleotide exchange of the ATPase domain, thereby affecting the binding of Hsp70 to substrate proteins (9, 10).
In addition to the basic chaperone function of Hsp70, recent literature has focused on the involvement of this protein in the control of tumor cell growth. These studies have shown that Hsp70 can produce stable complexes with the proteins of the apoptotic signaling pathway, including JNK (11, 12), AIF (13, 14), apoptotic protein activation factor 1 (15), and granzyme B (16). This activity is mainly directed to promote survival of the tumor cells.
Our previous studies revealed that Hsp70 interacted with Tag7; the gene for this protein was discovered in our laboratory (17–19). This evolutionarily conserved 20-kDa protein can recognize peptidoglycan and Gram-positive bacteria and is considered a component of the innate immunity system (20–24). In this role, Hsp70 is involved in antitumor defense; it forms a 1:1 complex with Tag7. The Tag7-Hsp70 complex induces apoptosis in some cultured tumor cells and markedly retards tumor growth in mice (17, 25). Furthermore, the Tag7-Hsp70 complex is produced in lymphokine-activated killer cells (LAK) of the CD8+ type and secreted by these lymphocytes upon contact with certain tumor-derived cells, being cytotoxic for other tumor cell lines.
Examination of the mechanism producing a Tag7-Hsp70 complex has revealed that Tag7 binds to the peptide-binding domain of Hsp70 and interacts with its 14-residue peptide first described by Multhoff et al. (26). It has also been found that the presence of the ATP-binding domain is prerequisite to assembly of a stable complex with maximal cytotoxic activity (17).
Logically, we hypothesized that HspBP1 could modulate the cytotoxic activity of Tag7-Hsp70 complex. The results reported here demonstrate that HspBP1 can interact not only with Hsp70 but also with Tag7. HspBP1 is released by CD8+ lymphocytes and down-regulates the cytotoxic activity of recombinant and lymphocytic Tag7-Hsp70 complexes.
EXPERIMENTAL PROCEDURES
Cells
Mouse L929 fibroblasts and human K562 erythroblastoid cells were cultured in RPMI 1640 supplemented with 2 mm l-glutamine and 10% fetal calf serum (Invitrogen, Carlsbad, CA). Human peripheral blood was obtained from healthy volunteers at the Cancer Research Center in Moscow to prepare LAK cultures. Mononuclear cells were isolated by Ficoll-Hypaque gradient centrifugation and cultivated for 6 days with 1000 units/ml of recombinant interleukin-2 (Sigma). CD8+ lymphocytes were isolated using a magnetic bead isolation kit (Dynal Biotech ASA, Oslo, Norway) by the manufacturer's protocols.
Proteins and Complexes
The human 70-kDa heat shock protein 1A (Hsp70, GenBankTM accession number NM_005345) was subcloned into pQE-30 (Qiagen) and expressed in Escherichia coli M15[pREP4] (Qiagen). Human HspBP1 (GenBankTM accession number NM_012267) and core domain of HspBP1 (amino acids 84–359) were subcloned into pET-28a (Novagen, Madison, WI) and expressed in E. coli as described by Raynes and Guerriero (8). The proteins were purified on Ni-NTA-agarose (Qiagen) as recommended by the manufacturer. Mouse rTag7 produced in yeast was kindly provided by Dr. S. V. Benevolensky. Unless otherwise specified, the standard incubation for formation of protein complexes was 30 min at 37 °C in PBS at pH 7.4. Secreted proteins were obtained by incubation of lymphocytes with K562 cells (100:1) for 18 h in serum-free RPMI 1640. The antibodies to HspBP1 were added at 10 μg/ml for inhibition experiments. In the control experiments, IgG from sheep serum were added at the same concentration. Cells were removed by centrifugation and the conditioned medium was tested for the presence of Tag7 and HspBP1 by enzyme-linked immunosorbent assays (ELISA) and in cytotoxic assays.
Antibodies, Immunoadsorption, and Immunoblotting
Anti-HspBP1 antibodies were raised in sheep as described by Raynes et al. (27). Rabbit anti-HspBP1 were from Delta Biolabs, Campbell, CA. Anti-human Tag7 antibodies were raised in mice by injection of electrophoretically purified human Tag7 (produced as in Ref. 28); mouse serum IgG were purified and used for immunoblotting at 1:20,000 dilution.
Anti-Tag7 resin and anti-HspBP1 resin were made by coupling antibodies to CNBr-activated Sepharose 4B (Amersham Biosciences) using the manufacturer's protocol. HspBP1 and Tag7 were preincubated in equimolar concentrations (10 nm) for 1 h at room temperature and passed through the columns. The columns were extensively washed with PBS plus 0.5 m NaCl, PBS, and eluted with 0.25 m triethylamine (TEA, Sigma) pH 12.
Eluted proteins were resolved by 10–15% SDS-PAGE. Proteins were transferred to PVDF membranes (Amersham Biosciences) by semidry transfer, blocked, and incubated with anti-Tag7 antibodies or sheep anti-HspBP1. Blots were incubated with horseradish peroxidase-conjugated secondary antibodies (Amersham Biosciences rabbit anti-mouse, 1:40,000 or donkey anti-sheep, 1:20,000). ECL Plus (Amersham Biosciences) was used for visualization according to the manufacturer's instructions.
The concentration of HspBP1 in the conditioned medium was determined using enzyme-linked immunosorbent assays (ELISA) as described by Raynes et al. (27). The content of Tag7 in the samples was measured by competitive EIA. Recombinant Tag7 (200 ng/ml) was adsorbed in wells of a 96-well plate. The reaction was carried out by adding 50 μl of polyclonal mouse anti-Tag7 antibodies (dilution 1:10,000) to 50 μl of analyzed fluid. Recombinant Tag7 at 1–100 ng/ml was used for plotting the calibration curve. The TMB liquid substrate system was from Sigma.
Microscopy
CD3+CD8+ lymphocytes were washed twice with PBS; fixation and permeabilization were carried out using FIX & PERM kit (Caltag Laboratories, Burlingame, CA). To visualize intracellular HspBP1, cells were stained with sheep anti-HspBP1 antibodies followed by Alexa488-conjugated donkey anti-sheep IgG (Invitrogen). After washing with PBS and 50 mm NH4Cl, specimens were mounted in ProLong Gold antifade reagent (Invitrogen). Fluorescence images were obtained with a Leica TCS SP2 confocal microscope, analyzed with Leica confocal software, and prepared in Photoshop CE (Adobe Systems).
Cytotoxicity
Proteins were preincubated at equimolar concentrations (10 nm) in various combinations and order. Cytotoxicity was measured in L929 cells cultured in 96-well plates at a density of 3 × 104 cells/well. Proteins were added (200 μl) and incubated for 20 h at 37 °C. Cells were stained with Trypan Blue, and coded samples were counted under the microscope; at least 100 cells were scored for each group (17). Cytotoxicity was calculated as Equation 1,
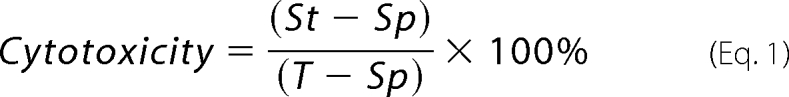 |
where St is the number of stained cells; Sp, spontaneously stained cells; T, total cells. In some cases cell death was determined with the CytoTox 96 Assay kit (Promega, Madison WI); the discrepancy between the two assays never exceeded 7%. Tag7, Hsp70, and HspBP1 alone were used as controls, and the cytotoxicity of each did not exceed 3%.
Flow Cytometry
To test membrane-bound extracellular HspBP1, lymphocytes were stained with R-Phycoerytrin-labeled monoclonal antibodies to CD8 (Beckman Coulter Inc.) followed by staining with sheep anti-HspBP1 antibodies and by FITC-conjugated donkey anti-sheep IgG (Sigma). To test intracellular HspBP1, CD8-stained lymphocytes were fixed and permeabilized using the FIX & PERM kit (Caltag Laboratories) and stained as above. Washed cell were fixed with 1% paraformaldehyde (Sigma). The samples (at least 104 cells each) were analyzed with a Cytomics FC500MPL flow cytometer (Beckman Coulter Inc.) in the logarithmic channel of fluorescence. The data were processed with CXP Software (version 2.2, Beckman Coulter, Inc.).
Mass Spectrometry
10 μg of rHspBP1 was digested with 0.25 μg of trypsin (Sigma) in 20 mm Tris-HCl, pH 6.8 for 1 h at 37 °C. The reaction was stopped by adding 0.25 μg of soybean trypsin inhibitor. The peptides were loaded on a Tag7-Sepharose column, extensively washed with PBS containing 0.5 m NaCl followed by PBS, and eluted with 0.25 m TEA, pH 12. The eluted peptides were analyzed by MALDI-TOF mass spectrometry, and selected mass values from the spectra were used to search the protein data base Swiss-Prot.
RESULTS
HspBP1 Interacts with Tag7
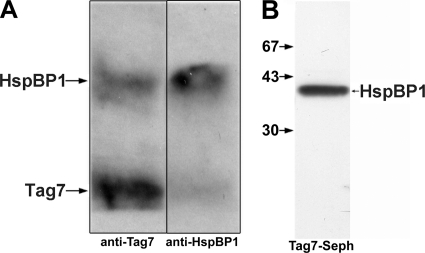
Before analyzing the effect of HspBP1 on the cytotoxic activity of the Tag7-Hsp70 complex, we had to test whether this co-chaperone could interact with both protein components of this complex. Binding of HspBP1 to Hsp70 was clearly demonstrated previously (8, 10). Here we examined the ability of HspBP1 and Tag7 to interact with each other. First, specific immunoadsorbent columns with antibodies against HspBP1 and Tag-7 were prepared and shown to be protein-specific (supplemental Fig. S1). Both proteins were co-incubated in equimolar concentrations (10−9 m), the mixtures were applied to the columns and the bound fractions were analyzed with SDS-PAGE. Tag7 and HspBP1 were bound to each column (Fig. 1A), indicating formation of a complex. This interaction was confirmed by HspBP1 binding to the column with immobilized Tag7 (Fig. 1B). HspBP1 was subjected to tryptic hydrolysis and the resulting peptides were applied to the column with immobilized Tag7. The bound peptides were eluted and analyzed by MALDI mass spectrometry. Tag7 was bound to the peptide corresponding to residues 246–265 of the HspBP1 amino acid sequence. These results establish that HspBP1 interacts with Tag7, and identify the specific interaction site on HspBP1.
FIGURE 1.
HspBP1 interacts with Tag7. A, Tag7 and HspBP1 were incubated for 30 min in PBS at 10−9 m at room temperature, then applied to anti-Tag7 and anti-HspBP1-Sepharose columns, and washed with PBS plus 0.5 m NaCl, with PBS, and water, followed by elution with 0.25 M TEA (pH 12.5). All eluates were dried, dissolved in a minimal volume of the SDS-PAGE sample buffer, and analyzed on 12% gels. After blotting onto nitrocellulose, the proteins were visualized using anti-Tag7 and anti-HspBP1 antibodies followed by either anti-mouse (Tag-7) or anti-sheep (HspBP1) antibodies and ECL Plus. B, HspBP1 was applied to Tag7-Sepharose, following the same procedures as in A. Western blot was visualized using anti-HspBP1 antibodies and ECL Plus.
HspBP1 Blocks the Cytotoxic Activity of the Tag7-Hsp70 Complex
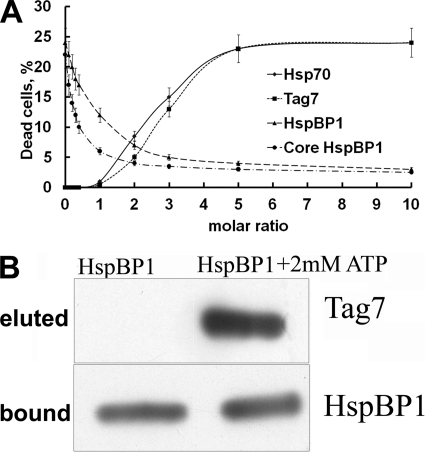
The interaction of HspBP1 with Hsp70 and Tag7 was examined for effects on cytotoxicity using the established assay for cytotoxic activity of the binary Tag7-Hsp70 complex (17). None of these proteins alone had a toxic effect on L-929 cells (supplemental Fig. S2). Moreover, cytotoxicity did not appear after co-incubation of all three proteins in equimolar concentrations (supplemental Fig. S2). Complexes were produced by incubating equimolar mixtures of the following protein pairs: [Tag7+Hsp70], [Hsp70+HspBP1], and [Tag7+HspBP1]. An aliquot of each complex was incubated with increasing amounts of the third component (HspBP1, Tag7, or Hsp70). All samples were analyzed for cytotoxic activity (Fig. 2A). One protein pair, Tag7+Hsp70, at 10−10 M demonstrated full activity (25% death of l-929 cells), while Hsp70+HspBP1 and Tag7+HspBP1 pairs showed no cytotoxicity. Addition of HspBP1 to the Tag7-Hsp70 complex resulted in a drop of cytotoxicity. An equimolar concentration of HspBP1 decreased the maximal cytotoxic activity by half, while a 5-fold greater concentration of this protein eliminated it completely. These data were corroborated by addition of the core domain of HspBP1 (amino acids 84–359) to Tag7+Hsp70. This truncated protein exhibited the properties of the full-length protein. Titration of inactive pairs with Tag7 and Hsp70 resulted in an increase in cytotoxicity that was half-maximal at a 2.5-fold excess of the titrant, while a 5-fold excess of the complementary protein resulted in nearly complete restoration of cytotoxicity. These results suggest competitive replacement of HspBP1 in an inactive pair by the active component (Tag7 or Hsp70), leading to formation of the cytotoxic Tag7-Hsp70 complex.
FIGURE 2.
HspBP1 inactivates the Tag7-Hsp70 complex. A, cytotoxic assay. Protein pairs [Tag7+HspBP1 (♦), Hsp70+HspBP1 (■), Tag7+Hsp70 (▴), Tag7+Hsp70 (●)] were incubated at equal molar concentrations (10−10 m) for 30 min at 20 °C under sterile conditions. Increasing amounts of the third protein were added to each pair, Hsp70 (♦), Tag7 (■), HspBP1(▴), or core fragment of HspBP1 (●). The mixtures was incubated for another 30 min and assayed for cytoxicity against L929 cells. The mean percentage of cell killing (± S.E., n = 5) by the protein mixture (final dilution 1:10) is plotted against increasing concentrations of the third added protein. B, dissociation of Tag7 from Tag7-Hsp70 complex by HspBP1. Tag7-Hsp70 complex was first applied to anti-Tag7-Sepharose to remove the free Hsp70. The bound complex was eluted with 0.25 M TEA (pH 12.5), dialyzed against PBS (pH 7.5) and applied to anti-Hsp70 Sepharose. The column was washed with PBS containing 0.5 m NaCl, with PBS, and H2O. HspBP1 or HspBP1 with 2 mm ATP in PBS were applied to the loaded material (upper lane indicates eluted proteins). Proteins remaining on the column were eluted with 0.25 M TEA (pH 12.5), separated by SDS-PAGE, blotted onto nitrocellulose, and visualized using anti-Tag7 and anti-HspBP1 antibodies as described under “Experimental Procedures.”
It can be hypothesized that the dramatic loss of cytotoxic activity of the Tag7-Hsp70 complex in the presence of HspBP1 is related to dissociation of Tag7 from the complex as a result of structural alteration of the peptide-binding domain. To check this point, we immobilized the Tag7-Hsp70 complex to a specific anti-Hsp70 column (supplemental Fig. S1) and processed this column with a 5-fold excess of HspBP1 (relative to the concentration of the complex). The bound and free fractions were assayed by SDS-PAGE. Fig. 2B (left lane) shows that under conditions that result in a complete loss of cytotoxic activity, HspBP1 is bound to the column but cannot dissociate Tag7 from the complex. No traces of Tag7 were detected in the fractions not bound to the column.
The effect of ATP on the three-protein complex was examined. It has been established that HspBP1 can accelerate nucleotide exchange and decrease the affinity of protein binding at the peptide-binding site (10). A 5-fold excess of HspBP1 and 2 mM ATP were added to the complex bound on the anti-Hsp70 column. Tag7 was released from the column (Fig. 2B, right lane). In control experiments, addition of 5 mm ATP without HspBP1 did not induce dissociation of Tag7 from the complex (supplemental Fig. S3). As reported previously, 50 mm ATP produced partial dissociation of the Tag7-Hsp70 complex (supplemental Fig. S3, Ref. 17). Elimination of the cytotoxic activity of Tag7-Hsp70 under the action of HspBP1 without ATP can be explained by binding of this inhibitor to the proteins of the complex (Fig. 2, A and B). This interaction can modify the structure of the active binary complex. Gel filtration of the active complex [Tag7-Hsp70] with a 5-fold excess of HspBP1 showed that all three proteins were aggregated, resulting in a broad peak with a maximum at 500 kDa (data not shown).
HspBP1 Down-regulates the Cytotoxicity of Tag7-Hsp70 Secreted by LAK Cells
Previously we established that after incubation of LAK cells with HLA-negative tumor cells, the conditioned medium acquired cytotoxic activity against other tumor cells that was completely inhibited by either anti-Tag7 or anti-Hsp70 antibodies (Ref. 17, supplemental Fig. S4). Under these conditions the lymphocytes secreted Tag7 complexed with Hsp70. These experiments did not detect free (not Hsp70-bound) Tag7 in the conditioned medium.3 This fact makes it possible to assess the concentration of the secreted Tag7-Hsp70 complex by measuring the concentration of Tag7 protein. The concentrations of Tag7 in the conditioned medium were determined by immunoenzyme assays. The time course of Tag7 during incubation of LAK cells with K562cells was measured for 30 h. Table 1 shows that appreciable amounts of Tag7 appeared as early as within 3 h, and gradually increased to a maximum at 30 h.
TABLE 1.
Tag7 and HspBP1 secretion by LAK cells during interaction with K562
PBMC were incubated with IL2 (1000 units/ml) for 6 days. CD8+ lymphocytes were obtained with commercially available magnetic bead isolation kits and were incubated with K562 cells for 30 h (100:1) in serum-free RPMI 1640 at 37 °C. Cells were removed by centrifugation and the conditioned medium was tested for the presence of Tag7 and HspBP1. Concentrations of Tag7 and HspBP1 in the condition medium were determined with an immunoenzyme assay.
| Time | Conc.Tag7 | Conc.HspBP1 | HspBP1/Tag7 ratio |
|---|---|---|---|
| h | nm | nm | |
| 1 | 0.055 ± 0.006 | 1.84 ± 0.2 | 33 |
| 3 | 0.095 ± 0.01 | 2.5 ± 0.2 | 26 |
| 6 | 0.15 ± 0.01 | 4.1 ± 0.3 | 27 |
| 18 | 0.34 ± 0.03 | 0.8 ± 0.1 | 2.3 |
| 30 | 0.4 ± 0.03 | 1.05 ± 0.1 | 2.6 |
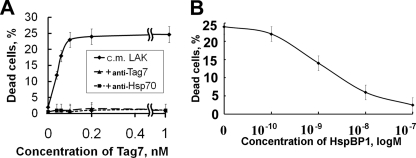
The cytotoxic activity of the conditioned medium was assessed on L-929 cells, which were most susceptible to the action of the Tag7-Hsp70 complex (Ref. 17, supplemental Fig. S4). Maximal cytotoxicity in the conditioned medium was observed at a Tag7 concentration of 10−10 m, and further elevation of Tag7 concentration did not increase the percentage of dead cells (Fig. 3A). Addition of HspBP1 to the conditioned medium inhibited the cytotoxicity of the Tag7-Hsp70 complex. However, this reaction developed at higher concentrations of HspBP1 as compared with the experiments with a Tag7-Hsp70 complex made of recombinant proteins. Half-maximal cytotoxicity was achieved with a 10-fold excess of HspBP1 (relative to the Tag7-Hsp70 complex, Fig. 3B), while a 5-fold excess of HspBP1 was sufficient to completely eliminate the cytotoxic activity of the recombinant Tag7-Hsp70 complex (Fig. 2A).
FIGURE 3.
Inhibition of LAK-released cytotoxic activity. A, cytotoxic activity of conditioned media from LAK cells (♦), with 100 nm anti-Tag7 (▴) or anti-Hsp70 antibodies (■). Conditioned media were concentrated using Centricons (Millipore) and dissolved by adding RPMI medium to achieve a needed concentration of Tag7. B, inhibition of cytotoxic activity of conditioned medium from LAK cells by addition of recombinant HspBP1.
HspBP1 Is Present in CD8+ Cytotoxic Lymphocytes
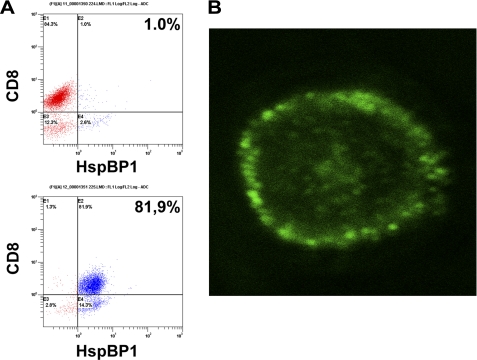
According to our preliminary data, only CD8+ lymphocytes in the cultured LAK cells can secrete the Tag7-Hsp70 complex during contact with tumor cells.4 Therefore, we isolated CD8+ cells from LAK cells and assayed HspBP1 in this fraction. Flow cytometry with specific antibodies revealed no HspBP1 on the surface of these cells. However, nearly 82% of CD8+ lymphocytes contained HspBP1 in their interior (Fig. 4A). This observation agrees with confocal microscopy analysis. The protein localized to granule-like structures situated at the cell periphery (Fig. 4B). When lymphocytes were co-incubated with K562 cells, the conditioned medium contained not only Tag7-Hsp70 complex, but also HspBP1 (Table 1). The concentration of HspBP1 in the conditioned medium was dependent on the duration of cell incubation. The maximum was at 6 h, which corresponded to an HspBP1/Tag7 ratio of 27. After incubation for 18–30 h, the HspBP1 concentration decreased, with a roughly 10-fold drop of the HspBP1/Tag7 ratio. It is noteworthy that although HspBP1 is available in K562 cells, they do not secrete this protein into the medium. In control experiments, lymphocytes not co-incubated with K562 cells released HspBP1 in significantly lower amounts, which remained constant ver 30 h of incubation (supplemental Table S1). Thus, secretion of HspBP1 was induced during the first hours of co-incubation of lymphocytes with K562 cells.
FIGURE 4.
HspBP1 is located under the lymphocyte surface. A, HspBP1 on the surface of LAK cells (top) and inside the cell (bottom) as determined by flow cytometry. B, confocal image of a permeabilized CD8+ cell stained with anti-HspBP1 antibodies. (10.0×/1.40 NA oil objective).
Tag7-Hsp70 Cytotoxic Activity Is Affected by the Presence of HspBP1 in Conditioned Medium
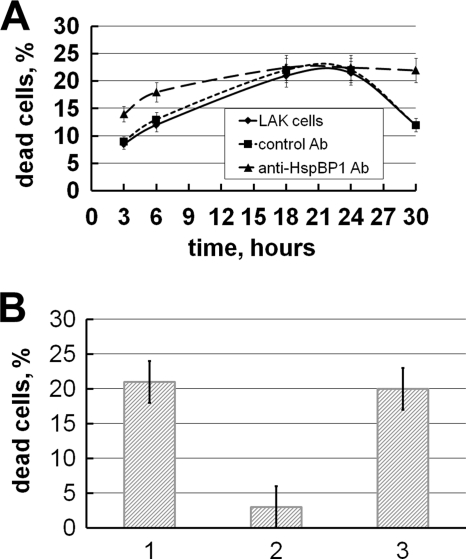
The effect of persistent presence of HspBP1 in the conditioned medium on the cytotoxic activity of the Tag7-Hsp70 complex secreted by lymphocytes was examined. As early as 6 h of incubation of lymphocytes with K562 cells, Tag7 concentration in the medium reached 0.15 nm, which was sufficient to evoke maximal cytotoxicity (Table 1). This concentration continued to increase for 30 h. Paradoxically, the plot of Tag7-Hsp70 cytotoxic activity against incubation time was bell-shaped (Fig. 5A), with maximal cytotoxicity observed in 18–24 h. Co-incubation of lymphocytes with K562 cells in the presence of antibodies against HspBP1 modified the shape of the plot. Cytotoxic activity increased in the interval from 3 to 18 h of incubation, and from 24 to 30 h and became gently sloping. Co-incubation of the cells with rabbit IgG produced no effect on the shape of the plot. This suggests that HspBP1 in the conditioned medium can suppress Tag7-Hsp70 cytotoxic activity. After 6 h of incubation, the concentration of HspBP1 in the conditioned medium was 27 times higher than that of Tag7, and the cytotoxity was inhibited although the concentration of Tag7 reached its maximal value. The results in Fig. 3B demonstrate that addition to Tag7-Hsp70 of a 27-fold excess of HspBP1 strongly inhibits the cytotoxic activity. After 18 h, the concentration of HspBp1 dramatically drops, which allows the Tag7-Hsp70 complex to show its maximal cytotoxic potential. However, after long storage (30 h) the cytotoxic activity of the Tag7-Hsp70 complex declines even at a low concentration of the inhibiting component (HspBP1) as a result of aggregation of the active complex. Previously we observed that after storage for 30 h, the conditioned medium of LAK cells completely lost its cytotoxic properties. The present data show that this medium contained 0.8 nm HspBP1 (Table 1). These data allow us to suggest that HspBP1 may by responsible for this effect, because storing the conditioned medium for 30 h in the presence of antibodies against HspBP1 spared its cytotoxic activity (Fig. 5B).
FIGURE 5.
HspBP1 antibody restores the cytotoxity of LAK cell supernatant. A, time-dependent cytotoxic activity of conditioned medium from LAK cells after incubation with K562 cells, (♦) conditioned medium alone, (▴) incubation with 100 nm anti-HspBP1 antibodies, and (■) incubation with 100 nm preimmune IgG. B, reactivation of cytoxicity of conditioned medium after 36 h. 1, Initial activity of conditioned medium of LAK cells; 2, activity after 30 h at +4 °C; 3, activity after 30 h in the presence of 100 nm anti-HspBP1 antibodies.
DISCUSSION
Our data show that co-chaperone HspBP1 interferes with the formation of active Tag7-Hsp70 as well as reduces the cytotoxicity of this complex, either formed of recombinant proteins in solution or released by cytotoxic lymphocytes. Moreover, we have found that HspBP1 is present in CD8+ lymphocytes and is released simultaneously with Tag7-Hsp70 during their contact with tumor cells.
Apparently, HspBP1 inhibits the cytotoxic activity of Tag7-Hsp70 because of its ability to interact with the constituent proteins of this complex: it can bind not only with Hsp70 but also with Tag7, the binding site of HspBP1 in both cases containing Lys-249 (10). According to our recent data (29), the Ca2+-binding protein Mts1 can also bind with Hsp70 and Tag7, this interaction resulting in Tag7 dissociation from the complex and, consequently, in complete loss of cytotoxic activity.
HspBP1 binding to Hsp70 stimulates nucleotide exchange, resulting in decreased affinity between Hsp70 and protein in the protein-binding site (10). HspBP1 binding to the Tag7-Hsp70 complex facilitated dissociation of Tag7 from the complex in the presence of ATP. In the absence of HspBP1, a 25-fold greater concentration of ATP was required to release Tag7 (17). Inhibition of cytotoxic activity of the Tag7-Hsp70 complex was also observed in the absence of ATP. HspBP1 bound to the complex fixed on the immunosorbent, but this did not lead to release of Tag7. Therefore, release of Tag7 is not required for inhibition of cytotoxicity. The data presented here suggest that HspBP1 binds to the Tag7-Hsp70 complex, modifies its structure, and eliminates its activity.
Hsp70 is a multifunctional protein with a variety of activities; therefore, the biological activity of HspBP1 is also multifaceted. For example, it has been shown that HspBP1 is involved in the control of proteasomal activity by inhibition of CHIP ubiquitin ligase when it is complexed with Hsp70 (30). Recently it has been found that binding of HspBP1 to Hsp70 prevents inhibition of cathepsin-dependent death of tumor cells. The cells with a high HspBP1/Hsp70 molar ratio possess greater sensitivity to antitumor drugs than the cells with low values of this ratio (31).
HspBP1 is also found in serum (27), which suggests its role as an extracellular protein. Following heat stress, HspBP1 co-localizes with Hsp70 in chromogranin A-positive granules and is secreted simultaneously with Hsp70 (32). In addition, HspBP1 binds to the brain tumor cell surface and can be internalized (33), providing further evidence for a function outside the cell. This report further widens the extracellular role of HspBP1 demonstrating its ability to inhibit the cytotoxicity of the lymphocytic Tag7-Hsp70 complex. Earlier we have found that the cytotoxic Tag7-Hsp70 complex is formed inside CD8+ lymphocytes and is released into the medium. This secretion occurs via the Golgi complex (17), and it seems that Fas-FasL interaction is essential for triggering this secretion. In cytotoxic activity, the secreted Tag7-Hsp70 complex does not differ from the one made of recombinant proteins in solution. Both complexes induce apoptotic death of the target cancer cells involving mitochondria and lysosomes. This fact allows us to suggest internalization of the cytotoxic Tag7-Hsp70 complex.3 Interestingly, HspBP1 was found inside cytotoxic CD8+ lymphocytes. This co-chaperone is released into the medium simultaneously with the Tag7-Hsp70 complex during the contact of lymphocytes with the tumor cells. However, taking into consideration the heterogeneity of the CD8+ subpopulation, it is not certain that the same lymphocytes secrete both the cytotoxic complex and its inhibitor. Further work is needed to clarify this point.
The concentration HspBP1 in the conditioned medium changed during cell incubation. The cytotoxic activity of the Tag7-Hsp70 complex was inhibited at a high concentration of HspBP1, but recovered when the HspBP1 concentration decreased. However, long-term incubation of Tag7-Hsp70 even at a low concentration of HspBP1 inactivated this complex. Thus, the cytotoxic activity of lymphocyte-secreted Tag7-Hsp70 complex was preserved for no longer than 30 h, which could be related to formation of inactive complexes or aggregates.
The biological importance of simultaneous release of the complex and its inhibitor cannot be explained unequivocally. This paradox is related to the old problem of how the cells secreting cytotoxic agents can protect themselves from the action of their own products (34, 35). It is common knowledge that blood serum contains both cytotoxic proteins and their inhibitors (36, 37). It cannot be excluded that accumulation of secreted Tag7-Hsp70 complex and its long-term presence in the blood could damage normal cells such as lymphocytes or endotheliocytes. As one can see from supplemental Fig. S4, 500× concentration of Tag7-Hsp70 complex can kill prime culture fibroblasts. In this case, the simultaneous release of HspBP1 may be a mechanism of cell protection from the cytotoxic effect of this complex.
This work was supported by the Russian Foundation for Basic Research, RAS program for Molecular and Cellular Biology, and International Centre for Genetic Engineering and Biotechnology (ICGEB).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
E. A. Dukhanina and D. V. Yashin, unpublished observations.
L. P. Sashchenko, unpublished observations.
- Hsp70
- heat shock-binding protein 70
- LAK
- lymphokine-activated killer cells
- HspBP
- heat shock-binding protein
- TEA
- triethylamine.
REFERENCES
- 1. Bukau B., Deuerling E., Pfund C., Craig E. A. (2000) Cell. 101, 119–122 [DOI] [PubMed] [Google Scholar]
- 2. Bukau B., Weissman J., Horwich A. (2006) Cell. 125, 443–451 [DOI] [PubMed] [Google Scholar]
- 3. DeLuca-Flaherty C., McKay D. B., Parham P., Hill B. L. (1990) Cell 62, 875–887 [DOI] [PubMed] [Google Scholar]
- 4. Bukau B., Horwich A. L. (1998) Cell. 92, 351–366 [DOI] [PubMed] [Google Scholar]
- 5. Greene L. E., Zinner R., Naficy S., Eisenberg E. (1995) J. Biol. Chem. 270, 2967–2973 [DOI] [PubMed] [Google Scholar]
- 6. Young J. C., Agashe V. R., Siegers K., Hartl F. U. (2004) Nat. Rev. Mol. Cell Biol. 5, 781–791 [DOI] [PubMed] [Google Scholar]
- 7. Mayer M. P., Bukau B. (2005) Cell Mol Life Sci. 62, 670–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Raynes D. A., Guerriero V., Jr. (1998) J. Biol. Chem. 273, 32883–32888 [DOI] [PubMed] [Google Scholar]
- 9. McLellan C. A., Raynes D. A., Guerriero V. (2003) J. Biol. Chem. 278, 19017–19022 [DOI] [PubMed] [Google Scholar]
- 10. Shomura Y., Dragovic Z., Chang H. C., Tzvetkov N., Young J. C., Brodsky J. L., Guerriero V., Hartl F. U., Bracher A. (2005) Mol. Cell 17, 367–379 [DOI] [PubMed] [Google Scholar]
- 11. Gabai V. L., Meriin A. B., Yaglom J. A., Volloch V. Z., Sherman M. Y. (1998) FEBS Lett. 438, 1–4 [DOI] [PubMed] [Google Scholar]
- 12. Park H. S., Lee J. S., Huh S. H., Seo J. S., Choi E. J. (2001) EMBO J. 20, 446–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ravagnan L., Gurbuxani S., Susin S. A., Maisse C., Daugas E., Zamzami N., Mak T., Jäättelä M., Penninger J. M., Garrido C., Kroemer G. (2001) Nat. Cell Biol. 3, 839–843 [DOI] [PubMed] [Google Scholar]
- 14. Gurbuxani S., Schmitt E., Cande C., Parcellier A., Hammann A., Daugas E., Kouranti I., Spahr C., Pance A., Kroemer G., Garrido C. (2003) Oncogene 22, 6669–6678 [DOI] [PubMed] [Google Scholar]
- 15. Beere H. M., Wolf B. B., Cain K., Mosser D. D., Mahboubi A., Kuwana T., Tailor P., Morimoto R. I., Cohen G. M., Green D. R. (2000) Nat. Cell Biol. 2, 469–475 [DOI] [PubMed] [Google Scholar]
- 16. Gross C., Koelch W., DeMaio A., Arispe N., Multhoff G. (2003) J. Biol. Chem. 278, 41173–41181 [DOI] [PubMed] [Google Scholar]
- 17. Sashchenko L. P., Dukhanina E. A., Yashin D. V., Shatalov Y. V., Romanova E. A., Korobko E. V., Demin A. V., Lukyanova T. I., Kabanova O. D., Khaidukov S. V., Kiselev S. L., Gabibov A. G., Gnuchev N. V., Georgiev G. P. (2004) J. Biol. Chem. 279, 2117–2124 [DOI] [PubMed] [Google Scholar]
- 18. Kustikova O. S., Kiselev S. L., Borodulina O. R., Senin V. M., Afanas'eva A. V., Kabishev A. A. (1996) Genetika. 32, 621–628 [PubMed] [Google Scholar]
- 19. Kiselev S. L., Kustikova O. S., Korobko E. V., Prokhortchouk E. B., Kabishev A. A., Lukanidin E. M., Georgiev G. P. (1998) J. Biol. Chem. 273, 18633–18639 [DOI] [PubMed] [Google Scholar]
- 20. Kang D., Liu G., Lundström A., Gelius E., Steiner H. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 10078–10082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dziarski R. (2004) Mol. Immunol. 40, 877–886 [DOI] [PubMed] [Google Scholar]
- 22. Michel T., Reichhart J. M., Hoffmann J. A., Royet J. (2001) Nature 414, 756–759 [DOI] [PubMed] [Google Scholar]
- 23. Dziarski R., Platt K. A., Gelius E., Steiner H., Gupta D. (2003) Blood 102, 689–697 [DOI] [PubMed] [Google Scholar]
- 24. Cho J. H., Fraser I. P., Fukase K., Kusumoto S., Fujimoto Y., Stahl G. L., Ezekowitz R. A. (2005) Blood 106, 2551–2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dukhanina E. A., Yashin D. V., Lukjanova T. I., Romanova E. A., Kabanova O. D., Shatalov Y. V., Sashchenko L. P., Gnuchev N. V. (2007) Dokl. Biol. Sci. 414, 246–248 [DOI] [PubMed] [Google Scholar]
- 26. Multhoff G., Pfister K., Gehrmann M., Hantschel M., Gross C., Hafner M., Hiddemann W. (2001) Cell Stress Chaperones 6, 337–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Raynes D. A., Thomson C. A., Stroster J., Newton T., Cuneo P., Guerriero V. (2006) J. Immunoassay Immunochem. 27, 251–264 [DOI] [PubMed] [Google Scholar]
- 28. Guan R., Wang Q., Sundberg E. J., Mariuzza R. A. (2005) J. Mol. Biol. 347, 683–691 [DOI] [PubMed] [Google Scholar]
- 29. Dukhanina E. A., Kabanova O. D., Lukyanova T. I., Shatalov Y. V., Yashin D. V., Romanova E. A., Gnuchev N. V., Galkin A. V., Georgiev G. P., Sashchenko L. P. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 13963–13967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alberti S., Böhse K., Arndt V., Schmitz A., Höhfeld J. (2004) Mol. Biol. Cell 15, 4003–4010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tanimura S., Hirano A. I., Hashizume J., Yasunaga M., Kawabata T., Ozaki K., Kohno M. (2007) J. Biol. Chem. 282, 35430–35439 [DOI] [PubMed] [Google Scholar]
- 32. Evdonin A., Kinev A., Tsupkina N., Guerriero V., Raynes D. A., Medvedeva N. (2009) Biol. Cell 101, 351–360 [DOI] [PubMed] [Google Scholar]
- 33. Graner M. W., Raynes D. A., Bigner D. D., Guerriero V. (2009) Cancer Science 100, 1871–1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Herberman R. B., Reynolds C. W., Ortaldo J. R. (1986) Annu. Rev. Immunol. 4, 651–680 [DOI] [PubMed] [Google Scholar]
- 35. Jiang S., Persechini P. M., Perussia B., Young J. D. (1989) J. Immunol. 143, 1453–1460 [PubMed] [Google Scholar]
- 36. Chertov O. Yu., Ermolaeva M. V., Satpaev D. K., Saschenko L. P., Kabanova O. D., Lukanidin E. M., Lukjianova T. I., Redchenko I. V., Blishchenko L. Yu., Gnuchev N. V. (1994) Immunol Lett. 42, 97–100 [DOI] [PubMed] [Google Scholar]
- 37. Karelin A. A., Phylippova M. M., Blishchenko E. Yu., Bovin N. V., Nasonov V. V., Shiyan S. D., Petrova E. E., Nesmeyanov V. A., Sashchenko L. P., Gnuchev N. V. (1994) Biochem. Mol Biol Int. 33, 73–80 [PubMed] [Google Scholar]