Abstract
Glucocorticoids (GCs) are known inhibitors of wound healing. In this study we report the novel finding that both keratinocytes in vitro and epidermis in vivo synthesize cortisol and how this synthesis regulates wound healing. We show that epidermis expresses enzymes essential for cortisol synthesis, including steroid 11 β-hydroxylase (CYP11B1), and an enzyme that controls negative feedback mechanism, 11β-hydroxysteroid dehydrogenase 2 (11βHSD2). We also found that cortisol synthesis in keratinocytes and skin can be stimulated by ACTH and inhibited by metyrapone (CYP11B1 enzyme inhibitor). Interestingly, IL-1β, the first epidermal signal of tissue injury, induces the expression of CYP11B1 and increases cortisol production by keratinocytes. Additionally, we found induction of CYP11B1 increased production of cortisol and activation of GR pathway during wound healing ex vivo and in vivo using human and porcine wound models, respectively. Conversely, inhibition of cortisol synthesis during wound healing increases IL-1β production, suggesting that cortisol synthesis in epidermis may serve as a local negative feedback to proinflammatory cytokines. Local GCs synthesis, therefore, may provide control of the initial proinflammatory response, preventing excessive inflammation upon tissue injury. Inhibition of GC synthesis accelerated wound closure in vivo, providing the evidence that modulation of cortisol synthesis in epidermis may be an important regulatory mechanism during wound healing.
Keywords: Epithelium, Inflammation, Interleukin, Skin, Steroid Hormone, Corticosteroid Synthesis, Epidermis, Keratinocytes, Wound Healing
Introduction
Acute wound healing is a complex biological process mediated by a network of signaling pathways that coordinate multiple cellular processes, including cellular migration and proliferation, ultimately leading to barrier restoration (1). Wound healing is a tightly spatiotemporally regulated process, and changes in any component of this process can be detrimental, leading to further tissue damage or impairment of healing (2, 3). For example, timing of inflammatory response is essential; pro-inflammatory signals are important in the early stage of healing, but they can be detrimental if they persist (4, 5), underscoring a need for tight control of both stimulators and inhibitors during the wound healing process.
Upon injury, keratinocytes are the first cells that respond (6, 7) by releasing pre-stored interleukin-1 (IL-1) (8). IL-1 has an autocrine and a paracrine function; that is, to activate keratinocytes and to alert the surrounding cells and tissues. The release of IL-1 by keratinocytes, with subsequent release of additional signaling molecules (9, 10), demarcates the proinflammatory phase of wound healing. In response to these signals, activated keratinocytes start migrating and proliferating (7, 8, 11). Successful repair after tissue injury requires resolution of inflammatory response, transitioning keratinocyte (HEK) from activated to differentiating phenotype. However, very little is known about endogenous epidermal signals that control keratinocyte activation cycle and inflammatory response.
Epidermal keratinocytes have important immunologic functions (8, 12, 13), many of which are affected by GCs3 (14). Glucocorticoid hormones are known products of the adrenal cortex, and their production is under control of the HPA axis (15). Synthesis of GCs, aldosterone, and androgens in adrenal gland proceeds through a shared pathway up to the final step. In this step, 11 β-hydroxylase (CYP11B1), a mitochondrial cytochrome P-450 enzyme specific for GCs, converts 11-deoxycortisol into cortisol. It has been documented that keratinocytes indeed express different steroidogenic enzymes, StAR protein, and functional MCR2 receptor as well as and a number of components of HPA axis (CRH-R1, POMC, ACTH) (16–23). In the acute phase of immune response to injury, pro-inflammatory cytokines like TNFα, IL-1, and IL-6 act on the hypothalamic, pituitary, or adrenal component of HPA axis causing increased release of cortisol. This cortisol in turn inhibits the synthesis of cytokines and inflammatory mediators, thus, forming a negative feedback loop (24). One additional local fine-tuning mechanism of cortisol activity is 11β-hydroxysteroid dehydrogenase (11β-HSD1/11β-HSD2) enzymes, which catalyze the interconversion of hormonally active cortisol and inactive cortisone and vice versa (25, 26). In vivo, 11β-HSD2 works predominantly as dehydrogenase, converting cortisol to inactive cortisone. Alteration in expression of these 11β-HSD isoenzymes in peripheral tissues can modify glucocorticoid action on the local level by prereceptor modulation of GCs action (27–29). We have found previously that epidermis expresses 11β-HSD2 and that treatment of keratinocytes with dexamethasone induces its expression (30). In addition, cholesterol, a precursor from which steroid hormones are synthesized, is produced by skin (31). All major components of HPA axis (CRH, CRH-R, urocortin, POMC, ACTH, MC-R) and critical enzymes necessary for GCs synthesis are produced and identified in skin (32–36). In addition, isolated hair follicles, dermal fibroblasts, and melanocytes secrete cortisol and display HPA axis-like regulatory feedback systems (37–39). However, cortisol synthesis in keratinocytes and epidermis as well as its biological role has not been previously established.
In this report we show that intact human epidermis expresses steroidogenic enzymes and exhibits endogenous activity of the GR-mediated pathway. Surprisingly, we found that both human keratinocytes and skin produce measurable amounts of cortisol. Cortisol synthesis can be regulated; that is, induced by ACTH or by substrate progesterone and blocked by CYP11B1 inhibitor, metyrapone. Additionally, we have found that proinflammatory cytokine IL-1 induced, whereas IGF-1 decreased production of cortisol in HEK due to changes in expression of CYP11B1. Finally, we show induction of steroidogenic enzymes, cortisol synthesis, and activation of GR in the epidermis of acute human wounds ex vivo and confirmed it in acute porcine wounds in vivo. Interestingly, inhibition of GC synthesis during wound healing increases expression of proinflammatory cytokine IL-1 and enhances the rate of epithelialization in comparison to untreated skin. To the best of our knowledge, our results for the first time identify endogenous glucocorticoid synthesis in keratinocytes and epidermis and demonstrate its biological importance in regulation of the wound healing process.
EXPERIMENTAL PROCEDURES
Human Specimens and Wounding Experiments
Human skin specimens were obtained from reduction mammoplasty and abdominoplasty in accordance to approved institutional protocol and used to generate ex vivo acute wounds. Human skin ex vivo wound model has been extensively used to study epidermal wound healing (40–44). A 3-mm biopsy punch was used to create an acute wound, and skin specimens were maintained at the air-liquid interface with DMEM (BioWhittaker), antibiotic/antimycotic, and delipidated fetal bovine serum (Gemimi Bio-Products) for 0, 4, 24, 48, and 96 h and 7 days. Where indicated, the specimens were maintained in the presence or absence of 1 μm dexamethasone (Sigma), 1 mm metyrapone (Sigma), or a combination. Specimens were fixed in 4% paraformaldehyde overnight (Sigma) at room temperature, dehydrated with ethanol, and embedded in paraffin.
Porcine studies were approved by the University of Miami Animal Use Committee. Two young female specific-pathogen-free pigs weighing 25–30 kg were kept in-house for 2 weeks before initiation of the experiments. The animals were fed a basal diet ad libitum and housed individually in the university accredited animal facilities, anesthetized, and prepared before the wounding as previously described (45). The partial-thickness wounds (10 × 7 × 0.3 mm) were made with a specialized electrokeratome and either left untreated or treated as follows: vehicle (100% ethyl alcohol), dexamethasone (1 mm), metyrapone (500 μm). Treatment was reapplied every day. Four wedge biopsies per condition were harvested, fixed, embedded in paraffin, and processed for routine hematoxylin and eosin staining. Images were collected using Nikon Eclipse E800 microscope, and a percent of wound epithelialized was measured using planimetry. Statistical significance was determined using Student's t test.
Immunohistochemistry
Six-μm thick tissue sections were serially cut on a microtome (HM 315, Carl Zeiss) and mounted on slides. Sections were de-waxed in xylene, re-hydrated, and washed with 1× PBS and either processed for routine hematoxylin and eosin staining or immunostainings. For antigen retrieval, paraffin sections were heated in 95 °C water bath in Target Retrieval Solution (DAKO Corp.) and washed. For peroxidase staining, histological slides were treated with 0.1% H2O2 in methanol for 30 min, rinsed with H2O, and blocked with normal rabbit serum for 30 min (Vectastain Kit Elite ABC, Vector Laboratories) or blocked with 5% BSA in PBS 30 min at room temperature for immunofluorescent staining. Sections were then incubated with anti-CYP11B antibody (Santa-Cruz, CA) (1:500) (46, 47) in a commercially available antibody diluent (DAKO antibody diluent with background reducing components, DAKO Corp.) or anti-phospho-Ser211 (GR-P) antibody (1:250) (48) in 5% BSA in PBS overnight at +4 °C. For peroxidase staining, a rabbit biotinylated secondary antibody was added, and the avidin-biotin complex was visualized using DAB (DAB peroxidase substrate kit, Vector Laboratories). Slides were counterstained with hematoxylin. For immunofluorescent staining, slides were incubated with Alexa Fluor 488-conjugated goat anti-rabbit antibody (Invitrogen) for 1 h at room temperature. As a negative control, 1× PBS was substituted for primary antibody, except for the GR-P staining, where preimmune serum was used. All slides were mounted with mounting media containing propidium iodide (Vector Laboratories) to visualize the cell nucleus. The slides were analyzed using a Nikon Eclipse E800 microscope, and digital images were collected using SPOT-Camera Advanced program.
Cell Culture
Normal human epidermal keratinocytes were grown as previously described (49). Cells were expanded through two passages before they were grown to 85% or 95% confluence. Cells were washed several times with 1× PBS and incubated in basal phenol red-free keratinocyte medium (Invitrogen) custom made to exclude hydrocortisone, thyroid hormone, and bovine pituitary extract. Where indicated, cells were incubated in the presence or absence of ACTH 0.1 μm, (Sigma), progesterone 1 μm (Sigma), metyrapone 1 mm (Sigma), IL-1β 10 ng/ml (R&D Systems), or IGF-1 100 ng/ml (Sigma) for 24 or 48 h followed by media collection.
ELISA Assay for Cortisol Detection
Cells were cultured to 90% of confluence as described above. Medium was collected at 24 or 48 h after switching cells to customized basal medium containing no glucocorticoids, thyroid hormone, phenol red, or bovine pituitary extract (30). Cortisol concentration in cultured media samples was normalized to the number of cells per plate or per gram of tissue assessed.
Human skin specimens were obtained as described above. 1-cm2 templates of skin were generated using a scalpel and incubated in customized basal media. Medium was collected after 24 h. For the acute wound experiment, a 3-mm biopsy punch was used to create an acute wound, and skin specimens were maintained in basal media with or without 0.1 μm ACTH (Sigma) for 8, 24, 48, and 96 h.
Control porcine skin specimens were incubated in customized basal media for 4 h and wounded skin specimens for 24 and 48 h without or with ACTH (0.1 μm) and metyrapone (1 mm). All skin specimens were weighed before experiment, and cortisol production was normalized per gram of tissue.
Release of cortisol levels in media, harvested from cultured cells or skin as well as media alone, were measured using ELISA (R&D Systems) following a commercial protocol (39) and using a Tecan Spectra Fluor Plus spectrophotometer (TECAN U.S. Inc.) with Magellan 6 software. The minimal detectable dose of this ELISA as determined by subtracting 2 S.D. from the mean optical density value of twenty zero standard replicates and calculating the corresponding concentration ranging from 0.030 to 0.111 ng/ml. Thus, samples measured in our experiments were 100-fold higher than the minimal detectable dose. Data are expressed as the mean ± S.D., where n indicates the number of samples. Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Dunnett's multiple comparison post hoc test for comparing treatment with control or Tukey test for pair wise comparison. Differences were considered significant when p < 0.05.
Protein Isolation and Western Blot
Proteins from human skin and epidermis were extracted using a Tissue-PE LB kit (Geno Technology, Inc.) with the addition of a Protease Inhibitor Mixture (Sigma) and Phosphatase Inhibitor Mixture Set III (Calbiochem) according to a commercial protocol. Protein extract from the human adrenal gland were purchased from Clontech. The soluble supernatants were normalized for total protein concentration using the Bradford protein assay, and the samples were stored at −20 °C. Tissue protein extracts were boiled for 5 min in 2× Laemmli sample buffer, separated by 10% SDS-PAGE, and transferred to nitrocellulose membranes (VWR) at 100 V for 1 h in Tris/glycine transfer buffer. The membranes were blocked for 1 h in 5% bovine serum albumin in Tris-buffered saline, pH 7.4, at room temperature and then incubated in blocking solution with primary antibody at 4 °C overnight using antibodies specific for Ser(P)-211 GR (Cell Signaling) (48) and CYP11B1 (Santa-Cruz) (46). Horseradish protein-conjugated anti-rabbit secondary antibody (Santa Cruz) was used, and immune complexes were visualized using Super Signal West Pico Chemiluminescent substrate (Pierce). The immunoblot was exposed on x-ray film (HyBlot CL, Denville, NJ) according to the manufacturer's instructions.
RNA from Laser Capture Microdissection of Porcine Acute Wound Epidermis
All procedures were carried out in an RNase-free environment. 8-μm-thick sections were stained immediately after sectioning before laser capture as follows; they were fixed with 75% ethanol for 30 s, washed in distilled water, stained using HistoGene Staining Solution (Molecular Devices, Sunnyvale, CA) for 20 s, washed in water, incubated in 75, 95, and 100% ethanol for 30 s, dehydrated with xylene, and stored in a vacuum desiccator until microdissection. Sections were microdissected using a Leica laser capture microdissection unit at the Imaging Laboratory, University of Miami Miller School of Medicine (Miami, FL). On average, ∼1200 keratinocytes were collected per slide. Keratinocytes from three slides for each condition were pooled. RNA from pooled keratinocytes for each time point was extracted using a PicoPure RNA isolation kit (Molecular Devices).
Real Time qPCR
RNA isolation and purification from human samples was performed using TRIzol (Invitrogen) extraction and subsequent Qiagen RNeasy kit column purification (Qiagen). For detection of melanocytes in HEK utilized in experiments, we used primers specific for tyrosinase: Tyr forward (CAATGTCCCAGGTACAGGGAT) and Tyr reverse (GTAGGATTCCCGGTTATGTCCA). Samples were separated by electrophoresis on 2% agarose gels containing 0.5 μg/ml ethidium bromide and were visualized under UV light. For detection and quantitative real time PCR, 0.5 μg of total RNA from skin, epidermis, adrenal gland, and acute wounds treated or untreated and 1 μg of total RNA from cells were reverse-transcribed using a Omniscript Reverse Transcription kit (Qiagen). Real time PCR (qPCR) was performed in triplicate using the iCycler iQ thermal cycler and detection system and an iQ SYBR Green Supermix (Bio-Rad). Relative expression was normalized for levels of hypoxanthine-guanine phosphoribosyltransferase (HPRT1). The primer sequences used were: HPRT1, forward (5′-AAAGGACCCCACGAAGTGTT-3′) and reverse primer (5′-TCAAGGGCATATCCTACAACAA-3′); CYP11B1, forward (5′-CTCTACCCTGTGGGTCTGTTC-3′) and reverse primer (5′-GGGTTATAGCGCTCAGGCC-3′); 11β-HSD2, forward primer (5′-GGCCACAATGAAGTAGTTGC-3′) and reverse primer (5′-CTCCCCACAGTCACGATG-3′); IL-1β, forward (5′-GGCTTATTACAGTGGCAATGAGGA-3′) and reverse primer (5′-TCCATGGCCACAACAACTGA-3′). Statistical comparisons of expression levels were performed using Student's t test. CYP11B1 PCR obtained products from skin, HEK, and adrenal gland were sequenced using the same primers used for PCR.
For RNA isolation of porcine skin samples, we modified the protocol for GE Healthcare Illustra RNAspin Mini kit. Samples were incubated for 1 h in 150 μl of proteinase K solution (120 μl of buffer (30 mm Tris, 10 mm EDTA, 1% SDS, pH 8) + 30 μl of proteinase K (Qiagen)). Next, 350 μl of RA1 buffer was added, and the sample was homogenized using a handheld homogenizer and disposable sterile pestle. The homogenate was centrifuged for 3 min at 16,100 relative centrifugal force, and clear lysate was removed and transferred to the red filter. The rest of the procedure was followed according to protocol.
The complete mRNA sequence for Sus scrofa CYP11B1 was found through UniProt and EMBL databases. Primers were then designed using Beacon Designer software, and the choice of primer was selected on basis of lowest self-dimer, hairpin, and cross-dimer energies. The primer sequence for CYP11B1 was forward (5′-GCGGAGGTGTCGGTAGGC-3′) and reverse (5′-CTTCCACCTTCTGACACCGCT-3′). Primers were diluted to 10 ng/μl, and 1 μl of RNA was used. Real time PCR (RT-qPCR) was performed in triplicate using the iCycler iQ thermal cycler and detection system and Qscript 1 step RT along with SYBR Green (Bio-Rad). Relative expression was normalized for levels of GAPDH forward (5′-ACATCATCCCTGCTTCTAC-3′) and reverse (5′-TGCTTCACCACCTTCTTG-3) and B2M forward (5′-CCTGCTCGGGCTGCTCTC-3′) and reverse (5′-GTGGCGTGAGTAAACCTGAACC-3′). We confirmed primers by cloning PCR products with TOPO-TA One Shot Chemical Transformation, isolated DNA using the QIAprep Spin Miniprep kit, and sequenced the plasmids through Genewiz.
RESULTS
Glucocorticoid Receptor Is Constitutively Active in Human Epidermis
To establish the level of activity of the GC pathway in epidermis, we stained six different specimens of normal human skin with antiphospho-Ser211 GR antibody that recognizes ligand-induced phosphorylation at Ser211 (50). Interestingly, ligand-activated Ser211-phosphorylated-GR (GR-P) was localized in the nuclei of epidermal keratinocytes, suggesting endogenous hormone-induced activation of the receptor. To analyze the activity of the GC pathway in isolated epidermis without an external source of GCs, we incubated normal human skin on an air-liquid interface in basal, serum-free medium (40) in the absence of hydrocortisone for 24 h. Skin specimens were stained with GR-P antibody, and the signal was found in more than 30% of the nuclei of the cells in the epidermis of untreated human skin (Fig. 1A). As expected, topical GC (positive control) further activates the receptor, as evident by positive staining in almost all nuclei. This result suggests that the local production of hormone in epidermis may contribute to endogenous activation of the GC pathway. To further explore this possibility, we used an inhibitor of cortisol synthesis, metyrapone (CYP11B1 inhibitor). Metyrapone eliminates the GR-P signal from the epidermis, suggesting that local glucocorticoid synthesis in situ may occur in the epidermis and lead to substantial endogenous activation of the GC pathway.
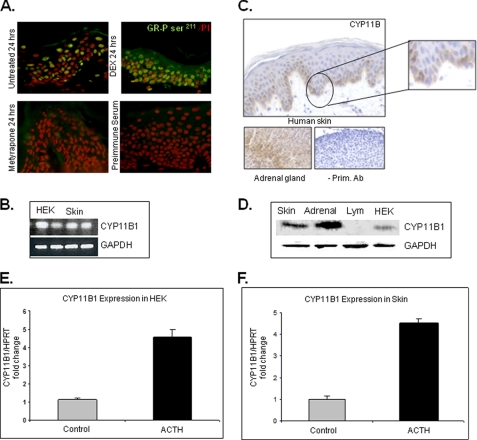
FIGURE 1.
GR receptor is activated in human epidermis. A, immunofluorescence staining with GR- phosphorylated Ser211 antibody of human skin explants maintained in the absence of GCs for 24 h revealed the nuclear presence of the receptor in keratinocytes. Dexamethasone (DEX)-treated skin, a positive control, confirmed nuclear translocation of GR after topical corticosteroid treatment. No staining is found in the absence of primary antibody. B, qRT- PCR confirms expression of CYP11B1 in HEK and skin. Expression levels were normalized to GAPDH. C, immunoperoxidase staining of human skin with CYP11B antibody (Ab) shows increased staining of basal and first suprabasal keratinocytes, whereas staining intensity decreases in upper epidermal layers. No staining was observed in sections incubated without primary antibody. As expected, signal was present in zona fasciculata of mouse adrenal gland. D, Western blot with CYP11B1 antibody and protein extracts from adrenal gland, skin, and HEK and lymphocytes (Lym) is shown. Both keratinocytes and skin showed significant protein levels of CYP11B1 in comparison to adrenal gland (positive control), whereas no CYP11B1 was detected in lymphocytes, which do not express CYP11B1 (52) and served as negative control. E, ACTH stimulates CYP11B1 expression in keratinocytes. HEK were incubated with/without ACTH (10−7 m) for 24 h (n = 4). Quantitative real time PCR for the expression of CYP11B1 is shown. Expression levels were normalized to HPRT1. Error bars represent S.D. F, ACTH stimulates CYP11B1 expression in skin. Skin specimens were incubated with/without ACTH (10−7 m) for 24 h (n = 4). Quantitative real time PCR for the expression of CYP11B1 is shown. Error bars represent S.D.
CYP11B1, an Enzyme Responsible for the Final Step in GC Synthesis, Is Expressed in Epidermis in Vivo and Keratinocytes in Vitro
To further investigate the possible GC synthesis in keratinocytes/epidermis, we focused on CYP11B1, the enzyme responsible for the conversion of 11-deoxycortisol to cortisol, the final step in GC synthesis. We utilized multiple approaches to determine whether CYP11B1 is present and expressed in the epidermis. We confirmed the expression of CYP11B1 in HEK using RT PCR (Fig. 1B). To determine protein levels we used both immunohistochemistry and Western blot. We found CYP11B present in the epidermis with a very specific distribution pattern; it was predominantly present in basal and first suprabasal keratinocyte layers (Fig. 1C). Cultured keratinocytes expressed significant amounts of CYP11B1 (Fig. 1D). Surprisingly, the level of enzyme detected in skin was comparable to the adrenal gland (51). Protein extracts from lymphocytes, which do not express CYP11B1 (52), were used as a negative control, and no signal was detected. We conclude that in addition to endogenously hormone-activated GR, skin and keratinocytes also express CYP11B1, supporting endogenous glucocorticoid synthesis.
To confirm functional significance of these results, we used real-time qPCR to analyze ACTH effects on CYP11B1 expression (Fig. 1, E and F). We found that ACTH treatment increases CYP11B1 mRNA levels more than 4-fold in both keratinocytes and skin explants.
To confirm that regulation of cortisol synthesis occurs in skin in vivo, we utilized the porcine model (45). Using RNA isolated from full thickness skin biopsies of intact (unwounded) porcine skin, we confirmed the expression of CYP11B1 with a real-time qPCR followed by cloning and sequencing. To further confirm CYP11B1 expression in epidermis in vivo, we performed laser capture microdissection. We laser-captured porcine epidermal keratinocytes, isolated RNA, and confirmed that porcine epidermal keratinocytes expresses CYP11B1, an enzyme indispensable for the synthesis of cortisol (please see Fig. 5).
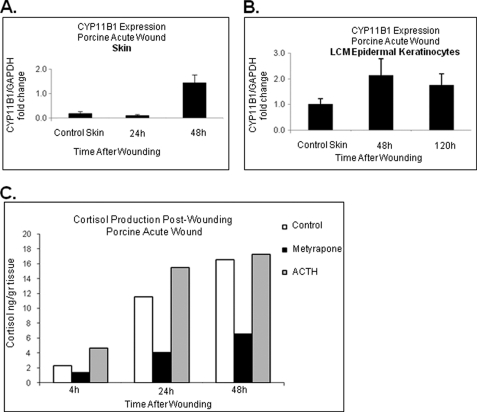
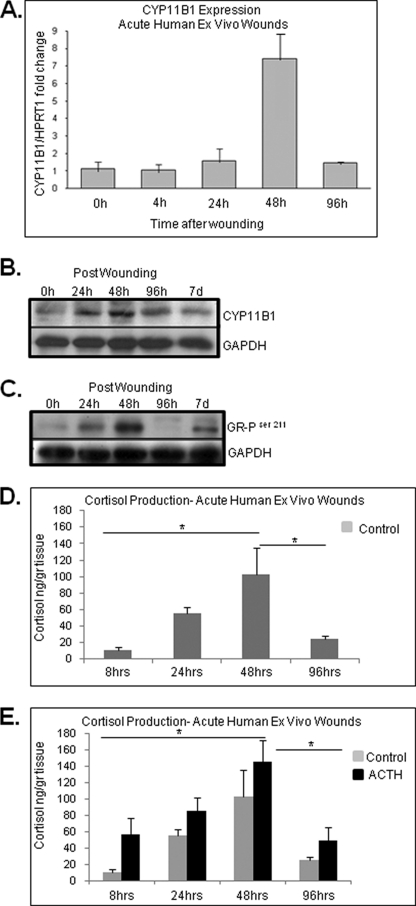
FIGURE 5.
Expression of CYP11B1 and cortisol synthesis during acute wound healing in vivo. A, CYP11B1 gene expression determined using real-time qPCR showed induction at 48 h post wounding as compared with normal porcine skin (n = 3). Error bars represent S.D. B, laser microdissected epidermal keratinocytes demonstrate induction of CYP11B1 enzyme 48 h post wounding. At the later time points CYP11B1 expression decreased back to the level of normal, unwounded skin (n = 3). Error bars represent S.D. C, porcine wound explants show the highest levels of cortisol production 48 h after the wounding (n = 2). Cortisol production is inhibited with metyrapone (10−3 m) and induced by ACTH (10−7 m).
Primary Keratinocytes and Skin Produce and Secrete Cortisol
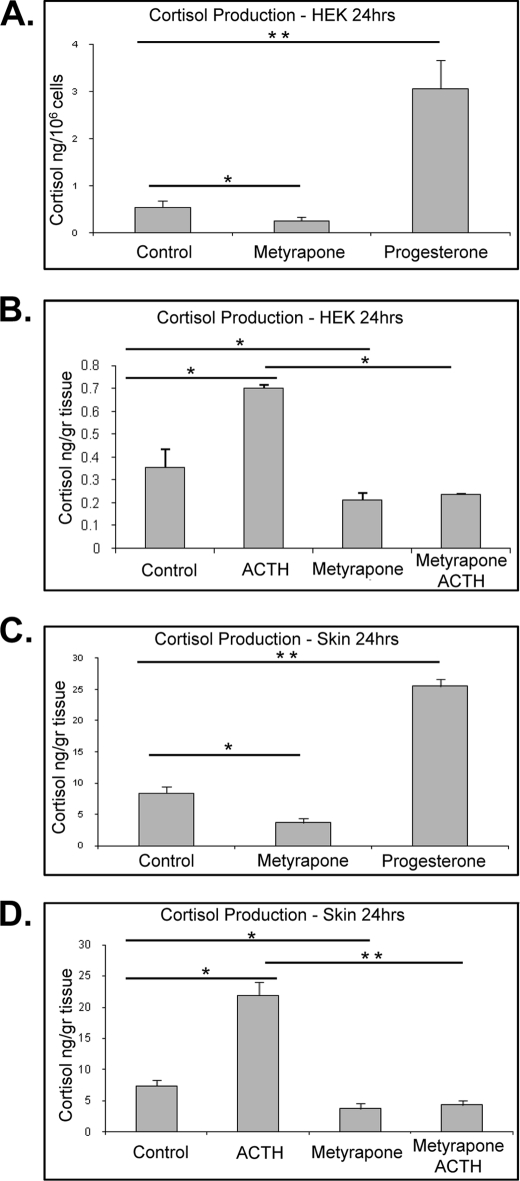
To confirm that keratinocytes indeed produce GCs, we collected medium from cultured cells grown in minimal media in the absence of any alternative GCs source and measured cortisol production by ELISA. To assure the purity of primary HEK cultures, we used RT-PCR to test for the presence of the melanocyte marker, tyrosinase (39). As expected, we detected tyrosinase expression only in the melanocytes (positive control) but not in the HEK, thus, confirming purity of HEK cultures (data not shown). The level of cortisol found produced by HEK after 24 h was 0.430 ng/million cells (Fig. 2, A and B). To confirm the production in tissue, we measured cortisol released from skin explant cultures. We measured 8.1 ng/ml cortisol released by 1 g of skin into 1 ml of basal media in the first 24 h. Interestingly, the addition of progesterone as a substrate for GC synthesis led to more than 3-fold (p < 0.01) induction of cortisol production. In contrast, blocking the CYP11B1 enzyme with metyrapone decreases endogenous levels in both HEK and skin explants (p < 0.05) (Fig. 2, A and C). To induce the cortisol synthesis, we stimulated keratinocytes and skin by exogenous ACTH. We found induction of cortisol levels in cultured HEK and skin explants 2- and 3-fold, respectively (p < 0.01) (Fig. 2, B and D). Simultaneous treatment with the inhibitor of CYP11B1, metyrapone, blocked induction of cortisol synthesis by ACTH in both skin and HEK.
FIGURE 2.
Cortisol levels in culture medium. A, effects of progesterone and metyrapone on cortisol synthesis in HEK are shown. Cells were incubated with progesterone (10−6 m) and/or metyrapone (10−3 m) for 24 h (n = 4). Progesterone as a substrate stimulates cortisol production (p < 0.01), and metyrapone inhibits it (p < 0.05). Data represent the mean ± S.D. and were analyzed by one-way ANOVA (F = 463.89; p < 0.0001) and Dunnett's post hoc test (*, p < 0.05; **, p < 0.01). B, ACTH stimulates cortisol production, and metyrapone inhibits it (p < 0.01). HEK were incubated with/without ACTH (10−7 m) for 24 h. Metyrapone co-treatment reverses the effect of ACTH (p < 0.01). Data represent the mean ± S.D. (n = 4) and were analyzed by one-way ANOVA (F = 73.85; p < 0.0001) and Tukey's post hoc test (*, p < 0.01). C, effects of progesterone and metyrapone on cortisol synthesis in skin are shown. Skin explants were incubated with progesterone (10−6 m) and/or metyrapone (10−3 m) for 24 h (n = 3). Progesterone as a substrate stimulates cortisol production (p < 0.01), and metyrapone inhibits it (p < 0.05). Data represent the mean ± S.D. and were analyzed by one-way ANOVA (F = 142.66; p < 0.0001) and Dunnett's post hoc test (*, p < 0.05; **, p < 0.01). D, ACTH stimulates cortisol production. Skin punches were incubated with/without ACTH (10−7 m) for 6 h (p < 0.01). Metyrapone co-treatment reverses the effect of ACTH (p < 0.001). Data represent the mean ± S.D. (n = 4) and were analyzed by one-way ANOVA (F = 176.27; p < 0.0001) and Tukey's post hoc test (*, p < 0.01; **, p < 0.001).
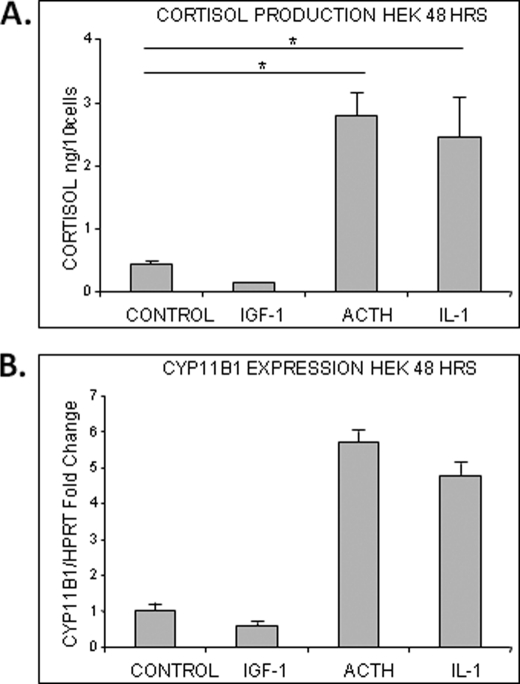
To determine the biological relevance of GC synthesis in epidermis, we decided to test if activation of keratinocytes and injury-related molecules affect cortisol synthesis. We treated HEK with two mediators of wound healing, IL-1β and IGF-1, along with ACTH (positive control). IL-1β is a primary injury-response proinflammatory cytokine, whereas IGF-1, known to modulate GC metabolism, is also an important regulator of wound healing (53, 54). We measured cortisol levels 48 h post treatment in cultured media and found a statistically significant (p < 0.01) increase in cortisol synthesis in the IL-1β-treated cells, which was comparable to ACTH induction. In contrast, IGF-1 inhibited cortisol production in cells (Fig. 3A). The expression of CYP11B1 in treated cells corresponded to cortisol production (Fig. 3B). IL-1β induces the expression of CYP11B1 similar to ACTH, whereas IGF-1 inhibits it. We conclude that injury-related molecules play a role in controlling the local production of cortisol in the epidermis by regulating the expression levels of the enzyme, which executes this process.
FIGURE 3.
IL-1β and IGF-1 modulate CYP11B1 expression and cortisol production in HEK. A, IL-1 and ACTH stimulate cortisol production in HEK (p < 0.01), whereas IGF-1 inhibits it. Cells were incubated with IL-1β (10 ng/ml), IGF-1 (100 ng/ml), or ACTH (10−7 m) for 24 h (n = 5). Data represent the mean ± S.D. and were analyzed by one-way ANOVA (F = 75.32; p < 0.0001) and Dunnett's post hoc test (*, p < 0.01). B, IL-1 stimulates CYP11B1 expression in HEK, and IGF-1 inhibits it. Cells were incubated with IL-1β (10 ng/ml), IGF-1(100 ng/ml), or ACTH (10−7 m) for 24 h (n = 3). Quantitative real time PCR for the expression of CYP11B1 is shown. Expression levels were normalized to HPRT1. Error bars represent S.D.
Cortisol Production Is Regulated during Acute Wound Healing
To further understand GC synthesis during wound healing, we used an established ex vivo human skin organ culture wound model (40). Acute wounds were maintained for 0, 4, 24, 48, and 96 h post wounding at the air-liquid interface, and the expression level of CYP11B1 was determined using real-time qPCR. We found a 6.5-fold induction of CYP11B1 48 h post wounding (Fig. 4A). The expression of CYP11B1 was decreased to the basal level 96 h after wounding. Expression data are confirmed on the protein level using Western blot. We found the highest levels of enzyme in the wound samples 48 h post-wounding. In addition, a slight increase was detected in the 24-h time point (Fig. 4B). To determine whether the induction of CYP11B1 leads to hormone-mediated activation of GR, we tested wound samples for the presence of GR-P using Western blot and GR-P-specific antibody. A prominent increase in GR-P levels was detected at 48 h followed by a decrease at 96-h post-wounding (Fig. 4C). Taken together, these data suggest an increase in cortisol synthesis in the first 2 days post wound healing, which is known as the inflammatory stage.
FIGURE 4.
Expression of CYP11B1 and cortisol production during acute wound healing. A, expression of CYP11B1 during acute wound healing is shown. Acute wounds were maintained for 0, 4, 24, 48, and 96 h post wounding at the air-liquid interface, and the expression levels of CYP11B1 were determined using quantitative real time PCR (n = 4). Expression levels were normalized to HPRT1. Error bars represent S.D. B, immunoblotting shows the gradual increase in CYP11B1 protein levels during acute wound healing, with a peak at 48 h post wounding. Protein extracts from acute wound explants were tested with anti-CYP11B1 antibody. Loading control with anti-GAPDH antibody is shown. C, immunoblotting is shown. Protein extracts from acute wound explants show an increase in GR-phosphorylated Ser211 48 h after the wounding. GAPDH was used as a loading control. D and E, cortisol levels in culture medium are shown. Media from acute wounds explants with/without ACTH was collected at 8, 24, 48, and 96 h post wounding and assessed with ELISA for presence of cortisol (n = 4). Characteristic induction of cortisol production was detected during acute wound healing peaking at 48 h post wounding (p < 0.05). Data represent the mean ± S.D. (n = 4) and were analyzed by one-way ANOVA (F = 5.4; p < 0.05) and Tukey's post hoc test (*, p < 0.05).
To confirm these data, we incubated acute wound skin explants in basal, GC-free media and measured the release of cortisol into media at 8, 24, 48, and 96 h post wounding or in the presence of ACTH (positive control) (Fig. 4D). Cortisol concentration rose gradually to a maximal 10-fold increase at 48 h and then decreased at 96 h. Therefore, GC synthesis, its regulation, and GR pathway activation occurs during the acute process of wound healing in a time response manner, reaching its peak at 48 h post injury. We concluded that epidermal injury by wounding leads to increased cortisol production.
To determine whether a similar process occurs in skin in vivo, we utilized a porcine partial thickness wound model (45) and found that wounding of porcine skin induces expression of CYP11B1. We collected biopsies of unwounded porcine skin and at 24 and 48 h post wounding. Gene expression analysis showed induction of the CYP11B1 enzyme at 48 h post wounding (Fig. 5A), thus, confirming data obtained in the ex vivo organ culture model. To confirm CYP11B1 regulation during early and later time points of acute wound healing, we performed laser capture microdissection. Epidermal keratinocytes from the edge of the wound showed induction of CYP11B1 during early time points and suppression at late time points, 120 h post wounding (Fig. 5B), confirming human ex vivo data in porcine wounds in vivo.
In addition we measured the release of cortisol at 4, 24, and 48 h post wounding in porcine wounds in the presence or absence of metyrapone. Similar to human wounds, cortisol levels increased gradually to maximal levels of 16.5 ng/g of tissue at 48 h post wounding. Metyrapone treatment blocked induction of cortisol, whereas ACTH promoted it (Fig. 5C). Therefore, we conclude that GC synthesis in vivo follows the pattern of human skin ex vivo, providing evidence of timed induction of cortisol synthesis in the first 48 h post-wounding.
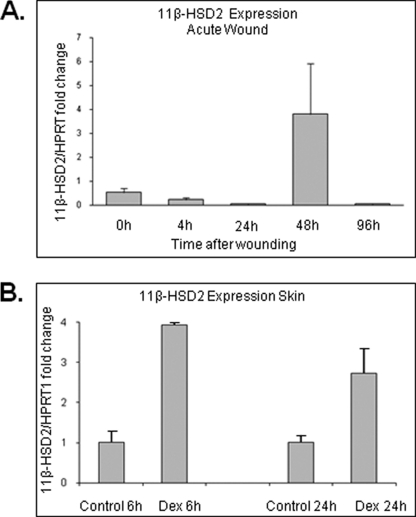
A characteristic increase of cortisol production and levels at 48 h post-wounding with normalization by 96 h (Fig. 4D) indicated that there is a possible negative feed-back mechanism that controls levels of GCs in epidermis. Thus, we determined expression levels of 11β-HSD2, an enzyme responsible for negative feedback and conversion of active cortisol to inactive cortisone, during acute wound healing. We detected a 4-fold induction of 11β-HSD2 at 48 h that was decreased to the basal level at 96 h post wounding (Fig. 6A). These data indicate that de novo synthesis of cortisol during wound healing is tightly controlled by a time-dependent feedback mechanism. In our previously published work using microarray analysis of dexamethasone-treated HEK, we found characteristic induction of 11β-HSD2 (30). To test the possibility that the surge of cortisol during wound healing elicits a fast self-limiting negative feedback mechanism, we treated the skin explants topically for 6 and 24 h with dexamethasone and utilized real-time qPCR to quantify the changes in 11β-HSD2 mRNA levels. GC treatment led to 2-fold increase in 11β-HSD2 expression (Fig. 6B).
FIGURE 6.
11β-HSD2 (negative feedback enzyme) is induced by glucocorticoids. A, expression of 11β-HSD2 during ex vivo human acute wound healing (n = 3) is shown. Induction of 11β-HSD2 48 h post wounding is shown. Quantitative real time PCR for the expression of 11β-HSD2 is shown. Expression levels were normalized to HPRT1. Error bars represent S.D. B, dexamethasone (Dex, 10−3 m) treatment for 6 h of human skin leads to a 4-fold induction of 11β-HSD2 (n = 3). Quantitative real time PCR for the expression of 11β-HSD2 is shown. Expression levels were normalized to HPRT1. Error bars represent S.D.
Endogenous GC Synthesis Regulates IL-1
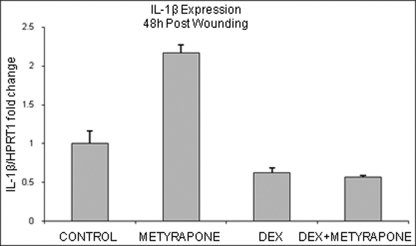
Both IL-1 and wounding (which triggers endogenous release of IL-1) stimulate the expression of CYP11B1 and cortisol production in HEK and skin, suggesting that increased endogenous synthesis of the cortisol in skin during wound healing process may serve as a feedback mechanism to curb proinflammatory cytokines. To test this possibility, we blocked cortisol synthesis in wounded skin explants with metyrapone and analyzed expression of IL-1β. Dexamethasone-treated wounds served as a control. Interestingly, 48 h post wounding, metyrapone treatment led to 2-fold increase in IL-1β expression compared with control (Fig. 7), whereas dexamethasone treatment, as expected, led to an ∼2-fold suppression, suggesting that endogenous cortisol synthesis regulates IL-1β during acute wound healing response.
FIGURE 7.
Inhibition of cortisol production during wound healing leads to induction of IL-1β expression. Acute wound explants were treated with metyrapone (10−3 m), dexamethasone (DEX, 10−3 m), or combination for 48 h (n = 3). Metyrapone treatment induces expression of IL-1β, whereas dexamethasone reverses that effect. Error bars represent S.D.
Endogenous Cortisol Levels Regulate the Rate of Epithelialization ex Vivo and in Vivo
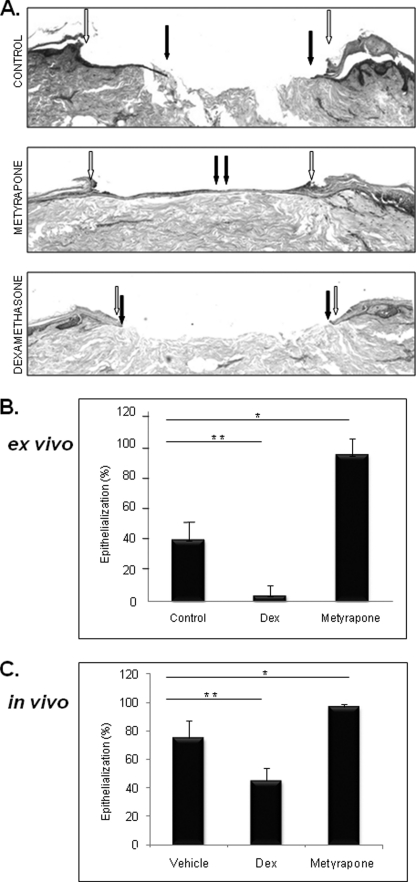
To determine whether modulation of local GCs production can affect clinical outcomes of wound healing, we treated acute wounds with metyrapone and examined the rate of epithelialization (Fig. 8). Dexamethasone treatment was used as a control. As expected, dexamethasone treatment inhibited epithelialization (p < 0.03) and wound closure. However, the addition of metyrapone promoted epithelialization (p < 0.05) and improved the rate of healing. Furthermore, in vivo in deep partial thickness porcine wounds, topical treatment with metyrapone significantly (p < 0.01) promoted epithelialization and improved the rate of healing (Fig. 8C). As expected, glucocorticoid treatment inhibited epithelialization and wound closure. Taken together these data suggest that modulation of local GC synthesis may improve clinical outcome of wound healing.
FIGURE 8.
Metyrapone promotes wound healing in ex vivo human and in vivo porcine wound models. A, hematoxylin and eosin staining demonstrates complete epithelialization after treatment of acute ex vivo wounds with metyrapone. Open arrows indicate wound edges after initial wounding, whereas solid arrows point to the epithelialized edges of the migrating fronts 4 days after the wounding. B, quantification of the rate of epithelialization 4 days after wounding is shown. Metyrapone (10−3 m) promotes epithelialization in a human skin organ culture model (*, p < 0.05). Topical dexamethasone (Dex, 10−3 m) treatment delayed epithelialization when compared with control untreated skin (**, p < 0.03) (n = 3). C, quantification of the epithelialization rate in a porcine deep partial thickness model is shown. Metyrapone treatment promotes epithelialization (*, p < 0.01), whereas dexamethasone inhibits it (**, p < 0.006) (n = 4). Error bars represent S.D.
DISCUSSION
In this report we show that epidermis represents an extra-adrenal site of glucocorticoid synthesis. Both epidermis and cultured keratinocytes synthesize cortisol in a tightly regulated manner. They express enzymes essential for its synthesis (CYP11B1), secrete cortisol, and in the intact state maintain a constitutive level of GR nuclear activity. Cortisol synthesis can be increased by stimulation with ACTH or proinflammatory cytokine IL-1β and decreased by IGF-1 or metyrapone. Furthermore, epidermal GC synthesis is induced during wound healing. Simultaneous increase in CYP11B1 expression, cortisol, and hormone-activated GR-P was found in acute wounds and may serve as a possible feedback loop that attenuates initial proinflammatory response, preventing excess inflammation that can lead to further tissue damage.
The idea of extra-adrenal sources of GC has emerged in recent years. Initially, it was found that the thymic epithelium can synthesize GCs in a paracrine manner, and more recently, intestinal mucosa and skin have been proposed to synthesize GCs (55–57). Although very little is known about steroidogenesis in non-adrenal tissues, the concept of skin as endocrine organ is well established; it produces other hormones such as estrogen, progesterone, retinoids, and vitamin D, all of which have significant impact on its physiology and pathology (20, 58–62). The presence of critical enzymes that participate in the steroidogenesis has been identified in aorta, intestine, and skin, suggesting the possibility of adrenal-independent GC synthesis in situ. Recently, cortisol production by fibroblasts, melanocytes, and isolated hair follicles was demonstrated in vitro (37–39).
CYP11B1 is predominantly localized to basal and first suprabasal layers of epidermis. These layers of epidermis are the major contributors to tissue repair, as they have the highest proliferative capacity (63) and, therefore, can be considered as primary targets for proinflammatory molecules. Interestingly, we found that Ca2+-induced differentiation of cultured HEK leads to decreased production of cortisol (data not shown). Our data support the notion that locally synthesized glucocorticoids may provide a specific subset of cells in the epidermis with a mechanism of negative feedback to limit the inflammation.
Skin serves as a barrier to the outside world, and it is constantly exposed to different types of injuries (UV light, mechanical and chemical trauma, microbes, stress). It is in regular contact not only with foreign antigens and commensal bacteria but also with potentially dangerous pathogenic bacteria, parasites, and viruses. Therefore, epidermis as the “first line of defense” has developed mechanisms of alarming the body when there is tissue damage either mechanical (wound), chemical, physical (UV), or biological (64). In addition, epidermis is one of the few tissues in the body without its own vascular supply, which in conjunction to its barrier maintenance function points to unique tissue characteristics. One such feature is the protective response, keratinocyte activation, which involves release of prestored IL-1β and a cascade of the timely regulated secretion of other proinflammatory cytokines and growth factors (7). In response, keratinocytes activate a number of processes such as proliferation and migration, differentiation, and DNA repair (11). However, very little is known about the mechanisms that control this rapid inflammatory response. One possibility is receptor unresponsiveness or down-regulation by a high concentration of ligand. Alternatively, factors such as IL-10, IL-RA, TGF-β1, or glucocorticoids may participate in resolution of inflammation and reversal of the activation. Glucocorticoids are known to attenuate the proinflammatory response in the body (30, 65). It was shown that reduction in local production of GCs in intestinal epithelium increases the susceptibility of mice to compound-induced experimental colitis due to augmented immune response (56). Therefore, it is possible that keratinocytes produce GCs to allow proinflammatory signaling in a controlled, timed fashion while preventing excess inflammation, which can lead to further tissue damage and weakened repair. In our previously published data we have shown that GC treatment of HEK inhibits synthesis or signaling pathways of most cytokines and growth factors involved in the inflammatory phase of wound healing in a precise timely fashion (TNFα, IL-1β, IL-4, IL-8, EGF, VEGF, INF-γ, TGFβ) (30). We have also shown that GCs utilize a complex molecular mechanism to inhibit the effects of EGF in wound healing (49, 66–68). Here we have shown that inhibition of endogenous cortisol synthesis with metyrapone during wound healing increases the expression of IL-1β compared with untreated wound. This result indicates that endogenous cortisol synthesis in skin can serve as a mechanism that limits inflammation during acute wound healing.
In view of our data, we propose that GC synthesis acts as paracrine mechanism to increase the tissue level of cortisol. It is well established that the immune response to injury affects ACTH-cortisol secretion, causing increased release of cortisol as proinflammatory cytokines such as TNFα, IL-1, and IL-6 act on the hypothalamic, pituitary, or adrenal component of HPA axis (24). Our results indicate the possibility that the similar mechanism exists locally in tissues such as epidermis. In addition, constitutive epidermal cortisol synthesis changes the paradigm of inflammatory response and brings a new concept; that is, proinflammatory signals need to overcome the endogenous basic levels of GC for inflammation to occur. This further raises a number of interesting possibilities, particularly in chronic inflammatory and stress-mediated skin disorders as well as auto-immune diseases with skin manifestations.
De Novo Synthesis of Glucocorticoids in Keratinocytes during Acute Wound Healing
Another surprising finding is the increased presence of CYP11B1 in the epidermis during the time course of wound healing. Interestingly, CYP11B1 protein levels were found to be gradually increasing, with the peak at 48 h post wounding. Increased levels of CYP11B1 at 48 h post wounding coupled with a similar increase of hormone-activated GR-P indicates a linked pattern where the induction of cortisol synthesis leads to the activation of the GR pathway. A possible explanation for this result is induction of cortisol synthesis by proinflammatory signals triggered by wounding.
Spatiotemporal cellular organization is especially crucial in the wound healing process; thus, a tight regulatory pattern is essential. Therefore, increased levels of CYP11B1 and cortisol are resolved by 96 h post wounding. Although GCs may be useful for tight control of proinflammatory signals, the prolonged presence of high levels of cortisol would slow down or inhibit wound healing. We showed that dexamethasone treatment blocks epithelialization, whereas metyrapone treatment promotes epithelialization compared with the control wound. This control of GC synthesis during wound healing occurs at two levels. It is induced by IL-1β upon wounding, whereas it is down-regulated by IGF-1, which is released later during wound healing. It is possible that other wound-healing-mediated signals regulate CYP11B1 expression as well. In addition, down-regulation of CYP11B1 occurs by its own negative feedback loop whereby cortisol induces HSD11B1 that converts active cortisol into an inactive form.
In summary, data presented in this paper describe de novo synthesis of cortisol by epidermal keratinocytes in vitro and in vivo. Epidermal keratinocytes synthesize and secrete cortisol, express CYP11B1, an enzyme important for the last step of cortisol synthesis, and show constitutive activation of the GR pathway. Furthermore, IL-1 induces the CYP11B1 enzyme and increases cortisol production by epidermis. In contrast, inhibition of cortisol synthesis increases IL-1. Cortisol levels increase upon acute epidermal injury by wounding in both human skin explants and porcine skin samples, possibly to limit initial proinflammatory response. Therefore, we propose that epidermal synthesis of cortisol in situ constitutes a negative feed-back mechanism to curb inflammatory response caused by epidermal injury.
Acknowledgments
We are grateful to Dr. Olivier Chabre for CYP11B antibody and Dr. Michael Garabedian for a GR-phosphorylated Ser211 antibody developed in laboratories. Special thanks to Sara Vukelic, Lena Pastar, Mia Maricic, and Tijana and Dimitrije Canic for continued support and true inspiration.
This work was supported, in whole or in part, by National Institutes of Health Grant NR008029. Drs. Tomic-Canic and Brem have submitted a patent application “De novo synthesis of glucocorticoids in the epidermis and its uses and applications.” This application has not yet been approved.
- GC
- glucocorticoid
- CYP11B1
- 11 β-hydroxylase
- ANOVA
- analysis of variance
- HPRT1
- hypoxanthine-guanine phosphoribosyltransferase
- qPCR
- quantitative PCR
- GR-P
- phosphorylated-GR
- HPA
- hypothalamic-pituitary-adrenal.
REFERENCES
- 1. Brem H., Tomic-Canic M. (2007) J. Clin. Invest. 117, 1219–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Braun S., Hanselmann C., Gassmann M. G., auf dem Keller U., Born-Berclaz C., Chan K., Kan Y. W., Werner S. (2002) Mol. Cell. Biol. 22, 5492–5505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Loots M. A., Lamme E. N., Zeegelaar J., Mekkes J. R., Bos J. D., Middelkoop E. (1998) J. Invest. Dermatol. 111, 850–857 [DOI] [PubMed] [Google Scholar]
- 4. Mast B. A., Schultz G. S. (1996) Wound Repair Regen. 4, 411–420 [DOI] [PubMed] [Google Scholar]
- 5. Robson M. C. (1997) Wound Repair Regen. 5, 12–17 [DOI] [PubMed] [Google Scholar]
- 6. Nickoloff B. J., Naidu Y. (1994) J. Am. Acad. Dermatol. 30, 535–546 [DOI] [PubMed] [Google Scholar]
- 7. Freedberg I. M., Tomic-Canic M., Komine M., Blumenberg M. (2001) J. Invest. Dermatol. 116, 633–640 [DOI] [PubMed] [Google Scholar]
- 8. Kupper T. S. (1990) J. Invest. Dermatol. 94 (Suppl. 6), 146S–150S [DOI] [PubMed] [Google Scholar]
- 9. Barrientos S., Stojadinovic O., Golinko M. S., Brem H., Tomic-Canic M. (2008) Wound Repair Regen. 16, 585–601 [DOI] [PubMed] [Google Scholar]
- 10. Pastar I., Stojadinovic O., Tomic-Canic M. (2008) Surg. Technol. Int. 17, 105–112 [PubMed] [Google Scholar]
- 11. Tomic-Canic M., Komine M., Freedberg I. M., Blumenberg M. (1998) J. Dermatol. Sci. 17, 167–181 [DOI] [PubMed] [Google Scholar]
- 12. Kondo S. (1999) J. Investig. Dermatol. Symp. Proc. 4, 177–183 [DOI] [PubMed] [Google Scholar]
- 13. Steinhoff M., Brzoska T., Luger T. A. (2001) Curr. Opin. Allergy Clin. Immunol. 1, 469–476 [DOI] [PubMed] [Google Scholar]
- 14. Lee B., Tomic-Canic M. (2002) in Molecular Mechanisms Of Action Of Steroid Hormone Receptors (Krstic - Demonacos M., Demonacos C. eds) pp. 1–25, Research Signpost, Kerala, India [Google Scholar]
- 15. Tsigos C., Chrousos G. P. (2002) J. Psychosom. Res. 53, 865–871 [DOI] [PubMed] [Google Scholar]
- 16. Rousseau K., Kauser S., Pritchard L. E., Warhurst A., Oliver R. L., Slominski A., Wei E. T., Thody A. J., Tobin D. J., White A. (2007) FASEB J. 21, 1844–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Slominski A., Pisarchik A., Tobin D. J., Mazurkiewicz J. E., Wortsman J. (2004) Endocrinology 145, 941–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Slominski A., Zbytek B., Zmijewski M., Slominski R. M., Kauser S., Wortsman J., Tobin D. J. (2006) Front. Biosci. 11, 2230–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Slominski A., Wortsman J., Pisarchik A., Zbytek B., Linton E. A., Mazurkiewicz J. E., Wei E. T. (2001) FASEB J. 15, 1678–1693 [DOI] [PubMed] [Google Scholar]
- 20. Zouboulis C. C. (2000) Horm. Res. 54, 230–242 [DOI] [PubMed] [Google Scholar]
- 21. Arck P. C., Slominski A., Theoharides T. C., Peters E. M., Paus R. (2006) J. Invest. Dermatol. 126, 1697–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tiala I., Suomela S., Huuhtanen J., Wakkinen J., Hölttä-Vuori M., Kainu K., Ranta S., Turpeinen U., Hämäläinen E., Jiao H., Karvonen S. L., Ikonen E., Kere J., Saarialho-Kere U., Elomaa O. (2007) J. Mol. Med. 85, 589–601 [DOI] [PubMed] [Google Scholar]
- 23. Rogoff D., Gomez-Sanchez C. E., Foecking M. F., Wortsman J., Slominski A. (2001) J. Steroid. Biochem. Mol. Biol. 78, 77–81 [DOI] [PubMed] [Google Scholar]
- 24. Rhen T., Cidlowski J. A. (2005) N. Engl. J. Med. 353, 1711–1723 [DOI] [PubMed] [Google Scholar]
- 25. Albiston A. L., Obeyesekere V. R., Smith R. E., Krozowski Z. S. (1994) Mol. Cell. Endocrinol. 105, R11–R17 [DOI] [PubMed] [Google Scholar]
- 26. Quinkler M., Stewart P. M. (2003) J. Clin. Endocrinol. Metab. 88, 2384–2392 [DOI] [PubMed] [Google Scholar]
- 27. Hardy R. S., Filer A., Cooper M. S., Parsonage G., Raza K., Hardie D. L., Rabbitt E. H., Stewart P. M., Buckley C. D., Hewison M. (2006) Arthritis Res. Ther. 8, R108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tomlinson J. W., Moore J., Cooper M. S., Bujalska I., Shahmanesh M., Burt C., Strain A., Hewison M., Stewart P. M. (2001) Endocrinology 142, 1982–1989 [DOI] [PubMed] [Google Scholar]
- 29. Tomlinson J. W., Sherlock M., Hughes B., Hughes S. V., Kilvington F., Bartlett W., Courtney R., Rejto P., Carley W., Stewart P. M. (2007) J. Clin. Endocrinol. Metab. 92, 857–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stojadinovic O., Lee B., Vouthounis C., Vukelic S., Pastar I., Blumenberg M., Brem H., Tomic-Canic M. (2007) J. Biol. Chem. 282, 4021–4034 [DOI] [PubMed] [Google Scholar]
- 31. Davidson V. L., Sittman D. B. (1994) Biochemistry, 3rd Ed., p. 433, Harwal Publishing, Philadelphia, PA [Google Scholar]
- 32. Thiboutot D., Jabara S., McAllister J. M., Sivarajah A., Gilliland K., Cong Z., Clawson G. (2003) J. Invest. Dermatol. 120, 905–914 [DOI] [PubMed] [Google Scholar]
- 33. Slominski A., Wortsman J., Tuckey R. C., Paus R. (2007) Mol. Cell. Endocrinol. 265–266, 143–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Slominski A., Gomez-Sanchez C. E., Foecking M. F., Wortsman J. (2000) Biochim. Biophys. Acta 1474, 1–4 [DOI] [PubMed] [Google Scholar]
- 35. Slominski A., Ermak G., Hwang J., Chakraborty A., Mazurkiewicz J. E., Mihm M. (1995) FEBS Lett. 374, 113–116 [DOI] [PubMed] [Google Scholar]
- 36. Ermak G., Slominski A. (1997) J. Invest. Dermatol. 108, 160–165 [DOI] [PubMed] [Google Scholar]
- 37. Ito N., Ito T., Kromminga A., Bettermann A., Takigawa M., Kees F., Straub R. H., Paus R. (2005) FASEB J. 19, 1332–1334 [DOI] [PubMed] [Google Scholar]
- 38. Slominski A., Zbytek B., Szczesniewski A., Wortsman J. (2006) J. Invest. Dermatol. 126, 1177–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Slominski A., Zbytek B., Szczesniewski A., Semak I., Kaminski J., Sweatman T., Wortsman J. (2005) Am. J. Physiol. Endocrinol. Metab. 288, E701–E706 [DOI] [PubMed] [Google Scholar]
- 40. Tomic-Canic M., Mamber S. W., Stojadinovic O., Lee B., Radoja N., McMichael J. (2007) Wound Repair Regen. 15, 71–79 [DOI] [PubMed] [Google Scholar]
- 41. Kratz G. (1998) Microsc. Res. Tech. 42, 345–350 [DOI] [PubMed] [Google Scholar]
- 42. Pastar I., Stojadinovic O., Krzyzanowska A., Barrientos S., Stuelten C., Zimmerman K., Blumenberg M., Brem H., Tomic-Canic M. (2010) Mol. Med. 16, 92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pullar C. E., Grahn J. C., Liu W., Isseroff R. R. (2006) FASEB J. 20, 76–86 [DOI] [PubMed] [Google Scholar]
- 44. Roupé K. M., Nybo M., Sjöbring U., Alberius P., Schmidtchen A., Sørensen O. E. (2010) J. Invest. Dermatol. 130, 1167–1177 [DOI] [PubMed] [Google Scholar]
- 45. Davis S. C., Mertz P. M. (2008) Ostomy Wound Manage. 54, 16–18, 20,, 22–25 [PubMed] [Google Scholar]
- 46. Chabre O., Portrat-Doyen S., Chaffanjon P., Vivier J., Liakos P., Labat-Moleur F., Chambaz E., Morel Y., Defaye G. (2000) J. Clin. Endocrinol. Metab. 85, 4060–4068 [DOI] [PubMed] [Google Scholar]
- 47. Defaye G., Monnier N., Guidicelli C., Chambaz E. M. (1982) Mol. Cell. Endocrinol. 27, 157–168 [DOI] [PubMed] [Google Scholar]
- 48. Ismaili N., Garabedian M. J. (2004) Ann. N.Y. Acad. Sci. 1024, 86–101 [DOI] [PubMed] [Google Scholar]
- 49. Stojadinovic O., Brem H., Vouthounis C., Lee B., Fallon J., Stallcup M., Merchant A., Galiano R. D., Tomic-Canic M. (2005) Am. J. Pathol. 167, 59–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang Z., Frederick J., Garabedian M. J. (2002) J. Biol. Chem. 277, 26573–26580 [DOI] [PubMed] [Google Scholar]
- 51. Fallo F., Pezzi V., Barzon L., Mulatero P., Veglio F., Sonino N., Mathis J. M. (2002) Eur. J. Endocrinol. 147, 795–802 [DOI] [PubMed] [Google Scholar]
- 52. Zhou Z., Shackleton C. H., Pahwa S., White P. C., Speiser P. W. (1998) Mol. Cell. Endocrinol. 138, 61–69 [DOI] [PubMed] [Google Scholar]
- 53. Rappolee D. A., Mark D., Banda M. J., Werb Z. (1988) Science 241, 708–712 [DOI] [PubMed] [Google Scholar]
- 54. Sharp L. L., Jameson J. M., Cauvi G., Havran W. L. (2005) Nat. Immunol. 6, 73–79 [DOI] [PubMed] [Google Scholar]
- 55. Vacchio M. S., Papadopoulos V., Ashwell J. D. (1994) J. Exp. Med. 179, 1835–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Coste A., Dubuquoy L., Barnouin R., Annicotte J. S., Magnier B., Notti M., Corazza N., Antal M. C., Metzger D., Desreumaux P., Brunner T., Auwerx J., Schoonjans K. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 13098–13103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mueller M., Cima I., Noti M., Fuhrer A., Jakob S., Dubuquoy L., Schoonjans K., Brunner T. (2006) J. Exp. Med. 203, 2057–2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kayes-Wandover K. M., White P. C. (2000) J. Clin. Endocrinol. Metab. 85, 2519–2525 [DOI] [PubMed] [Google Scholar]
- 59. Young M. J., Clyne C. D., Cole T. J., Funder J. W. (2001) J. Clin. Endocrinol. Metab. 86, 5121–5126 [DOI] [PubMed] [Google Scholar]
- 60. Reichrath J., Lehmann B., Carlberg C., Varani J., Zouboulis C. C. (2007) Horm. Metab. Res. 39, 71–84 [DOI] [PubMed] [Google Scholar]
- 61. Zouboulis C. C., Chen W. C., Thornton M. J., Qin K., Rosenfield R. (2007) Horm. Metab. Res. 39, 85–95 [DOI] [PubMed] [Google Scholar]
- 62. Haselbeck R. J., Ang H. L., Duester G. (1997) Dev. Dyn. 208, 447–453 [DOI] [PubMed] [Google Scholar]
- 63. Li A., Pouliot N., Redvers R., Kaur P. (2004) J. Clin. Invest. 113, 390–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tomic-Canic M., Magnus S. A., Oscar M. A. (2004) in The Epidermis in Wound Healing (David T. Rovee, Maibach H. I. eds) pp. 25–57, CRC Press LLC, Boca Raton, FL [Google Scholar]
- 65. Sapolsky R. M., Romero L. M., Munck A. U. (2000) Endocr. Rev. 21, 55–89 [DOI] [PubMed] [Google Scholar]
- 66. Radoja N., Komine M., Jho S. H., Blumenberg M., Tomic-Canic M. (2000) Mol. Cell. Biol. 20, 4328–4339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jho S. H., Vouthounis C., Lee B., Stojadinovic O., Im M. J., Brem H., Merchant A., Chau K., Tomic-Canic M. (2005) J. Invest. Dermatol. 124, 1034–1043 [DOI] [PubMed] [Google Scholar]
- 68. Lee B., Vouthounis C., Stojadinovic O., Brem H., Im M., Tomic-Canic M. (2005) J. Mol. Biol. 345, 1083–1097 [DOI] [PubMed] [Google Scholar]