Abstract
The mechanism of action of clofazimine (CFZ), an antimycobacterial drug with a long history, is not well understood. The present study describes a redox cycling pathway that involves the enzymatic reduction of CFZ by NDH-2, the primary respiratory chain NADH:quinone oxidoreductase of mycobacteria and nonenzymatic oxidation of reduced CFZ by O2 yielding CFZ and reactive oxygen species (ROS). This pathway was demonstrated using isolated membranes and purified recombinant NDH-2. The reduction and oxidation of CFZ was measured spectrally, and the production of ROS was measured using a coupled assay system with Amplex Red. Supporting the ROS-based killing mechanism, bacteria grown in the presence of antioxidants are more resistant to CFZ. CFZ-mediated increase in NADH oxidation and ROS production were not observed in membranes from three different Gram-negative bacteria but was observed in Staphylococcus aureus and Saccharomyces cerevisiae, which is consistent with the known antimicrobial specificity of CFZ. A more soluble analog of CFZ, KS6, was synthesized and was shown to have the same activities as CFZ. These studies describe a pathway for a continuous and high rate of reactive oxygen species production in Mycobacterium smegmatis treated with CFZ and a CFZ analog as well as evidence that cell death produced by these agents are related to the production of these radical species.
Keywords: Amyloid, Blood, Brain, RNA Binding Protein, RNA Folding, Translation Control
Introduction
The riminophenazine derivative, clofazimine, (CFZ2; [3-(4-chloroanilino)-10-(4-chlorophenyl)-2,10-dihydro-2-(isopropylimino)-phenazine]; Fig. 1) was synthesized >50 years ago in an effort to produce a strong antimycobacterial antibiotic (1–3). Although able to kill most mycobacteria in vitro including Mycobacterium tuberculosis, CFZ was ineffective in animal models of tuberculosis (1–3). CFZ achieved status as an antibiotic in the treatment of leprosy and is currently part of the three-drug treatment regimen approved for multibacillary disease by the World Health Organization (3).
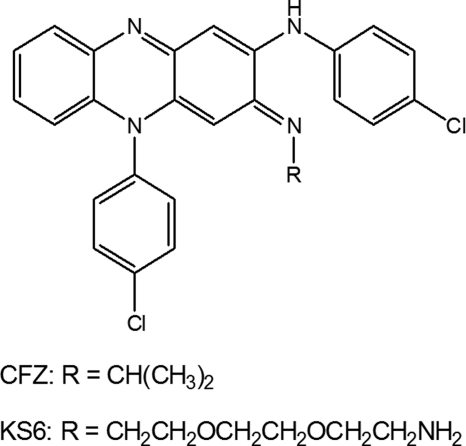
FIGURE 1.
Structures of clofazimine and a new, more soluble analog, KS6.
The apparent inability of most mycobacteria, including isolates of M. tuberculosis, to develop resistance to CFZ (3) has led to a resurgence of interest in the drug. Currently, new CFZ analogs are being tested for treatment of Mycobacterium avium infections associated with AIDS and for multidrug-resistant tuberculosis (3–6). Although primarily antimycobacterial, experimental evidence suggests a broader based activity toward Gram-positive bacteria (7, 8) and strains of yeast (9). Apart from its antibiotic properties, CFZ appears to have anti-inflammatory properties exhibiting the ability to suppress neutrophil and lymphocyte activity (10–13). This immunosuppressive effect currently is being explored in treatments of autoimmune disorders, including, multiple sclerosis, lupus, and psoriasis (12, 13). Lastly, CFZ may have anti-cancer activity (14, 15).
The mechanism(s) of action of CFZ has remained elusive. The drug is extremely hydrophobic (cLogP = 7.5), suggesting that it functions in association with membranes (2, 16). Several studies have pointed to inhibition of K+ transport. However, it is not clear whether inhibition results from a specific interaction(s) or is a consequence of membrane disruption (17, 18). CFZ also is reported to bind to DNA. Again the mechanism of this interaction is unclear and does not appear to be through base intercalation like other dyes that disrupt DNA function (19).
The first published study on CFZ noted that it was a redox active compound and that it was reduced and oxidized within mycobacteria, likely in conjunction with respiratory chain activity (1). Because reduced forms of dyes such as CFZ were known to produce reactive oxygen species (ROS) upon reoxidation in air, it was argued that the generation of ROS was an important aspect of the mechanism of action of the drug. In support of this argument, it was noted that mycobacteria deficient in catalase activity showed a greater sensitivity to CFZ than wild type bacteria.
In the current study, we characterize the interaction of CFZ and a more soluble analog of CFZ with the respiratory chain in isolated M. smegmatis membranes and describe a pathway for the NADH-dependent redox cycling of the dye resulting in ROS production and mycobacterial death.
EXPERIMENTAL PROCEDURES
Materials
CFZ, HRP, and bovine liver superoxide dismutase were from Sigma-Aldrich. Trifluoperazine (TPZ) was from LKT Laboratories (St. Paul, MN), and Amplex Red was from Invitrogen. The bioluminescence ATP detection kit CLS II and proteinase inhibitor mixture (complete EDTA-free) were from Roche Applied Science. Culture media and agar were from Difco. BCA kit for protein measurement was from Pierce. TALON resin was from BD Biosciences. Big CHAP was from Anatrace (Maumee, OH).
Growth of Bacteria and Isolation of Membranes
Typically, M. smegmatis (strain mc2 155 from ATCC) was grown aerobically in a batch method at 37 °C in 7H9 media supplemented with 0.2% glycerol and 0.1% Tween 80. Growths were monitored at 600 nm; bacteria reaching roughly 0.8 OD were in mid-log phase and those reaching 1.2–1.5 OD were in stationary phase under the conditions and media used in these experiments. Bacteria were harvested by centrifugation at 9,600 × g using a JA 10 rotor and Beckman-Coulter J25 centrifuge. Pellets ranging from 12–15 g were then suspended in 100 ml of buffer (10 mm HEPES, pH 7.0, 50 mm KCl, 5.0 mm MgCl2, and 10% glycerol) containing protease inhibitors and 1.5 mg/ml lysozyme. After incubation at 37 °C for 1 h, the bacteria were lysed in a French Press (three passes) at roughly 1500 psi. Heavy debris was removed by centrifugation at 9.600 × g in a JLA 16.250 rotor (20 min at 4 °C), and lighter materials were removed by centrifugation of the resulting supernatant at 39,000 × g in a JA 25.50 rotor (20 min at 4 °C). Membranes in the supernatant obtained above were pelleted by ultracentrifugation at 125,000 × g using a 50.2Ti rotor and Beckman L8–80 M preparative ultracentrifuge. The brownish membrane pellet was resuspended in 3–6 ml of buffer, and aliquots of the mixture were snap frozen and stored in liquid nitrogen.
Membrane preparations had the following characteristics after resuspension. The protein concentration of resuspended membranes ranged from 7.5–20 mg/ml as determined by the BCA method. Difference spectra (dithionite reduced minus air oxidized) of membranes showed absorbance peaks characteristic of cytochromes b, c, and a (20). Based on the absorbance of cytochrome a + a3, the concentration of cytochrome aa3 oxidase was between 2–5 μm. Membrane preparations exhibited an active oxidative phosphorylation pathway, catalyzing the oxidation of NADH and the reduction of O2. Under appropriate conditions, they were able to catalyze the phosphorylation of ADP to ATP using either NADH or succinate as an electron donor. ATP synthase activity was measured using the luciferin/luciferase bioluminescence detection system.
Membranes from Escherichia coli, Paracoccus denitrificans, Pseudomonas aeruginosa, and Staphylococcus aureus were prepared in a similar manner to those from M. smegmatis except that S. aureus was treated with lysostaphin instead of lysozyme to make spheroplasts. All were grown aerobically in LB media at 37 °C to midlog phase. S. cerevisiae was grown in YPD media, and mitochondria and submitochondrial membranes were prepared according to published procedures (21, 22). Rat liver mitochondria were prepared according to published procedures (23).
Synthesis of KS6
KS6 is a more soluble analog of CFZ. Data and properties of KS6 are presented in the supplementary material.
Isolation of Recombinant NDH-2
Construction of the recombinant expression system of M. tuberculosis type II NADH oxidoreductase (NDH-2) in M. smegmatis and purification of the intact enzyme were described previously (24). In this study, extraction of NDH-2 from membranes and chromatography on TALON resin were performed using Big CHAP instead of cholate. We found that Big CHAP provided greater enzyme stability than cholate. SDS-PAGE of the purified enzyme demonstrated one major band with an apparent mass of 50 kDa.
Enzyme Assays
In assays of NADH oxidation, buffer and NADH concentrations were typically 0.1 m HEPES/Na+, pH 7.0, and 250 μm, respectively. The NADH concentration was roughly 10-fold more than the apparent Km for membrane catalysis of NADH oxidation. Stock membrane preparations (7.5–20 mg/ml) were typically diluted >50-fold in NADH oxidase assays. (Specific protein concentrations in assays are in the figure legends.) A 5.0 mm CFZ stock was made in 100% Me2SO. Additions to assay mixtures were made so that the final Me2SO concentration was <2%. KCN (2.0 m stock) added to reactions was made fresh in 1.0 m HEPES, pH 7.0. The final KCN concentration of 10–20 mm in reactions inhibited >90% of the membrane catalyzed NADH oxidase activity.
Extinction coefficients (mm−1 cm−1) used in conversions were 6.22 for NADH consumption at 340 nm; 54 for resorufin formation at 563 nm; 35 for CFZ at 452 nm in 100% EtOH, and 33 for cytochrome aa3 at 604 nm (25). Absorbance and spectra were recorded using a Beckman DU640 UV-visible spectrophotometer. ROS production was assayed using the Tecan Infinite M1000 plate reader.
Measurement of ROS
The detection of ROS followed the procedure described by Invitrogen. The detection system consisted of the dye, Amplex Red, HRP, and superoxide dismutase mixture, which was added to reactions with membranes or purified NDH-2. In this system, HRP catalyzes the stoichiometric reaction of Amplex Red + H2O2 → water + resorufin; the product, resorufin, is monitored at 563 nm. Reactions measuring ROS contained buffer (50 mm HEPES, pH 7.0, and 2.0 mm MgCl2), 50 μm Amplex Red, 80 units/ml superoxide dismutase, and 2 units/ml HRP. Reduced CFZ most likely reacts with O2 to produce the ROS species O2− (O2 + e− → O2−), which is converted to hydrogen peroxide in the presence of superoxide dismutase according to the following reaction: 2O2− + 2H+ → H2O2 + O2 (Amplex Red + H2O2 → water + resorufin). The oxidation of Amplex Red is a two-electron transfer; thus, oxidation of one molecule of NADH (2e−) can produce one molecule of resorufin.
Measurement of M. smegmatis Growth Inhibition by CFZ and INH
M. smegmatis strain mc2 155 (ATCC) was grown overnight in Difco Middlebrook 7H9 broth, supplemented with 0.2% glycerol and 0.1% Tween 80. The culture was then adjusted to an A600 nm of 0.8 and by four successive 10-fold dilution steps, diluted to predicted final A600 nm of 0.00008. Fifty μl of the final dilution was spread over 6.0-cm diameter plates containing 7.0 ml of 7H9/0.2% glycerol/0.1% Tween 80 agar. Growth inhibitors and/or antioxidants were added to the agar mixture before solidification. Data reported are the number of colonies on the plate after 4 days of incubation at 37 °C.
RESULTS
Distribution and Charge of CFZ in Reactions with Isolated Bacterial Membranes
The reactivity of CFZ is difficult to study because of its insolubility in water. The dye is a weak base of neutral charge. The calculated octanol/water partition ratio (cLogP) for the base is extremely high at 7.5, indicating that it is highly lipophilic and will dissolve much better in hydrophobic than aqueous environments. The binding of a proton produces a cationic species, which could alter solubility properties. Important to our study, charged and uncharged species were shown to differ in redox potential (Em = −950 mV versus −180 mV for uncharged and cationic species, respectively) with only the cationic species predicted to be redox active under physiological conditions (26). Therefore, to better understand the state of CFZ in our reactions containing isolated bacterial membranes and in live bacteria, the solubility and species composition of CFZ in water, detergents, and membranes were characterized as a function of pH (details in supplemental material).
Solubility of CFZ was determined using centrifugation to separate aggregated/precipitated from soluble material and spectroscopy to quantify CFZ. CFZ added to buffer was initially solubilized in Me2SO and diluted 100-fold into water containing buffer, detergents, or membranes. Consistent with dissociation of a proton to produce a neutral form, CFZ solubility in water decreased as the pH was raised from 3.5 to 8.0. At pH 7.0, soluble CFZ was near the limit of detection (absorbance of 0.025 at 492 nm). Below pH 7.0, the visible spectrum of buffer-soluble CFZ reflected that reported for the cationic species (9, 19). In agreement with a previous study (27), CFZ solubility increased markedly in the presence of a neutral (Triton X-100) and an anionic detergent (lauroyl sarcosine). A concentration of at least 50 μm CFZ was soluble in 0.5–1.0% (w/v) detergents over the pH range of 5.0–10.0. Two spectral species consistent with cationic and neutral forms of CFZ were observed in Triton-X 100, and their proportion appeared modulated by the titration of a group with pKa of 6.8. In contrast to Triton X-100, cationic CFZ dominated in anionic detergents until pH 10.0, indicating its stabilization by roughly 3.0 pH units relative to Triton X-100. Addition of isolated membranes from M. smegmatis or E. coli to solutions also increased the solubility of CFZ. Similar to anionic detergents, membranes at pH 7.0 preferred to bind cationic CFZ especially at low CFZ/membrane ratios (see supplemental Fig. S3), suggesting stabilization by negatively charged lipids. Solubilization and stabilization of cationic species describes how the redox active form can exist at pH 7. 0. The amount of CFZ incorporated into M. smegmatis membranes was substantial and estimated from our studies to be as high as 0.5 μg/μg membrane protein at pH 7.0. Because CFZ will be mostly membrane bound in our reactions, the reporting of a CFZ concentration in reactions would be misleading. Therefore, in studies we report CFZ as μg added to a reaction volume. In reactions, CFZ did not exceed its predicted membrane saturation level unless noted.
Effect of CFZ on NADH Oxidation Catalyzed by Isolated Bacterial Membranes
Early work with CFZ reported partial restoration of O2 consumption when the compound was added to cultures of M. tuberculosis treated with KCN; a result suggesting the reduction of CFZ by the respiratory chain (1). To further investigate this possibility, the effect of CFZ on the respiratory chain activity of membranes isolated from M. smegmatis and E. coli were examined.
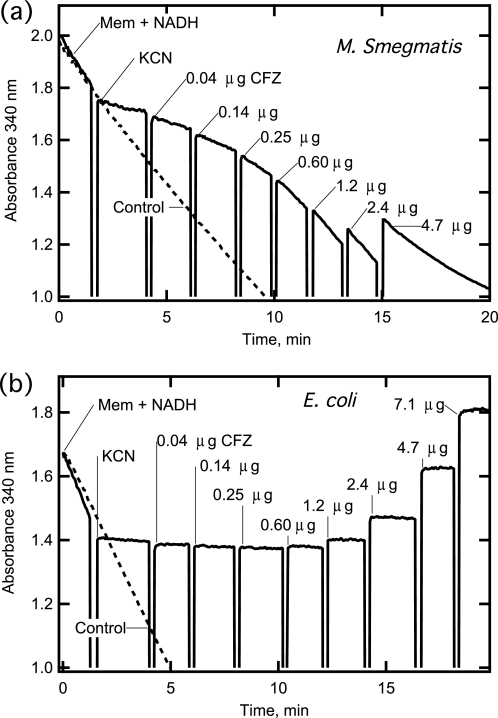
Membranes from both M. smegmatis and E. coli catalyzed the oxidation of NADH measured by loss of absorbance at 340 nm (Fig. 2). NADH oxidation was ≥90% inhibited by KCN addition, indicating that most of the activity was mediated by respiratory chain enzymes transferring electrons to O2. Addition of CFZ to inhibited M. smegmatis membranes restored a significant level of NADH oxidation, as shown by increases in the rate of absorbance decrease relative to that remaining after KCN addition (Fig. 2a). NADH (250 μm) in the reaction was in molar excess of CFZ, which was equivalent to 40 μm after all additions. Despite the difference, NADH was continuously consumed until it was fully oxidized when reactions were monitored for longer times. Thus, CFZ acted in a catalytic manner to provide a new path for electrons from NADH to exit the respiratory chain. By comparison, cyanide-inhibited E. coli membranes showed no restoration of NADH oxidation upon CFZ addition, consistent with the bacterial specificity of CFZ (Fig. 2b).
FIGURE 2.
Effect of CFZ on NADH oxidation catalyzed by isolated M. smegmatis and E. coli membranes. a, NADH oxidation catalyzed by M. smegmatis membranes (Mem) was monitored at 340 nm in a cuvette. Reactions were performed in total volume of 250 μl containing 10.0 μg membrane protein, 250 μm NADH with 20 mm KCN and CFZ added as indicated. Breaks in the trace indicate additions to the reaction; the amounts of CFZ reported are cumulative values. Dashed line indicates “control” activity measured in the absence of KCN and CFZ. The sharp absorbance increases at ≥4.7 μg/ml CFZ are due in part to CFZ absorbance and possibly to light scattering upon saturation of membranes with CFZ. b, NADH oxidation by E. coli membranes was measured similarly. Reaction conditions were the same, except membrane protein added was 4.0 μg membrane protein and KCN concentration was 5.0 mm.
The CFZ-related increases in NADH oxidase activity in Fig. 2a and similar experiments with different membrane preparations were quantified, and the data were presented in Fig. 3a as the fraction of NADH activity restored by addition of CFZ. NADH oxidation rates were estimated from the slopes of lines generated in studies similar to those in Fig. 2a. The points at 0.0 CFZ is the NADH oxidase activity remaining after addition of KCN. Membranes in reactions had roughly the same starting NADH oxidase activity; that is, the amount of membrane added in reactions produced a decrease in 340 nm absorbance of ∼0.1/min. A relatively large range of CFZ additions was used to study effects on NADH oxidase activity as membrane levels approached (and possibly slightly exceeded) saturation. We estimate that saturation was approached at ∼5.0 μg of CFZ.
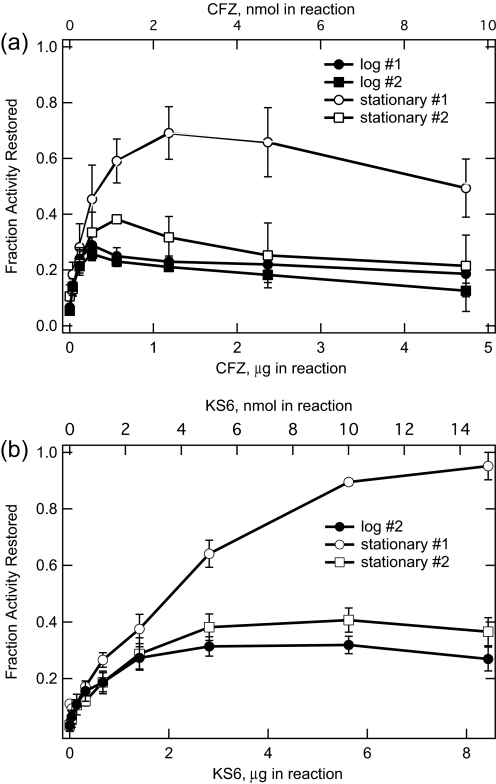
FIGURE 3.
Ability of CFZ (a) or KS6 (b) to restore NADH oxidase activity in isolated M. smegmatis membrane preparations treated with KCN. a, NADH oxidase activity as a function of the amount of CFZ added to reactions was determined from time courses performed as in Fig. 2. Oxidation rates were determined from the slopes of the lines associated with the various additions. Fraction activity restored is the ratio of the NADH oxidation rates produced by CFZ addition to membrane preparations after KCN addition as in Fig. 2, divided by the NADH oxidation rate before the addition of KCN. All rates were measured as ΔA340 nm/min. The rate at 0.0 CFZ is the fractional activity remaining after KCN addition. Sets 1 and 2 refer to membrane preparations in two time-separated studies (approximately 1 year apart). The amount of membrane added to reactions was standardized to have an activity in the range of 0.1 ΔA/min at 340 nm; the measured control NADH oxidase rates averaged 0.130 ± 0.5 (n = 13) for log membranes and 0.114 ± 0.04 (n = 15) for stationary membranes. Standardized to protein, stationary phase membranes had less activity than log phase membrane; thus, to achieve similar activities, log phase reactions contained an average of 3.8 ± 1.6 μg membrane protein, and stationary phase reactions contained an average of 13.0 ± 5.5 μg membrane protein. b, experimental conditions were similar to those in a, except that KS6 was added to reactions instead of CFZ. Data are the average and S.D. of three measurements. In the case of set 2 membranes, membrane preparations from the three separate log phase growths were each assayed once, and the results were averaged, and membrane preparations from the three stationary phase growths were each assayed once, and the results were averaged. In the case of set 1, only stationary stage membranes were assayed, two different membrane preparations were assayed separately, and the results were averaged; the data reported are the average values, and the error bars are the deviation from the average. The mass of KS6 is greater than that of CFZ (562 versus 473 Da), which accounts for the difference in x axis values in the two panels.
M. smegmatis membranes derived from log and stationary phase growth was studied to determine whether stage of growth affected membrane reactivity with CFZ. Sets 1 and 2 in Fig. 3a refer to chronologically separated studies involving several membrane preparations derived from time-monitored growths. Set 1 consists of three preparations: two from cultures grown into stationary phase and one from a culture grown into mid-log phase. The data presented for the stationary phase membranes is the average ± S.E. of five measurements (three with one preparation and two with the other). The data shown in Fig. 2a is included in the above measurements. The log growth data is the average ± S.D. of three measurements made with the one mid-log phase growth. Set 2 consisted of six separate membrane preparations; three were grown into stationary phase, and three were grown into mid-log phase. Both the stationary and log data presented are the average ± S.D. of three measurements, one with each membrane preparation.
All membrane preparations showed significant restoration of NADH oxidase activity. Although the extent of activity restoration differed between log and stationary phase membranes, profiles were similar exhibiting a progressive increase to a maximum followed by a plateau or slight decline. We suggest that the progressive increase is due to the rapid accumulation of the cationic CFZ species (redox active) in membranes at low saturation levels (see supplemental material), whereas the flattening/decline may be the result of a limit on membrane incorporation of the cationic form coupled with possible adverse effects on membrane integrity (fluidity and or porosity) affecting enzyme reactions. It is important to note that even at high amounts of added CFZ, NADH oxidase activity continues.
As described in the supplemental material, we synthesized a more soluble form of CFZ (referred to as KS6) by replacement of the R-imino isopropyl group with an aminoethoxylethoxyethyl group (Fig. 1). The modification was not expected to change the redox characteristics, and as shown in Fig. 3b, KS6 restores NADH oxidation in reactions with KCN-treated membranes. NADH oxidase activity generated by KS6 increased more gradually than CFZ, and the dose-dependent profile appeared more characteristic of an enzyme-substrate interaction, perhaps reflecting its greater water solubility.
The ability of log phase membranes from both studies (sets 1 and 2) to restore NADH oxidase activity was similar (maximum of 0.25). Restoration levels derived from stationary phases were higher (maximum of 0.40–0.70) but varied. The reason for membrane variation in extent of restoration is unclear. The transition from log to stationary phase does not completely explain the variation, though it may contribute. It cannot be discounted that the high pressure method needed to disrupt mycobacteria may lead to variable extents of membrane damage and compositional differences among preparations. Variation in NADH oxidase activity restoration levels was also noted in preparations where growth phase was not carefully documented. In these preparations, restoration was never less than that observed for log phase membranes and sometimes approached that of the most active stationary phase. Regardless of the reason for the variation, the rate of CFZ-mediated NADH oxidase activity in all membrane preparations was on scale with the rate of the electron transport chain at saturating levels of NADH. Thus, CFZ-mediated NADH oxidase activity could have consequential affects on mycobacterial physiology.
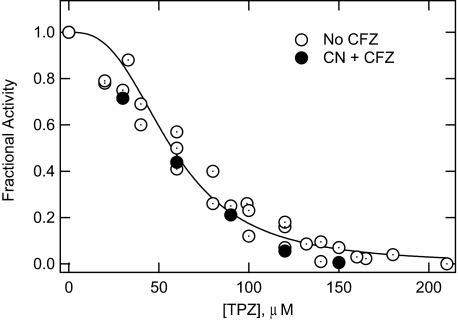
Based on previous studies, it was tacitly assumed that the CFZ-mediated NADH oxidase activity involved a respiratory chain oxidoreductase. However, so far, the data do not rule out a nonrespiratory chain enzyme because control NADH oxidase activity measurements were based on the ability to transfer electrons/H+ to O2 and would not measure the activity of an NADH-dependent oxidoreductase requiring a different electron acceptor to function. To directly implicate a respiratory chain enzyme(s), the effect of the drug TPZ on NADH oxidase activity and CFZ-mediated NADH oxidase activity was investigated (Fig. 4). Previously, we showed that TPZ specifically inhibits the mycobacterial respiratory chain enzyme, NDH-2 (20, 24). NDH-2 is the primary NADH:quinone oxidoreduction expressed by M. smegmatis as well as other mycobacteria (20, 24) and therefore is responsible for the transfer of electrons from NADH into the electron transport chain. As shown in Fig. 4, TPZ inhibited CFZ-mediated NADH oxidase activity in log and stationary membranes similarly to control NADH activity, strongly indicating that a respiratory chain enzyme(s) was interacting with CFZ.
FIGURE 4.
TPZ inhibition of M. smegmatis NADH oxidase activity in the absence and presence of KCN/CFZ. Reactions, 250 μl total volume, contained 0.1 m HEPES pH 7.0, 225 μm NADH, and M. smegmatis membranes (10 μg membrane protein) from a preparation highly reactive with CFZ (see Fig. 2). NADH oxidase rates (loss/min of absorbance at 340 nm) were measured from time-courses as shown in Fig. 1. TPZ was added to reactions without (open circles) or with KCN/CFZ (20 mm/0.5 μg, filled circles). Fractional activity is the rate of absorbance loss in presence of TPZ relative to the control without TPZ. Line fit through the data were obtained using the Hill equation with an exponent of 3.0.
Reduction and Oxidation of CFZ by Isolated M. smegmatis Membranes
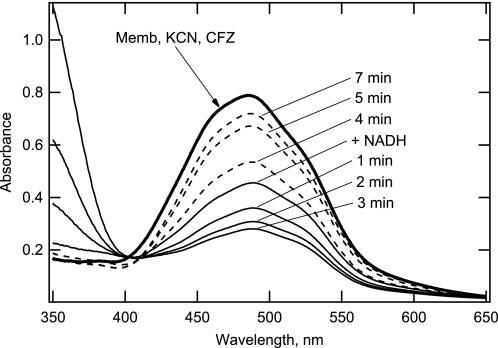
Given the reported redox properties of CFZ (1, 2, 26), NADH oxidation mediated by CFZ likely involves the reduction of CFZ itself and then its spontaneous reoxidation by O2. Evidence for this mechanism of action is presented in Figs. 5 and 6. The reduction of CFZ during NADH oxidation was demonstrated by the bleaching of the drug during reactions with KCN-treated membranes (Fig. 5). In this study, a relatively large amount of M. smegmatis membranes from a preparation that showed high CFZ restoration of NADH oxidase activity was first incubated with KCN and CFZ. The absorption spectrum shown in Fig. 5 for these treated membranes reflected that of the redox active cationic form of CFZ (Fig. 5, thick line); the spectrum did not change with time. NADH was then added to the incubation, and spectra were recorded at the times shown. The NADH absorption peak at 340 nm does not overlap with that of CFZ (400–600 nm) in the visible region but is the major contributor to the spectra from 350 to 400 nm. A marked loss of CFZ absorbance was observed after addition of NADH that continued for ∼3 min until the NADH became depleted (Fig. 5, solid lines). After this time, the absorbance in the CFZ region of the spectrum began to increase returning to near that of the starting spectrum after 4–7 min (Fig. 5, dashed lines). The bleaching of the visible absorbance spectrum is consistent with the reduction of CFZ, and the return of absorbance is consistent with spontaneous reoxidation of reduced CFZ.
FIGURE 5.
Reduction and re-oxidation of CFZ during a reaction with KCN-treated M. smegmatis membranes. Spectra were obtained from a single reaction in a total volume of 500 μl monitored over time; background absorbance due to membranes was subtracted. Membranes (Memb, 80 μg membrane protein) from a highly CFZ-responsive preparation were preincubated with 10 mm KCN and 4.8 μg of CFZ; the spectrum did not change with time (thick line). NADH (225 μm) was then added, and the spectrum was recorded immediately (+ NADH). Additional spectra were then recorded at ∼1.0-min intervals. Absorbance in the region of 350–400 nm is from NADH and that from 400–550 nm is from CFZ. Spectra depicted with a solid line show change in CFZ (reduction phase) correlating with decrease in NADH absorbance, and those depicted with a dotted line show CFZ absorbance change (reoxidation phase) after virtually complete oxidation of NADH.
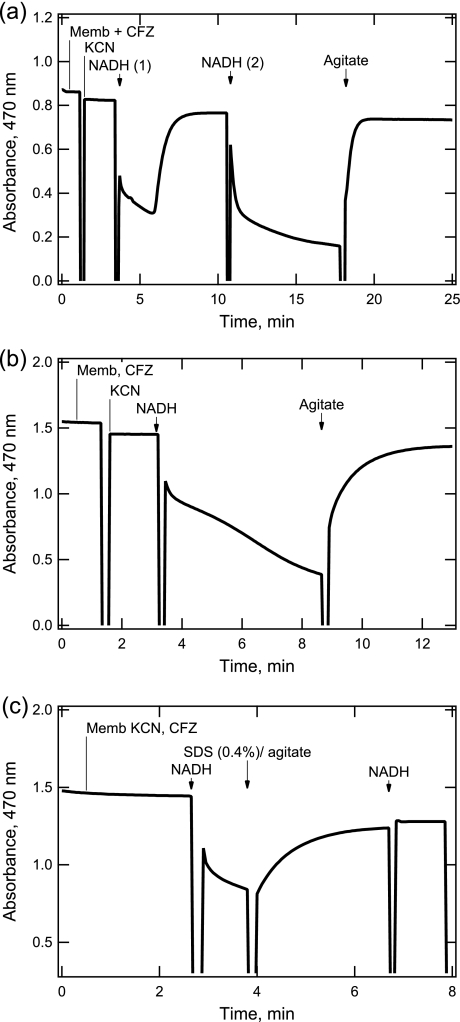
FIGURE 6.
Characterization of CFZ reduction and reoxidation in reactions with KCN-treated M. smegmatis membranes. a, time course showing reduction (absorbance decrease) and spontaneous oxidation of CFZ. Reaction conditions were similar to those in Fig. 5, except that absorbance was monitored continuously at a single wavelength of 470 nm. Breaks in the trace are due to raising/closing the door of the spectrophotometer to make additions. The order of addition to the 250-μl reaction were 1) membranes (40 μg membrane protein) and 2.4 μg CFZ, 2) 20 mm KCN, 3) 225 μm NADH, 4) second addition of 225 μm NADH after return of absorbance, and 5) manual agitation of the reaction by vigorous shaking of the cuvette. b, the reactions was performed similar to a, except that the NADH and CFZ concentrations were increased. The order of addition to the 250 μl reaction were 1) membranes (Memb, 85 μg membrane protein) and 6.0 μg CFZ, 2) 20 mm KCN, 3) 500 μm NADH, and 4) agitation of the cuvette. c, same as b, except for the addition of SDS during the slow phase of absorbance loss. Additions to reaction were 1) membranes, KCN, and CFZ as in b, 2) 225 μm NADH, 3) SDS (0.4% final) with agitation, and 4) second addition of NADH.
The reduction and oxidation of CFZ was characterized further in time course studies monitoring absorbance at 470 nm, the isosbestic point of the cationic and/neutral forms of CFZ (see supplemental material). As shown in Fig. 6a, the absorbance change followed a time course similar to that shown in Fig. 4. There was a rapid decrease in absorbance upon NADH addition, which slowed and then returned to near the starting absorbance. The reversibility of the redox change was demonstrated when a second addition of NADH added to reoxidized CFZ induced an absorbance decrease. The second decrease did not reverse until the cuvette containing the reaction was manually shaken. Agitation likely raised the O2 concentration in the reaction, which was depleted during the first cycle of reduction/oxidation. A similar change in absorbance was observed when the reactions were performed under anaerobic conditions and then exposed to air (data not shown).
To drive the O2 concentration lower during the reaction, an incubation with a 2-fold higher concentration of NADH was incubated with twice the CFZ as shown in Fig. 6a. The higher concentration of NADH produced a prolonged slow phase with no spontaneous reoxidation until the cuvette was agitated. The return of absorbance after agitation fitted well to a first (pseudo) order process with an observed rate constant of 1.25 ± 0.01 min−1 (error in fit). Assuming that agitation saturates the reaction with O2 (250 μm at 1 atm pressure), the second order rate constant calculated for the reoxidation was 83 m−1 s−1.
Reduced forms of phenazines tend to react spontaneously with O2 (1, 2). The nonenzymatic oxidation of reduced CFZ in our reaction is shown in Fig. 6c, where SDS is introduced into the reaction to denature all enzymes. This reaction was performed as in Fig. 6a except that before reoxidation occurred, 0.5% SDS (final) was added to denature the system. As shown, SDS did not inhibit the reoxidation initiated by agitation upon the addition of the detergent. The reoxidation fitted well to a first order process with an observed rate constant 1.40 ± 0.01 min−1, which is similar to that observed in the absence of SDS. CFZ reoxidized in the presence of SDS did not show an absorbance loss upon a further addition of NADH, confirming the denaturation of membrane enzymes. Similarly, SDS added at the beginning of a new reaction blocked CFZ reduction by NADH (data not shown). Reoxidation also was observed when CFZ, which had been chemically reduced by dithionite in absence of membranes, was exposed to air (data not shown).
NADH Oxidation by Membranes in Presence of CFZ Produces ROS
The spontaneous reoxidation of reduced CFZ by O2 is likely to produce ROS, probably O2−. To show ROS was generated in our membrane assays, a coupled assay was devised to detect ROS. The detection system consisted of superoxide dismutase, HRP, and Amplex Red, a dye. Superoxide dismutase converts O2− to H2O2 which reacts via HRP to oxidize Amplex Red to resorufin; the latter compound is quantified by absorbance at 563 nm. Because this coupled reaction measures product formation, it was not necessary to add KCN (KCN also inhibits HRP) to inhibit the electron transport chain. Thus, these studies also provide evidence on the ability of CFZ to abstract electrons from the respiratory chain while it is functioning.
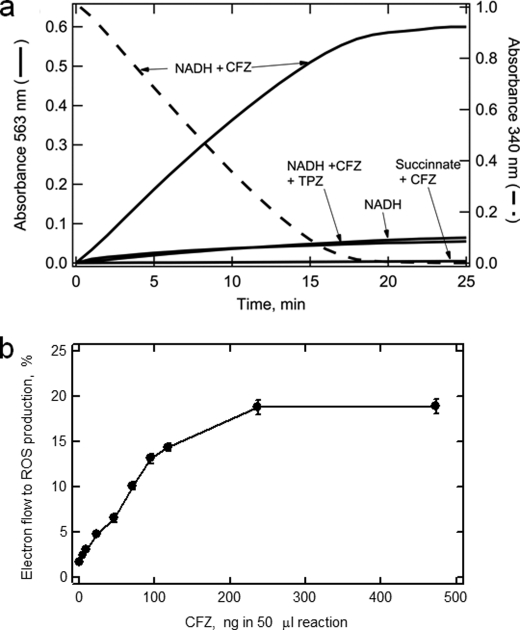
As shown in Fig. 7a, NADH oxidation by log-phase membranes in the absence of CFZ showed a low rate of ROS production. The rate of ROS production increased markedly (11.5-fold) over the background rate upon addition of CFZ to the reaction. The rate of ROS production was similar to the rate of NADH oxidation measured in a parallel reaction monitored at 340 nm. In these reactions, the rate of electron flux through the respiratory chain was near maximal because the NADH concentration was close to saturating for the system. ROS production was sensitive to TPZ, consistent with the involvement of a respiratory chain enzyme in CFZ-mediated ROS production. Thus, CFZ is able to withdraw electrons from a normally functioning electron transport chain to produce ROS.
FIGURE 7.
ROS production catalyzed by M. smegmatis log-phase membranes in the absence and presence of CFZ. a, demonstration of CFZ-mediated ROS production. NADH oxidation and ROS production were measured in separate reactions following absorbance at either 340 nm (right axis, dashed line) or 563 nm (left axis, solid lines), respectively. Reactions were preformed in 1.0 ml containing 20 μg membrane protein, either NADH (0.2 mm) or succinate (1.0 mm) as electron donors, CFZ (0.5 μg) or TPZ (0.2 mm), and ROS detection reagents as described under “Experimental Procedures.” b, dependence of ROS production on the amount of CFZ. This study was performed in 50 μl total volume using a plate reader (Tecan Corp.). The reaction contained 1.0 μg of log-phase membranes and different amounts of CFZ as indicated in the panel. Parallel reactions were monitored at 340 nm and 563 nm. At each concentration, initial velocities for the rate of NADH consumption and resorufin production were used to calculate flux of electrons producing resorufin relative to NADH oxidation rate (y axis) as described under “Experimental Procedures.”
In Fig. 7b, the dependence of ROS production on the amount of CFZ in the reaction is shown. Rates of reaction in the absence and presence of CFZ were measured as in Fig. 7a. The data is plotted to show the proportion of the electron flux from NADH oxidation that went to the production of ROS. The percentage of NADH electrons producing resorufin was estimated using the extinction coefficients (listed under “Experimental Procedures”) for NADH oxidation, a two-electron transfer, and resorufin production from Amplex Red, also a two-electron transfer. The rate of ROS production appears to increase progressively before attaining a maximum at a CFZ/membrane protein ratio of 0.1–0.2 μg CFZ/μg membrane protein. Based on this maximum value, the resorufin production was ∼18% of the rate of NADH consumption. Given that NADH oxidation by the respiratory chain is a continual process, this estimate suggests that significant levels of ROS production will occur within M. smegmatis upon accumulation of CFZ in the membrane.
Other Reductants
Menaquinone is a respiratory chain component that shuttles electrons/H+ derived from the oxidation of NADH, or citric acid cycle intermediates succinate and malate, to the respiratory chain oxidoreductases. In M. smegmatis, menaquinone is reduced by at least three reactions: 1) NADH via NDH-2, 2) succinate via succinate dehydrogenase (producing fumarate which is the oxidized product), and malate via malate:menaquinone oxidoreductase (producing oxaloacetate which is the oxidized product). The ability of NADH to reduce CFZ indicates that NDH-2 has a role in the reduction but does not eliminate the other respiratory chain enzymes (cytochrome bc1 complex and terminal oxidases). If respiratory chain oxidoreductases other than NDH-2 reduce CFZ, succinate should be able to drive CFZ reduction. When succinate was substituted for NADH in our reactions, neither CFZ reduction nor ROS production was observed. In experiments similar to those described in Fig. 6, 10 mm succinate did not produce CFZ reduction. The inability of succinate to drive ROS production is shown in Fig. 7a. Succinate dehydrogenase was active in isolated membranes as succinate could drive O2 reduction and ATP synthesis. These results along with the inhibition CFZ-mediated NADH oxidation and ROS production by TPZ (Figs. 4 and 7) strongly suggest that NDH-2, rather than the downstream oxidoreductase cytochrome bc1 complex, cytochrome aa3, or bd oxidase, is the respiratory chain oxidoreductase catalyzing CFZ reduction.
Interaction of Purified NDH-2 with CFZ
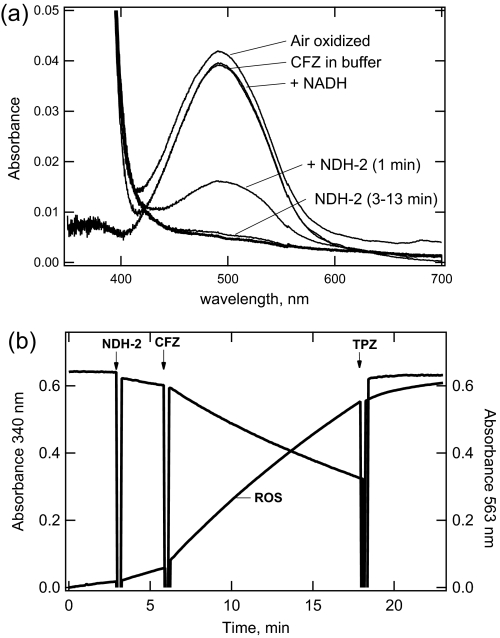
To more conclusively show that NDH-2 is the respiratory enzyme able to reduce CFZ, studies were performed using purified recombinant NDH-2 instead of isolated membranes. Previously, we reported the expression and purification of recombinant M. tuberculosis NDH-2 (24). In Fig. 8a, the reduction of CFZ by purified NDH-2 is shown. This study was performed in a manner similar to that shown in Fig. 5, except that the incubation was performed under anaerobic conditions, and the reaction pH was lowered to 6.5 to increase CFZ solubility.
FIGURE 8.
Reduction of CFZ (a) and ROS production (b) catalyzed by purified recombinant M. tuberculosis NDH-2. a, spectra showing reduction of CFZ by NDH-2 under anaerobic conditions and subsequent reoxidation. Stock CFZ in Me2SO was added to a solution of 50 mm MOPS/Na+, pH 6.5, 2.0 mm MgCl2 to produce a final concentration of 1.18 μg/ml. Based on spectra taken during a 40-min period (CFZ in buffer), ∼70% of the CFZ remained in solution exhibiting a spectrum consistent with cationic CFZ. NADH (200 μm) was then added; it did not produce a significant change during a 10-min incubation period (+ NADH). Purified NDH-2 (2.7 μg/ml, 0.01% Big CHAPS at final) was then added to start the reaction (1 min), and additional spectra were taken at the indicated times. After the absorbance loss was complete, the cuvette was exposed to air for 20 min after which a spectrum was recorded (air-oxidized). b, production of ROS by purified NDH-2. NADH oxidation (340 nm absorbance decrease) and ROS generation (absorbance increase, 563 nm) catalyzed by NDH-2 was measured under aerobic conditions similar to the reactions in Fig. 7. Reactions were performed in a total volume of 1.0 ml and contained 200 μm NADH with an ROS detection system. At the indicated times, NDH-2 was added to final concentration of 2.0 μg/ml, followed by CFZ to a final of 1.0 μg/ml or TPZ to a final of 200 μm.
To perform this reaction (Fig. 8b), CFZ was added to the reaction buffer to achieve a concentration equivalent to 1.2 μg/ml. After a 40-min incubation, the absorption spectrum (CFZ in buffer) was taken. About two-thirds of CFZ remained soluble with spectra reflecting the cationic species. NADH was then added to the reaction to a final concentration of 0.2 mm. A spectrum taken after 10 min of incubation (+ NADH) showed no change other than that attributed to NADH below 400 nm. The reaction was started by addition of purified NDH-2, which produced a rapid decrease in the visible spectrum, indicating reduction of CFZ. After 13 min of further incubation, the bleaching appeared complete. The cuvette was then exposed to air for 20 min. The spectrum after this incubation (air-oxidized) was consistent with the return of the oxidized CFZ. Finally, 0.1% SDS was added to the cuvette. This addition solubilized the precipitated material, which was in the oxidized form (data not shown). Thus, all of the CFZ was accounted for as oxidized after exposure to air.
In Fig. 8b, the generation of ROS by the incubation of purified recombinant NDH-2 with NADH and CFZ is shown. ROS formation was inhibited by TPZ added during the reaction. Taken together, both studies in Fig. 8 demonstrate that purified NDH-2 reduces CFZ similar to reactions with membranes.
Effect of Riminophenazines on Respiratory Chain of Other Bacteria, Yeast, and Mammalian Mitochondria
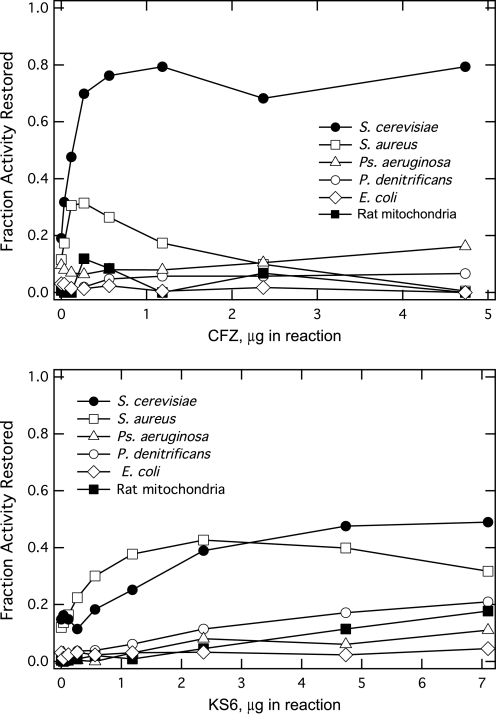
In Fig. 2, we showed that CFZ could not restore NADH oxidase activity in E. coli membranes inhibited with KCN addition, indicating that CFZ was not reactive with membranes from all bacteria. To further investigate the species specificity of CFZ, studies similar to those shown in Fig. 2 were carried out using membranes from three Gram-negative bacteria, E. coli, P. denitrificans, and P. aeruginosa, a Gram-positive bacterium, S. aureus, and submitochondrial membranes from S. cerevisiae and rat mitochondria. Membranes prepared from the three Gram-negative organisms and rat mitochondria showed little, if any, restoration of NADH oxidation over background upon addition of CFZ (Fig. 9a) or KS6 (Fig. 9b). In contrast, membranes from S. aureus and submitochondrial membranes from S. cerevisiae demonstrated significant restoration of NADH oxidation over background. Similar results were also shown for ROS production using the assay described in Fig. 7 (data not shown).
FIGURE 9.
CFZ-mediated (a) and KS6-mediated (b) restoration of NADH oxidation by membranes isolated from nonmycobacterial organisms. Measurements and data reported were made as described in Figs. 2 and 3. Bacterial membranes in the assays had a starting NADH oxidase activity of ∼0.1 ΔA/min at 340 nm/min. The mitochondrial preparations had an activity of 0.05 ΔA/min at 340 nm.
The growth of S. aureus and S. cerevisiae are reported to be sensitive to CFZ (7, 9). In the case of S. aureus, Fig. 9 shows that high levels of CFZ were inhibitory to CFZ-mediated NADH oxidation. This inhibitory effect was not seen with more soluble KS6 nor was it this dramatic with M. smegmatis membranes. The difference suggests that CFZ binding to some bacterial membranes can have significant disruptive effects on membrane enzyme activities.
Effect of CFZ and Antioxidants on Growth of M. smegmatis
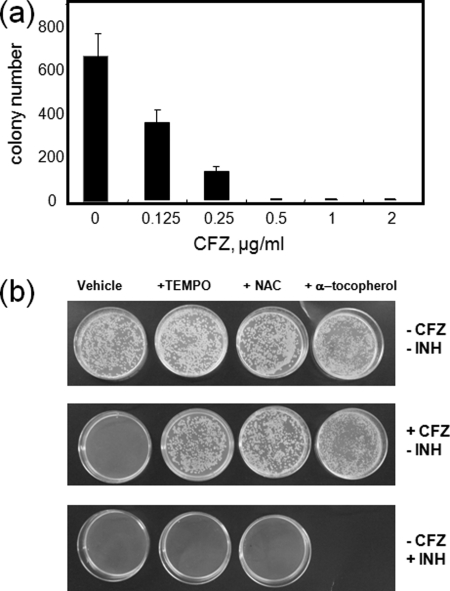
To show that ROS generation is responsible for inhibition of M. smegmatis growth in culture, the effect of antioxidants on CFZ-mediated growth inhibition was investigated. As shown in Fig. 10a, incorporation of CFZ into solid medium inhibits the growth of M. smegmatis in a dose-dependent manner. Virtually no colonies appeared on plates of agar containing the equivalent of ≥0.5 μg/ml CFZ after ≥4 days of incubation, whereas control plates with no CFZ averaged 670 ± 100 colonies (Fig. 10a). As shown in Fig. 10b, growth inhibition at this CFZ concentration was prevented by addition of three different antioxidants, α-tocopherol (12.5 μg/ml), 4-hydroxy-TEMPO (5 mm), and N-acetylcysteine (5 and 10 mm). INH, an antibiotic used in the treatment of tuberculosis also completely inhibited M. smegmatis growth in this assay at a concentration of 10 μg/ml. In contrast to CFZ, the inhibitory effect of INH was not sensitive to antioxidants at the concentrations used in this study. Because INH activity is based on mycolic acid synthesis inhibition, these studies strongly implicate the production of ROS in the mechanism of action of CFZ.
FIGURE 10.
Inhibition of M. smegmatis growth by CFZ and INH and effect of antioxidants. a, effect of CFZ on colony counts of wild type M. smegmatis mc2 155 plated on 7H9 agar. Data at each concentration represent the average colony number on three plates ± S.D. b, effect of free radical scavengers on CFZ inhibition of M. smegmatis growth. M. smegmatis was grown on agar plates containing various antioxidants (5.0 mm 4-hydroxy-TEMPO, 5.0 mm N-acetylcysteine (NAC), and 12.5 μg/ml α-tocopherol) with or without 0.5 μg/ml CFZ or 10 μg/ml INH. Representative plates demonstrating bacterial growth under the various conditions are shown.
DISCUSSION
CFZ is a phenazine derivative originally developed in 1957 as an antitubercular drug (1). Its ineffectiveness in guinea pig and monkey models of tuberculosis diminished interest in developing CFZ further for a role in treating this disease. However, CFZ is used currently in the treatment of leprosy, another mycobacterial disease (2, 4). The mechanism of action of CFZ has remained unclear. Our results advance a bactericidal mechanism of action that is dependent on the redox activity of the drug.
CFZ reduction by mycobacteria was noted in the first study on the drug (1). The study reported that M. tuberculosis strain H37Rv incubated with CFZ under aerobic conditions became reddish in keeping with the color of the dye but turned colorless when switched to anaerobic conditions, indicating its reduction. Reduction appeared to involve a respiratory chain component because addition of CFZ to cultures could partially overcome cyanide inhibition of O2 consumption. Reduced forms of dyes such as CFZ were known to react with molecular O2 to form ROS, raising speculation that CFZ antimicrobial activity was related to the intracellular redox activity of the dye. Supporting this mechanism of action, isoniazid-resistant, catalase-negative mutants of M. tuberculosis were reported to be more susceptible to CFZ than wild type bacteria. Many of the same redox properties were reiterated in a 1973 study describing CFZ growth inhibition of yeast strain S. cerevisiae (9).
In the current study, we describe a pathway in M. smegmatis for ROS generation coupled to the redox cycling of CFZ (Fig. 11). Although very sparingly soluble in water at pH 7.0, we show that CFZ will concentrate in membranes and that this association stabilizes to a significant extent the cationic (protonated) form of the drug, which is reported to be the redox active form (26). Addition of CFZ to cyanide-inhibited membranes restored a significant fraction of the NADH oxidase activity. Restoration was shown to involve reduction of CFZ catalyzed by the respiratory oxidoreductase NDH-2. This reaction resulted in the direct transfer of electrons/H+ from NADH to CFZ. Reduced CFZ was unstable and spontaneously reacted with O2. A rough estimate suggests that the second order rate constant for this reaction was in the range of 100 m−1 s−1. CFZ-mediated NADH oxidation by membranes in the absence of KCN produced ROS as demonstrated using a coupled assay system to detect ROS. Taken together, the above results indicate that CFZ treatment of mycobacteria will result in the generation of a cyclical pathway to produce ROS that is fueled by NADH and O2 (Fig. 11). This cycle will continually run because NADH is a key substrate in generating ATP and is continually produced by the citric acid cycle and/or β-oxidation of fatty acids. On the other hand, the rate of the cycle could vary depending on the O2 tension in various lung microenvironments where M. tuberculosis exists in tuberculosis (28–30).
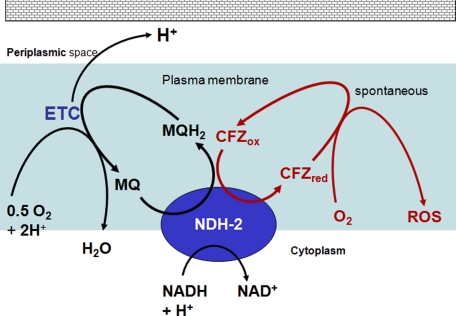
FIGURE 11.
Depiction of CFZ-mediated redox cycling and ROS production. Diagram depicting menaquinone (MQ) of the respiratory chain and CFZ as competing substrates of NDH-2. The electron transport chain (ETC) in M. smegmatis is primarily composed of two oxidoreductases in addition to NDH-2: cytochrome bc1 complex, which is reduced by menaquinol, and cytochrome aa3, which obtains electrons from cytochrome bc1 and transfers them to O2 in a coupled reaction that produces water and the translocation of protons from the cytoplasm to periplasmic space. Oxidation of reduced CFZ by oxygen occurs nonenzymatically and produces ROS. The cell wall of M. smegmatis is much thicker than depicted in the diagram.
NDH-2 is a membrane bound protein of ∼50 kDa containing a single FAD moiety (20, 31). It typically catalyzes the transfer of electrons from NADH to menaquinone, the only quinone type in mycobacteria, thereby contributing to respiratory chain activity in mycobacteria. Menaquinol continues the transfer by supplying electrons/H+ to downstream respiratory chain oxidoreductases, which in turn use the electrons/H+ to reduce O2. The catalytic reduction of CFZ by NDH-2 and generation of ROS was confirmed using purified recombinant M. tuberculosis NDH-2. Succinate dehydrogenase, a citric acid cycle oxidoreductase, also transfers electrons to menaquinone upon oxidation of succinate to fumarate. Because succinate dehydrogenase could not drive CFZ reduction, it appears that NDH-2 is the only respiratory chain oxidoreductase capable of reducing CFZ. We presume that CFZ competes with menaquinone for electrons carried by the FAD moiety of reduced NDH-2, although such competition was not documented in this study. The catalytic reduction of CFZ by NDH-2 is the first demonstration of a specific interaction between CFZ and a bacterial constituent.
To produce cell death, ROS generation must increase to a level that overcomes detoxifying pathways (32, 33). Experimental evidence suggests intracellular hydrogen peroxide levels need to be in the range of 1.0 mm for ROS to be cytotoxic/bactericidal (32, 34, 35). Such levels apparently are obtained by treatment of cells/bacteria with naphthoquinones (e.g. plumbagin and menadione) which are routinely used to place cells and bacteria under oxidative stress (36, 37). Naphthoquinones are well established redox cycling agents, with reduction/oxidation mediated by a quinone-like structural element. They are reduced by a number of enzymes, including soluble NADPH:cytochrome P450 reductases found in the cytoplasm of most cells, and complex I, the mitochondrial NADH:quinone oxidoreduction referred to as NDH-1. Like CFZ, reduced forms of naphthoquinones are nonenzymatically oxidized by molecular O2 to produce ROS.
Although described as quinoid-like (26), CFZ differs markedly in structure from naphthoquinones. It has no oxygen substituents and reduction likely involves nitrogen groups on the phenazine three-member ring in conjunction with a single imino group substituent. CFZ does not appear to be as toxic as naphthoquinones quite possibly because structural differences with more classical quinones limits reduction to specific enzymes such as NDH-2. Mitochondria and bacteria that predominantly express respiratory chain NADH:quinone oxidoreductases of the type I variety do not reduce CFZ as will be discussed below. Moreover, the administration of CFZ to humans for treatment of leprosy and other disorders does not in general produce dramatic side effects other than skin discoloration (2, 3, 12).
Although CFZ is a more selective substrate than naphthoquinones, the coupling of ROS generation to a respiratory chain oxidoreductase may provide the high levels of intracellular ROS needed to affect bacterial growth. At least in M. smegmatis and M. tuberculosis, we have shown that NDH-2 is the only oxidoreductase mediating the transfer of NADH electrons/H+ to the respiratory chain (20, 31); thus, the entire respiratory chain flux from NADH is dependent on this oxidoreductase. Studies with KCN-treated membranes showed that CFZ-mediated NADH oxidase rates in membrane preparations were relatively fast attaining 25–70% of the oxidation rate measured in the absence of KCN with saturating NADH (Fig. 3a). Under the conditions where electron flow in the respiratory chain of membranes was not inhibited by KCN addition, CFZ-mediated ROS production attained a rate that was ∼18% of the rate of the electron transport chain with near saturating NADH.
Strong support for a mechanism of action involving ROS was obtained in studies measuring bacterial growth. Colony formation on agar containing 0.5 μg/ml CFZ was undetectable. This apparent bactericidal activity was prevented by the addition of antioxidants to the medium. Three structurally different antioxidants, α-tocopherol, N-hydroxy-TEMPO, and N-acetylcysteine nullified the growth inhibitory activity of CFZ. Furthermore, their effect was specific for CFZ as INH inhibition of M. smegmatis growth was not altered by the same antioxidants. INH-mediated growth inhibition is primarily based on inhibition of mycolic acid synthesis (34, 38). The activation of INH in mycobacteria is believed to be mediated by catalase activity, implying a requirement for peroxides (34, 38). Because antioxidants should reduce cellular peroxide levels, the lack of an effect on INH inhibition is unclear. We suggest that although lower in the presence of antioxidants, the peroxide level was still sufficient to activate INH by catalase. In contrast, the same low cellular ROS level is probably not adequate to be bactericidal.
CFZ affects a spectrum of Gram-positive but not Gram-negative bacteria (8). Gram-positive species reported sensitive to CFZ other than mycobacteria include Staphylococcus, Streptococcus, Enterococcus, and Listeria. In contrast to our results, a study characterizing the effect of CFZ on the above bacteria suggested a mechanism of action not involving ROS (8). In their study, minimum inhibitory concentration values were about equal under aerobic and anaerobic conditions and unchanged in comparable strains lacking catalase activity. Although α-tocopherol antagonized the inhibitory activity of CFZ, two other antioxidants, DTT and ascorbic acid, showed no effect. Moreover, the results of tests measuring drug killing effectiveness appeared to point to a bacteriostatic rather than bactericidal mechanism of action.
Although limited in extent, our study characterizing the interaction of CFZ with S. aureus membranes suggested a response to the drug somewhat different than observed for M. smegmatis. CFZ addition to S. aureus membranes inhibited by KCN addition demonstrated a return of NADH oxidase activity (Fig. 10). However, restoration of this activity became rapidly inhibited as the CFZ dose increased. A parallel study with KS6, a more soluble form of CFZ, did not show the inhibitory activity, suggesting that the binding of CFZ to S. aureus membranes may be more structurally disruptive than to M. smegmatis membranes.
CFZ may have more than one mechanism of action due to its lipophilic nature and preference to accumulate in membranes. Stabilization of cationic CFZ, presumably by negatively charged membrane lipids could be particularly disruptive to membrane integrity (39–42). Such disruption could affect membrane functions such as K+ transport as mentioned in the Introduction. The interaction of CFZ with DNA probably also reflects the hydrophobicity of the drug. However, it is unclear why mechanisms of action based on binding to membranes or DNA should be limited to Gram-positive bacteria. We have found that low levels of CFZ inhibit ATP synthesis activity in isolated inverted bacterial membranes.3 The inhibition occurs substantially below the point of membrane saturation and might represent a second mechanism of action of a specific nature.
Gram-negative bacteria appear generally insensitive to CFZ (8). As shown here, membranes isolated from three different Gram-negative bacteria, E. coli, P. denitrificans, and P. aeruginosa did not exhibit significant CFZ-mediated NADH oxidation or ROS production. They also did not react significantly with the more soluble CFZ analog KS6. Three NADH:quinone oxidoreductases that function in the respiratory chain of bacteria have been described: NDH-1, NDH-2, and a sodium-pumping NDH (43, 44). Mycobacteria are unusual in that only NDH-2 is functional. E. coli expresses NDH-1 and NDH-2 (44), and we found both were active in our preparations of isolated membranes. Based on the use of NDH-1 specific substrate, deamino-NADH, ∼40% of the total membrane NADH oxidase activity was due to NDH-2. NDH-1 is the only functional NADH:quinone oxidoreductase associated with the respiratory chain of P. denitrificans, a bacterium used as a model system for mitochondrial respiration (45–47) and P. aeruginosa expresses three oxidoreductases in undetermined proportion (44). The negative results with these bacteria indicate that NDH-1 does not interact with CFZ and that the ability to reduce CFZ is a property of some, but not all, NDH-2 oxidoreductases. The inability of rat mitochondria to reduce CFZ is consistent with the inability of NDH-1 to reduce CFZ.
Although membranes from the above Gram-negative bacteria could not generate ROS, membranes isolated from S. aureus, a Gram-positive bacteria and submitochondrial particles isolated from S. cerevisiae were able to oxidize NADH and generate ROS in the presence of CFZ. It might appear inconsistent for S. cerevisiae, a eukaryotic organism, to show sensitivity to CFZ; however, this yeast does not express NDH-1 but uses NDH-2 type oxidoreductases in the respiratory chain (48). Our inspection of the S. aureus genome identified a gene coding for an NDH-2 type oxidoreductase, but no evidence for an operon encoding the numerous subunits composing NDH-1.
CFZ is active against multidrug-resistant strains of M. tuberculosis in vitro, and M. tuberculosis in general shows very little ability to develop resistance to the drug (3). The need for new antibiotics to combat resistant strains has renewed interest CFZ and in the development of new CFZ derivatives (4, 6, 49). In this study, we describe a more soluble analog of CFZ with a similar redox potential. Studies are underway to determine the effectiveness of this analog against tuberculosis in bacterial culture and animal models. The results should provide information on the design and development of new derivatives.
As stated in the Introduction, CFZ is reported to have anti-inflammatory effects on neutrophils, macrophages, and lymphocytes (11, 13, 49–51) and has been used as an immunosuppressant in a number of diseases. Physiological changes reported in these cells include increased ROS production. So far, the direct reduction of CFZ in these cells has not been demonstrated. This finding may indicate that an enzyme(s) other than NDH-2 is capable of efficiently reducing CFZ at least in leukocytes. In future studies, we plan to investigate further the specificity of CFZ reduction in bacteria and in leukocytes.
Acknowledgments
We thank Khisi Mdluli, Zhenkun Ma, and Takushi Kaneko of the Global Alliance for New Tuberculosis Drug Development for intellectual and financial support of this work, whose interest in reinvestigating clofazimine as a tuberculosis drug played a large role in the initiation of this work. We also thank Val Bratinov, Jennifer Yano, and Yashashree Angadi for excellent technical support throughout this study.
This work was supported, in whole or in part, by National Institutes of Health Grant R01-AI068942-02. This work was also supported by grants from the Global Alliance for New Tuberculosis Drug Development and the Institute for the Translational Medicine and Therapeutics from the University of Pennsylvania.

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Experimental Procedures,” Equation 1, Figs. S1–S8, and additional references.
T. Yano, S. Kassovska-Bratinova, J. S. Teh, J. Winkler, K. Sullivan, A. Isaacs, N. M. Schechter, and H. Rubin, unpublished observations.
- CFZ
- clofazimine
- INH
- isoniazid
- NDH-2
- NADH-quinone oxidoreductase type 2
- TPZ
- trifluoperazine
- TEMPO
- 2,2′,6,6′-tetramethylpiperidinyl-1-oxy
- KCN
- potassium cyanide.
REFERENCES
- 1. Barry V. C., Belton J. G., Conalty M. L., Denneny J. M., Edward D. W., O'Sullivan J. F., Twomey D., Winder F. (1957) Nature 179, 1013–1015 [DOI] [PubMed] [Google Scholar]
- 2. O'Connor R., O'Sullivan J. F., O'Kennedy R. (1995) Drug Metab. Rev. 27, 591–614 [DOI] [PubMed] [Google Scholar]
- 3. Reddy V. M., O'Sullivan J. F., Gangadharam P. R. J. (1999) J. Antimicrob. Chemother. 43, 615–623 [DOI] [PubMed] [Google Scholar]
- 4. Reddy V. M., Nadadhur G., Daneluzzi D., O'Sullivan J. F., Gangadharam P. R. J. (1996) Antimicrob. Agents Chemother. 40, 633–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sano K., Tomioka H., Sato K., Sano C., Kawauchi H., Cai S., Shimizu T. (2004) Antimicrob. Agents Chemother. 48, 2132–2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Van Rensburg C. E. J., Jooné G. K., Sirgel F. A., Matlola N. M., O'Sullivan J. F. (2000) Chemother. 46, 43–48 [DOI] [PubMed] [Google Scholar]
- 7. Oliva B., O'Neill A. J., Miller K., Stubbings W., Chopra I. (2004) J. Antimicrob. Chemother. 53, 435–440 [DOI] [PubMed] [Google Scholar]
- 8. Van Rensburg C. E. J., Jooné G. K., O'Sullivan J. F., Anderson R. (1992) Antimicrob. Agents Chemother. 36, 2729–2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rhodes P. M., Wilkie D. (1973) Biochem. Pharmacol. 22, 1047–1056 [DOI] [PubMed] [Google Scholar]
- 10. Krajewska M. M., Anderson R., O'Sullivan J. F. (1993) Int. J. Immunopharmac. 15, 99–111 [DOI] [PubMed] [Google Scholar]
- 11. Zeis B. M., Anderson R. (1986) Int. J. Immunopharmac. 8, 731–739 [DOI] [PubMed] [Google Scholar]
- 12. Sanchez M. R. (2000) Clin. Dermatol. 18, 131–145 [DOI] [PubMed] [Google Scholar]
- 13. Ren Y. R., Pan F., Parvez S., Fleig A., Chong C. R., Xu J., Dang Y., Zhang J., Jiang H., Penner R., Liu J. O. (2008) PLoS ONE 3, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van Rensburg C. E., Van Staden A. M., Anderson R. (1993) Cancer Res. 53, 318–323 [PubMed] [Google Scholar]
- 15. Durandt C., van Rensburg C. E. (2001) Int. J. Oncol. 19, 579–583 [DOI] [PubMed] [Google Scholar]
- 16. Franzblau S. G., White K. E., O'Sullivan J. F. (1989) Antimicrob. Agents Chemother. 33, 2004–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bopape M. C., Steel H. C., Cockeran R., Matlola N. M., Fourie P. B., Anderson R. (2004) J. Antimicrob. Chemother. 53, 971–974 [DOI] [PubMed] [Google Scholar]
- 18. Cholo M. C., Boshoff H. I., Steel H. C., Cockeran R., Matlola N. M., Downing K. J., Mizrahi V., Anderson R. (2006) J. Antimicrob. Chemother. 57, 79–84 [DOI] [PubMed] [Google Scholar]
- 19. Morrison N. E., Marley G. M. (1976) Int. J. Lepr. 44, 475–481 [PubMed] [Google Scholar]
- 20. Weinstein E. A., Yano T., Li L. S., Avarbock D., Avarbock A., Helm D., McColm A. A., Duncan K., Lonsdale J. T., Rubin H. (2005) Proc. Nat. Acad. Sci. U.S.A. 102, 4548–4553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meisinger C., Pfanner N., Truscott K. N. (2006) Meth. Mol. Biol. 313, 33039. [DOI] [PubMed] [Google Scholar]
- 22. Pon L., Moll T., Vestweber D., Marshallsay B., Schatz G. (1989) J. Cell Biol. 109, 2603–2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnson D., Lardy H. (1967) Methods Enzymol. 10, 94–101 [Google Scholar]
- 24. Yano T., Li L. S., Weinstein E., Teh J. S., Rubin H. (2006) J. Biol. Chem. 281, 11456–11463 [DOI] [PubMed] [Google Scholar]
- 25. Mochizuki M., Aoyama H., Shinzawa-Itoh K., Usui T., Tsukihara T., Yoshikawa S. (1999) J. Biol. Chem. 274, 33409–33411 [DOI] [PubMed] [Google Scholar]
- 26. Kovacic P., Ames J. R., Ryan M. D. (1989) Bioelectrochem. and Bioenerg. 21, 269–278 [Google Scholar]
- 27. Fahelelbom K. M. S., Timoney R. F., Corrigan O. I. (1993) Pharma. Res. 10, 631–634 [DOI] [PubMed] [Google Scholar]
- 28. Lenaerts A. J., Degroote M. A., Orme I. M. (2008) Trends Microbiol. 16, 48–54 [DOI] [PubMed] [Google Scholar]
- 29. Hoff D. R., Caraway M. L., Brooks E. J., Driver E. R., Ryan G. J., Peloquin C. A., Orme I. M., Basaraba R. J., Lenaerts A. J. (2008) Antimicrob. Agents Chemother. 52, 4137–4140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Russell D. G., Cardona P. J., Kim M. J., Allain S., Altare F. (2009) Nat. Immunol. 10, 943–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Teh J. S., Yano T., Rubin H. (2007) Inf. Disord. Drug Targets 7, 169–181 [DOI] [PubMed] [Google Scholar]
- 32. Keyer K., Imlay J. A. (1996) Proc. Nat. Acad. Sci. 93, 13635–13640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Raha S., Robinson B. H. (2000) Trends Biochem. Sci. 25, 502–508 [DOI] [PubMed] [Google Scholar]
- 34. Rosner J. L., Storz G. (1994) Antimicrob. Agents Chemother. 38, 1829–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kohanski M. A., Dwyer D. J., Hayete B., Lawrence C. A., Collins J. J. (2007) Cell 130, 797–810 [DOI] [PubMed] [Google Scholar]
- 36. Inbaraj J. J., Chignell C. F. (2004) Chem. Res. Toxicol. 17, 55–62 [DOI] [PubMed] [Google Scholar]
- 37. Castro F. A., Mariani D., Panek A. D., Eleutherio E. C., Pereira M. D. (2008) PLoS ONE 3, e3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Timmins G. S., Deretic V. (2006) Mol. Microbiol. 62, 1220–1227 [DOI] [PubMed] [Google Scholar]
- 39. Yang L., Gordon V. D., Mishra A., Som A., Purdy K. R., Davis M. A., Tew G. N., Wong G. C. L. (2007) J. Am. Chem. Soc 129, 12141–12147 [DOI] [PubMed] [Google Scholar]
- 40. Epand R. F., Savage P. B., Epand R. M. (2007) Biochem. Biophys. ACTA 1768, 2500–2509 [DOI] [PubMed] [Google Scholar]
- 41. Epand R. M., Rotem S., Mor A., Berno B., Epand R. F. (2008) J. Am. Chem. Soc. 130, 14346–14352 [DOI] [PubMed] [Google Scholar]
- 42. Lai X. Z., Feng Y., Pollard J., Chin J. N., Rybak M. J., Bucki R., Epand R. F., Epand R. M., Savage P. B. (2008) Acc. Chem. Res. 41, 1233–1240 [DOI] [PubMed] [Google Scholar]
- 43. Yano T., Ohnishi T. (2001) J. Bioenerg. Biomembr. 33, 213–222 [DOI] [PubMed] [Google Scholar]
- 44. Melo A. M., Bandeiras T. M., Teixeira M. (2004) Microbiol. Mol. Biol. Rev. 68, 603–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. John P., Whatley F. R. (1975) Nature 254, 495–498 [DOI] [PubMed] [Google Scholar]
- 46. Yagi T., Yano T., Di Bernardo S., Matsuno-Yagi A. (1998) Biochem. Biophys. ACTA 1364, 125–133 [DOI] [PubMed] [Google Scholar]
- 47. Baker S. C., Ferguson S. J., Ludwig B., Page M. D., Richter O. M., van Spanning R. J. (1998) Microbiol. Mol. Biol. Rev. 62, 1046–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. de Vries S., Grivell L. A. (1988) Eur. J. Biochem. 176, 377–384 [DOI] [PubMed] [Google Scholar]
- 49. Zeis B. M., Anderson R., O'Sullivan J. F. (1987) Antimicrob. Agents Chemother. 31, 789–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. van Rensburg C. E., Gatner E. M., Imkamp F. M., Anderson R. (1982) Antimicrob. Agents Chemother. 21, 693–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Niwa Y., Sakane T., Miyachi Y., Ozaki M. (1984) J. Clin. Microbiol. 20, 837–842 [DOI] [PMC free article] [PubMed] [Google Scholar]