Abstract
Dishevelled (Dvl) is a key component in the Wnt/β-catenin signaling pathway. Dvl can multimerize to form dynamic protein aggregates, which are required for the activation of downstream signaling. Upon pathway activation by Wnts, Dvl becomes phosphorylated to yield phosphorylated and shifted (PS) Dvl. Both activation of Dvl in Wnt/β-catenin signaling and Wnt-induced PS-Dvl formation are dependent on casein kinase 1 (CK1) δ/ϵ activity. However, the overexpression of CK1 was shown to dissolve Dvl aggregates, and endogenous PS-Dvl forms irrespective of whether or not the activating Wnt triggers the Wnt/β-catenin pathway. Using a combination of gain-of-function, loss-of-function, and domain mapping approaches, we attempted to solve this discrepancy regarding the role of CK1ϵ in Dvl biology. We analyzed mutual interaction of CK1δ/ϵ and two other Dvl kinases, CK2 and PAR1, in the Wnt/β-catenin pathway. We show that CK2 acts as a constitutive kinase whose activity is required for the further action of CK1ϵ. Furthermore, we demonstrate that the two consequences of CK1ϵ phosphorylation are separated both spatially and functionally; first, CK1ϵ-mediated induction of TCF/LEF-driven transcription (associated with dynamic recruitment of Axin1) is mediated via a PDZ-proline-rich region of Dvl. Second, CK1ϵ-mediated formation of PS-Dvl is mediated by the Dvl3 C terminus. Furthermore, we demonstrate with several methods that PS-Dvl has decreased ability to polymerize with other Dvls and could, thus, act as the inactive signaling intermediate. We propose a multistep and multikinase model for Dvl activation in the Wnt/β-catenin pathway that uncovers a built-in de-activation mechanism that is triggered by activating phosphorylation of Dvl by CK1δ/ϵ.
Keywords: Protein Kinases, Protein Phosphorylation, Protein-Protein Interactions, Signal Transduction, Wnt Pathway, Dishevelled
Introduction
The Wnt signaling pathway is a well conserved signaling pathway necessary during embryonic development whose dysregulation in adult tissues is linked to cancer (1, 2). Wnts are secreted glycoproteins that can activate several downstream cascades including the Wnt/β-catenin (canonical) pathway and other (noncanonical) pathways. These noncanonical pathways include planar cell polarity, the Wnt/Ca2+ pathway, and other less-defined signaling cascades. The Wnt/β-catenin pathway initiates with the binding of Wnt to its receptor Frizzled (3) and the co-receptor LRP5/6. Upon LRP5/6 phosphorylation, the complex of Frizzled and LRP5/6 recruits Axin to the cell membrane. The β-catenin destruction complex, composed of Axin, adenomatosis polyposis coli, and glycogen synthase kinase 3β, is subsequently disrupted. β-Catenin accumulates and is then translocated to the nucleus where it binds to the TCF55/LEF family of transcription factors. The binding of β-catenin to TCF/LEF removes transcriptional repressors such as Groucho and initiates the transcription of TCF/LEF target genes (1, 4).
The cytoplasmic protein Dishevelled (Dvl; Dvl1, Dvl2, Dvl3 in mammalian cells) is critically required for Wnt/β-catenin signal transduction. Dvl acts as a scaffolding protein, which interacts with many proteins and serves as a key signaling intermediate between the WNT receptor Frizzled and downstream components in the Wnt/β-catenin and non-canonical Wnt pathways (5–8). The current model of Wnt/β-catenin signal transduction proposes Dvl as a core protein of dynamic protein assemblies called signalosomes (9). The signalosome hypothesis proposes that upon stimulation of the Wnt pathway, Dvl protein multimerizes via its DIX domains and forms a platform that recruits other proteins including Axin and that is required for the phosphorylation of LRP6 (9–11). When Dvl is overexpressed in mammalian cells, it is present in dynamic protein aggregates and is visible as Dvl punctae, which most likely represents Dvl multimers (12, 13). Although Dvl multimerization has yet to be convincingly demonstrated at the endogenous level, it is clear that endogenous Dvl becomes phosphorylated after the addition of Wnt. Wnt-induced activation of Dvl is visible as a Wnt-induced shift in the electrophoretic mobility of all three Dvl isoforms, forming the so called phosphorylated and shifted (PS) Dvl (14–16). It has been clearly demonstrated that both the activation of Dvl in the Wnt/β-catenin pathway (17–21) and Wnt-induced PS-Dvl formation are dependent on casein kinase 1 (CK1) δ/ϵ activity (14, 16). It is, thus, conceivable that CK1δ/ϵ phosphorylation of Dvl promotes the formation of signalosomes/Dvl aggregates, which is unique for the Wnt/β-catenin pathway. However, these predictions have not been supported by experimental data. First, overexpression of CK1 dissolved (and not promoted) Dvl aggregates in all tested cell types (14, 20, 22). Second, endogenous PS-Dvl formed irrespective of whether or not the activating Wnt triggered the Wnt/β-catenin pathway (14, 15, 23).
In the present study we attempted to solve this obvious conundrum in our current understanding of the role of CK1ϵ in Dvl biology. We analyzed the mutual interaction of CK1δ/ϵ, and two other Dvl kinases, CK2 and PAR1, in the Wnt/β-catenin pathway. Using a combination of gain-of-function, loss-of-function, and domain mapping approaches, we proposed a multistep and multikinase model for Dvl activation in the Wnt/β-catenin pathway. This approach uncovered a built-in negative feedback loop triggered after activating phosphorylation of Dvl by CK1δ/ϵ.
MATERIALS AND METHODS
Cell Culture, Transfection, and Treatments
WT mouse embryonal fibroblasts (MEFs) and HEK-293T cells were propagated in DMEM, 10% FCS, 2 mm l-glutamine, 50 units/ml penicillin, and 50 units/ml streptomycin. Cells (40,000–60,000 per well) were seeded in 24-well plates either directly (for biochemical analysis) or on sterile coverslips (for microscopy). The next day cells were transfected using polyethyleneimine in a stoichiometry of 2.5 μl per 1 μg of DNA. Cells were harvested for immunoblotting or immunocytochemistry 24 h after transfection. The following plasmids have been described previously: Dvl2-Myc (42), ca-β-catenin (43), FLAG-Dvl3 deletion constructs (32), Xenopus (x) Dsh and xPAR1 constructs (25), xCK2 constructs (44), xCK1ϵ constructs (45), GST-DSH (36), and FLAG-Dvl1 (46). Treatment with the D4476, IC261, TBBt, or TBCA (Calbiochem) dissolved in DMSO was done in 24-well plates in the presence of 1 μl per well FuGENE 6 reagent to increase cell penetration. For analysis of cellular signaling, the cells were stimulated with mouse Wnt-3a or -5a (R&D Systems, Minneapolis, MN) for 2 h if not otherwise stated. Control stimulations were done with 0.1% BSA in PBS.
RNA Interference
MEF or HEK-293T cells were transfected with siRNA using neofection according to the manufacturer's instructions (Ambion). In brief, siRNAs (0.55 μl of 20 μm siRNA) were mixed with Lipofectamine 2000 (1.45 μl; Invitrogen) and Opti-MEM (48 μl; Invitrogen) and incubated for 20 min at room temperature. The transfection mixture (50 μl) was added to the 24-well plate and mixed with a suspension of freshly trypsinized cells (25,000 cells/well in 350 μl of complete media) resulting in a final concentration of 30 nm siRNA. When a combination of two different siRNAs was used, each siRNA was used at 30 nm, and the control siRNA was at 60 nm. The transfection was terminated after 5 h by changing the culture media. Cells were then treated with Wnt-3a or -5a inhibitors or transfected according to the scheme used. The used siRNAs were from Ambion (mCK1ϵ catalog no. 188528, mCK1δ catalog no. 88388) and from Santa Cruz Biotechnology (hCK1ϵ sc 29914, hCK1δ sc 29910, hCK2α sc-29918, mCK2α sc-29919, control siRNA sc-37007).
Dual-Luciferase Assay, Western Blotting, Solubility Assay, Dephosphorylation Assay, and Immunoprecipitation
For the luciferase reporter assay, cells were transfected with 0.1 μg of Super8X TopFlash construct and 0.1 μg of Renilla luciferase construct per well in a 24-well plate 24 h after seeding. For the TopFlash assay, a Promega Dual-Luciferase assay kit was used according to the manufacturer's instructions. Relative luciferase units were measured on a MLX luminometer (Dynex Technologies) and normalized to the Renilla luciferase expression 24 h post-transfection. Results were shown as the means with S.D. of at least three independent experiments.
Immunoblotting and sample preparations were performed as previously described (14). For solubility assays, cells were seeded out on 12-well plates and transfected according to the scheme. Twenty-four hours post-transfection, cells were scraped into 200 μl of buffer containing 50 mm Tris, pH 8.5, 150 mm NaCl, 1 mm MgCl2, and protease inhibitors (Roche Applied Science, 11836145001) at 4 °C, and lysis was carried out for 15 min. Cells were then subjected to three freeze/thaw cycles. The lysates were centrifuged at 16,100 × g, and the supernatant was collected. The pellet was then resuspended in 200 μl of lysis buffer. Both supernatant and pellet were prepared for Western blotting by adding 40 μl of 5× Laemmli buffer, sonicated, and boiled at 95 °C for 5 min. The antibodies used include phospho-LRP6 (Ser-1490, #2568), Dvl3 (#3218), Dvl2 (#3224), Axin1 (#2087) from Cell Signaling Technologies, actin (C-11, sc-1615), Dvl3 (sc-8027), Dvl2 (sc-8026), CK1ϵ (sc-6471), and Myc (sc-40) from Santa Cruz Biotechnology, CK2α (#611610) from BD Transduction Laboratories, and FLAG M2 (F1804) from Sigma.
For dephosphorylation assay cells were washed with buffer containing 100 mm Tris, pH 8.5, 150 mm NaCl, 0.2 mm EDTA. Then they were scraped into dephosphorylation buffer containing 50 mm Tris, pH 8.5, 150 mm NaCl, 1 mm MgCl2, and protease inhibitors (Roche Applied Science, 11836145001). Cells were lysed by a 22-gauge needle and then spun down at 16,100 × g. Supernatant was divided into two parts. The pellet was resuspended in dephosphorylation buffer and also divided into two parts. One part of the supernatant and pellet was treated for 1 h by 80 units of alkaline phosphatase (Sigma P8361). 5× Laemmli buffer was added to each sample, and samples were sonicated and boiled for 5 min.
Immunoprecipitation was performed as previously described (22). The antibodies used for immunoprecipitation were anti-FLAG (F1804; Sigma), anti-CK1ϵ (sc-6471), anti-Dvl3 (sc-8027), anti-Dvl2 (sc-8026), and mouse IgG (sc-2025) (all Santa Cruz Biotechnology).
GST Pulldown Assay
Production of recombinant GST-Dsh (36) was induced by adding 0.2 mm isopropyl 1-thio-β-d-galactopyranoside and grown for 4 h at 37 °C. The bacteria were spun down at 4000 × g at 4 °C for 10 min, and the pellet was resuspended in 10 ml of GST lysis buffer (50 mm Tris-Cl, pH 7.5, 150 mm NaCl, 5 mm MgCl2, protease inhibitors (Roche Applied Science)) and stored at −80 °C. Then the solution was thawed, sonicated 3 × 30 s, and spun down 15,000 × g at 4 °C for 15 min, and the supernatant was then used for incubation with GST beads for 2 h on the rotator (100 μl of beads per 100 ml of original bacterial culture). After incubation beads were washed 3 times with 1 ml of GST lysis buffer and frozen at −80 °C as 25% slurry in GST lysis buffer + 20% glycerol.
HEK-293T cells were transfected according to the scheme, grown for 24 or 48 h, and lysed in 0.5% Nonidet P-40 lysis buffer (0.5% Nonidet P-40, 50 mm Tris, pH 7.4, 1 mm EDTA, 150 mm NaCl) with added protease inhibitors (Roche Applied Science), phosphatase inhibitors (Calbiochem), and 10 mm N-ethylmaleimide (Sigma). The lysate was spun down at 16,100 × g at 4 °C for 10 min, and the supernatant was used for overnight incubation with 15 μl of solid GST beads containing GST recombinant proteins on the rotator. After the incubation samples were washed with 800 μl of 0.5% Nonidet P-40 lysis buffer, the GST beads were collected at 0.1 × g at 4 °C for 1 min, the supernatant was aspirated; this washing was repeated 6 times. Proteins were eluted with 45 μl of 2× Laemmli buffer.
Immunocytochemistry
HEK-293 cells were seeded at ∼2 × 105 cells/well on collagen-coated coverslips in 24-well plates. Cells were then transfected the next day, and 24 h later they were fixed in fresh 4% paraformaldehyde, permeabilized with 0.05% Triton X-100, blocked with 3% BSA, 0,25% Triton, 0. 01% NaN3 for 1 h, and incubated overnight with primary antibodies. The next day coverslips were washed in PBS and incubated with secondary antibodies Cy2 and Cy3 (Jackson ImmunoResearch), washed with PBS, stained with DAPI (1:5000), and mounted on coverslips. Cells were visualized using a Zeiss LSM 710 confocal microscope. Two hundred positive cells per coverslip were analyzed and scored as described previously (14). Graphs show percentages of individual localization patterns.
Förster Resonance Electron Transfer (FRET)
HEK-293 cells were seeded in a 24-well plate on sterile glass coverslips and grown overnight (62,500 cells per well). Cells were transfected with 0.1 μg of Dvl2-GFP, 0.1 μg of FLAG-Dvl3, and either 0.25 μg of pcDNA 3.1 or CK1ϵ using the calcium phosphate method. The next day, cells were fixed in 4% paraformaldehyde in PBS for 15 min. Indirect immunostaining was done after blocking using anti-FLAG M2 (Sigma, catalogue no. F1804) and donkey anti-mouse Cy3-conjugated secondary antibody (Jackson ImmunoResearch). To control for co-transfection efficiency of Dvl isoforms and CK1ϵ, separate samples were also stained for CK1ϵ (Santa Cruz, catalogue no. sc-6471) and donkey anti-goat Cy-5-conjugated secondary antibody. Glass coverslips were mounted on microscopy slides using glycerol gelatin (Sigma). FRET between Dvl2-GFP and FLAG-Dvl3-Cy3 was performed on a Zeiss LSM710 confocal microscope and determined by photo acceptor bleaching employing 100% laser power of 561 nm diode laser for 20 s. Signal intensity was routinely reduced by 80–90%. Images were acquired before and after photo bleaching with excitation/emission range 488/493–545 nm (GFP) and 561/562–681 nm (Cy3). Quantification of GFP emission pre- and post-bleaching was determined with region of interest analysis in at least 15 individual cells per experiment and condition, employing the ZEN 2009 software from ZEISS. Data were normalized to the GFP intensity before bleaching. For statistical analysis, Student's t test was performed.
Mass Spectrometry (MS/MS) Analysis
Protein pellets from MEF cells (5 × 15-cm-diameter confluent plates/sample) were stored at −80 °C until analysis. After thawing they were immunoprecipitated using 5 μg of anti-Dvl2 (sc-8026, Santa Cruz Biotechnology) and anti-Dvl3 (sc-8027, Santa Cruz Biotechnology) antibodies. Proteomic grade trypsin (Sigma) was added at a concentration of 100 ng/μl per sample. For label-free quantitative analysis, yeast alcohol dehydrogenase (Sigma) was added as a standard protein, with final concentrations of 50 fmol/μl per sample. After adding trypsin, samples were incubated at 37 °C for 12 h. Digested peptides were desalted using ZipTip C18 pipette tips (Millipore Corp.) according to the manufacturer's protocol. MS analysis was performed on a NanoAcquity UPLC (Waters) on-line coupled to an electrospray ionization Q-Tof Premier (Waters) mass spectrometer. 1 μl of sample was diluted in water and loaded onto a 180-μm × 20-mm nanoAcquity UPLC Symmetry trap column (Waters) packed with 5-μm BEH C-18 beads. After 1 min of trapping, peptides were eluted through a 75-μm × 150-mm nanoAcquity (Waters) analytical column packed with 1.7-μm BEH C-18 beads at a flow rate of 400 nl/min using a gradient of 3–40% acetonitrile with 0.1% formic acid for 35 min at a temperature of 35 °C. Effluent was directly fed into the electrospray ionization source of the mass spectrometer. Raw data were acquired in data independent MSE Identity (Waters) mode. Precursor ion spectra were acquired with collision energy of 5 V, and fragment ion spectra were acquired with collision energy of 20–35-V ramp in alternating 1-s scans. Peptide spectra and fragment spectra were acquired with 2 and 5 ppm tolerance, respectively. Raw data were then subjected to a data base search using species specific Uniprot and NCBI mouse protein data base by the PLGS2.3 software (Waters). Acetyl N-terminal, deamidation N and Q, carbamidomethyl C, and oxidation M were set as variable modifications. Identification of three consecutive y- or b-ions was required for a positive peptide match. Protein quantification is based on peak intensities of at least three positively identified peptides belonging to one protein compared with peak intensities of the standard protein (Dvl2). Quantitative analysis was performed by the PLGS2.3 software (Waters).
RESULTS
CK2α, PAR1, and CK1ϵ Promote Dvl-dependent Wnt/ β-Catenin Signaling via Distinct Mechanisms
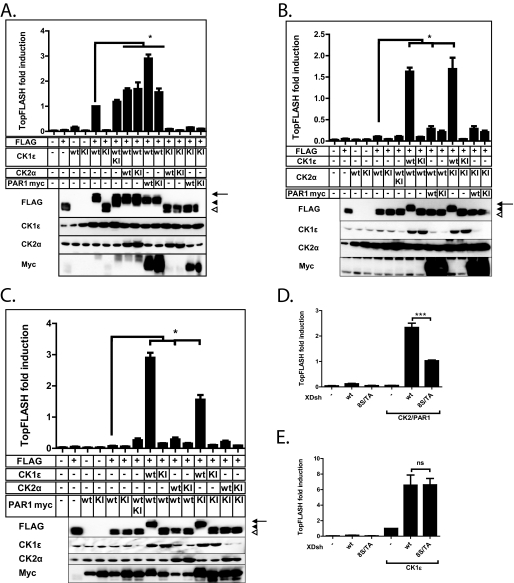
Three Dvl kinases, CK1ϵ, CK2α, and PAR1, have been previously shown to play a positive role in Wnt/β-catenin signaling (17, 24, 25). We addressed the question of the interplay between these three kinases and Dvl by coexpressing them alone (as wild type or kinase inactive variants) or in combination with FLAG-Dvl3 in HEK-293 cells. For CK2 overexpression, we co-expressed both kinase subunits (CK2α and CK2β) if not mentioned otherwise. We used TCF/LEF reporter (TopFlash) (26) activity and electrophoretic migration of FLAG-Dvl3 as a readout (Fig. 1). FLAG-Dvl3 can be detected on Western blot as two bands that we call Dvl and P (phosphorylated)-Dvl. Only CK1ϵ, but not CK2 or PAR-1, was able to promote the formation of novel, even more slowly migrating forms of Dvl3 labeled as PS-Dvl (see the Western blot panels in Fig. 1, A–C). Such changes in the electrophoretic mobility of FLAG-Dvl3 induced by CK1ϵ co-expression correlated with robust activation of the TopFlash reporter, which reflected activation of the Wnt/β-catenin pathway (see the graphs in Fig. 1, A–C). CK2 was able to partially activate the TopFlash reporter, whereas the activation of the reporter by PAR1 was negligible. However, co-expression of PAR1 was sufficient to significantly increase the reporter activation induced solely by either CK1ϵ or CK2 (see Fig. 1, A and B). The most prominent TopFlash induction was in the sample in which CK1ϵ and PAR1 were coexpressed together (highest bar in Fig. 1A). PAR1 in combination with CK2 was able to shift Dvl3 electrophoretic migration from Dvl to P-Dvl (Fig. 1C, lane 9) but never to PS-Dvl (Fig. 1, A–C, all samples with CK1ϵ). This difference suggested that the effects of CK2/PAR1 were distinct from the effects of CK1ϵ. As CK2 and PAR1 kinases were shown to mainly phosphorylate Dvl in the basic region/PDZ region (24, 25), this observation would suggest that they cooperate or were redundant in the phosphorylation of Dvl in the Wnt/β-catenin pathway. In line with these observations, when the Ser/Thr cluster in the basic region preceding PDZ domain of Dishevelled was mutated to alanines (25), it had a reduced capacity to activate the TopFlash reporter after phosphorylation by CK2, PAR1, or the combination of both (Fig. 1D, supplemental Fig. 1). It was, however, fully active after phosphorylation by CK1ϵ (Fig. 1E). These results demonstrate that CK1ϵ and CK2/PAR1 were both capable of activating the Wnt/β-catenin pathway in a Dvl-dependent manner, but the mechanisms were distinct. Efficient activation of downstream signaling accompanied by the formation of PS-Dvl can only be achieved by the phosphorylation by CK1ϵ. Because we have not observed any functional differences between CK2 and PAR1, we used the combination of the two kinases in the further work.
FIGURE 1.
A–C, cotransfection of CK1ϵ, CK2α/β, and PAR1 kinases with FLAG-Dvl3. FLAG-Dvl3 (0.1 μg) plasmid was cotransfected with 0.4 μg of each kinase or a corresponding amount of pcDNA3 as control. Samples were analyzed for the activation of TCF/LEF-dependent transcription using the TopFlash reporter system (graphs summarize three independent experiments) and for the analysis of electrophoretic migration of FLAG-Dvl3. Western blot analysis of CK1ϵ, CK2α, and PAR1-Myc kinases provides a control for efficiency of transfection. All the measurements have been performed in three independent replicates. The arrow indicates PS-Dvl3, the filled arrowhead indicates P-Dvl3, and the open arrowhead indicates non-modified Dvl3. D and E, HEK-293 cells were transfected with wild type Xenopus Dsh (XDsh) or XDsh with eight serines in the b-region mutated to alanines (8S/TA) and corresponding kinases. XDsh-8S/A does not lack the TopFlash-inducing potential for CK1ϵ but fails to induce TopFlash to the same extent in CK2/PAR1-transfected cells. Data represent the means ± S.D. *, p < 0.05; **, p < 0.01; ***, p < 0.001, one-way analysis of variance, Tukey post test. ns, not significant.
Down-regulation of CK1δ/ϵ but Not of CK2-diminished Formation of PS-Dvl in MEF Cells after Wnt Stimulation
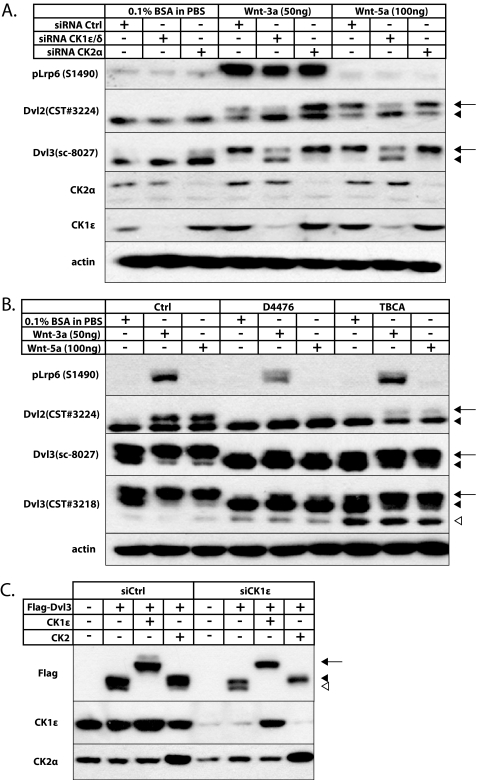
Gain-of-function experiments suggested that CK1 and CK2/PAR1 phosphorylated Dvl differently. To test the requirement of CK1 and CK2 for endogenous PS-Dvl formation, we used a loss-of-function approach in MEFs. MEFs represent a very suitable system for the analysis of Dvl biology because they respond efficiently to Wnt stimulation by the formation of PS-Dvl. We have used several antibodies against endogenous Dvl2 and Dvl3 that show slightly different sensitivity against individual phospho-variants of Dvl. We down-regulated CK1ϵ and -δ, which are two isoforms previously shown to be redundant and required for PS-Dvl formation (14), or CK2α using siRNA-mediated knockdown in MEF cells. Stimulation with recombinant mouse (rm) Wnt3a (50 ng/ml) or rmWnt5a (100 ng/ml) for 2 h efficiently promoted PS-Dvl formation in MEF cells (Fig. 2A). Consistent with the earlier findings, the knockdown of CK1δ/ϵ blocked the formation of PS-Dvl after Wnt3a or Wnt5a treatment (Fig. 2A). However, we failed to see any effect of CK2α knockdown on the Wnt-induced PS-Dvl formation. This experiment suggested that CK1δ/ϵ was indeed necessary for the dynamic Wnt-induced formation of PS-Dvl and that CK2 was not.
FIGURE 2.
A, MEF cells were transfected with siRNA for CK1ϵ and -δ or CK2α. 24 h post-transfection cells were treated with recombinant mouse Wnt3a (50 ng/ml) or Wnt5a (100 ng/ml). Cell lysates were harvested 2 h after treatment. After Wnt 3a/5a treatment, CK1ϵ/δ siRNA diminishes the formation of PS-Dvl, whereas CK2α siRNA does not. Western blot analysis for CK1ϵ and CK2α demonstrates knockdown efficiency; phosphorylation of Lrp6 was used to determine the Wnt3a activity. B, MEF cells were pretreated with the CK1 (D4476 100 μm) and CK2 (TBCA 100 μm) inhibitors. Cells were treated with rmWnt3a (50 ng/ml) or Wnt5a (100 ng/ml) after 30 min of pretreatment. Cell lysates were harvested 2 h after treatment. The CK1 inhibitor D4476 was able to diminish PS-Dvl formation after Wnt treatment, whereas CK2 inhibitors were not able to do so. Unlike CK1 inhibitors, CK2 inhibitors were able to cause the formation of lowest molecular weight form of Dvl3. C, HEK-293 cells were transfected with siRNA against CK1ϵ and after 24 h transfected with corresponding constructs. In the absence of endogenous CK1ϵ, CK2 clearly promotes P-Dvl, whereas PS-Dvl formation is only induced by CK1ϵ. The arrow indicates PS-Dvl3, the filled arrowhead indicates P-Dvl3, and the open arrowhead indicates non-modified Dvl3.
To confirm the knockdown data, we pretreated MEF cells with CK1 (100 μm D4476 and 50 μm IC261)- and CK2 (50 μm TBCA, 100 μm TBBt)-specific inhibitors for 30 min and subsequently with rmWnt3a (50 ng/ml) or rmWnt5a (100 ng/ml) for 2 h. As we show in Fig. 2B (D4476 and TBCA) and supplemental Fig. 2 (IC261 and TBBt), CK1-specific inhibitors were able to block the Wnt-induced formation of PS-Dvl. In contrast, the CK2 inhibitors failed to block or diminish the formation of the Wnt-induced PS-Dvl band. On the other hand, they were able to generally decrease Dvl phosphorylation, which also resulted in the appearance of a third-fastest migrating band (Dvl3, antibody CST#3218). The CK2α activity toward Dvl has been shown to be constitutive (24, 27). Thus, we propose that the CK2 inhibitor-induced third band was a non-modified Dvl3, as the molecular mass corresponded to the in silico-determined mass of Dvl3. Interestingly, treatment of cell lysates with alkaline phosphatase completely removed PS-Dvl but only partially promoted appearance of Dvl (supplemental Fig. 2B). This suggests that CK2-induced modification of Dvl is not solely phosphorylation but may be more complex and involve other modifications such as mono-ubiquitination. It is also worth noting that CK1 inhibition decreased Wnt3a-induced phosphorylation of LRP6 at Ser-1490, which is known to be a Dvl-dependent process (9, 28). These loss-of-function experiments suggest that endogenous Dvl is constitutively modified (but not only phosphorylated) in a CK2-dependent manner (P-Dvl) and after Wnt stimulation is further phosphorylated by CK1δ/ϵ to form PS-Dvl. To demonstrate gradual modification of Dvl3 by CK2 (to P-Dvl) and CK1δ/ϵ (to PS-Dvl), we first eliminated endogenous CK1ϵ by siRNA and overexpressed FLAG-Dvl3 with Xenopus CK2 and CK1ϵ, which were not targeted by siRNA. As we show in Fig. 2C, under these conditions (right side of the panel) exogenous CK2 clearly shifts Dvl to P-Dvl (filled arrowhead), and CK1ϵ to PS-Dvl (full arrow). The combination of results shown in Figs. 1 and 2, thus, suggests that CK2/PAR1 and CK1ϵ may phosphorylate Dvl sequentially with CK2 activity being constitutive and CK1ϵ activity being induced by Wnts.
Both CK1 and CK2 Kinase Activities Are Irreplaceably Required for Wnt/β-Catenin Activation via Dvl3
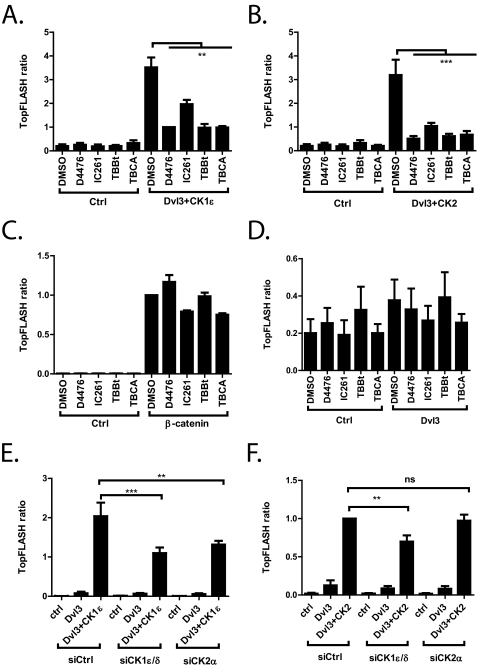
We hypothesized that phosphorylations by CK2 and CK1 represent distinct, although sequential steps in the chain of events required for Dvl activation in the Wnt/β-catenin pathway. To test this hypothesis, we induced TCF/LEF transcription by co-expressing FLAG-Dvl3 and CK1ϵ or FLAG-Dvl3 and CK2/PAR1, and we tested the effects of CK1 and CK2 inhibition, respectively. As we show in Fig. 3, A and B, the CK1ϵ inhibitors D4476 and IC261 were able to diminish TopFlash induction by both FLAG-Dvl3+CK1ϵ and FLAG-Dvl3+CK2/PAR1. Strikingly, the two CK2 inhibitors TBCA and TBBt showed very similar effects and blocked a Dvl3-dependent increase in TCF/LEF-driven transcription induced by both CK1ϵ and CK2/PAR1. Importantly, inhibition of casein kinases did not show a consistently negative effect on TCF/LEF-mediated transcription induced by constitutively active (S33A) β-catenin (Fig. 3C). This result was also not observed in the absence of FLAG-Dvl3, which demonstrates that (i) the inhibitors did not interfere with the signaling downstream of β-catenin, and (ii) the observed effects were Dvl3-dependent.
FIGURE 3.
A–D, HEK-293 cells were transfected with the indicated constructs and TopFlash reporter. Twenty-four hours post-transfection, cells were treated with CK1 (D4476 100 μm, IC261 50 μm) and CK2 (TBCA 50 μm, TBBt 100 μm) inhibitors. Both CK1 and CK2 inhibitors were able to significantly block TCF/LEF-driven transcription in both FLAG-Dvl3 + CK1ϵ (A)- or FLAG-Dvl3+CK2/PAR1 (B)-transfected cells. The inhibitors did not show any effect in cells transfected with constitutively active β-catenin (C) or in the absence of overexpressed Dvl3 (D). E and F, HEK-293 cells were transfected with siRNA against CK1ϵ or CK2α. After 24 h they were transfected with the corresponding constructs and TopFlash + Renilla constructs. siRNA against CK1ϵ and CK2α were able to diminish Dvl+CK1ϵ-induced TopFlash, whereas Dvl+CK2/PAR1-induced TopFlash could not be diminished by the siRNA. Data represent the means ± S.D. **, p < 0.01; ***, p < 0.001; one-way analysis of variance, Tukey post test. ns, not significant.
To demonstrate how endogenous CK1ϵ and CK2α contributed to the activation of the TCF/LEF transcription induced by overexpressed kinases, we down-regulated endogenous CK1ϵ and CK2α in HEK-293 cells using siRNAs. Used siRNAs do not target exogenous xCK1ϵ or xCK2/xPAR1 kinases, which were together with FLAG-Dvl3 overexpressed in cells depleted of CK1ϵ or CK2α (Fig. 3, E and F). Knockdown of CK1ϵ and CK2α decreased TopFlash induction in FLAG-Dvl3+xCK1ϵ, which suggested that endogenous CK2α activity was required for the action of overexpressed CK1ϵ. Furthermore, down-regulation of endogenous CK1ϵ was able to diminish the xCK2/xPAR1-induced TopFlash. CK2α siRNA did not show any effect in this assay, which suggests that xCK2α was able to fully rescue the lack of endogenous CK2α kinase. These results suggested that the phosphorylation of Dvl3 by CK1 and CK2 represented individual steps in the sequence of events. Accordingly, the activation of the Wnt pathway and interruption of any of these steps completely interfered with the ability of Dvl3 to promote Wnt/β-catenin signaling.
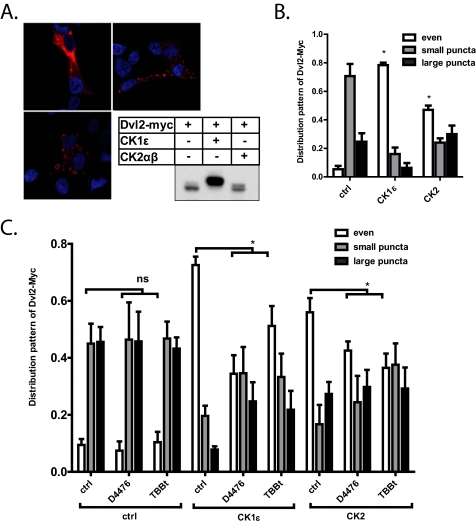
Both CK1ϵ and CK2 Promote Even Intracellular Distribution of Dvl
Dvl was usually found to be present in dynamic multiprotein aggregates (12, 13) called Dvl dots or puncta. Based on the cellular context, overexpressed Dvl2 or Dvl3 was usually present either in dots or was evenly distributed (Dvl2 exampled in Fig. 4A). CK1ϵ has been shown earlier to strongly promote even localization of Dvl (14, 20). Based on these findings, in the next step we tested the role of CK2 on Dvl distribution. Dvl2-Myc was overexpressed with the indicated kinases in HEK-293 cells, and the cells were scored for their pattern of subcellular localization of Dvl2. As we show in Fig. 4B, CK1ϵ promoted even localization of Dvl, as previously reported. Interestingly, overexpression of CK2 showed very similar, albeit somewhat weaker, effects than CK1ϵ. These effects are not due to reduced levels of Dvl2-Myc after kinase coexpression (inset in Fig. 4A). Importantly, CK1 inhibition by D4476 and CK2 inhibition by TBBt were both able to reduce the effects of either CK1ϵ or CK2 (Fig. 4C). These results suggested that the even distribution of Dvl was similar to the TCF/LEF-dependent transcription promoted by a mechanism that was non-redundantly dependent on both CK2 and CK1ϵ.
FIGURE 4.
HEK-293 cells were transfected with the indicated constructs and were then subjected to immunocytochemistry. A, shown is a typical localization pattern of Dvl2-Myc, which was scored in the experimental samples. The inset shows levels and electrophoretic migration of Dvl2-Myc in the analyzed cells. B, both kinases were able to change Dvl intracellular distribution from dotted to even. This effect can be reduced by treatment with CK1 (D4476) and CK2 (TBBt) inhibitors (C). Quantification is based on three independent replicates. *. p < 0.05, one-way analysis of variance (cells with even distribution), Tukey post test. ns, not significant.
Analysis of Dvl Domains Required for the Effects of CK1ϵ and CK2
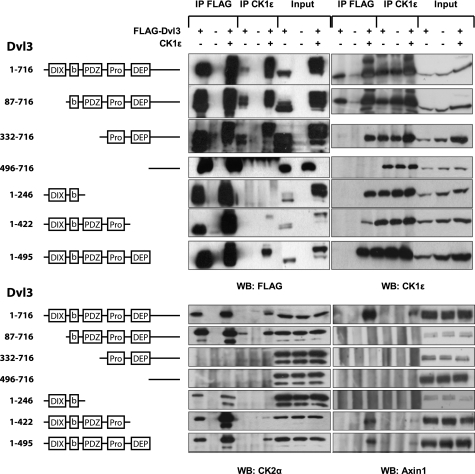
Dvl proteins have three very well conserved domains (see the scheme in Fig. 5): the N-terminal DIX domain, the PDZ domain, and the DEP domain. Apart from these main domains, there are two well conserved stretches of amino acids: a basic region (b) preceding the PDZ domain and a proline-rich cluster (Pro) between the PDZ and DEP domains. The DIX domain has been shown to function mainly in the canonical Wnt/β-catenin pathway (29), whereas the function of the DEP domain has been mostly associated with noncanonical pathways (30, 31). The PDZ domain serves as a scaffold for many cooperating proteins and has been shown to be necessary in both pathways. To test the roles of and mutual relationship between CK1ϵ and CK2 in Dvl biology, we analyzed a set of diverse human Dvl3 mutants. We took advantage of the previously described deletion mutants of Dvl3 (32) and analyzed the function of individual domains for Dvl3 behavior, which was modified by CK1 or CK2/PAR1. The parameters examined after CK1ϵ and in some cases after CK2 overexpression included (i) the ability to bind CK1ϵ (endogenous and overexpressed), endogenous CK2α, and endogenous Axin1, (ii) the ability to promote TCF/LEF-dependent transcription (see Fig. 3), (iii) intracellular localization of Dvl3 (see Fig. 4), and (iv) electrophoretic mobility shift of Dvl3 (see Fig. 1).
FIGURE 5.
HEK-293 cells were cotransfected with the indicated combinations of FLAG-Dvl3 mutants and CK1ϵ. The amino acids present in the mutants and the domain structure is schematized (b, basic region; Pro, proline-rich region). Two days later cells were lysed and subjected to immunoprecipitation (IP) with the corresponding antibodies (FLAG M2, CK1ϵ). The presence of Dvl3, CK1ϵ, CK2α, and Axin1 in the pull-down assay was analyzed by Western blotting (WB). FLAG-Dvl3 constructs used in this experiment are schematized on the left hand side of the figure.
As we show in the protein-protein interaction analysis (Fig. 5), full-length Dvl3 was able to bind both endogenous and overexpressed CK1ϵ, endogenous CK2α, and upon CK1ϵ overexpression, full-length Dvl3 also bound endogenous Axin1. Moreover, full-length Dvl3 acted as a scaffold, which was required for the co-immunoprecipitation of CK1ϵ with endogenous CK2α and Axin1. Analysis of mutants showed that CK1ϵ could be co-immunoprecipitated with at least two distinct domains of Dvl3. For high affinity interactions, which were indicated by the presence of endogenous CK1ϵ in the Dvl3 pulldown assay, only a DIX domain was dispensable (see aa 82–716 mutant). However, overexpressed CK1ϵ could be also found in association with all other mutants tested, with the exception of the extreme C terminus (mutant aa 496–716). Our results confirm previous findings that map the binding domains of CK1 to the DEP domain or its close proximity (compare aa 332–716 and 496–716 mutants and Kishida et al. (19)) and to the basic region preceding the PDZ domain (see mutant aa 1–246 and Klein et al. (33) and Klimowski et al. (34)). Endogenous CK2α could be co-immunoprecipitated with all the mutants of Dvl3, which contained a basic region between the DIX and PDZ domains,. This finding was in agreement with our data in Fig. 1D and with a previously published report (24). Interestingly, the binding of CK2 to Dvl3 was promoted by the co-expression of CK1ϵ (see e.g. aa 1–422 and 1–495 mutants). In line with this observation, CK1ϵ could be precipitated with endogenous CK2 in a Dvl3-dependent manner only when the basic region/PDZ (CK2 binding site) and the proline-rich-DEP domain (CK1 binding site) were present (WT, aa 80–716 and 1–495 mutants). We also, for the first time, showed that CK1ϵ overexpression could induce the recruitment of endogenous Axin1 to Dvl3 (Fig. 5, panel WB: Axin1), which is very relevant for the inactivation of the destruction complex and for further downstream β-catenin-dependent signaling. Axin1 bound only Dvl3 mutants containing both a DIX domain, which was previously reported as necessary for binding to Axin1 (29), and a PDZ-Pro-rich region, which probably contains crucial CK1ϵ phosphorylation sites required for Axin1 recruitment.
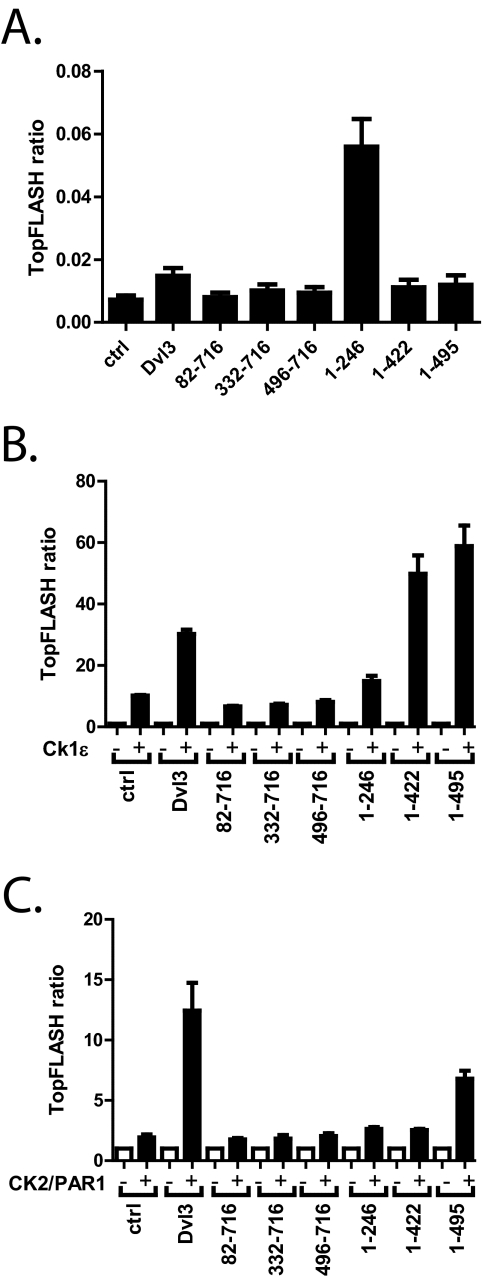
In the next step we tested the functional properties of Dvl3 mutants with respect to the effects of CK1 and CK2. Dvl3 and its mutants were transfected into HEK-293T cells and tested for their ability to induce TCF/LEF-dependent transcription. As we show in Fig. 6A, the ability of Dvl3 truncations to induce transcription was very weak, with only 2-fold induction of TopFlash by WT and aa 1–422 and 1–495 mutants. There was no induction by mutants lacking the DIX domain, but there was approximately an 8-fold induction by mutant aa 1–246, which contained the DIX domain and a basic region. Expression of CK1ϵ alone increased the TCF/LEF reporter ∼8-fold in this experimental setup (Fig. 6B), which we believe represented the effects of CK1ϵ on endogenous Dvl or on other endogenous components of the Wnt pathway. CK1ϵ together with DIX-deficient mutants (and also mutant aa 1–246) did not activate TopFlash above this level. In contrast, the activation capacity of both mutants, which contain a DIX, PDZ, and proline-rich region (aa 1–422 and 1–495) but lacked the C terminus, was even higher than the full-length Dvl after phosphorylation by CK1ϵ (Fig. 6B). This effect does not seem to be unique to Dvl3 because the Dvl1 mutant lacking the C terminus (Dvl1 aa 1–502) behaves very similarly (supplemental Fig. 3A). CK2/PAR1 increased TopFlash activation to a lesser extent than CK1ϵ, and the activity of none of the mutants, with the exception of mutant aa 1–495, which lacked only the C terminus, was potentiated more than 3-fold (Fig. 6C).
FIGURE 6.
HEK-293 cells were transfected with the indicated constructs and a TopFlash reporter. A, the ability of FLAG-Dvl3 constructs to promote TCF/LEF-dependent transcription is shown. B and C, FLAG-Dvl3 constructs were co-transfected with CK1ϵ (B) or CK2/PAR1 (C). The graphs show -fold change increase in the TopFlash reporter for each particular construct induced by the co-expressed kinase. Graphs summarize data from three independent experiments (means ± S.D.).
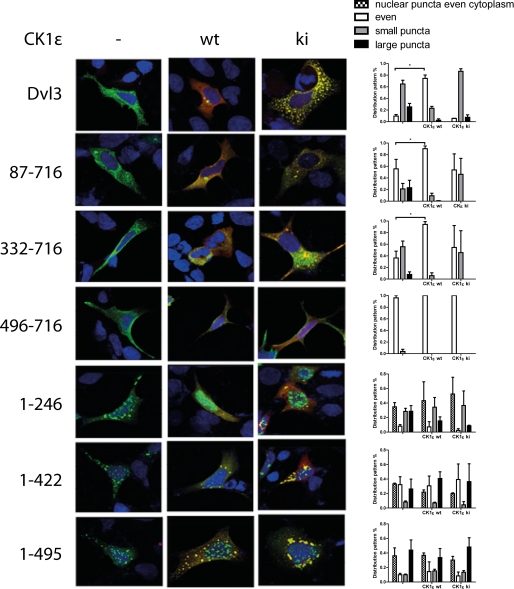
Individual deletion mutants intrinsically differ in other basic parameters than in its ability to promote the Wnt pathway. The presence of the DIX domain, which was shown to be required for polymerization of Dvl (11), strongly determined the solubility and localization of Dvl. Mutants containing the DIX domain were insoluble when mildly lysed by freezing/thawing (supplemental Fig. 3B) and formed large aggregates when they were visualized by immunocytochemistry (Fig. 7). On the other hand, mutants lacking the DIX domain were more soluble (supplemental Fig. 3B) and were more evenly localized (Fig. 7). Our data were also in good agreement with the previous report, which mapped the nuclear export signal C-terminal from the DEP domain (35) because the normally cytoplasmic Dvl3 was also found in the nucleus after deletion of the C terminus (supplemental Fig. 4A). As we demonstrated above, CK1ϵ and CK2/PAR1 changed the distribution of full-length Dvl3 from punctate to uniform (Fig. 4). The positive effects of CK1ϵ and CK2/PAR1 on even localization of Dvl3 were conserved in the aa 82–716 and 332–716 mutants (Fig. 7, supplemental Fig. 4B). Under basal conditions, these two mutants were localized more uniformly than WT, and because they lacked their own DIX domain, we speculate that they aggregated with endogenous Dvl isoforms. Importantly, the distribution pattern of DIX-containing mutants, which also lacked the N terminus, was not modulated by CK1ϵ (Fig. 7) or CK2/PAR1 (not shown). The ability of CK1ϵ to homogenize the localization of Dvl3 correlated well with its ability to promote the efficient formation of hyperphosphorylated Dvl3 (probably corresponding to PS-Dvl3). This form in the overexpressed FLAG-Dvl3 was visible as the third, most slowly migrating band, induced only in the conditions where CK1 was co-expressed (Fig. 5, or in more detail, supplemental Fig. 5). CK1ϵ and CK2 also caused the formation of a shifted form of Dvl3 in other mutants with the exception of aa 496–716 (C terminus). However, in these cases it promoted predominantly, although not exclusively, the ratio of P-Dvl/Dvl bands, which were also present after Dvl3 overexpression without the kinase (supplemental Fig. 5).
FIGURE 7.
HEK-293 cells were transfected with corresponding constructs and then subjected to immunocytochemistry. Intracellular distribution of Dvl was observed and quantified 24 h after transfection. Typical subcellular distribution of Dvl3 mutants and quantitative analysis of distribution pattern (200 cells in each of three independent experiments) is shown. wt, wild type; ki, kinase inactive.
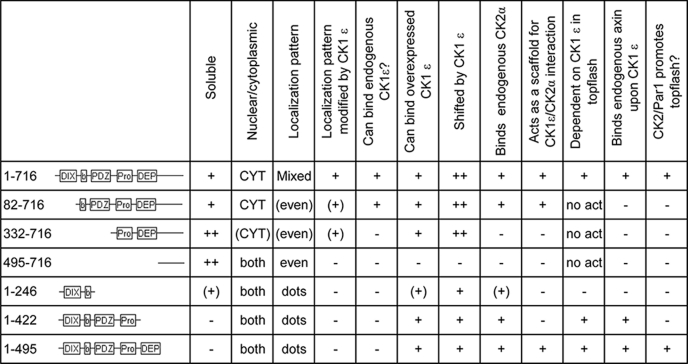
All the results regarding the deletion mutants of Dvl3 are summarized for clarity in Table 1. Based on the analysis of deletion mutants, we concluded the following previously unpublished information; (i) the roles of CK1ϵ and CK2 were distinct and involved different regions of Dishevelled, (ii) the C terminus showed an inhibitory function and was required for CK1ϵ-induced change in the subcellular localization from punctate to even, (iii) the PDZ and Pro-rich rich region were required for CK1ϵ-induced activation of Dvl in the Wnt/β-catenin pathway.
TABLE 1.
Summary of deletion mutants of Dvl3
CK1ϵ-phosphorylated PS-Dvl Has Decreased Ability to Polymerize with Other Dvls
The findings above suggested that CK1ϵ has apart from its clear positive role in Dvl/β-catenin signaling also a subsequent negative role, which is at least in part mediated by formation of PS-Dvl and the Dvl-C terminus. Because CK1ϵ has primarily a strong positive role, it is not possible to analyze the negative consequences of CK1ϵ phosphorylation by looking globally at downstream functional readouts. It is more likely that if indeed CK1ϵ affects Dvl signaling negatively, it will interfere with the properties of Dvl required for efficient downstream signaling in the β-catenin pathway. A suitable measure of this function is the ability of Dvls to polymerize via their DIX domains, which was shown to be crucial for Dvl function. In addition, polymerization-deficient mutants of Dvl were shown to be inactive (9, 11).
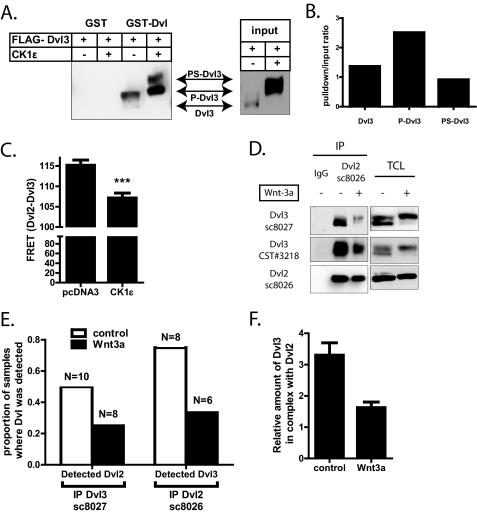
To test the ability of PS-Dvl phosphorylated by CK1ϵ to bind other Dvls, we have performed GST pulldown assays with GST-Dvl (36) as bait. As we show in Fig. 8A, GST-Dvl is able to efficiently pull down FLAG-tagged Dvl3. Importantly, P-Dvl binds to GST-Dvl most efficiently, whereas non-modified Dvl or PS-Dvl triggered by CK1ϵ show much lower affinity to bacterially produced GST-Dvl (see Fig. 8B for quantification).
FIGURE 8.
A and B, FLAG-Dvl3 was overexpressed either alone or with CK1ϵ in HEK-293 cells and subjected to GST pulldown assays with GST-Dvl (or GST tag only as the negative control). FLAG-Dvl3 in the pulldown and input (TCL) was visualized by Western blotting using FLAG antibody. The ratio of individual Dvl forms (pulldown/input) in the shown experiment was quantified by densitometry (B). C, Dvl2-GFP and FLAG-Dvl3 were overexpressed in HEK-293 cells, fixed, and stained for FLAG (Cy3-conjugated secondary antibody). As a measure of proximity between Dvl2-GFP and FLAG-Dvl3, FRET between GFP and Cy3 was determined by photoacceptor bleaching in cells co-transfected with pcDNA3.1 or CK1ϵ. The results from 30 individual cells are summarized in a bar graph (C). GFP fluorescence before photobleaching was set to 100% for normalization. Statistical significance was analyses by Student's t test. ***, p < 0.0001. D, MEF cells were either treated with control or Wnt3a-conditioned medium for 3 h, lysed, and subjected to immunoprecipitation (IP) with Dvl2-specific antibody. Unspecific antibody (IgG) was used as a control. The amount of Dvl2 and Dvl3 in the immunoprecipitates and in the input (TCL) was analyzed by Western blotting using several antibodies specific for individual Dvl isoforms. E and F, MEF cells were treated with control and Wnt3a-conditioned media, and endogenous Dvl2 or Dvl3 (baits) was immunoprecipitated. Composition of protein mixtures was analyzed by MS/MS. Graphs (E) indicate the proportion of samples where Dvl2 was found in complex with Dvl3 (bait), and Dvl3 was found in complex with Dvl2 (bait). Bait was identified in 100% of samples in this analysis. N indicates the number of experiments. F, relative amounts of endogenous Dvl3 in samples immunoprecipitated with anti-Dvl2 antibody are shown. Quantification is based on peak intensities with relative bait abundance set to 10.
Results of GST pulldown might be affected by protein modifications appearing in the cell extract after lysis. To avoid possible lysis artifacts, we performed FRET experiments in HEK-293 cells expressing Dvl2-GFP and FLAG-Dvl3 with and without overexpression of CK1ϵ. After fixation and indirect immunostaining of FLAG-Dvl3 with a Cy3-coupled secondary antibody, the electron acceptor (Cy3) was photobleached. Due to the destruction of the electron acceptor, FRET does not occur, and emission by the electron donor (GFP) increases compared with the prebleach situation when the two FRET partners are in close proximity to each other. In fact, FRET was measurable after photo acceptor bleaching with 115.8 ± 1.2% (mean ± S.E., n = 30) in Dvl2-GFP- and FLAG-Dvl3-expressing cells, which showed a predominant punctate distribution of the two Dvl isoforms. With CK1ϵ overexpression, Dvl2-GFP and FLAG-Dvl3 showed a more even distribution as reported previously, and the GFP emission after photobleaching remained unchanged compared with the GFP emission before bleaching (107.0 ± 1.2%; mean ± S.E., n = 30) (Fig. 8C). Typical appearance of cells used for FRET is shown in supplemental Fig. 6. We concluded that reduced FRET indicates the dissociation of the two Dvl isoforms upon CK1ϵ overexpression.
The results above suggested that persistent phosphorylation of Dvl by CK1ϵ, which leads to the formation of PS-Dvl, causes decreased ability of PS-Dvl to multimerize. However, PS-Dvl is also formed after Wnt3a stimulation (see Fig. 2). To exclude possible artifacts of overexpression, we decided to test whether endogenous PS-Dvl has also decreased ability to polymerize. Therefore, we stimulated MEF cells with Wnt3a, immunoprecipitated endogenous Dvl2, and analyzed the amount of Dvl3 in the pulldown assay by Western blotting. The antibody used for precipitation does not cross-react with endogenous Dvl1 and Dvl3 (Ref. 37 and data not shown). As we show in Fig. 8D, the amount of Dvl3 in complex with Dvl2 decreased after 2 h of Wnt3a stimulation, when PS-Dvl represented a dominant Dvl form.
To further confirm this finding and to avoid possible cross-reactivity of used antibodies, we have repeated the co-immunoprecipitation experiment with endogenous Dvl2 and Dvl3 as bait and analyzed the composition of the complexes by MS/MS. First, we have noticed that the number of samples where Dvl2 co-immunoprecipitated with Dvl3 and Dvl3 co-immunoprecipitated with Dvl2 dramatically decreased after 3 h of Wnt3a stimulation (Fig. 8E). Please note that only samples where the bait was detected with a significant score were considered for the analysis. We conclude from this non-quantitative analysis that the amount of the respective Dvl that co-immunoprecipitated with the bait after Wnt3a treatment was generally lower and more frequently below the detection limit of MS/MS technology. We could also perform MS/MS-based relative quantification of individual Dvls in a few Wnt3a-treated samples where Dvl2 was used as bait and where Dvl3 was detected in sufficient peptide quality and in sufficient number of technical replicates (see “Materials and Methods” for details). The amount of Dvl2 (bait) was set to 10 arbitrary units, and Dvl3 in the samples was quantified relatively to Dvl2. The results of this analysis showed that the amount of endogenous Dvl3 in complex with Dvl2 is indeed lower after Wnt3a stimulation (Fig. 8F). These results are in agreement with observations obtained by Western blot detection and by the non-quantitative MS/MS analysis. In summary, our findings shown in Fig. 8 demonstrate that PS-Dvl has decreased ability to bind other Dvls and suggest that PS-Dvl represents a negative signaling intermediate with decreased ability to polymerize.
DISCUSSION
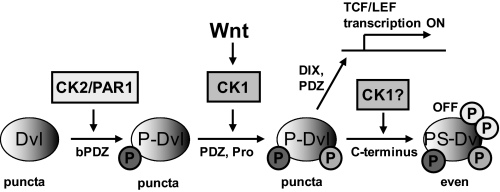
In this study we attempted to answer two main questions related to the Wnt/β-catenin signaling pathway; that is, how Dvl integrates phosphorylation by individual kinases and how CK1ϵ at the same time strongly induces Wnt/β-catenin signaling and dissolves Dvl puncta, which are required for signal activation. Based on our results, we propose a model that is consistent with the experimental evidence brought by us and others. This model sheds light on some of the so far controversial issues of Dvl biology and is schematized in Fig. 9.
FIGURE 9.
Model for the function of casein kinases in Dvl biology. Casein kinase 2 (and probably also PAR1 and its mammalian homologues) behave like constitutively active kinases. Under normal conditions CK2 phosphorylates Dvl in the basic domain/PDZ (bPDZ) to trigger formation of P-Dvl. P- Dvl was then subject to phosphorylation by CK1δ/ϵ, which becomes activated upon Wnt stimulation. CK1δ/ϵ phosphorylation of P-Dvl in the PDZ and/or Pro-rich region of Dvl allows recruitment of axin and phosphorylation of Lrp6 (usually detected after 10–15 min) and an executive phase of pathway activation (TCF/LEF-driven transcription), which is dependent on the DIX domains. Dvl in these functional states was capable of polymerization with an electrophoretic migration that resembles P-Dvl. Further phosphorylation of Dvl by CK1 led to the formation of PS-Dvl (usually detected 30–45 min after Wnt stimulation) and ultimately resulted in the inactivation of Dvl in the β-catenin pathway. This functional state of Dvl, which requires the C terminus of Dvl, leads to Dvl depolymerization and uniform subcellular distribution. Using this mechanism, CK1δ/ϵ can control both the activation and the termination of the pathway.
Our functional data show that CK2/PAR1 and CK1ϵ cannot replace each other. The effects of either kinase on Dvl-dependent induction of TCF/LEF-driven transcription can be blocked by inhibition/knockdown of the second kinase. This suggests that CK1ϵ and CK2 (or mammalian homologues of PAR1 from the MARK family of kinases, which may have similar function) act sequentially during the process of Dvl activation. Activity of CK1ϵ is required for the effects of CK2 to take place, and vice versa, the activity of CK2 is required for the effects of CK1ϵ to take place. In line with some earlier reports (24, 27), our data suggest that CK2 activity is constitutive or at least high enough to keep endogenous Dvl predominantly modified in the CK2-dependent manner in untreated cells. It remains to be tested as to the significance of the Wnt-induced increase in CK2 activity reported earlier by Gao and Wang (38). As a consequence, the levels of endogenous dephosphorylated Dvl were very low and only increased upon inhibition of CK2. In contrast, CK1ϵ seems to be a true Wnt-activated kinase required for the formation of PS-Dvl, which is the only hallmark of the activation of endogenous Dvl known to date. Our data suggest that CK1ϵ acts on Dvl modified by a CK2-dependent process. Furthermore, inhibition or low levels of CK2 can block or reduce the ability of CK1 to further phosphorylate Dvl and activate TCF/LEF-mediated transcription. The gradual effects of CK2 and CK1 toward Dvl can be visualized as a phosphorylation-dependent shift both in endogenous and exogenous Dvl (partially phosphorylated by CK2; Fig. 2C). It should be noted, however, that P-Dvl cannot be fully transformed to Dvl by phosphatase treatment, and thus, posttranslational modifications other than phosphorylation triggered by CK2 might be involved.
The effects of CK1ϵ-mediated phosphorylation of Dvl have been well described earlier in many cellular models. The most prominent CK1ϵ-induced changes involve (i) the ability of CK1ϵ to activate Dvl-dependent TCF/LEF-driven transcription (17–20) and (ii) the ability to dissolve Dvl polymers visible as Dvl puncta (14, 20). We show in this study that these two consequences of CK1ϵ phosphorylation are separated both spatially and functionally. First, CK1ϵ-mediated induction of TopFlash associated with dynamic recruitment of Axin1 was mediated via the PDZ-Pro-rich region of Dvl (given that DIX domain as the required prerequisite was present). Meanwhile, the ability of CK1ϵ to promote even localization of Dvl3 was mediated by the Dvl3 C terminus. The C-terminal part of Dvl was required for even localization of Dvl, which likely corresponds to the inability to form signalosomes. Dvl3 mutants lacking C terminus were hyperactive, showed punctate localization, and got less hyperphosphorylated after CK1ϵ co-expression, at least as assessed by the mobility shift. However, our data do not allow a direct link of electrophoretic migration of Dvl and Dvl localization. Although there is a strong correlation between these two features of Dvl (both strongly promoted by C terminus), there are few notable exceptions from this rule; for example, CK2 is unable to promote Dvl3 hyperphosphorylation but still efficiently shifts Dvl localization from punctate to even. However, our findings clearly demonstrated that the ability of CK1 to activate downstream signaling and promote even localization of Dvl are separate events with distinct functions.
This observation sheds light on some of the unclear issues of Dishevelled biology. It is well established that CK1ϵ is required for signaling downstream of Dvl, which involves Dvl polymerization and subsequent formation of signalosomes, Lrp6 phosphorylation, and stabilization of β-catenin (9, 11). However, activation of Dvl-mediated (and CK1-dependent) downstream signaling measured either as the formation of signalosomes (9), phosphorylation of LRP6 (39), internalization of LRP6 (40), or dephosphorylation of β-catenin (16) appears in less than 10–15 min, whereas the earliest formation of PS-Dvl is detected first after 30 min (14–16, 41). Functional separation of CK1-triggered downstream signaling and Dvl depolymerization allowed us to formulate the hypothesis that hyperphosphorylated Dvl (PS-Dvl), which is predominantly evenly distributed, is in an inactive form. Furthermore, our data suggest that PS-Dvl is part of a Dvl-inactivating mechanism maintained by CK1ϵ. CK1ϵ can, thus, control both the initiation and the termination of signaling (see Fig. 9).
According to our results the C-terminal part of Dvl carries the ability to switch off the activity of Dvl in the Wnt/β-catenin signaling upon CK1ϵ phosphorylation. In line with our findings, the C terminus of Dvl was shown to be an important region for the interaction with known negative regulators of the Wnt/β-catenin pathway. For example, KLHL12-cullin 3 system, which triggers ubiquitination and degradation of Dvl, binds the Dvl3 C terminus (32). The other inhibitor of the Wnt/β-catenin pathway, atypical receptor-tyrosine kinase Ror2, binds to the C terminus of Dvl3 and is the only known Dvl interaction partner that binds specifically to PS-Dvl after CK1 phosphorylation (41). Moreover, we have shown recently that negative effects mediated by the Dvl3 C terminus are dependent on Ror2 (41). Our work, thus, suggests that PS-Dvl, which is the only universal marker of Dvl activation in both canonical and non-canonical Wnt pathways, may be a general inactive signaling intermediate. This view was supported by the fact that PS-Dvl formed irrespective of whether or not activated Wnt triggered the Wnt/β-catenin pathway (14–16). We have also shown before that PS-Dvl, triggered by Wnt3a and Wnt5a, was indistinguishable in terms of its dynamics, the electrophoretic migration, and the kinases involved in its formation (14). Although it is becoming clear that the C terminus of Dvl is an important functional domain of PS-Dvl, most likely by providing an interaction interface for negative regulators of Wnt/β-catenin pathway, future work is required to uncover the molecular details of this phenomenon.
Acknowledgments
We thank Dr. S. Yanagawa (Kyoto University, Japan), Dr. Randy Moon (University of Washington, Seattle), Dr. S. Byers (Georgetown University, Washington D. C.), Drs. Olga Ossipova, Sergei Sokol, and Marek Mlodzik (Mount Sinai School of Medicine), Dr. Isabel Dominguez (Boston University School of Medicine), Dr. Madelone Maurice (UMC Utrecht), and Dr. J. M. Graff (University of Texas, Dallas) for providing plasmids. LWnt3A cells (ATCC#CRL-2647) were provided by Dr. Vladimir Korinek (Institute of Molecular Genetics, Prague).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–6.
- TCF
- T-cell-specific transcription factor
- PS
- phosphorylated and shifted
- CK1
- casein kinase 1
- MEF
- mouse embryonal fibroblast
- rm
- recombinant mouse
- x
- Xenopus
- Dvl
- Dishevelled
- LEF
- lymphoid enhancer binding factor
- TBCA
- (E)-3-(2,3,4,5-tetrabromophenyl)acrylic acid
- TBBt
- tetrabromobenzotriazole
- aa
- amino acids.
REFERENCES
- 1. Clevers H. (2006) Cell 127, 469–480 [DOI] [PubMed] [Google Scholar]
- 2. Malanchi I., Huelsken J. (2009) Curr. Opin. Oncol. 21, 41–46 [DOI] [PubMed] [Google Scholar]
- 3. Schulte G., Bryja V. (2007) Trends Pharmacol. Sci. 28, 518–525 [DOI] [PubMed] [Google Scholar]
- 4. Angers S., Moon R. T. (2009) Nat. Rev. Mol. Cell Biol. 10, 468–477 [DOI] [PubMed] [Google Scholar]
- 5. Malbon C. C., Wang H. Y. (2006) Curr. Top. Dev. Biol. 72, 153–166 [DOI] [PubMed] [Google Scholar]
- 6. Wallingford J. B., Habas R. (2005) Development 132, 4421–4436 [DOI] [PubMed] [Google Scholar]
- 7. Gao C., Chen Y. G. (2010) Cell. Signal. 22, 717–727 [DOI] [PubMed] [Google Scholar]
- 8. Wharton K. A., Jr. (2003) Dev. Biol. 253, 1–17 [DOI] [PubMed] [Google Scholar]
- 9. Bilic J., Huang Y. L., Davidson G., Zimmermann T., Cruciat C. M., Bienz M., Niehrs C. (2007) Science 316, 1619–1622 [DOI] [PubMed] [Google Scholar]
- 10. Schwarz-Romond T., Metcalfe C., Bienz M. (2007) J. Cell Sci. 120, 2402–2412 [DOI] [PubMed] [Google Scholar]
- 11. Schwarz-Romond T., Fiedler M., Shibata N., Butler P. J., Kikuchi A., Higuchi Y., Bienz M. (2007) Nat. Struct. Mol. Biol. 14, 484–492 [DOI] [PubMed] [Google Scholar]
- 12. Schwarz-Romond T., Merrifield C., Nichols B. J., Bienz M. (2005) J. Cell Sci. 118, 5269–5277 [DOI] [PubMed] [Google Scholar]
- 13. Smalley M. J., Signoret N., Robertson D., Tilley A., Hann A., Ewan K., Ding Y., Paterson H., Dale T. C. (2005) J. Cell Sci. 118, 5279–5289 [DOI] [PubMed] [Google Scholar]
- 14. Bryja V., Schulte G., Rawal N., Grahn A., Arenas E. (2007) J. Cell Sci. 120, 586–595 [DOI] [PubMed] [Google Scholar]
- 15. González-Sancho J. M., Brennan K. R., Castelo-Soccio L. A., Brown A. M. (2004) Mol. Cell. Biol. 24, 4757–4768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bryja V., Schulte G., Arenas E. (2007) Cell. Signal. 19, 610–616 [DOI] [PubMed] [Google Scholar]
- 17. Peters J. M., McKay R. M., McKay J. P., Graff J. M. (1999) Nature 401, 345–350 [DOI] [PubMed] [Google Scholar]
- 18. Sakanaka C., Leong P., Xu L., Harrison S. D., Williams L. T. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 12548–12552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kishida M., Hino Si., Michiue T., Yamamoto H., Kishida S., Fukui A., Asashima M., Kikuchi A. (2001) J. Biol. Chem. 276, 33147–33155 [DOI] [PubMed] [Google Scholar]
- 20. Cong F., Schweizer L., Varmus H. (2004) Mol. Cell. Biol. 24, 2000–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Foldynová-Trantírková S., Sekyrová P., Tmejová K., Brumovská E., Bernatík O., Blankenfeldt W., Krejcí P., Kozubík A., Dolezal T., Trantírek L., Bryja V. (2010) Breast Cancer Res. 12, R30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bryja V., Gradl D., Schambony A., Arenas E., Schulte G. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 6690–6695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bryja V., Schambony A., Cajánek L., Dominguez I., Arenas E., Schulte G. (2008) EMBO Rep. 9, 1244–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Willert K., Brink M., Wodarz A., Varmus H., Nusse R. (1997) EMBO J. 16, 3089–3096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ossipova O., Dhawan S., Sokol S., Green J. B. (2005) Dev. Cell 8, 829–841 [DOI] [PubMed] [Google Scholar]
- 26. Korinek V., Barker N., Morin P. J., van Wichen D., de Weger R., Kinzler K. W., Vogelstein B., Clevers H. (1997) Science 275, 1784–1787 [DOI] [PubMed] [Google Scholar]
- 27. Song D. H., Sussman D. J., Seldin D. C. (2000) J. Biol. Chem. 275, 23790–23797 [DOI] [PubMed] [Google Scholar]
- 28. Zeng X., Huang H., Tamai K., Zhang X., Harada Y., Yokota C., Almeida K., Wang J., Doble B., Woodgett J., Wynshaw-Boris A., Hsieh J. C., He X. (2008) Development 135, 367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kishida S., Yamamoto H., Hino S., Ikeda S., Kishida M., Kikuchi A. (1999) Mol. Cell. Biol. 19, 4414–4422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li L., Yuan H., Xie W., Mao J., Caruso A. M., McMahon A., Sussman D. J., Wu D. (1999) J. Biol. Chem. 274, 129–134 [DOI] [PubMed] [Google Scholar]
- 31. Boutros M., Paricio N., Strutt D. I., Mlodzik M. (1998) Cell 94, 109–118 [DOI] [PubMed] [Google Scholar]
- 32. Angers S., Thorpe C. J., Biechele T. L., Goldenberg S. J., Zheng N., MacCoss M. J., Moon R. T. (2006) Nat. Cell Biol. 8, 348–357 [DOI] [PubMed] [Google Scholar]
- 33. Klein T. J., Jenny A., Djiane A., Mlodzik M. (2006) Curr. Biol. 16, 1337–1343 [DOI] [PubMed] [Google Scholar]
- 34. Klimowski L. K., Garcia B. A., Shabanowitz J., Hunt D. F., Virshup D. M. (2006) FEBS J. 273, 4594–4602 [DOI] [PubMed] [Google Scholar]
- 35. Itoh K., Brott B. K., Bae G. U., Ratcliffe M. J., Sokol S. Y. (2005) J. Biol. 4, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jenny A., Reynolds-Kenneally J., Das G., Burnett M., Mlodzik M. (2005) Nat. Cell Biol. 7, 691–697 [DOI] [PubMed] [Google Scholar]
- 37. Lee Y. N., Gao Y., Wang H. Y. (2008) Cell. Signal. 20, 443–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gao Y., Wang H. Y. (2006) J. Biol. Chem. 281, 18394–18400 [DOI] [PubMed] [Google Scholar]
- 39. Khan Z., Vijayakumar S., de la Torre T. V., Rotolo S., Bafico A. (2007) Mol. Cell. Biol. 27, 7291–7301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yamamoto H., Komekado H., Kikuchi A. (2006) Dev. Cell 11, 213–223 [DOI] [PubMed] [Google Scholar]
- 41. Witte F., Bernatik O., Kirchner K., Masek J., Mahl A., Krejci P., Mundlos S., Schambony A., Bryja V., Stricker S. (2010) FASEB J. 24, 2417–2426 [DOI] [PubMed] [Google Scholar]
- 42. Lee J. S., Ishimoto A., Yanagawa S. (1999) J. Biol. Chem. 274, 21464–21470 [DOI] [PubMed] [Google Scholar]
- 43. Orford K., Crockett C., Jensen J. P., Weissman A. M., Byers S. W. (1997) J. Biol. Chem. 272, 24735–24738 [DOI] [PubMed] [Google Scholar]
- 44. Dominguez I., Mizuno J., Wu H., Imbrie G. A., Symes K., Seldin D. C. (2005) Mol. Cell. Biochem. 274, 125–131 [DOI] [PubMed] [Google Scholar]
- 45. McKay R. M., Peters J. M., Graff J. M. (2001) Dev. Biol. 235, 388–396 [DOI] [PubMed] [Google Scholar]
- 46. Tauriello D. V., Haegebarth A., Kuper I., Edelmann M. J., Henraat M., Canninga-van Dijk M. R., Kessler B. M., Clevers H., Maurice M. M. (2010) Mol. Cell 37, 607–619 [DOI] [PubMed] [Google Scholar]