Abstract
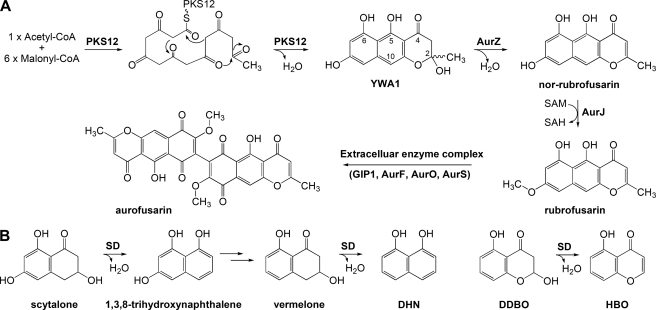
Previous studies have reported the functional characterization of 9 out of 11 genes found in the gene cluster responsible for biosynthesis of the polyketide pigment aurofusarin in Fusarium graminearum. Here we reanalyze the function of a putative aurofusarin pump (AurT) and the two remaining orphan genes, aurZ and aurS. Targeted gene replacement of aurZ resulted in the discovery that the compound YWA1, rather than nor-rubrofusarin, is the primary product of F. graminearum polyketide synthase 12 (FgPKS12). AurZ is the first representative of a novel class of dehydratases that act on hydroxylated γ-pyrones. Replacement of the aurS gene resulted in accumulation of rubrofusarin, an intermediate that also accumulates when the GIP1, aurF, or aurO genes in the aurofusarin cluster are deleted. Based on the shared phenotype and predicted subcellular localization, we propose that AurS is a member of an extracellular enzyme complex (GIP1-AurF-AurO-AurS) responsible for converting rubrofusarin into aurofusarin. This implies that rubrofusarin, rather than aurofusarin, is pumped across the plasma membrane. Replacement of the putative aurofusarin pump aurT increased the rubrofusarin-to- aurofusarin ratio, supporting that rubrofusarin is normally pumped across the plasma membrane. These results provide functional information on two novel classes of proteins and their contribution to polyketide pigment biosynthesis.
Keywords: Enzyme Catalysis, Fungi, Gene Knockout, Membrane Proteins, Quinones, Aurofusarin, Dehydratase, Orphan Genes, Polyketide Synthase, YWA1
Introduction
The plant pathogenic fungus Fusarium graminearum (teleomorph Gibberella zeae) is capable of producing a plethora of secondary metabolites, many of which belong to the polyketide class of compounds, such as the mycotoxins zearalenone, fusarin C, and aurofusarin (1). The F. graminearum genome encodes 15 polyketide synthases (PKS)2 (2, 3), of which FgPKS12 is the progenitor of nor-rubrofusarin/rubrofusarin/aurofusarin (4, 5), FgPKS4 and FgPKS13 are the progenitors of zearalenone (1, 6), FgPKS10 is the progenitor of fusarin C (7, 8), and FgPKS3 is the progenitor of an uncharacterized black/purple perithecial pigment (8). PKS genes are typically found in gene clusters that comprise genes encoding transcription factor, transporters, and tailoring enzymes required for biosynthesis of the final product (1, 4–6). In addition to genes encoding well characterized classes of enzymes, the clusters typically also include orphan genes that encode proteins annotated as “hypothetical” or “conserved hypothetical proteins,” signifying that no similarity to any previously characterized protein has been found. In the case of F. graminearum, more than half of all gene models found in the Fusarium graminearum Genome Database from the Munich Information Center for Protein Sequences (MIPS) fall into either of these two categories (9).
Functional characterization of hypothetical proteins is challenging, but those belonging to secondary metabolite gene clusters provide a unique opportunity because their location suggests a function in the respective biosynthetic pathways. The PKS12 gene cluster in F. graminearum, which is responsible for biosynthesis of the red mycelium pigment aurofusarin (4, 10), consists of 11 co-regulated genes. Nine of these have been functionally characterized, all encoding well described classes of enzymes such as laccases (GIP1 and AurL2), polyketide synthase (PKS12), monooxygenase (AurF), O-methyltransferase (AurJ), oxidoreductase (AurO), major facilitator pump (AurT), and transcription factors (AurR1 and AurR2) (5, 11). However, the gene cluster also includes two genes, aurZ (FG02325) and aurS (FG02329), annotated as encoding “hypothetical proteins.” They are both regulated by the cluster-specific transcription factor AurR1, and their expression coincides with the remaining genes in the cluster (5), suggesting that they play a role in aurofusarin biosynthesis.
In the present study, we wish to gain a fuller understanding of the aurofusarin pathway and assign functions to the two hypothetical proteins, AurZ and AurS. The role of the putative aurofusarin pump (AurT) is reassessed, and a new hypothesis for the aurofusarin biosynthetic pathway is presented.
EXPERIMENTAL PROCEDURES
Bioinformatics
Sequences were retrieved from the genetic sequence database (GenBankTM) from the National Center for Biotechnology Information, the Fusarium Comparative Database from Broad Institute, and the MIPS Fusarium graminearum Genome Database (9). Multiple sequence alignments were constructed using ClustalW version 2 with default settings (12). Ad hoc ab initio gene modeling was performed using FGENESH and FGENESH+ (SoftBerry). Motifs in unaligned sequences were identified using Meta-MEME version 4.1.1 and MAST (13, 14). Primer design and standard sequence manipulations were performed using Vector NTI 11 (Invitrogen). Identification of known motifs was performed using the Conserved Domain Database (15). SignalP 3.0 and TargetP 1.1 were used for prediction of signal peptides and subcellular localization (16, 17). TMHMM (version 2.0) was used to predict transmembrane helices (18). Predictions of glycosylphosphatidylinositol anchor locations were performed with big-PI Fungal Predictor (19). Secondary structure predictions were performed with DSSP (20) and PSIPRED (21) using PRALINE (22). HHpred 1.6.0.0 (23) was used to identify proteins that share secondary structure features similar to those predicted for AurZ, by searching the pdb70_2Oct10 database with default settings.
Targeted Gene Replacement
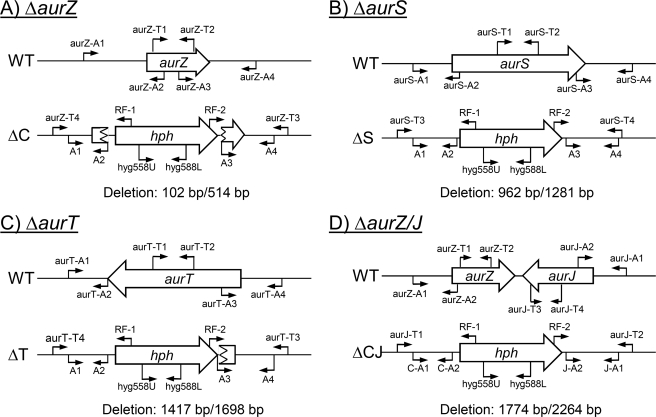
Binary vectors for targeted replacement of aurT, aurZ, and aurS were constructed by four-fragment In-Fusion cloning (Clontech) (24). The recombination flanks (geneX-A1/A2 and geneX-A3/A4) (Fig. 1) were amplified by PCR using Phusion DNA polymerase (Finnzyme) with gDNA from F. graminearum PH-1 as template. The pAg1-H3 vector was prepared for cloning by sequential digestion with SmaI and SwaI. The primer pairs (supplemental Table S1) used for amplification of the inserts included 30–34-bp-long 5′-overhangs that introduced terminal extensions into the amplicons, identical to the sequences surrounding the used restriction enzyme sites in the vector. The inserts in the replacement vectors pAg1-H3:ΔaurT, pAg1-H3:ΔaurS, and pAg1-H3:ΔaurZ were sequenced by GATC Biotech (Constance, Germany) to verify correct insertion events. The aurZ (FG02325.1) and aurJ (FG02326.1) loci are neighbors in the aurofusarin gene cluster, separated by 319 bp, allowing replacement of both genes in a single experiment. The replacement vector was constructed using the aurZ-A1/A2 (this study) and the aurJ-A1/A2 (5) recombination flanks. The aurZ-A1/A2 fragment was cloned into the SmaI site of the vector by In-Fusion cloning followed by ligase-dependent cloning of the aurJ-A1/A2 fragment into the SpeI/SalI digested vector, as described in Ref. 5. The verified replacement vectors were transformed into F. graminearum PH-1 by Agrobacterium tumefaciens-mediated transformation as described in Refs. 4 and 5. Correct aurT, aurZ, and aurS replacement strains were identified by diagnostic PCR, using four primer pairs: Hyg588U/L, geneX-T1/T2, and different combination of the RF-1 and RF-2 primers with geneX-T3 and geneX-T4 primers as specified in supplemental Table S1 and Fig. 1. The ΔaurZ/J strain was analyzed using: Hyg588U/L, aurZ-T1/T2, aurJ-T1/T2, aurJ-T1/RF1, and aurJ-T2/RF-2. Transformants that proved correct by the PCR analysis were verified by Southern blot analysis, using the 588-bp-long fragment of the hph gene as probe, amplified from pAg1-H3 using the primers Hyg588U/L. Genomic DNA from the wild type and the different deletion strains were digested with SalI (ΔaurZ:8575 bp, WT:6101 bp), NsiI (ΔaurT:10915 bp, WT:4321 bp), NheI (ΔaurS:9740, WT:8137 bp), and BplI (ΔaurZ/J: 8787 bp, WT: 8047 bp). Southern blotting was performed as described in Ref. 5.
FIGURE 1.
Strategy for genetic modifications. A–D, construction of targeted gene replacement strains and placement of primers for verification of the strains: A, ΔaurZ; B, ΔaurS; C, ΔaurT; D, ΔaurZ/J. The figure is not drawn to scale. Primer sequences are listed in supplemental Table S1. The size of the deletion is listed as the number of removed base pair positions when compared with the size of the coding sequence.
Initial Chemical Analysis of the Mutants
The wild type, ΔaurT, ΔaurS, ΔaurZ, ΔaurZ/J, ΔaurJ, and ΔaurF strains were grown for 10 days on defined Fusarium medium in darkness at 25 °C (4). Metabolites were extracted using methanol:dichloromethane:ethyl acetate 1:2:3 (v/v) and, after 1% formic acid was added, analyzed by reversed-phase high-performance liquid chromatography-diode array detection (HPLC-DAD), as described in Ref. 5. To monitor aurofusarin accumulation in the ΔaurT strain as a function of time, the mutant and wild type were grown on defined Fusarium medium in darkness at 28 °C for 4, 5, 6, and 9 days, at which time points 4-mm diameter agar plugs were cut out, covering the area from the center to the edge of the plates. The plugs were stored at −20 °C for later analysis. Metabolites were extracted as described above, and the ergosterol content was determined by HPLC-DAD using a GROM-SIL 120 ODS-5ST, 3 μm, 60 × 4.6-mm inner diameter column (Grom Analytik +HPLC GmbH). Analytes were eluted with methanol with 0.1% phosphoric acid using a flow rate of 1 ml/min for 12 min as described previously (25, 26). A 100 μg/ml ergosterol standard (Sigma-Aldrich) was used to identify and verify the ergosterol peak in the chromatograms based on retention time and UV spectrum (26). The samples were adjusted to similar ergosterol contents by dilution with methanol, and the aurofusarin and rubrofusarin contents were measured by HPLC-DAD as described in Ref. 5. The metabolite experiments were performed with at least three biological replicates.
Purification and Structural Characterization of the aurZ-specific Compound YWA1
The ΔaurZ mutant was grown at room temperature in darkness for 14 days on agar plates containing Bells medium (27) with maltose as the carbon source and urea as the nitrogen source as described in Ref. 4. The plates were freeze-dried, and the material (25 g) was extracted for 4 h at room temperature with 1 liter of methanol. The brown greenish extract was filtered and concentrated in vacuo to yield a dark brown oily residue. 100 of ml brine and ∼1 liter of ethyl acetate were added to the condensed extract. The collected organic phase was dried over MgSO4, filtered through a plug of Celite, and concentrated in vacuo to yield a black oily substance. The fungal material was extracted twice using this method to yield ∼500 mg of crude extract. The crude extract was subjected to flash chromatography using a 9-g KP-SIL column (150 × 12 mm, 40–63 μm, 60 Å, Biotage AB, Uppsala, Sweden) and a Biotage Quad 3 Flash Collector (Biotage AB). The analytes were eluted with dichloromethane:methanol: acetic acid 95:5:0.1. Fractions were assessed for content of YWA1 by liquid chromatography-mass spectrometry (LC-MS) on a system described elsewhere (28) and by reversed-phase HPLC using a Merck-Hitachi system (Hitachi, Ltd., Tokyo, Japan) consisting of a D-7000 interface, an L-7100 pump, an L-7200 autosampler, an L-7300 column oven, and an L-7450 DAD and controlled by LaChrom analysis software. The column used was a Zorbax, Eclipse XDB-C18, 4.6- × 75-mm inner diameter, 3.5 μm (Rockland Technologies, Hewlett Packard) kept at 40 °C. The separations were performed at 0.5 ml/min with water:methanol:trifluoroacetic acid 90:10:0.1 (mobile phase A) and methanol:water:trifluoroacetic acid 90:10:0.1 (mobile phase B) using the following linear gradient elution profile: 0 min, 20% B; 2 min, 20% B; 12 min, 80% B; 13 min, 20% B; 15 min, 20% B. Fractions containing almost pure YWA1 were combined, whereas other fractions containing YWA1 were rechromatographed as described above but with dichloromethane:methanol:acetic acid 93:7:0.1 as eluent. YWA1-containing fractions from both separations were combined to yield 20 mg of material. The target compound was further purified by preparative scale HPLC using a Waters system described elsewhere (28). The column used was a 100 × 19 mm-inner diameter, 5 μm, Phenomenex Gemini C-18 (Phenomenex Inc., Torrance, CA) operated at room temperature. The separations were performed at 10 ml/min with water:trifluoroacetic acid 1000:1 (mobile phase A) and acetonitrile:trifluoroacetic acid 1000:1 (mobile phase B) using the following linear gradient elution profile: 0 min, 5% B; 2 min, 5% B; 25 min, 95% B; 27 min, 5% B; 30 min, 5% B, resulting in isolation of 9 mg of pure YWA1. High-resolution MS for exact mass determination was performed on the above instrument. NMR experiments were performed at ambient temperature on a Bruker Avance NMR spectrometer (1H frequency of 300.13 MHz) equipped with a 5-mm broad band observe probe or a Bruker Avance III NMR spectrometer (1H frequency of 799.96 MHz) equipped with a 5-mm cryogenic triple cooled inverse probe. Samples were prepared in dimethyl sulfoxide-d6 and transferred to a 2.5-mm NMR tube.
RESULTS
aurT, aurZ, and aurS Are All Involved in Aurofusarin Biosynthesis
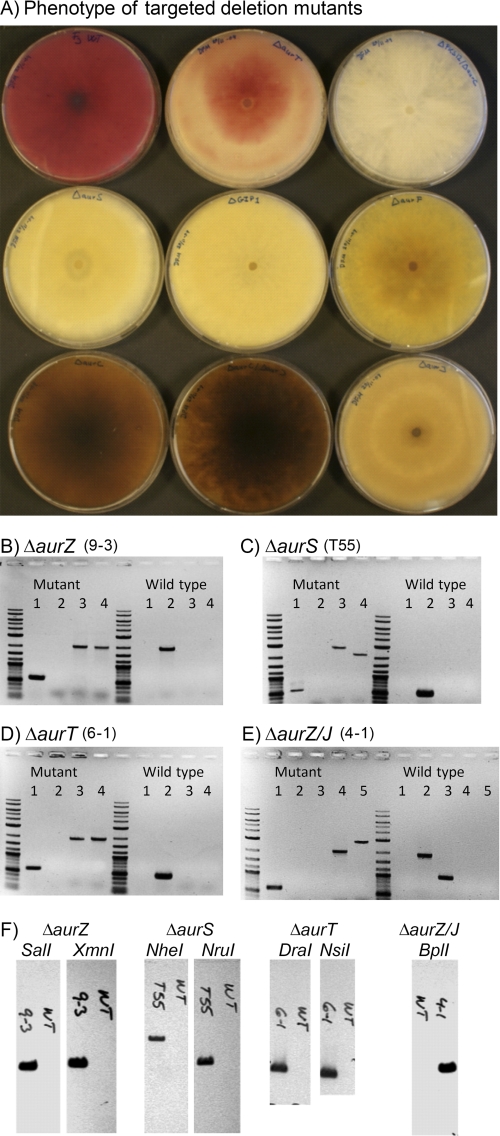
Construction of binary replacement vectors was performed by single-step four-fragment In-Fusion cloning, resulting in 10–20% correct E. coli transformants. Agrobacterium tumefaciens mediated transformation of F. graminearum with the constructed vectors resulted in 36 aurZ, 36 aurT, and 107 aurS hygromycin B-resistant transformants, with a double crossover frequency of 11, 78, and 74% based on the diagnostic PCR analysis. Five transformants for each of the deletion constructs were analyzed by Southern blot analysis, which showed that all selected transformants contained just a single tDNA copy (Fig. 2). The altered pigmentation of the verified replacement strains indicated that all three genes were involved in aurofusarin biosynthesis. The ΔaurZ strain displayed a brown/green mycelium pigmentation not observed in previous mutants (Fig. 2). The ΔaurS strain displayed a yellow pigmentation similar to that observed for the ΔGIP1 and ΔaurF strains, suggesting accumulation of the intermediate rubrofusarin. The color phenotype of the ΔaurT strain was both time-dependent and temperature-dependent, but under all analyzed incubation conditions, it differed from that of the wild type. In young ΔaurT cultures, the mycelium was pink, whereas it turned red in older cultures, suggesting a delayed production of aurofusarin.
FIGURE 2.
Analysis of the generated strains. A, phenotype of the constructed mutants (*) and previously constructed strains (8) grown for 10 days on defined Fusarium medium. Top left to right bottom, Fg PH-1 wild type, ΔAurT(*), ΔPKS12/ΔaurZ(*), ΔaurS(*), ΔGIP1, ΔaurF, ΔaurZ(*), ΔaurZ/J(*), and ΔaurJ. B–F, verification of genetically modified strains. B, PCR analysis of ΔaurZ. C, PCR analysis of ΔaurS. D, PCR analysis of ΔaurT. E, PCR analysis of ΔaurZ/J. F, Southern blot analysis of hph/tDNA copy number in the selected transformants. Lanes in B–D: lane 1 = hyg588U/L; lane 2 = check for target CDS; lane 3 = check of crossover in left flank; and lane 4 = crossover in right flank. Lanes in E: lane 1 = hph588U/L; lane 2 = aurZ-T1/T2; lane 3 = aurJ-T1/T2; and lanes 4 and 5, similar to 3 and 4 in B–D.
aurZ Homologs Are Associated with Polyketide Synthases
The 514-bp-long aurZ gene (FG02325) consists of two exons, 398 and 61 bp, separated by a 55-bp-long intron. The resulting 152-amino acid (aa) protein did not show identity to any previously characterized proteins and did not contain any described catalytic or structural domains. The available annotated fungal genome sequences were searched for AurZ homologs by position-specific iterative basic local alignment search tool (PSI-BLAST) with five recurring rounds. A total of 37 sequences with a high level of similarity were obtained from 19 ascomycetes and 2 basidiomycetes (Table 1). Analysis of the genomic regions surrounding the hits showed that 23 of the 38 AurZ homologs were located less than 10 genes away from PKS-encoding genes. 17 of the 23 PKS genes encoded non-reducing polyketide synthases similar to PKS12 in the aurofusarin gene cluster.
TABLE 1.
Identified AurZ homologs and their relative position to the nearest PKS encoding gene
Listed after succeeding identity (I) to AurZ, similarity (S), and length (L). Not found (–).
| AurZ homologs |
Closest PKS encoding gene on genome |
|||||
|---|---|---|---|---|---|---|
| Hit | Species | Accession No. | I/S/L | Distance | Accession No. | PKS domains |
| 0 | Fusarium graminearum | FG02325.1 | –/–/152 | + 1 (0.5 kb) | FG12040 | KS-AT-ACP-CYC |
| 1 | Talaromyces stipitatus | TSTA_058090 | 69/53/183 | + 2 (1.5 kb) | TSTA_058110 | AMP dependent CoA ligase |
| 2 | Pyrenophora tritici-repentis | PTRG_02729 | 69/48/186 | – | – | – |
| 3 | Microsporum canis | MCYG_05053 | 68/50/137 | + 1 (0.3 kb) | MCYG_05054.1 | KS-AT-ACP-ACP-CYC |
| 4 | Neosartorya fischeri | NFIA_101890 | 67/51/146 | + 7 (12 kb) | NFIA_101810 | KS-AT-ACP |
| 5 | Talaromyces stipitatus | TSTA_126440 | 66/57/152 | – | – | – |
| 6 | Penicillium marneffei | PMAA_100350 | 66/56/187 | + 1 (0.2 kb) | PMAA_100360 | KS-AT-ACP |
| 7 | Aspergillus fumigatus | Afu4g14470 | 66/51/142 | + 9 (12 kb) | Afu4g14560 | KS-AT-ACP |
| 8 | Penicillium marneffei | PMAA_031690 | 65/55/180 | + 5 (11 kb) | PMAA_031640 | KS-AT-ACP |
| 9 | Microsporum canis | MCYG_06698 | 65/51/188 | + 8 (12 kb) | MCYG_06706 | KS |
| 10 | Neosartorya fischeri | NFIA_101630 | 63/57/151 | + 3 (2.5 kb) | NFIA_101660 | KS-AT-ACP |
| 11 | Aspergillus terreus | ATEG_08457 | 62/47/142 | + 6 (8 kb) | ATEG_08451 | KS-AT-ACP |
| 12 | Aspergillus nidulans | ANID_11847.1 | 60/55/139 | + 1 (0.3 kb) | ANID_00150.1 | KS-AT-ACP |
| 13 | Talaromyces stipitatus | TSTA_008960 | 60/54/167 | + 1 (1 kb) | TSTA_008950 | KS-AT-ACP-CYC |
| 14 | Aspergillus oryzae | AO090001000323 | 59/50/137 | – | – | – |
| 15 | Aspergillus flavus | AFL2G_07546 | 59/50/137 | – | – | – |
| 16 | Chaetomium globosum | CHGG_08978 | 56/50/147 | – | – | – |
| 17 | Penicillium chrysogenum | Pc16g10800 | 55/53/143 | + 5 (?) | Pc16g10750 | KS-AT-ACP |
| 18 | Aspergillus niger | e_gw1_14.245 | 54/56/133 | + 1 (0.8 kb) | e_gw1_14.257 | KS-AT-ACP-ACP-CYC |
| 19 | Talaromyces stipitatus | TSTA_083280 | 51/55/133 | + 3 (3.7 kb) | TSTA_083310 | KS-AT-ACP-CYC |
| 20 | Penicillium marneffei | PMAA_063590 | 49/55/130 | + 3 (4.1 kb) | PMAA_063620 | KS-AT-ACP-CYC |
| 21 | Phaeosphaeria nodorum | SNOG_08275 | 46/54/127 | + 1 (1.8 kb) | SNOG_08274 | KS-AT-ACP-CYC |
| 22 | Aspergillus nidulans | AN1088.2 | 45/50/127 | – | – | – |
| 23 | Penicillium marneffei | PMAA_043660 | 39/53/139 | – | – | – |
| 24 | Aspergillus niger | An11g06450 | 38/50/165 | + 1 (1 kb) | An11g06460 | KS-AT-DH-KR-ACP |
| 25 | Magnaporthe grisea | MGG_14687.6 | 37/61/126 | + 4 (7.8 kb) | MGG_00428.6 | KS-AT-ACP-CYC |
| 26 | Pyrenophora tritici-repentis | PTRG_10876 | 36/57/144 | – | – | – |
| 27 | Aspergillus niger | An02g08300 | 36/54/164 | + 1 (0.8 kb) | An02g08290 | KS-AT-DH-KR |
| 28 | Fusarium graminearum | FGSG_13420.3a | 35/65/183 | + 8 (35 kb) | FGSG_08208.3 | KS-AT-DH-ER-KR-ACP |
| 29 | Fusarium oxysporum | FOXG_16598.2 | 35/63/180 | + 29 (71 kb) | FOXG_16568.2 | AMP dependent CoA ligase |
| 30 | Moniliophthora perniciosa | MPER_10734 | 35/56/200 | – | – | – |
| 31 | Nectria haematococca | EEU41176.1 | 35/51/161 | – | – | – |
| 32 | Ustilago maydis | UM05965.1 | 34/65/153 | – | – | – |
| 33 | Talaromyces stipitatus | TSTA_000620 | 34/54/131 | – | – | – |
| 34 | Fusarium graminearum | FGSG_03463.3 | 33/53/177 | – | – | – |
| 35 | Fusarium verticillioides | FVEG_01729.3 | 29/71/184 | + 6 (14 kb) | FVEG_01736.3 | KS-AT-DH-ER-KR-(no ACP) |
| 36 | Fusarium oxysporum | FOXG_02891 | 29/69/184 | + 7 (10 kb) | FOXG_02884.2 | KS-AT-DH-KR |
| 37 | Fusarium verticillioides | FVEG_13655.3 | 29/54/147 | +14 (45 kb) | FVEG_13670.3 | AMP dependent CoA ligase |
a Modified gene models.
A multiple sequence alignment of the 20 proteins that showed the highest level of similarity to AurZ revealed that the sequences shared 8 fully conserved sequence positions and 33 positions with similar properties (supplemental Fig. S1). The search for conserved sequence motifs, using Meta-MEME, revealed four conserved domains with high information content and a total of 24 highly or fully conserved sequence positions (supplemental Fig. S2). The analyzed sequences were predicted to have very similar secondary structures including four helices and four strands, as seen by multiple alignment based on secondary structure (supplemental Fig. S3).
The search for structurally related proteins based on similarities in secondary structure resulted in over 50 high scoring hits in the Protein Data Bank (PDB) covering the entire primary sequence of AurZ (Table 2). The proteins all displayed very limited identity on the primary sequence level but a highly similar tertiary structure belonging to the split α-β sandwich group (ferredoxin-like fold) of the α+β fold class of proteins. Alignment guided by the secondary structure of AurZ and enzymes, for which biochemical data were available, showed that the enzymes did not share any conserved residues in the known active sites of the characterized enzymes (supplemental Fig. S3).
TABLE 2.
Top 20 proteins and proteins with known function displaying similar secondary structure as predicted for AurZ
SS = secondary structure score for how well the PSIPRED-predicted (three-state) or actual DSSP-determined (eight-state) secondary structure sequence agree with each other. I = identity on primary sequence level for aligned region.
| Hit | PDB identifier | Predicted function (origin) | SS | Coverage | I |
|---|---|---|---|---|---|
| 1 | 3HF5_A; 3HDS_A; 3HFK_A; 2IFX_A (MLMI) | 4-Methylmuconolactone methylisomerase (Pseudomonas reinekei) | 11.0 | 108 aa | 15% |
| 2 | 2FTR_A | Unknown (Bacillus halodurans) | 11.9 | 106 aa | 14% |
| 3 | 3BF4_A | Ethyl TERT-butyl ether degradation (Ralstonia eutropha) | 11.5 | 103 aa | 10% |
| 4 | 1TR0_A; 1SI9_A | Stress protein (unknown function) (Populus tremula) | 13.7 | 106 aa | 10% |
| 5 | 2JDJ_A (HapK) | Prodigiosin biosynthesis (unknown) (Hahella chejuensis) | 10.2 | 82 aa | 11% |
| 6 | 3BB5_A | Stress responsive alpha-beta protein (Jannaschia sp) | 12.6 | 103 aa | 10% |
| 7 | 2FB0_A | Unknown (Bacteroides thetaiotaomicron) | 10.5 | 78 aa | 8% |
| 8 | 1Q4R_A; 1Q53_A;2Q3P_A | Unknown (Arabidopsis thaliana) | 11.6 | 106 aa | 15% |
| 9 | 2OMO_A | Predicted oxidoreductase (Nitrosomonas europaea) | 11.0 | 121 aa | 14% |
| 10 | 3FMB_A | Unknown (Bacteroides fragilis) | 13.3 | 101 aa | 13% |
| 11 | 2GYC_A | Unknown (Bordetella bronchiseptica) | 12.7 | 96 aa | 10% |
| 12 | 3BDE_A | Unknown (Mesorhizobium loti) | 12.0 | 97 aa | 18% |
| 13 | 3LO3_A | Unknown (Colwellia psychrerythraea) | 8.2 | 66 aa | 8% |
| 14 | 3E8O_A | Unknown (Deinococcus radiodurans) | 11.6 | 105 aa | 11% |
| 15 | 3BN7_A | Unknown (Caulobacter crescentus) | 12.0 | 86 aa | 14% |
| 16 | 3KG0_A; 3KG1_A; 3KNG_A (SnoB) | Anthracycline monooxygenase (Streptomyces nogalater) | 9.7 | 98 aa | 18% |
| 17 | 1IUJ_A (TT1380)) | Unknown (Thermus thermophilus) | 9.5 | 100 aa | 13% |
| 18 | 1X7V_A (PA3566) | Unknown (Pseudomonas aeruginosa) | 11.3 | 82 aa | 13% |
| 19 | 1Q8B_A | Unknown (Bacillus subtilis) | 9.1 | 95 aa | 7% |
| 20 | 3BM7_A | Unknown (Caulobacter crescentus) | 10.0 | 79 aa | 14% |
| 28 | 1TUV_A; 1R6Y_A (YgiN) | Menadione oxidase (Escherichia coli) | 11.8 | 86 aa | 10% |
| 35 | 1SQE_A; 2ZDP_A; 3LGM_A; 3LGN_A (IsdG/I) | Heme-degrading enzyme (Staphylococcus aureus) | 6.3 | 75 aa | 11% |
| 37 | 1Y0H_A | Monooxygenase (Mycobacterium tuberculosis) | 7.8 | 102 aa | 9% |
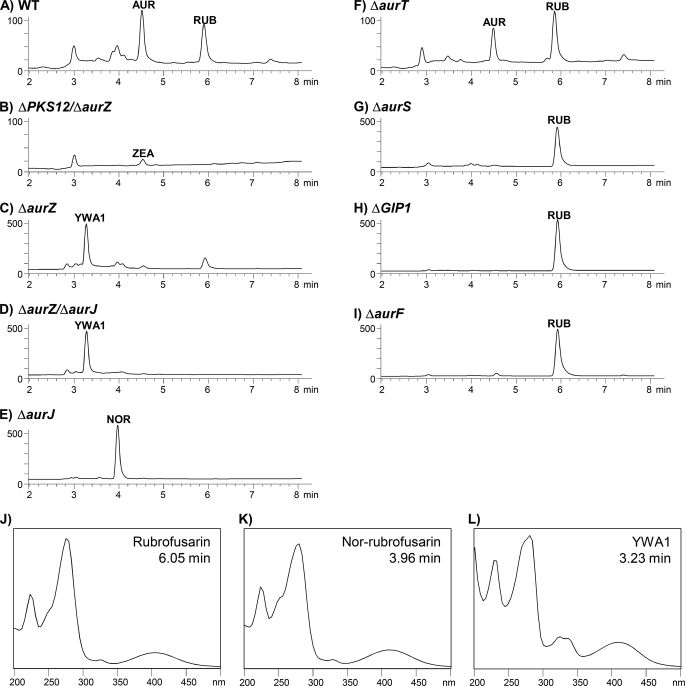
The reversed-phase HPLC-DAD analysis of the targeted aurZ deletion mutant showed that it accumulated a new compound (retention time 3.23 min) not found in the wild type or any of the previously described aurofusarin-deficient mutants (Fig. 3C). The UV-visible spectrum for the compound displayed a high level of similarity to that of rubrofusarin and nor-rubrofusarin, suggesting that the compounds were structurally related with respect to their conjugated ring systems (Fig. 3, J–L). The metabolite eluted ∼45 s prior to nor-rubrofusarin and 170 s prior to rubrofusarin, showing that the compound was more hydrophilic than any of the known intermediates in the aurofusarin pathway. High-resolution-MS analysis showed that it had an m/z of 277.0712 (= [M+H]+) and 259.0617 (= [M+H-H2O]+), equivalent to a molecular formula of C14H12O6, which implies that the compound had two more hydrogen and one more oxygen atom than nor-rubrofusarin. The similarity of the new ΔaurZ metabolite to an Aspergillus spore pigment (29) with respect to 1H NMR (supplemental Table S2) and UV-spectral data established its identity as YWA1. The presence of an additional hydroxyl group in YWA1 when compared with nor-rubrofusarin coincides with a shorter retention time as observed for the new metabolite. Equilibrium between YWA1 and the ring-opened diketo tautomer was observed when YWA1 was dissolved in dipolar aprotic solvents such as dimethyl sulfoxide. The 1H NMR resonances of the ring-opened form of YWA1 (supplemental Table S2) are comparable with those found for the related structure 2-acetyl-1,3,6,8-tetrahydroxynaphtalene (30), except for H-10, whose resonance frequency will be affected by the hydrogen-bonding scheme for its neighboring OH group.
FIGURE 3.
Reversed-phase HPLC-DAD analysis of genetically modified F. graminearum strains. A–I, chromatograms showing UV absorption (milliabsorbance units, 230 nm) as a function of retention time (min). A, wild type. AUR, aurofusarin; RUB, rubrofusarin. B, ΔaurPKS12/aurZ. ZEA, zearalenone. C, ΔaurZ. D, ΔaurZ/J. E, ΔaurJ. NOR, nor-rubrofusarin. F, ΔaurT. G, ΔaurS. H, ΔGIP1. I, ΔaurJ. J–L, UV-visible absorption spectra. J, rubrofusarin. K, nor-rubrofusarin. L, YWA1. Note the different y axes in the chromatograms.
AurZ Acts as the First Tailoring Enzyme in the Aurofusarin Biosynthetic Pathway
Simultaneous replacement of aurZ and aurJ resulted in 24 transformants with a double crossover frequency of 62.5%. The double replacement strain ΔaurZ/J accumulated YWA1, like the ΔaurZ strain, and not nor-rubrofusarin, as found in the ΔaurJ stain, showing that AurZ functions prior to AurJ during aurofusarin biosynthesis (Fig. 3, C–E). This finding suggests that the primary product of PKS12 is YWA1 and not nor-rubrofusarin as reported previously (5).
Targeted replacement of aurZ resulted in three different color phenotypes: red, yellow/green, and white mycelium. The PCR-based analysis showed that red transformants represented single crossover events where aurZ had not been replaced, and the yellow/green and white transformants were true aurZ replacements lacking the aurZ gene. Sequencing of the upstream recombination flank that extends into the PKS12 locus revealed that a single-nucleotide substitution of guanine (G) to thymine (T) had occurred in the white mutants. The substitution was located inside the predicted malonyltransferase (MAT) domain of PKS12 (693G→T), changing Ala193 to Val193 and most likely rendering the resulting enzyme dysfunctional. The lack of YWA1 accumulation in the resulting ΔPKS12/ΔaurZ strain shows that PKS12 synthesizes the compound that accumulates in the ΔaurZ strain. The base substitution was located in the terminal part of the replacement tDNA, allowing for the generation of both correct ΔaurZ mutants, by crossover close to the hygromycin resistance gene in the tDNA, and double mutants, by crossover close to the terminal end of the recombination flank in the tDNA.
aurS Mutants Accumulate Rubrofusarin
The 1281-bp-long aurS gene consists of two exons separated by a 48-bp intron and encodes a 407-aa-long protein. AurS did not show homology to any previously characterized proteins in GenBank; however, the search for conserved domains revealed two fasciclin domains (COG2335) at positions 52–192 and 195–365.
30 sequences displaying a high level of identity to AurS were identified by blastp, all of which contained two fasciclin domains (Table 3). Multiple sequence alignment revealed that the identified sequences shared 27 fully conserved positions and 117 positions with similar properties (supplemental Fig. S4). The search for conserved sequence motifs in the unaligned sequences (Meta-MEME analysis) showed that the proteins varied considerably in the N-terminal region with respect to both sequence and length but that they shared similar motifs and motif architecture in the C-terminal end (∼350 aa). The conserved C-terminal region was described by eight motifs covering 248 amino acid positions, including 82 highly or fully conserved positions (supplemental. Fig. S5). The novel conserved motifs that were identified by this Meta-MEME analysis are shown in supplemental Fig. S6. Analysis of the genomic region adjacent to the identified AurS homologs showed that they did not co-localize with PKS or non-ribosomal peptide synthetase encoding genes.
TABLE 3.
Identified AurS homologs
I = identity, S = similarity and E = expected value. The locations of the two fasciclin domains are shown in the right hand column.
| Hit | Species | Accession no. | I | S | E | Fasciclin domains |
|---|---|---|---|---|---|---|
| 1 | Microsporum canis | MCYG_08538 | 160/404 | 235/404 | 6e-72 | 93–193; 207–352 |
| 2 | Ajellomyces capsulatus | HCAG_03186 | 107/359 | 171/359 | 2e-36 | 142–256; 271–414 |
| 3 | Sclerotinia sclerotiorum | SS1G_05049 | 126/377 | 186/377 | 6e-36 | 156–278; 296–451 |
| 4 | Ajellomyces capsulatus | HCBG_05627 | 108/358 | 174/358 | 8e-36 | 158–260; 274–417 |
| 5 | Ajellomyces dermatitidis | BDCG_02276 | 106/334 | 157/334 | 1e-34 | 166–269; 283–426 |
| 6 | Ajellomyces dermatitidis | BDBG_06929 | 106/334 | 157/334 | 1e-34 | 166–269; 282–426 |
| 7 | Ajellomyces capsulatus | HCDG_08956 | 102/326 | 158/326 | 4e-34 | 158–260; 274–417 |
| 8 | Botryotinia fuckeliana | BC1G_09860 | 115/367 | 179/367 | 2e-32 | 155–279; 297–452 |
| 9 | Coccidioides posadasii | CPC735_024720A | 109/370 | 172/370 | 3e-32 | 121–247; 271–403 |
| 10 | Coccidioides immitis | CIMG_05852 | 108/370 | 171/370 | 1e-31 | 124–247; 271–403 |
| 11 | Aspergillus terreus | ATEG_00632 | 109/381 | 184/381 | 2e-31 | 133–264; 278–429 |
| 12 | Penicillium marneffei | PMAA_005700 | 109/368 | 174/368 | 4e-31 | 226–328; 342–488 |
| 13 | Uncinocarpus reesii | UREG_04495 | 107/356 | 167/356 | 6e-31 | 129–255; 270–412 |
| 14 | Pyrenophora tritici-repentis | PTRG_06147 | 106/369 | 174/369 | 8e-30 | 162–288; 302–451 |
| 15 | Phaeosphaeria nodorum | SNOG_07701 | 103/361 | 173/361 | 1e-29 | 141–267; 281–431 |
| 16 | Aspergillus fumigatus | AFUB_013850A | 107/375 | 169/375 | 3e-27 | 142–279; 298–446 |
| 17 | Aspergillus clavatus | ACLA_021110 | 109/383 | 171/383 | 5e-27 | 145–289; 337–456 |
| 18 | Talaromyces stipitatus | TSTA_104300 | 104/364 | 176/364 | 8e-27 | 200–330; 344–493 |
| 19 | Aspergillus fumigatus | AfA10A1.015 | 103/377 | 168/377 | 1e-26 | 142–279; 298–446 |
| 20 | Neosartorya fischeri | NFIA_011110 | 105/371 | 168/371 | 2e-26 | 142–282; 297–444 |
| 21 | Nectria haematococca | NECHADRAFT_92290 | 100/369 | 177/369 | 2e-26 | 100–228; 242–389 |
| 22 | Aspergillus flavus | AFLA_042090 | 108/378 | 172/378 | 5e-24 | 132–260; 275–421 |
| 23 | Penicillium chrysogenum | Pc16g12870 | 100/352 | 161/352 | 6e-24 | 282–386; 399–561 |
| 24 | Aspergillus oryzae | AO090011000381 | 104/350 | 160/350 | 2e-23 | 132–260; 275–421 |
| 25 | Aspergillus nidulans | AN0768.2 | 104/391 | 170/391 | 2e-23 | 131–278; 300–447 |
| 26 | Gibberella zeae | FG02859 | 103/380 | 174/380 | 7e-23 | 114–241; 255–397 |
| 27 | Podospora anserina | PODANSg4381 | 99/343 | 154/343 | 8e-23 | 115–245; 260–404 |
| 28 | Aspergillus niger | An15g02640 | 96/366 | 154/366 | 2e-19 | 57–179; 196–366 |
The MIPS annotation of AurS includes the prediction of a transmembrane domain located from positions 7 to 29, and an analysis using SignalP suggests that this N-terminal sequence contains a signal peptide, which is cleaved between Ser36 and Gln37. This combined with a TargetP prediction suggests that the signal peptide induces secretion of the protein to the extracellular surface of the cell (score: 0.899). Fungal fasciclin-like proteins have been reported to be tethered to the outer leaflet of the plasma membrane by glycosylphosphatidylinositol anchors (31), but a search for glycosylphosphatidylinositol anchor sites in AurS and homologs did not find any sites with a significant score. Prediction of subcellular localization for the identified homologs showed that 28 of the 30 analyzed sequences are probably excreted. Targeted replacement of aurS resulted in an aurofusarin-deficient strain that accumulated rubrofusarin (Fig. 3G), which previously has been demonstrated to accumulate in ΔGIP1, ΔaurO, and ΔaurF strains (Fig. 3, H and I) (5).
AurT Encodes a Major Facilitator-type Efflux Pump
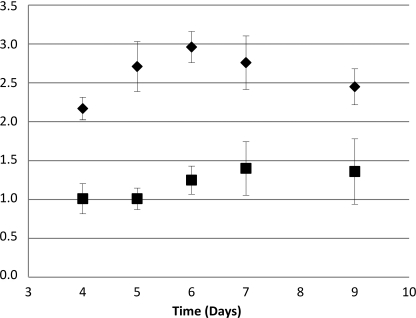
The aurT (FG02322) gene consists of two exons, 629 and 1006 bp, separated by a 63-bp-long intron, resulting in a total coding sequence of 1635 bp, equivalent to a protein of 544 amino acids. The deduced amino acid sequence shows significant similarity to major facilitator-type efflux pumps. The highest similarity was found to the hypothetical protein nh_02161 from Nectria haematococca mpVI (identity = 55.6%), and among the characterized proteins, the highest similarity was found to the sterigmatocystin (aflatoxin) efflux pump AflT from Aspergillus nidulans (I = 42%). The AurT sequence included 14 predicted transmembrane domains, and the N-terminal secretion signal is predicted to target the protein to the plasma or endoplasmic reticulum membrane. Targeted replacement of aurT resulted in a reduction in aurofusarin production and a doubling of the rubrofusarin-to-aurofusarin ratio when compared with that observed in the wild type (Fig. 4). Aurofusarin was continuously being synthesized, but at a lower rate than in the wild type (Fig. 3F).
FIGURE 4.
Rubrofusarin-to-aurofusarin ratio. A plot of the rubrofusarin-to-aurofusarin ratio in the wild type (squares) and ΔaurT (diamonds) strains is shown (each point is supported by three biological replicates). Error bars indicate S.E.
DISCUSSION
Fungal secondary metabolite gene organization facilitates the characterization of the respective pathways by stepwise dissection. However, a full understanding of the pathways is still challenging, particularly when novel hypothetical proteins are involved. The detailed knowledge of the aurofusarin pathway was exploited to address the function of two novel classes of proteins. This provides important new biological insight and knowledge of the molecular machinery required for the biosynthesis of a fungal pigment.
AurZ Is Required for Dehydration of YWA1 to Nor-rubrofusarin
In previous models for aurofusarin biosynthesis, FgPKS12 was predicted to form the naphthopyrone, nor-rubrofusarin (4, 5). Here we show that AurZ is required for nor-rubrofusarin biosynthesis, which implies that FgPKS12 synthesizes a precursor to nor-rubrofusarin, YWA1. This is consistent with results reported for the closely related wA PKS from A. nidulans (32). Accordingly, the new model for aurofusarin biosynthesis includes YWA1 as the first stable intermediate in the pathway (Fig. 5A). The C2 hydroxyl group in the pyrone ring of YWA1 is probably formed during ring closure by an aldol-type cyclization reaction as described for wA in A. nidulans (33). The accumulation of YWA1 in the ΔaurZ strain suggests that AurZ is responsible for catalyzing the dehydration reaction that converts the novel intermediate into nor-rubrofusarin (Fig. 5A). As AurZ does not show significant sequence similarity to any previously characterized enzymes, including dehydratases, it is the first identified member of a novel family of dehydratases. However, further experimental data are required to establish whether the enzyme is capable of performing the dehydration by itself or whether it is dependent on additional co-factors.
FIGURE 5.
Action of the dehydratase AurZ. A, a revised model for the initial step of aurofusarin biosynthesis, including the suggested dehydration reaction of YWA1 catalyzed by AurZ. B, the action of SD in 1,8-dihydroxynaphthalene (DHN) melanin biosynthesis and with an alternative naphthopyrone substrate. S-adenosylmethionine (SAM), S-adenosyl-homocysteine (SAH), 2,3-dihydro-2,5-dihydroxy-4H-benzopyran-4-one (DDBO), 5-hydroxy-4H-1-benzopyran-4-one (HBO).
The 1,8-dihydroxynaphthalene melanin pathway found in Wangiella dermatitidis, Sordaria macrospora, Colletotrichum lagenarium, Magnaporthe grisea, Neurospora crassa, Alternaria alternata, and several Aspergillus species is currently the best described fungal mycelium pigment biosynthetic pathway (34, 35). The pathway includes two dehydration reactions catalyzed by scytalone dehydratase (SD) (EC 4.2.1.94) (Fig. 5B) (36). The ligand-binding pocket of the enzyme is a cone-shaped α+β barrel, consisting of eight β strands and four α helixes sandwiched together. The enzyme acts independently of co-factors, prosthetic groups, or metal ions (37). In addition to its natural substrates, scytalone and vermelone, SD is also able to act on the pyrone 2,3-dihydro-2,5-dihydroxy-4H-benzopyran-4-one (DDBO), which resembles the structure of YWA1 (38). Although both SD and AurZ catalyze dehydration reactions of related polycyclic substrates, they have limited primary sequence identity (13% when compared with SD from M. grisea) but a high level of similarity in the predicted secondary structure, suggesting a similar tertiary structure (47% when compared with SD from M. grisea). Comparison of the characterized active site residues of SD (Tyr30, Tyr50, His85, and His110) and AurZ, by secondary structure alignment, showed that these residues were not conserved in AurZ. This suggests that AurZ and SD have different catalytic mechanisms. The predicted secondary structure of AurZ and the homologs listed in Table 1 displayed a high level of similarity to several proteins that have been structurally characterized (Table 2). Common to these proteins is a ferredoxin-like fold, consisting of a β-α-β-β-α-α-β motif with the antiparallel β strands forming the floor of the active site and the three α helixes arching the β sheet structure creating a reactive cavity. This group of structurally related proteins includes enzymes responsible for 4-methylmuconolactone methyl degradation (isomerase, Pseudomonas reinekei) (39), ethyl tert-butyl ether degradation (Ralstonia eutropha) (PDB id 3BF4), polyketide biosynthesis (anthracycline monooxygenase, Streptomyces nogalater) (40), redox cycling (menadione oxidase, Escherichia coli) (41), and heme degradation (Staphylococcus aureus) (42). Although the enzymes share structural features, they catalyze very diverse types of reactions and display different active site residues. They all act independently of co-factors, prosthetic group and metals. The substrates vary greatly in size, which is reflected in the size of the active site cavities of the enzymes (43). The fact that substrates such as heme groups (porphyrin structure), anthracycline (four aromatic rings), and actinorhodin (three aromatic rings) can be accommodated by this type of protein fold makes it likely that AurZ can bind YWA1 in a similar fashion.
The double targeted replacement strains ΔaurZ/J and PKS12/aurZ support the proposed order of reactions in the revised model for aurofusarin biosynthesis (Fig. 5A) as the PKS12 mutation was epistatic to the aurZ mutation, which in turn was epistatic to the aurJ mutation (Figs. 2 and 4). The close genetic linkage between AurZ homologs and non-reducing PKS genes in different fungal genomes suggests that this novel class of dehydratases is an essential part of other polyketide biosynthetic pathways that lead to the formation of aromatic compounds in fungi.
What Function Does AurS Have in the Biosynthesis of Aurofusarin?
AurS does not show homology to any previously characterized proteins but does contain two fasciclin domains. Enzymes containing fasciclin domains have been described from a wide range of eukaryotic organisms and have typically been shown to facilitate cell-cell adhesion via protein-protein interactions (44–47). This domain type was initially identified as a crucial cell adhesion protein in the nervous system of Drosophila. In the fungi Lentinula edodes and Magnaporthe oryzae, they were shown to be required for morphogenesis and tissue differentiation (48, 49). However, apart from the presence of fasciclin domains, these two proteins do not show homology to AurS. The search for AurS homologs identified AfA10A1.015 (Afu4g14470) from Aspergillus fumigatus as a homolog (Table 3), a protein that previously has been suggested to mediate host-pathogen interaction (50). Our results are the first to indicate that proteins with fasciclin domains play a role in pigment biosynthesis.
Resolution of the steps leading to formation of rubrofusarin has been possible due to the accumulation of unique pathway intermediates after replacement of individual gene cluster members. Mutations in later steps of the pathway have all resulted in the accumulation of rubrofusarin and a lack of aurofusarin. These results are best explained by the existence of a protein complex that becomes inactive or disintegrates if any of its members are missing (5). Based on the accumulation of rubrofusarin in deletion mutants, the aurofusarin biosynthetic protein complex is predicted to include AurO, AurF, Gip1, and AurS. Three of these four proteins contain N-terminal signal peptides for export to the plasma membrane via the endoplasmic reticulum. We propose that this extracellular protein complex is responsible for converting rubrofusarin to aurofusarin (Fig. 5A).
There is evidence to suggest that many secondary metabolite biosynthetic pathways in fungi are restricted to specific organelles, as shown for cyclosporine, penicillin, and aflatoxin biosynthesis (51–56). Recently specific synthesis vesicles, aflatoxisomes (57), were discovered in Aspergillus parasiticus. In the case of aurofusarin biosynthesis, the members of the putative enzyme complex all lack known retention signals, which suggests that part of this pathway is completed outside the cell.
We have recently shown that the conversion of rubrofusarin to aurofusarin is dependent on the generic trans-plasma membrane redox system (58), which suggests that conversion of rubrofusarin to aurofusarin is catalyzed outside the cell in relation to the surface of the plasma membrane. The indication that AurT is a rubrofusarin-specific pump further supports that rubrofusarin is transported across the plasma membrane for enzymatic processing. Previous reports did not detect any effect of deleting aurT (11), but this could be explained by the nonspecific action of one or more of the 307 putative major facilitator superfamily efflux pumps present in the F. graminearum genome or differences in the culture conditions used.
In conclusion, our results suggest that the hypothetical protein AurZ is the first member of a previously unknown class of dehydratases acting on hydroxylated naphthopyrone ring structures. AurS is the first fasciclin domain-containing protein to be associated with fungal pigment biosynthesis. AurT is predicted to facilitate the transfer of rubrofusarin to the plasma membrane surface for conversion into aurofusarin. The results have led to a revised model for aurofusarin biosynthesis in which an additional step is included.
This work was supported by a grant from the Danish Ministry for Food, Agriculture and Fisheries and by The Danish Research Council for Technology and Production Sciences (Grant 274-06-0371).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S6 and Tables S1 and S2.
- PKS
- polyketide synthase
- HPLC-DAD
- high-performance liquid chromatography-diode array detection
- aa
- amino acid
- SD
- scytalone dehydratase.
REFERENCES
- 1. Lysøe E., Klemsdal S. S., Bone K. R., Frandsen R. J., Johansen T., Thrane U., Giese H. (2006) Appl. Environ. Microbiol. 72, 3924–3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Varga J., Kocsubé S., Tóth B., Mesterházy A. (2005) Acta. Biol. Hung. 56, 375–388 [DOI] [PubMed] [Google Scholar]
- 3. Tobiasen C., Aahman J., Ravnholt K. S., Bjerrum M. J., Grell M. N., Giese H. (2007) Curr. Genet. 51, 43–58 [DOI] [PubMed] [Google Scholar]
- 4. Malz S., Grell M. N., Thrane C., Maier F. J., Rosager P., Felk A., Albertsen K. S., Salomon S., Bohn L., Schäfer W., Giese H. (2005) Fungal Genet. Biol. 42, 420–433 [DOI] [PubMed] [Google Scholar]
- 5. Frandsen R. J., Nielsen N. J., Maolanon N., Sørensen J. C., Olsson S., Nielsen J., Giese H. (2006) Mol. Microbiol. 61, 1069–1080 [DOI] [PubMed] [Google Scholar]
- 6. Kim Y. T., Lee Y. R., Jin J., Han K. H., Kim H., Kim J. C., Lee T., Yun S. H., Lee Y. W. (2005) Mol. Microbiol. 58, 1102–1113 [DOI] [PubMed] [Google Scholar]
- 7. Song Z., Cox R. J., Lazarus C. M., Simpson T. J. (2004) ChemBioChem 5, 1196–1203 [DOI] [PubMed] [Google Scholar]
- 8. Gaffoor I., Brown D. W., Plattner R., Proctor R. H., Qi W., Trail F. (2005) Eukaryotic Cell 4, 1926–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Güldener U., Mannhaupt G., Münsterkötter M., Haase D., Oesterheld M., Stümpflen V., Mewes H. W., Adam G. (2006) Nucleic Acids Res. 34, D456–D458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim J. E., Han K. H., Jin J., Kim H., Kim J. C., Yun S. H., Lee Y. W. (2005) Appl. Environ. Microbiol. 71, 1701–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim J. E., Kim J. C., Jin J. M., Yun S. H., Lee Y. W. (2008) Plant Pathol. J. 24, 8–16 [Google Scholar]
- 12. Larkin M. A., Blackshields G., Brown N. P., Chenna R., McGettigan P. A., McWilliam H., Valentin F., Wallace I. M., Wilm A., Lopez R., Thompson J. D., Gibson T. J., Higgins D. G. (2007) Bioinformatics 23, 2947–2948 [DOI] [PubMed] [Google Scholar]
- 13. Bailey T. L., Elkan C. (1994) in Proceedings of the Second International Conference on Intelligent Systems for Molecular Biology (Altman R., Brutlag D., Karp P., Lathrop R., Searls D. eds) p. 36, The AAAI Press, Menlo Park, CA [Google Scholar]
- 14. Bailey T. L., Gribskov M. (1998) Bioinformatics 14, 48–54 [DOI] [PubMed] [Google Scholar]
- 15. Marchler-Bauer A., Anderson J. B., Derbyshire M. K., DeWeese-Scott C., Gonzales N. R., Gwadz M., Hao L., He S., Hurwitz D. I., Jackson J. D., Ke Z., Krylov D., Lanczycki C. J., Liebert C. A., Liu C., Lu F., Lu S., Marchler G. H., Mullokandov M., Song J. S., Thanki N., Yamashita R. A., Yin J. J., Zhang D., Bryant S. H. (2007) Nucleic Acids Res. 35, D237–D240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bendtsen J. D., Nielsen H., von Heijne G., Brunak S. (2004) J. Mol. Biol. 340, 783–795 [DOI] [PubMed] [Google Scholar]
- 17. Emanuelsson O., Nielsen H., Brunak S., von Heijne G. (2000) J. Mol. Biol. 300, 1005–1016 [DOI] [PubMed] [Google Scholar]
- 18. Krogh A., Larsson B., von Heijne G., Sonnhammer E. L. (2001) J. Mol. Biol. 305, 567–580 [DOI] [PubMed] [Google Scholar]
- 19. Eisenhaber B., Schneider G., Wildpaner M., Eisenhaber F. (2004) J. Mol. Biol. 337, 243–253 [DOI] [PubMed] [Google Scholar]
- 20. Kabsch W., Sander C. (1983) Biopolymers. 22, 2577–2637 [DOI] [PubMed] [Google Scholar]
- 21. Jones D. T. (1999) J. Mol. Biol. 292, 195–202 [DOI] [PubMed] [Google Scholar]
- 22. Simossis V. A., Heringa J. (2005) Nucleic Acids Res. 33, W289–W294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Söding J. (2005) Bioinformatics 21, 951–960 [DOI] [PubMed] [Google Scholar]
- 24. Zhu B., Cai G., Hall E. O., Freeman G. J. (2007) BioTechniques 43, 354–359 [DOI] [PubMed] [Google Scholar]
- 25. Bills C. E., McDonald F. G., BeMiller L. N., Steel G. E., Nussmeier M. (1931) J. Biol. Chem. 93, 775–785 [Google Scholar]
- 26. Pruess L. M., Peterson W. H., Fred E. B. (1932) J. Biol. Chem. 97, 483–489 [Google Scholar]
- 27. Bell A. A., Wheeler M. H., Liu J., Stipanovic R. D., Puckhaber L. S., Orta H. (2003) Pest Manag. Sci. 59, 736–747 [DOI] [PubMed] [Google Scholar]
- 28. Nielsen N. J., Nielsen J., Staerk D. (2010) J. Agric. Food Chem. 58, 5509–5514 [DOI] [PubMed] [Google Scholar]
- 29. Watanabe A., Fujii I., Sankawa U., Mayorga M. E., Timberlake W. E., Ebizuka Y. (1999) Tetrahedron Lett. 40, 91–94 [Google Scholar]
- 30. Watanabe A., Ebizuka Y. (2002) Tetrahedron Lett. 43, 843–846 [Google Scholar]
- 31. Chatterjee S., Mayor S. (2001) Cell. Mol. Life Sci. 58, 1969–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mayorga M. E., Timberlake W. E. (1990) Genetics. 126, 73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Standforth S. P. (2006) Natural Product Chemistry at a Glance, pp. 38–40, Wiley-Blackwell Publishing, Oxford [Google Scholar]
- 34. Engh I., Nowrousian M., Kück U. (2007) FEMS Microbiol. Lett. 275, 62–70 [DOI] [PubMed] [Google Scholar]
- 35. Langfelder K., Streibel M., Jahn B., Haase G., Brakhage A. A. (2003) Fungal Genet. Biol. 38, 143–158 [DOI] [PubMed] [Google Scholar]
- 36. Jordan D. B., Zheng Y. J., Lockett B. A., Basarab G. S. (2000) Biochemistry 39, 2276–2282 [DOI] [PubMed] [Google Scholar]
- 37. Basarab G. S., Steffens J. J., Wawrzak Z., Schwartz R. S., Lundqvist T., Jordan D. B. (1999) Biochemistry 38, 6012–6024 [DOI] [PubMed] [Google Scholar]
- 38. Thompson J. E., Basarab G. S., Pierce J., Hodge C. N., Jordan D. B. (1998) Anal. Biochem. 256, 1–6 [DOI] [PubMed] [Google Scholar]
- 39. Marín M., Heinz D. W., Pieper D. H., Klink B. U. (2009) J. Biol. Chem. 284, 32709–32716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Grocholski T., Koskiniemi H., Lindqvist Y., Mäntsälä P., Niemi J., Schneider G. (2010) Biochemistry. 49, 934–944 [DOI] [PubMed] [Google Scholar]
- 41. Adams M. A., Jia Z. C. (2005) J. Biol. Chem. 280, 8358–8363 [DOI] [PubMed] [Google Scholar]
- 42. Lee W. C., Reniere M. L., Skaar E. P., Murphy M. E. (2008) J. Biol. Chem. 283, 30957–30963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lemieux M. J., Ference C., Cherney M. M., Wang M., Garen C., James M. N. (2005) J. Struct. Funct. Genomics 6, 245–257 [DOI] [PubMed] [Google Scholar]
- 44. Huber O., Sumper M. (1994) EMBO J. 13, 4212–4222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kawamoto T., Noshiro M., Shen M., Nakamasu K., Hashimoto K., Kawashima-Ohya Y., Gotoh O., Kato Y. (1998) Biochim. Biophys. Acta. 1395, 288–292 [DOI] [PubMed] [Google Scholar]
- 46. Kim J. E., Kim S. J., Lee B. H., Park R. W., Kim K. S., Kim I. S. (2000) J. Biol. Chem. 275, 30907–30915 [DOI] [PubMed] [Google Scholar]
- 47. Sato K., Nishi N., Nomizu M. (2004) Arch. Biochem. Biophys. 424, 1–10 [DOI] [PubMed] [Google Scholar]
- 48. Miyazaki Y., Kaneko S., Sunagawa M., Shishido K., Yamazaki T., Nakamura M., Babasaki K. (2007) Curr. Genet. 51, 367–375 [DOI] [PubMed] [Google Scholar]
- 49. Liu T. B., Chen G. Q., Min H., Lin F. C. (2009) J. Zhejiang Univ. Sci. B. 10, 434–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pain A., Woodward J., Quail M. A., Anderson M. J., Clark R., Collins M., Fosker N., Fraser A., Harris D., Larke N., Murphy L., Humphray S., O'Neil S., Pertea M., Price C., Rabbinowitsch E., Rajandream M. A., Salzberg S., Saunders D., Seeger K., Sharp S., Warren T., Denning D. W., Barrell B., Hall N. (2004) Fungal Genet. Biol. 41, 443–453 [DOI] [PubMed] [Google Scholar]
- 51. Hoppert M., Gentzsch C., Schörgendorfer K. (2001) Arch. Microbiol. 176, 285–293 [DOI] [PubMed] [Google Scholar]
- 52. Lendenfeld T., Ghali D., Wolschek M., Kubicek-Pranz E. M., Kubicek C. P. (1993) J. Biol. Chem. 268, 665–671 [PubMed] [Google Scholar]
- 53. Evers M. E., Trip H., van den Berg M. A., Bovenberg R. A., Driessen A. J. M. (2004) Adv. Biochem. Eng. Biotechnol. 88, 111–135 [DOI] [PubMed] [Google Scholar]
- 54. van de Kamp M., Driessen A. J., Konings W. N. (1999) Antonie Van Leeuwenhoek 75, 41–78 [DOI] [PubMed] [Google Scholar]
- 55. Hong S. Y., Linz J. E. (2008) Appl. Environ. Microbiol. 74, 6385–6396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hong S. Y., Linz J. E. (2009) Mycol. Res. 113, 591–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Roze L. V., Chanda A., Linz J. E. (2011) Fungal Genet. Biol. 48, 35–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Frandsen R. J., Albertsen K. S., Stougaard P., Sørensen J. L., Nielsen K. F., Olsson S., Giese H. (2010) Eukaryotic Cell 9, 1225–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]