Abstract
In the adaptation of avian viruses to mammalian hosts, mutations in the viral polymerase, notably in the PB2 subunit, play an important role. A PB2 C-terminal domain rich in putative host adaptation residues has been shown to bind importin α nuclear import receptors. Adaptation has been proposed to involve binding of PB2 to importins of the new host. To date PB2-importin complexes have been characterized semiquantitatively with no precise measurement of binding parameters. To investigate the effects of adaptive mutations on importin interaction and selectivity, surface plasmon resonance was used to compare the binding rate constants and affinities of avian H5N1 and human H3N2 PB2 C-terminal variants with importin isoforms human α 1, 3, 5 and 7, and avian α 1. Using purified proteins eliminates host environment effects and permits measurement of intrinsic affinities and rates of complex formation and dissociation. Two effects were observed: first, adaptive mutations D701N, R702K, and S714R in the nuclear localization signal domain increased 2–4-fold the association rates with avian and human importins; second, measurement of different structural forms of the PB2 C terminus demonstrated that the upstream 627 domain reduced binding affinity, consistent with a steric clash predicted from crystal structures. From these kinetic data, structural analyses, and the data of others, a model is proposed in which an increase in charged surface residues during host adaptation increases the association rate of PB2 to cytoplasmic importins and where the C-terminal 627-nuclear localization signal domain may reorganize upon importin binding, consistent with a role in active polymerase assembly.
Keywords: Nuclear Transport, Protein Structure, Protein-Protein Interactions, Surface Plasmon Resonance (SPR), Viral Polymerase, PB2 Subunit, Host Adaptation, Influenza
Introduction
Adaptation of avian influenza viruses to human hosts is a major health concern. To date, the currently circulating H5N1 strains are highly pathogenic with mortality rates of approximately 70%, but they do not sustain transmission between humans. This limited compatibility between hosts and virus strains is due to a species barrier that must be overcome during a process of host adaptation. The mutagenic mechanisms are well understood and involve a gradual accumulation of mutations in the viral genes due to the lack of proofreading activity in the viral RNA-dependent RNA polymerase, termed genetic drift. Furthermore, reassortment of genome segments between coinfecting viruses can permit sudden adaptation of animal viruses to humans (1).
The molecular mechanisms of influenza A virus adaptation to new host species are only partly understood. Most studied are the roles of hemagglutinin (HA) and neuraminidase subtypes in cell surface receptor specificity leading to viral uptake and shedding, respectively (2–4). Comparative sequence analyses have identified with high confidence host signatures that are correlated with adaptation of avian viruses to mammalian hosts and pathogenicity (5–9; for review, see Refs. 10, 11): 2 in PA, 3 in PB1, and 13 in PB2 (9). Thus, the highest density of host determinant residues is found in the latter subunit that contains the mRNA cap-binding activity necessary for cap snatching (12).
For most of these PB2 mutations, little is known about their mode of action with the exception of residue 627 that is almost invariably glutamic acid in avian viruses and lysine in those that have adapted to humans. The mutation E627K in an otherwise non-human-infective avian virus can be sufficient to confer host adaptation (8) and increased virulence (13). Structural studies have revealed how this mutation generates a strong basic surface patch on the domain that is presumed to modulate molecular interactions (14, 15). It has been suggested that Glu627 avian variants are bound by a host-restricting factor present in human cells (16) or, conversely, that Glu627 interacts with a factor in avian cells that is not present, or binds with low affinity, in human cells (17); currently the identity of these putative partners is unknown. Some functional data are also available for the surface located PB2 positions 701 and 714 that have been shown to increase polymerase activity in mammalian cells in an additive manner (18), whereas the single amino acid change D701N in PB2 is sufficient for infection of mice by avian viruses (19) and partially compensates for Glu627 in humans (20, 21).
Influenza polymerase subunits are produced in the cytoplasm and subsequently imported into the nucleus for assembly into functional trimer (22, 23). Nuclear import is mediated by the classical importin α pathway where the cargo is bound via a nuclear localization signal (NLS).2 Such signals have been identified in PA (24), PB1 (25), PB2 (26, 27), and NP (28–30). PB2 is translocated in the absence of other polymerase subunits, possibly in complex with host factors such as Hsp90 (31) and CCT (32). The association with importin α isoforms is necessary for nuclear transport, and it seems possible that host adaptation could improve binding of PB2 to the importins of the new host animal. Indeed, a general observation is that the affinity of cargoes to their importins correlates clearly with the efficiency of nuclear localization (33–36). In support of this mechanism for adaptation, it was shown that human importin α (Hu Imp-α) isoforms associated efficiently with the PB2 protein of a H3N2 human virus but bound to diminished and variable extents to PB2 from H5N1 avian strains (37). Furthermore, the single PB2 adaptive mutation D701N was observed to enhance 4-fold the binding of importin α1 (Imp-α1) in mammalian cells but not avian cells (38), suggesting that improved binding to specific importins could result from single adaptive mutations, as suggested by structural studies of the PB2 NLS domain–Imp-α5 complex (27). Importins have been proposed as having a second function beyond nuclear localization in mediating nuclear assembly of active trimeric polymerase (37). The high density of host adaptation residues in the Imp-α-binding C-terminal region of PB2 (14) suggests that some of these may have a role in increasing binding potential to importins of the new host species, thereby increasing nuclear accumulation and polymerase assembly.
From our previous structural studies on the PB2 C-terminal region of a human H3N2 isolate (14, 27), two soluble constructs were characterized: a domain bearing the NLS (NLS domain) and an upstream domain containing residue 627 (627 domain). Both are soluble in isolation, but when fused, they form a single unit (627-NLS domain) where the separate domains pack together via a hydrophilic interface, stabilized by ionic interactions. The availability of milligram quantities of purified domains from the structural studies provides a framework for the analysis of mutants in vitro using biophysical methods. Here, we used surface plasmon resonance (SPR) to measure with high accuracy the kinetic and thermodynamic binding parameters of a series of importin α isoforms to purified C-terminal PB2 constructs containing naturally occurring sequence variations. These were cloned from human H3N2 and avian H5N1 strains that differ by 10 mutations in the 627-NLS domain, all of which have been proposed to be adaptive (supplemental Fig. S1) (6–9, 39). The overall aims were (i) to see whether PB2-importin interactions exhibited selectivity along host species lines and whether adaptive mutations in the C-terminal region affected these interactions and (ii) to determine whether the domain architecture influenced this interaction because the structural studies predict a clash between the 627 domain and the importin.
These biophysical measurements complement those made in complex cell-based systems (e.g. by coimmunoprecipitation and in nuclear translocation and polymerase activity assays) but have distinct advantages. First, the interactions can be studied in the absence of host factors, permitting direct determination of the intrinsic properties of the interaction. Second, cell-based studies of interactions are often only semiquantitative (e.g. immunoprecipitation assesses the persistence of complexes through long wash steps) or measure indirect effects of complex formation such as transcription rates or replication efficiency. By contrast, we measure here with high accuracy not only the intrinsic affinities of the complexes, but their component rate constants. The latter provide more biological insight than a simple affinity measurement because they determine the rate of complex formation as well as its stability once formed (half-life). The severalfold differences in association between the various PB2 forms and importins observed here are of a comparable magnitude to the increases in nuclear accumulation observed in cellular studies (18, 38, 40), suggestive of a meaningful correlation between in vitro and in vivo results.
EXPERIMENTAL PROCEDURES
Protein Expression and Purification
A codon-optimized human H3N2 pb2 synthetic gene encoding amino acids 538–759 from A/Victoria/3/1975 (41) and a typical avian H5N1 isolate exemplified by A/duck/Shantou/4610/2003 were synthesized to facilitate expression in Escherichia coli (Geneart, Regensburg, Germany). A panel of Imp-α protein isoforms lacking the autoinhibitory N-terminal region IBB domain (ΔIBB) were produced using the same methodology; Hu Imp-α1 (KPNA2; Uniprot: P52292, amino acids 60–529), Hu Imp-α3 (KPNA4; Uniprot: O00629, amino acids 59–521), Hu Imp-α5 (KPNA1; Uniprot: P52294, amino acids 66–512), Hu Imp-α7 (KPNA6; Uniprot: O60684, amino acids 59–536), and Av Imp-α1 (KPNA2; Uniprot: Q5ZMA9, amino acids 59–528). All constructs were subcloned into a pET9a-derived vector with N-terminal hexahistidine tag and TEV protease cleavage sequence (MGHHHHHHDYDIPTTENLYFQG). For expression of the MBP-NLSbipartite construct, a DNA cassette encoding the PB2 NLS with two linking serines (SSKRKRDSSILTDSQTATKRIRMAIN; NLS regions underlined) was fused directly to the 3′ end of the malE gene via the SacI/BamHI sites of pMAL-c2g (New England Biolabs); this cassette was then expressed from a pET9a vector with an N-terminal TEV-cleavable hexahistidine tag. Proteins were expressed in E. coli BL21 AI (Invitrogen) supplemented with RIL plasmid (Stratagene) in TB medium. Protein expression was induced by the addition of 0.2% w/v arabinose for 20 h at 25 °C. After centrifugation, bacterial pellets were lysed using sonication in 20 mm Tris-HCl, pH 7.5, containing 200 mm NaCl and complete protease inhibitor (Roche Applied Science). Purification of proteins was achieved using Ni2+-affinity chromatography and 20 mm Tris-HCl, pH 7.5, containing 200 mm NaCl and 0.5 m imidazole. The hexahistidine tag was then removed by overnight incubation at 20 °C with TEV protease. After dialysis against 20 mm HEPES, pH 7.5, with 200 mm NaCl, the cleaved protein was purified through an additional Ni2+-affinity chromatography step to remove unwanted material. Proteins were purified to homogeneity by an additional gel filtration step on Superdex 75 or 200 (GE Healthcare), yielding highly pure quality samples and monodisperse as assessed by SDS-PAGE and dynamic light scattering, respectively.
Ligand Immobilization and SPR Measurements
SPR measurements were conducted in a BIAcore X instrument using CM5 sensor chips (BIAcore AB) at 20 °C. Purified C-terminal PB2 recombinant proteins and variants were covalently coupled to sensor chips CM5 using standard amine-coupling protocols. Briefly, the carboxylated dextran matrix was activated by injecting a mixture of 0.1 m N-hydroxysuccinimide and 0.4 m EDC in a 1:1 ratio. Recombinant proteins (1–15 μg/ml) were diluted in 10 mm sodium acetate buffer, pH 5.0, and then injected until binding of 650–800 response units was reached. Blocking of remaining active esters was performed by a 7-min injection of 1 m ethanolamine hydrochloride at pH 8.5. One of the flow cells was used as a reference to monitor the response due to buffer effects and unspecific interactions. This flow cell was treated in the same way as the other during the immobilization procedure, but omitting the ligand immobilization step. All proteins were diluted with the HBS-EP (10 mm HEPES, 150 mm NaCl, 3 mm EDTA, 0.005% Tween 20) running buffer. Samples (1–100 nm) were injected over the immobilized C-terminal PB2 forms and reference surfaces for an association phase of 210 s at a flow rate of 20 μl/min followed by a 290-s dissociation phase. Nonspecific binding to the matrix was controlled by subtracting the signal obtained on the control surface. Regeneration of the surface was achieved using a short injection of 10 mm glycine, pH 2.5, or 10 mm NaOH at 100 μl/min. Each concentration was analyzed in duplicate. Kinetic parameters were determined by curve fitting with BIAevaluation (version 4.1) software using a simple 1:1 binding model and standard variation calculated for each binding parameters. Injections at different flow rates (15–100 μl/min) showed that mass transfer effects were insignificant.
RESULTS
Binding of Human Single NLS Domain and Variants to Hu and Av Imp-α1 Isoforms
We first optimized conditions for affinity measurements of the Hu NLS domain binding to Hu Imp-α1 by using SPR. After immobilizing the NLS domain to the CM5 sensor chip and upon injection of various concentrations of Hu Imp-α1, a specific signal increased in a concentration-dependent manner, confirming a specific interaction with the NLS domain. Sensorgrams displayed a clear association phase followed by a slow dissociation phase that is characteristic of a high affinity. Binding curves were fitted using the simple Langmuir binding model in agreement with a 1:1 stoichiometric interaction and low nanomolar affinity.
An affinity of 3.8 nm was calculated for the complex between the NLS domain and Hu Imp-α1 with a nearly 4-fold higher affinity for Av Imp-α1. We then tested the effects of three previously studied host range determinants within the single NLS domain: D701N, R702K, and S714R for their affinities to either Hu Imp-α1 or Av Imp-α1. Detailed binding constants are shown in Table 1. Av Imp-α1 binds the unmodified, human NLS domain (Asp701, Arg702, and Ser714) with a KD of 1.1 nm, 3-fold more tightly than Hu Imp-α1 (KD = 3.8 nm) (Fig. 1A). The adaptive mutation S714R and the avian-like R702K behaved similarly; in fact, all three mutations increased the association rate to both importins (Fig. 1B). For S714R and R702K, this was accompanied by an approximate 3-fold affinity increase to Hu Imp-α1, but not Av Imp-α1. The adaptive mutant D701N also bound more rapidly to both importins than the human PB2 domain (Fig. 1B), but then dissociated faster (Fig. 1C), resulting in a slightly lower affinity (Fig. 1A).
TABLE 1.
Kinetic constants and calculated binding constants for PB2 C-terminal binding to importin α isoforms
| Ligands | Analytes |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
kon (m−1 s−1) × 105 |
koff (s−1) × 10−3 |
KD (nm)a |
|||||||||||||
| Hu Imp |
Av Imp | Hu Imp |
Av Imp | Hu Imp |
Av Imp | ||||||||||
| α1 | α3 | α5 | α7 | α1 | α1 | α3 | α5 | α7 | α1 | α1 | α3 | α5 | α7 | α1 | |
| Hu NLS | 3.3 | NDb | 1.6 | ND | 8.5 | 1.2 | ND | 1.2 | ND | 0.9 | 3.8 | ND | 8.5 | ND | 1.1 |
| Hu NLS (D701N) | 7.7 | ND | 4.9 | ND | 13.1 | 3.6 | ND | 2.3 | ND | 3.1 | 4.7 | ND | 5.1 | ND | 2.5 |
| Hu NLS (R702K) | 13.1 | ND | 4.2 | ND | 21.2 | 1.6 | ND | 2.7 | ND | 2.2 | 1.3 | ND | 7.0 | ND | 1.1 |
| Hu NLS (S714R) | 10.4 | ND | 5.2 | ND | 23.2 | 1.5 | ND | 2.0 | ND | 1.5 | 1.5 | ND | 4.3 | ND | 0.7 |
| Hu 627-NLS | 1.7 | 2.9 | 2.2 | 2.5 | 4.5 | 1.1 | 0.4 | 1.2 | 1.4 | 1.1 | 6.9 | 1.9 | 6.2 | 6.1 | 3.2 |
| Av 627-NLS | 1.9 | 2.9 | 1.9 | 5.2 | 8.8 | 1.2 | 0.7 | 1.2 | 2.9 | 1.1 | 7.1 | 2.6 | 7.0 | 6.4 | 1.4 |
| MBP-NLSbipartite | 3.4 | 4.2 | ND | 4.9 | 10.6 | 0.5 | 0.6 | ND | 0.3 | 0.8 | 1.6 | 1.8 | ND | 0.8 | 0.8 |
a Quality of curve fitting to experimentally determined sensorgrams available in supplementary Fig. S6.
b ND, not determined.
FIGURE 1.
Rate and affinity values for NLS domain variants binding to Imp-α1 isoforms. Hu and Av Imp-α1 (1–100 mm) were injected over immobilized NLS domain and NLS domain variants. Rate and affinity constants were extracted from sensorgrams and are displayed as histograms: A, KD; B, kon; and C, koff. Experiments were performed in duplicate, and error bars indicate the range of the two values.
Binding of Human Double 627-NLS Domain and Variants to Hu and Av Imp-α1 Isoforms
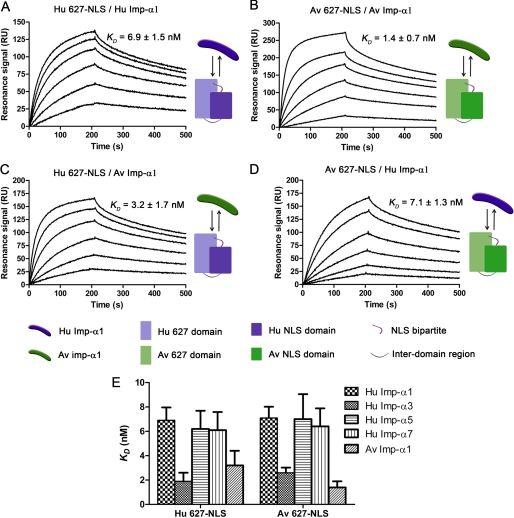
To assess whether host determinant residues present on the surface of the larger 627-NLS domain (14) affect importin α binding, we measured the affinities of a typical human 627-NLS (H3N2) and a fully avian 627-NLS (H5N1) for both Hu and Av Imp-α1 isoforms. The sequences of these 627-NLS domains varied at 10 residues, all of which have been identified as host signatures (5, 9): D567N, A588I, V613T, E627K, A661T, A674T, T676I, G682S, A684S, and K702R (supplemental Fig. S1A). SPR results revealed formation of tight complexes with KD values of 6.9 nm and 1.4 nm for human and avian pairs, respectively (Fig. 2, A and B; Table 1). For interspecies complexes, the Hu 627-NLS-Av Imp-α1 pair interacted with an affinity of 3.2 nm whereas the Av 627-NLS-Hu Imp-α1 pair was 7.1 nm (Fig. 2, C and D). In all cases, Av Imp-α1 binds both Av and Hu 627-NLS domains (and NLS domains) with a higher affinity than Hu Imp-α1. It is clear that Hu Imp-α1 binds a fully avian PB2 subunit containing no adaptive mutations without impairing the affinity (Fig. 2, A and D). Similarly, these 10 combined adaptive mutations did not affect strongly the affinity of Av Imp-α1 for Hu 627-NLS (Fig. 2, B and C). All results taken together suggest that, in the context of the double 627-NLS domain, the 10 host range residues tested only affect to a minor extent importin α1 binding, and no selectivity along species lines was observed.
FIGURE 2.
Affinity determination for 627-NLS domains binding to Imp-α1 isoforms. The host origins of the Imp-α1 and virus from which the 627-NLS domain was obtained are shown to the right of the sensorgrams. Both intra- and interspecies permutations were tested (A and B; C and D, respectively). E, KD values for Hu Imp-α1, α3, α5, and α7 and Av Imp-α1 isoforms binding to Hu and Av 627-NLS domains compared as a histogram. Experiments were performed in duplicate, and error bars indicate the range of the two values.
Selectivity of Hu and Av 627-NLS Domains toward Other Importin Isoforms
To determine whether there was a preference for specific importins found in human cells, we cloned, expressed, and purified human isoforms Imp-α3, Imp-α5, and Imp-α7 with the aim of measuring their affinities to the C-terminal 627-NLS domain (supplemental Fig. S2). SPR data showed that all pairs tested resulted in stable complexes with KD values ranging from 1.9 to 6.9 nm (Fig. 2E). For 627-NLS domains of both avian and human viral origin, Av Imp-α1 and Hu Imp-α3 exhibited the highest affinities, interacting 3–4-fold tighter than the other isoforms (Table 1 and supplemental Figs. S3 and S4).
Influence of C-terminal PB2 Domain Architecture on Importin Binding
Superimposition of the crystal structure of Hu 627-NLS (14) onto the NLS domain-Imp-α5 complex (27) revealed a strong clash between the 627 domain and the body of Imp-α5 (Fig. 3). This implies that although the 627 domain may interact with importin, it may be in a negative manner, producing steric hindrance rather than enhancing binding via intermolecular bonds. Thus, we compared the affinity of three forms of the C-terminal:region 627-NLS domain, NLS domain, and MBP-NLSbipartite (maltose-binding protein and the PB2 bipartite signal peptide) (Fig. 4A).
FIGURE 3.
Predicted clash between 627-NLS domain and importin. The NLS domain-Hu Imp-α5 crystal structure (Protein Data Bank ID code 2JDQ; dark gray and yellow) (A) was superimposed with the 627-NLS domain (Protein Data Bank ID code 2VY6; dark gray and light gray) (B) revealing a strong clash between the 627 domain (light gray) and the importin, suggesting that the folded region of PB2 beyond the anchored bipartite NLS peptide must rotate to another position or dislocate at the polar interface between the 627 and NLS domains.
FIGURE 4.
Binding of NLS-containing proteins to Hu and Av Imp-α1. A, Imp-α1 (1–100 mm) was injected over immobilized proteins containing the bipartite NLS peptide and additional NLS domain, 627-NLS domain or MBP. B–D, histograms showing values of KD, kon, and koff, respectively. Error bars indicate the average of two separate experiments.
For Hu Imp-α1, the larger Hu 627-NLS construct bound with KD = 6.9 nm, whereas the NLS domain alone and MBP-NLSbipartite showed a 2–4-fold higher affinity (KD = 3.8 nm and 1.6 nm, respectively; Fig. 4B). The reduced affinity observed for Hu 627-NLS binding Av Imp-α1 is solely due to a slower on-rate (Fig. 4C); however, once bound, the dissociation rate (koff) is almost identical to the Hu NLS domain (Fig. 4C) supporting the idea of slower binding due to a steric clash between the 627 domain and the body of the importin. Av Imp-α1 also exhibits a slower association rate for Hu 627-NLS domain compared with the NLS-domain alone, but this effect is not apparent for the Av 627-NLS domain, which binds as fast as the NLS domain (Fig. 4C). Thus, the steric clash predicted for the 627-NLS domain gives measurable effects for the weaker binding Hu variant binding to both importins, but not for the Av variant that binds with a higher overall affinity to Av Imp-α1.
We observed nearly identical kon values for the NLS domain and MBP-NLSbipartite (3.3 and 3.4 × 105 m−1 s−1), but the MBP-NLSbipartite formed a more stable complex due to a slower dissociation rate (Fig. 4D). This might be explained by MBP interacting nonspecifically with the partner, an established property of MBP fusion proteins often exploited for stabilizing fused partners. These data suggest that, in the context of the longer two-domain 627-NLS construct, neither 627 domain nor NLS domain contributes positively to importin binding whereas the presence of the 627 domain in the larger 627-NLS domain construct actually reduces binding, consistent with the molecular clash predicted by structural superimposition (Fig. 5). This effect is most pronounced with the weaker binding Hu importin whereas the tighter binding Av importin is less affected.
FIGURE 5.
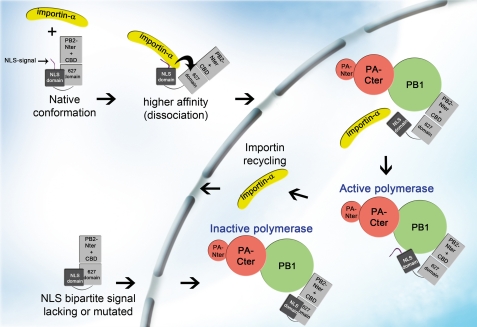
Model for binding of importin α to 627-NLS domain. Structural studies indicate a clash between the 627 domain and importin based on the superposition of the crystal structure of 627-NLS domains (unbound) and NLS domain (importin-bound). This observation is in agreement with a reduced affinity of 627-NLS due to a lower on-rate for the human importin compared with the NLS domain alone, suggesting a molecular reorganization of the 627-NLS domain upon importin binding. This molecular rearrangement may involve a rotation or dissociation of the 627 domain from NLS domain at the polar interface (14, 27). According to this model importin binding induces structural changes in PB2 which may be essential for correct assembly of PB2 into an active polymerase complex (37). CBD, cap binding domain.
DISCUSSION
We have shown previously that the C-terminal region of PB2 is responsible for nuclear transport of this polymerase subunit via a bipartite NLS peptide (amino acids 737–759) appended to a small domain forming a highly soluble structural unit (NLS domain) (27). In a subsequent study, we showed that the NLS domain was packed against an adjacent domain (627 domain) forming a single unit, the 627-NLS domain (14). The interdomain interface observed in this and a subsequent structure (15) is highly polar and stabilized by salt bridges rather than buried hydrophobic interactions. It thus seems possible that the crystal structure represents a closed conformation of a two domain construct that could exist equally in solvent in an open form comprising the two domains joined by a flexible linker. The function of the 627-NLS domain beyond importin binding remains unclear, although it has been suggested to bind RNA (15) and is rich in surface-exposed host determinant residues identified through experimental and bioinformatic studies (for review, see Ref. 10). A signature of adaptation from avian to human hosts is the increase in the basic surface charge of the 627-NLS domain (14, 42).
The superimposition of the double 627-NLS domain on the isolated NLS domain bound to importin α5 predicts a clash between the 627 domain and the importin that would appear incompatible with complex formation (Fig. 3). However, gel filtration (14, 27), nuclear transport assays (27), and immunoprecipitation experiments (37, 38) have all shown clearly that the double domain, both in isolation and in the context of the full-length subunit, binds importin, forming a complex stable enough to resist long experimental steps. This implies that some type of domain movement must occur relative to the importin to relieve the clash. A second aspect of this structural clash is that it puts the 627 domain in direct proximity to the importin molecule, thereby supporting the idea that host-determinant residues in this region may mediate selective importin binding in a host-specific manner.
To investigate quantitatively the effect of PB2 host adaptive surface residues and domain architecture on importin binding, we performed SPR experiments using highly purified importin and PB2 domains (supplemental Fig. S2). This permitted precise measurements of intrinsic affinities in an environment devoid of additional cellular factors. A second feature of the SPR method is that the association and dissociation phases of the binding reaction are measured independently, permitting deconvolution of the KD value (KD = koff/kon) into its component rate constants, offering potential mechanistic insights.
In the first experiments, we observed the NLS domain forming a tight complex with Hu and Av Imp-α1 (KD = 3.3 and 1.1 nm, respectively). This is consistent with affinities measured for importin α binding to peptides containing physiological high affinity NLS signals and cargo-NLS proteins using SPR and fluorescence depolarization/anisotropy (36, 43, 44).
Effect of Host-adaptive Residues in the Small NLS Domain on Importin Binding
The residue at position 701 of the NLS domain has been identified as a host-adaptive locus (18), with the D701N mutation increasing viral replication in mice (19, 45) and transmission in guinea pigs (21). Selection of the PB2-D701N mutation during human infections has been observed, for example, in the (nonpandemic) European swine H1N1 viruses (46) as well as in some highly pathogenic H5N1 avian viruses (20). It was reported that the D701N variation residing in the NLS domain contributed 4–7-fold enhanced binding to mammalian Imp-α1 over avian forms and improved nuclear transport of PB2 in mammalian cells (38). From structural analyses of free and importin-bound NLS domain, D701N has been proposed to disrupt a salt bridge with Arg753 in the bipartite NLS peptide (27, 38) that might increase the association rate and thus favor importin α binding as observed (38). We measured the effect of the D701N mutation on binding to Imp-α1 and also R702K, a back mutation of a residue that is commonly Lys in avian viruses and Arg in human strains and S714R that was selected in mouse adaptation experiments and shown to increase polymerase activity in an additive manner with D701N (18). All mutations increased the rate of association with both Hu and Av Imp-α1 relative to the original human-adapted virus residue (Table 1 and Fig. 1B).
Effect of Host-adaptive Residues in the Longer 627-NLS Domain on Importin Binding
Following analysis of variants of the shorter NLS domain, we measured the affinity of fully human (H3N2) and fully avian (H5N1) 627-NLS domains for Hu and Av Imp-α1 that differed between them by 10 variant positions, all identified previously as high confidence adaptive mutations (supplemental Fig. S1) (5, 9). The two domains did not vary at positions 701 and 714 because neither has been highlighted in sequence comparison studies of natural isolates, only in clinical or laboratory adaptation experiments. The Av Imp-α1 bound more tightly than the Hu Imp-α1 to both 627-NLS domains; this was solely due to increased association rates for Av Imp-α1 (Table 1 and Fig. 2), whereas the dissociation rates were identical. For a given importin, similar affinities and rate constants were measured for Hu and Av 627-NLS domains (Table 1 and Fig. 2E). Thus, it does not seem that any of the variant positions in the context of the 627-NLS domain strongly affect Imp-α1 selectivity in a host-specific manner, but Av Imp-α1 was consistently tighter binding to both PB2 domains.
Interaction of PB2 C-terminal Domains with Multiple Importin Isoforms
Seven importin α isoforms have been reported in humans (α1, α3, α4, α5, α6, α7, and α8) (47). Importins α1, α5, and α7 have all been shown to bind PB2 (14, 27, 37, 38), but no detailed quantitative measurements have been made. We therefore measured rates and affinities for human isoforms Imp-α1, α3, α5, and α7 binding to avian- and human-adapted 627-NLS domains. All bound tightly with affinities ranging from 1.9 to 6.9 nm (Fig. 2E) with association rates of 1.7–2.5 m−1 s−1 × 105 for isoforms α1, α3, and α5 and 5.2 m−1 s−1 × 105 for Imp-α7 (Table 1). The binding of PB2 to all isoforms tested is in apparent contradiction to coimmunoprecipitation experiments from cell lysates where human H3N2 and H5N1 PB2 proteins were observed to bind importin α isoforms in a selective manner (37). This may be a consequence of the differential abundance of various importin isoforms in the cell; our data indicate that any such preferences are not a consequence of very different intrinsic affinities of importin isoforms for this cargo. The absence of selectivity illustrates a redundant system in which PB2 cargoes may use multiple importins for nuclear transport as shown for nucleoprotein (28, 48, 49).
From a structural perspective, it seems unsurprising that binding to all importins was observed here. We previously showed by x-ray crystallography that the NLS domain bound Imp-α5 in an extended conformation (27, 50) characteristic of the classical cargo binding mode (51). Importin α isoforms are alike (∼80% overall sequence identity within subfamilies and 50% sequence identity between subfamilies) (47) (supplemental Fig. S5) with high similarity in NLS binding regions (52) (supplemental Fig. S1A). Likewise, the bipartite NLS of PB2 is strictly conserved within influenza A viruses, so a commonality in binding of this cargo to different isoforms might be expected.
Domain Architecture and Rearrangement upon Importin Binding
Our initial hypothesis was that host adaptation may occur due to strengthening of interactions between PB2 and importins of the new host. However, we noticed that there was a general difference in binding between the NLS domain and the 627-NLS domain: the presence of the 627 domain appeared to reduce the affinity of the importin interaction. The superposition of 627-NLS domain (Protein Data Bank ID code 2VY6) with the NLS domain-Hu Imp-α5 crystal structure (Protein Data Bank ID code 2JDQ) reveals a strong clash between the 627 domain and importin molecule (Fig. 3), suggesting that the folded region of PB2 upstream of the anchored bipartite NLS peptide must move to another position or dislocate at the polar interface between the 627 and NLS domains. Because the interface is polar (14), the molecular rearrangement would not compromise the solubility of the open and closed protein forms, which can both be expressed independently.
The SPR assay was used to compare importin binding by different forms of the PB2 C-terminal region, all containing at least the bipartite NLS peptide, but differing in the appended domains: 627-NLS domain, NLS domain alone, and control protein (MBP) fused to the NLS peptide (Fig. 4). MBP-NLS fusions have been used previously to assess the efficiency of importin binding in vitro (53) and in vivo (54). With Hu Imp-α1, the affinity increased across a 4-fold range: Av 627-NLS (7.1 nm) ≈ Hu 627-NLS (6.9 nm) < Hu NLS domain (3.8 nm) < MBP-NLSbipartite (1.6 nm) (Fig. 4A). The 2-fold lower affinities of the proteins containing the 627 domain were a consequence of a slower association rate (Fig. 4C), but once bound, dissociation rates were roughly equivalent (Fig. 4D). A similar tendency was observed with Av Imp-α, although binding was tighter for all cargoes than Hu Imp-α, and the presence of the 627 domain did not inhibit binding of the Av 627-NLS domain to the same level as for the Hu 627-NLS domain (Fig. 4, B–D).
This suggests that the predicted clash reduces the rate of complex formation. Tight binding of PB2 to importin α may require an energetically disfavorable rearrangement of the obscuring 627 region to produce a stable complex. The effect of this may be more pronounced with the weaker binding Hu importins than the tight binding Av importins.
Biological Significance of Results
The affinity of importin-NLS interactions has been proposed as a general parameter in determining nuclear import efficiency (33–36, 52). Host adaptation mutants appear to modulate the efficiency of PB2-importin binding, nuclear import, active polymerase assembly, and replication in mammalian cells (18, 27, 40). To date, in vivo studies on importin α and the viral cycle provide only a limited explanation of adaptive mutations due to the complexity of the cell and difficulties in obtaining accurate kinetic measurements. In vitro experiments complement in vivo studies because the intrinsic parameters (association and dissociation rate constants, affinity constants) of an interaction can be measured directly on highly purified components. In the importin system, we argue that the natural dissociation rate is of limited importance for high affinity, stable cargoes such as PB2 (half-life measured here of 9–10 min) because importin α-cargo complexes are quickly transported (∼1–2 min) (36). The cargo is then actively dissociated upon binding of nuclear Ran GTP to importin β (55, 56). Accordingly, the encounter rate of cargo and importin in the cytoplasm will most affect levels of nuclear accumulation; this is dependent on both cytoplasmic concentrations and the intrinsic association rate (not the affinity).
The SPR method used here permits direct measurement of the association rate constant and so provides a clearer understanding of this potentially important determinant of import efficiency. The two effects apparent from this study, increased rate of cargo binding mediated by some adaptive mutants and steric hindrance by the 627 domain, may both act together. Recent experiments have suggested that importins may have a role beyond nuclear import, being necessary for formation of an active trimeric complex because non-importin-binding NLS mutants of PB2 translocate into the nucleus passively, but form inactive trimers (37). Importins would therefore seem to have a role in loading PB2 into a functional trimer whereas inactive trimers likely contain subunits associating via the structurally characterized interdomain regions (57–59), but with some crucial aspect absent. Deletion experiments have identified that the region corresponding closely to our 627-NLS domain interacts tightly with PB1 (60). We therefore propose a model (Fig. 5) from those two studies and from our kinetic binding data in which importin α binds PB2, induces C-terminal reorganization leading to PB1 binding and active trimer formation.
In this study, the observed changes in association rates and other binding parameters are in the order of 2–4-fold. These are smaller than loss-of-function mutants and reflect how all PB2 variants in this study originate from viable infective viruses. Similar changes in PB2 importin binding and nuclear accumulation have been observed in cells (38). One limitation of this study is that isolated domains are being studied and not full-length PB2 because it cannot be purified. It is possible that full-length PB2 may interact with host proteins in a manner affected by adaptive mutations, indirectly affecting importin binding. Recent advances in the study of polymerase import mechanisms and kinetics using fluorescence cross-correlation spectroscopy and quantitative confocal microscopy (61) may provide the link between in vivo and in vitro observations.
Association rates are strongly influenced by long range electrostatic interactions (charge-charge Coulombic forces) that attract and align interacting molecules during binding events (62–64). Many of the 627-NLS adaptive mutations increase the basic nature of the domain e.g. D567N, E627K, D701N, S714R, and 590-SR (14, 42), suggesting a general mode of host adaptation through enhancement of binding to acidic importins. More efficient nuclear import may allow the virus to replicate more rapidly and out-compete the innate immune system.
Acknowledgments
We thank Ulrich Gohlke and Anja Schütz for initial guidance in the setup of the SPR assay, Stephen Cusack for critical reading of the manuscript, Rob Ruigrok for helpful discussions.
This work was supported by the Fondation Innovations en Infectiologie (FINOVI) and European Union FLUPOL Contract SP5B-CT-2007-044263.

The on-line version of this article (available at http://www. jbc.org) contains supplemental Figs. S1–S6.
- NLS
- nuclear localization signal
- EDC
- N-ethyl-N′-(3-diethylaminopropyl)carbodiimide)
- Av Imp-α
- avian importin α
- Hu Imp-α
- human importin α
- SPR
- surface plasmon resonance
- TEV
- tobacco etch virus.
REFERENCES
- 1. Noah D. L., Krug R. M. (2005) Adv. Virus Res. 65, 121–145 [DOI] [PubMed] [Google Scholar]
- 2. Chen H., Bright R. A., Subbarao K., Smith C., Cox N. J., Katz J. M., Matsuoka Y. (2007) Virus Res. 128, 159–163 [DOI] [PubMed] [Google Scholar]
- 3. Kobasa D., Takada A., Shinya K., Hatta M., Halfmann P., Theriault S., Suzuki H., Nishimura H., Mitamura K., Sugaya N., Usui T., Murata T., Maeda Y., Watanabe S., Suresh M., Suzuki T., Suzuki Y., Feldmann H., Kawaoka Y. (2004) Nature 431, 703–707 [DOI] [PubMed] [Google Scholar]
- 4. Suzuki Y., Ito T., Suzuki T., Holland R. E., Jr., Chambers T. M., Kiso M., Ishida H., Kawaoka Y. (2000) J. Virol. 74, 11825–11831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miotto O., Heiny A., Tan T. W., August J. T., Brusic V. (2008) BMC Bioinformatics 9, S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen G. W., Chang S. C., Mok C. K., Lo Y. L., Kung Y. N., Huang J. H., Shih Y. H., Wang J. Y., Chiang C., Chen C. J., Shih S. R. (2006) Emerg. Infect. Dis. 12, 1353–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Finkelstein D. B., Mukatira S., Mehta P. K., Obenauer J. C., Su X., Webster R. G., Naeve C. W. (2007) J. Virol. 81, 10292–10299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Naffakh N., Massin P., Escriou N., Crescenzo-Chaigne B., van der Werf S. (2000) J. Gen. Virol. 81, 1283–1291 [DOI] [PubMed] [Google Scholar]
- 9. Tamuri A. U., Dos Reis M., Hay A. J., Goldstein R. A. (2009) PLoS Comput. Biol. 5, e1000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boivin S., Cusack S., Ruigrok R. W., Hart D. J. (2010) J. Biol. Chem. 285, 28411–28417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Naffakh N., Tomoiu A., Rameix-Welti M. A., van der Werf S. (2008) Annu. Rev. Microbiol. 62, 403–424 [DOI] [PubMed] [Google Scholar]
- 12. Guilligay D., Tarendeau F., Resa-Infante P., Coloma R., Crepin T., Sehr P., Lewis J., Ruigrok R. W., Ortin J., Hart D. J., Cusack S. (2008) Nat. Struct. Mol. Biol. 15, 500–506 [DOI] [PubMed] [Google Scholar]
- 13. Hatta M., Gao P., Halfmann P., Kawaoka Y. (2001) Science 293, 1840–1842 [DOI] [PubMed] [Google Scholar]
- 14. Tarendeau F., Crepin T., Guilligay D., Ruigrok R. W., Cusack S., Hart D. J. (2008) PLoS Pathog. 4, e1000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuzuhara T., Kise D., Yoshida H., Horita T., Murazaki Y., Nishimura A., Echigo N., Utsunomiya H., Tsuge H. (2009) J. Biol. Chem. 284, 6855–6860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mehle A., Doudna J. A. (2008) Cell Host Microbe 4, 111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moncorgé O., Mura M., Barclay W. S. (2010) J. Virol. 84, 9978–9986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gabriel G., Dauber B., Wolff T., Planz O., Klenk H. D., Stech J. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 18590–18595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Z., Chen H., Jiao P., Deng G., Tian G., Li Y., Hoffmann E., Webster R. G., Matsuoka Y., Yu K. (2005) J. Virol. 79, 12058–12064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Jong M. D., Simmons C. P., Thanh T. T., Hien V. M., Smith G. J., Chau T. N., Hoang D. M., Chau N. V., Khanh T. H., Dong V. C., Qui P. T., Cam B. V., Ha do Q., Guan Y., Peiris J. S., Chinh N. T., Hien T. T., Farrar J. (2006) Nat. Med. 12, 1203–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Steel J., Lowen A. C., Mubareka S., Palese P. (2009) PLoS Pathog. 5, e1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boulo S., Akarsu H., Ruigrok R. W., Baudin F. (2007) Virus Res. 124, 12–21 [DOI] [PubMed] [Google Scholar]
- 23. Honda A., Ishihama A. (1997) Biol. Chem. 378, 483–488 [PubMed] [Google Scholar]
- 24. Nieto A., de la Luna S., Bárcena J., Portela A., Ortín J. (1994) J. Gen. Virol. 75, 29–36 [DOI] [PubMed] [Google Scholar]
- 25. Nath S. T., Nayak D. P. (1990) Mol. Cell. Biol. 10, 4139–4145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mukaigawa J., Nayak D. P. (1991) J. Virol. 65, 245–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tarendeau F., Boudet J., Guilligay D., Mas P. J., Bougault C. M., Boulo S., Baudin F., Ruigrok R. W., Daigle N., Ellenberg J., Cusack S., Simorre J. P., Hart D. J. (2007) Nat. Struct. Mol. Biol. 14, 229–233 [DOI] [PubMed] [Google Scholar]
- 28. O'Neill R. E., Palese P. (1995) Virology 206, 116–125 [DOI] [PubMed] [Google Scholar]
- 29. Neumann G., Castrucci M. R., Kawaoka Y. (1997) J. Virol. 71, 9690–9700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weber F., Kochs G., Gruber S., Haller O. (1998) Virology 250, 9–18 [DOI] [PubMed] [Google Scholar]
- 31. Naito T., Momose F., Kawaguchi A., Nagata K. (2007) J. Virol. 81, 1339–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fislová T., Thomas B., Graef K. M., Fodor E. (2010) J. Virol. 84, 8691–8699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hodel A. E., Harreman M. T., Pulliam K. F., Harben M. E., Holmes J. S., Hodel M. R., Berland K. M., Corbett A. H. (2006) J. Biol. Chem. 281, 23545–23556 [DOI] [PubMed] [Google Scholar]
- 34. Hu W., Jans D. A. (1999) J. Biol. Chem. 274, 15820–15827 [DOI] [PubMed] [Google Scholar]
- 35. Hübner S., Xiao C. Y., Jans D. A. (1997) J. Biol. Chem. 272, 17191–17195 [DOI] [PubMed] [Google Scholar]
- 36. Yang S. N., Takeda A. A., Fontes M. R., Harris J. M., Jans D. A., Kobe B. (2010) J. Biol. Chem. 285, 19935–19946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Resa-Infante P., Jorba N., Zamarreño N., Fernández Y., Juárez S., Ortín J. (2008) PLoS ONE 3, e3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gabriel G., Herwig A., Klenk H. D. (2008) PLoS Pathog. 4, e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miotto O., Heiny A. T., Albrecht R., García-Sastre A., Tan T. W., August J. T., Brusic V. (2010) PLoS ONE 5, e9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gabriel G., Abram M., Keiner B., Wagner R., Klenk H. D., Stech J. (2007) J. Virol. 81, 9601–9604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. de la Luna S., Martínez C., Ortín J. (1989) Virus Res. 13, 143–155 [DOI] [PubMed] [Google Scholar]
- 42. Mehle A., Doudna J. A. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 21312–21316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Catimel B., Teh T., Fontes M. R., Jennings I. G., Jans D. A., Howlett G. J., Nice E. C., Kobe B. (2001) J. Biol. Chem. 276, 34189–34198 [DOI] [PubMed] [Google Scholar]
- 44. Fanara P., Hodel M. R., Corbett A. H., Hodel A. E. (2000) J. Biol. Chem. 275, 21218–21223 [DOI] [PubMed] [Google Scholar]
- 45. Le Q. M., Sakai-Tagawa Y., Ozawa M., Ito M., Kawaoka Y. (2009) J. Virol. 83, 5278–5281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dunham E. J., Dugan V. G., Kaser E. K., Perkins S. E., Brown I. H., Holmes E. C., Taubenberger J. K. (2009) J. Virol. 83, 5485–5494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tejomurtula J., Lee K. B., Tripurani S. K., Smith G. W., Yao J. (2009) Biol. Reprod. 81, 333–342 [DOI] [PubMed] [Google Scholar]
- 48. Wang P., Palese P., O'Neill R. E. (1997) J. Virol. 71, 1850–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Melen K., Fagerlund R., Franke J., Kohler M., Kinnunen L., Julkunen I. (2003) J. Biol. Chem. 278, 28193–28200 [DOI] [PubMed] [Google Scholar]
- 50. Conti E., Uy M., Leighton L., Blobel G., Kuriyan J. (1998) Cell 94, 193–204 [DOI] [PubMed] [Google Scholar]
- 51. Conti E., Kuriyan J. (2000) Structure 8, 329–338 [DOI] [PubMed] [Google Scholar]
- 52. Jans D. A., Xiao C. Y., Lam M. H. (2000) Bioessays 22, 532–544 [DOI] [PubMed] [Google Scholar]
- 53. Schwab M. S., Dreyer C. (1997) Eur. J. Cell Biol. 73, 287–297 [PubMed] [Google Scholar]
- 54. Fouchier R. A., Meyer B. E., Simon J. H., Fischer U., Malim M. H. (1997) EMBO J. 16, 4531–4539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Clarke P. R., Zhang C. (2001) Trends Cell Biol. 11, 366–371 [DOI] [PubMed] [Google Scholar]
- 56. Nakielny S., Dreyfuss G. (1999) Cell 99, 677–690 [DOI] [PubMed] [Google Scholar]
- 57. Sugiyama K., Obayashi E., Kawaguchi A., Suzuki Y., Tame J. R., Nagata K., Park S. Y. (2009) EMBO J. 28, 1803–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. He X., Zhou J., Bartlam M., Zhang R., Ma J., Lou Z., Li X., Li J., Joachimiak A., Zeng Z., Ge R., Rao Z., Liu Y. (2008) Nature 454, 1123–1126 [DOI] [PubMed] [Google Scholar]
- 59. Obayashi E., Yoshida H., Kawai F., Shibayama N., Kawaguchi A., Nagata K., Tame J. R., Park S. Y. (2008) Nature 454, 1127–1131 [DOI] [PubMed] [Google Scholar]
- 60. Poole E., Elton D., Medcalf L., Digard P. (2004) Virology 321, 120–133 [DOI] [PubMed] [Google Scholar]
- 61. Huet S., Avilov S. V., Ferbitz L., Daigle N., Cusack S., Ellenberg J. (2010) J. Virol. 84, 1254–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhou H. X. (2005) Phys. Biol. 2, R1–R25 [DOI] [PubMed] [Google Scholar]
- 63. Zhou H. X. (1993) Biophys. J. 64, 1711–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tworowski D., Safro M. (2003) Protein Sci. 12, 1247–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]