Abstract
Polyubiquitin chains on substrates are assembled through any of seven lysine residues or the N terminus of ubiquitin (Ub), generating diverse linkages in the chain structure. PolyUb linkages regulate the fate of modified substrates, but their abundance and function in mammalian cells are not well studied. We present a mass spectrometry-based method to measure polyUb linkages directly from total lysate of mammalian cells. In HEK293 cells, the level of polyUb linkages was found to be 52% (Lys48), 38% (Lys63), 8% (Lys29), 2% (Lys11), and 0.5% or less for linear, Lys6, Lys27, and Lys33 linkages. Tissue specificity of these linkages was examined in mice fully labeled by heavy stable isotopes (i.e. SILAC mice). Moreover, we profiled the Ub linkages in brain tissues from patients of Alzheimer disease with or without concurrent Lewy body disease as well as three cellular models of proteolytic stress: proteasome deficiency, lysosome deficiency, and heat shock. The data support that polyUb chains linked through Lys6, Lys11, Lys27, Lys29, and Lys48 mediate proteasomal degradation, whereas Lys63 chains are preferentially involved in the lysosomal pathway. Mixed linkages, including Lys48, may also contribute to lysosomal targeting, as both Lys63 and Lys48 linkages are colocalized in LC3-labeled autophagosomes. Interestingly, heat shock treatment augments Lys11, Lys48, and Lys63 but not Lys29 linkages, and this unique pattern is similar to that in the profiled neurodegenerative cases. We conclude that different polyUb linkages play distinct roles under the three proteolytic stress conditions, and protein folding capacity in the heat shock responsive pathway might be more affected in Alzheimer disease.
Keywords: Alzheimer Disease, Autophagy, Lysosomes, Mass Spectrometry (MS), Parkinson Disease, Proteasome, Proteomics, Ubiquitin
Introduction
Posttranslational modification by monoUb3 or polyUb chains regulates a variety of cellular processes in eukaryotic cells (1, 2). The specificity of Ub signaling in these processes is mediated by E3 ligase-substrate interaction, the recognition of diverse Ub chains by Ub receptors (3), and selective disassembly of Ub chains by deubiquitinating enzymes (4). Recent developments in MS have greatly enhanced the analysis capacity of protein ubiquitination, leading to unexpected findings that any of eight amine groups (on the N terminus and seven Lys residues) in the Ub sequence can be modified by additional Ub molecules to elongate Ub chains (5–7), increasing the known diversity of polyUb chain structures.
Complex polyUb chains may provide structural diversity for different signaling events mediated by modified protein substrates (8). Conventional Lys48 polyUb linkages of at least four Ub moieties rapidly direct substrates to the 26 S proteasome for degradation (9, 10). A single mutation of Lys48 (11) or sextuple mutation of all non-Lys48 Lys sites in Ub (7) results in cellular lethality, indicating that Lys48 chains are essential but not sufficient to maintain cell viability. Unconventional polyUb linkages formed on the N-terminal half of Ub (Lys6, Lys11, Lys27, Lys29, and Lys33) are less characterized but may also contribute to proteasomal targeting (7, 12, 13). For example, Lys11 chains play a role in endoplasmic reticulum associated degradation in yeast (7) as well as in cell cycle regulation (14–16). However, Lys63 linkages and monoUb modification are mainly viewed as a nondegradation signal for protein sorting (17), DNA repair (18), and inflammation (19). Finally, linear polyUb chains are formed via the Ub N-terminal α amino group, but their role in proteolysis is controversial (6, 20, 21). Considering a potentially large number of proteins that are modified by different polyUb linkages, we are still in the early stage of studying the function of these linkages in cellular events and in disease development.
Because Ub-positive inclusions have been long identified in a number of neurodegenerative diseases, and genetic mutations in a few E3 enzyme genes (e.g. parkin) have been identified in familial cases, aberrant Ub signaling is proposed to participate in pathogenesis (22, 23). Proteolytic stress in neurodegeneration may be induced by an imbalance in the protein homeostasis network, including proteins assisting in protein folding, as well as the Ub-proteasome system and autophagy-lysosome degradation. Indeed, enhancement of protein folding capacity in neurodegenerative models of Drosophila or Caenorhabditis elegans attenuates neuronal toxicity (24), and defects in either the proteasomal or lysosomal degradation pathways in mice result in the formation of protein inclusions and some other neurodegenerative phenotypes (25, 26). Thus, the involvement of ubiquitin in protein homeostasis prompted us to systematically investigate different polyUb chains in neurodegeneration.
Here, we present a refined MS approach to detect major polyUb linkages directly from total cell lysate without pre-enrichment. We have used this approach to measure the level of these linkages in Alzheimer disease tissues and in cellular models of proteolytic stress. The analysis reveals that polyUb chains in mouse and human are primarily composed of Lys48, Lys63, Lys29, and Lys11 linkages. Moreover, the profiled polyUb patterns in the neurodegenerative disease and in models of altered protein homeostasis capacity suggest the differential regulatory functions of these linkages and possible etiology of Alzheimer disease.
EXPERIMENTAL PROCEDURES
Reagents
Reagents used in this study included antibodies against actin (catalog no. sc-1615, Santa Cruz Biotechnology, Santa Cruz, CA), ubiquitin (catalog no. mAb1510, Chemicon Millipore, Billerica, MA), Lys63 polyUb monoclonal Ab (HWA4C4, eBioscience, San Diego, CA); LC3 (catalog no. NB100-2331, Novus Biologicals, Littleton, CO); humanized anti-Lys48 and anti-Lys63 antibodies (27) (a kind gift of V. Dixit); MG-132 and epoxomicin (Boston Biochem., Cambridge, MA); riluzole (Tocris, Ellisville, MO); and 6-aminonicotinamide and bafilomycin A1 (Sigma).
Mouse and Human Tissues
Mouse chow with heavy stable isotope-labeled lysine (13C6, Cambridge Isotope Labs, Cambridge, MA) was used to feed wild type mice (CBAxC57BL/6) from parents through F2 generations according to the reported protocol (28). A female F2 mouse was sacrificed after weaning (21 days after birth), and tissues were collected for MS analysis. Frozen human frontal cortex was obtained through the Emory Neuropathology Core Facility (see supplemental Table S1).
Cell Culture
HEK293 cells were cultured in Dulbecco's modified Eagle's medium plus 4.5 g/liter glucose and glutamine (Lonza, Allendale, NJ) with 10% fetal bovine serum (Invitrogen) or, during stable isotope labeling, SILAC DMEM plus 2% dialyzed serum (Thermo Fisher Scientific) and 0.26 mm arginine (+10 Da) and lysine (+8 Da) (Cambridge Isotope Laboratories), as well as 200 mg/liter unlabeled l-proline (Sigma) to prevent arginine conversion to proline (29). Labeling was performed over the course of a minimum of 10 doublings. The cells were treated under various conditions and then analyzed by immunostaining or MS.
Embryonic primary cortical neurons were dissected from pups (C57BL/6 mouse, E18, Charles River Laboratories, Cambridge, MA), plated at a density of 50,000 cells/cm2 in 12-well plates coated with poly-l-lysine. Cells were maintained in neurobasal medium (Invitrogen) plus 5% B-27 supplement, 1% penicillin/streptomycin, and 1% l-glutamine for 2 days before the medium was changed. All treatments proceeded in biological duplicate on day 3. For consistency, treatment concentrations of chemicals were identical to those used for HEK293 cells.
Immunostaining of Tissues and Cells
Immunohistochemistry was performed as described previously (30). For cell staining, cultured cells were fixed in −20 °C methanol, blocked, incubated with primary antibody and secondary antibody, and finally visualized under an LSM 510 confocal microscope (Zeiss, Thornwood, NY). For volume determination of autophagosomes (LC3-positive structures), diameter was estimated as the average of measured height and width for each structure using LSM image examiner software and averaged for 10 such structures. Z-axis depth was adjusted to maximize the diameter of most nuclei.
Protein Extraction from Tissues and Cells
Tissue lysate was prepared via homogenization in urea/SDS buffer (10 mm Tris, pH 7.4, 8 m urea, 2% SDS, 4% glycerol, 150 mm NaCl, 5 mm EDTA, 10 mm iodoacetamide) with protease inhibitor mixture (Roche Diagnostics), with 20 strokes in a Dounce tissue grinder with loose pestle, and then 20 strokes with tight pestle (Kontes Glass, Vineland, NJ), followed by cycles of sonication for a 2-s pulse and 30-s cooling on ice, until DNA shearing was apparent by a loss of viscosity. Likewise, total cell lysate of HEK293 and cultured neurons was prepared in the urea/SDS buffer but without homogenization.
For differential protein extraction from HEK293 cells after heat shock response, the cells were lysed with modified radioimmune precipitation assay buffer (50 mm Tris, pH 7.4, 150 mm NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 5 mm EDTA, 10 mm iodoacetamide, and protease inhibitor mixture). Detergent insoluble pellet was obtained by centrifugation at 20,800 × g for 10 min and was solubilized in 50 μl of urea/SDS buffer. All protein concentrations were determined by bicinchoninic acid assay (Pierce) in the absence of reducing agents.
Targeted MS Measurement by LC-SRM
The quantification of polyUb linkages and selected proteins (E1 and Rpn2) was performed by MS using stable isotope-labeled internal standards, following a previously reported protocol (13, 31). The internal standards included labeled, synthetic GG-linked Ub peptides for absolute quantification (7), and metabolically labeled HEK293 cells (Lys +8.0142 Da and Arg +10.0083 Da) or mouse tissues (Lys +6.0201 Da) for relative quantification. Labeled cell lysate (20 μg) was prepared in the urea/SDS buffer, spiked into unlabeled lysates mostly at a 1:1 ratio, and resolved on a one-dimensional SDS gel. The gel region above 80 kDa containing the vast majority of polyUb species was used for in-gel trypsin digestion unless indicated differently. This produced pairs of light and heavy peptides. When synthetic peptides were used, they were spiked into gel pieces prior to digestion. Finally, digested peptide pairs were separated by reverse phase LC followed by MS, in which peptide ion pairs of interest were selected for fragmentation and quantified by intensity ratio of coeluting, related product ion pairs, a process termed selected reaction monitoring (SRM) or multiple reaction monitoring. The LC-SRM analysis was performed on a hybrid LTQ-Orbitrap mass spectrometer (Thermo Finnigan, San Jose, CA). The optimized parameters were included in the supplemental materials (see supplemental Table S2).
Fluorescent Protease Activity Assays
The assays were performed using caspase-3/7 substrates (Suc-LLVY-AMC or Suc-DEVD-AMC, respectively) with fluorescence activated by proteolysis (Anaspec, Fremont, CA) essentially as described (32). The 100-μl reactions in 96-well plates included 50 μl of total cell lyate (12.5 μg) in lysis buffer (50 mm HEPES, pH 7.4, 100 mm NaCl, 0.1% CHAPS, 0.1 mm EDTA) and 50 μl of 100 mm substrate (in the lysis buffer plus 2 mm DTT) at 37 °C for up to 60 min. Fluorescence measurements were taken every 3 min to calculate protease activity.
RESULTS
Improved MS Strategy for Measurement of Ub-Ub Linkages in Mammalian Cells and Tissues
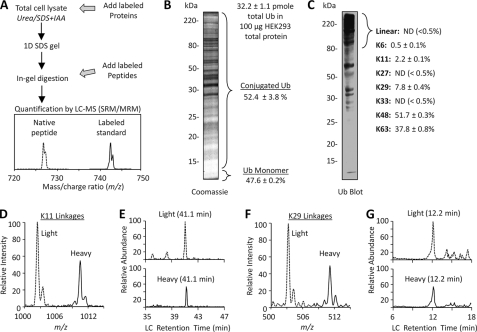
We employed an MS-based approach to measure the level of polyUb linkages, which is represented by the signature Gly-Gly-tagged peptides generated by trypsin digestion of ubiquitinated proteins (31, 33). A key step in this method is the addition of heavy isotope-labeled proteins/peptides (at a known amount) as internal standards for quantifying an unknown amount of unlabeled (light) Gly-Gly-tagged peptides (Fig. 1A). The internal standard proteins/peptides have identical chemical properties to the corresponding native peptides, but they are distinguished by MS to achieve quantitation. Moreover, the method can be used for measuring the abundance of any protein/peptide as long as its labeled internal standard is available. We have used the method with success in yeast (7) and now report its use with mammalian cells and tissues.
FIGURE 1.
Mass spectrometry enables measurement of ubiquitin linkages in mammalian cells. A, workflow for LC-MS quantification using stable isotope-labeled proteins or peptides. After digestion, the labeled peptides and their unlabeled counterparts were co-eluted in reverse phase LC followed by MS analysis in the mode of SRM, also termed multiple reaction monitoring (MRM). SRM/multiple reaction monitoring settings monitor specific product ions for known precursor ions, where the light/heavy intensity ratio of product ions is a surrogate for relative peptide abundance in the mixture. B, quantification of total Ub and the level of conjugation in HEK293 cells. C, Western blotting shows that the majority of Ub conjugates was above 80 kDa, which were excised and further quantified by MS. Note that a direct comparison of the abundance of polyUb to monoUb using using Western blotting is not possible, as it is known that polyUb is preferentially detected and usually generates much stronger signals than monoUb during Western blotting. This may be due to the difference in epitope exposure because the ubiquitin monomer is a highly stable protein that readily refolds. The percentage calculation of different linkages was based on the absolute quantification using synthetic GG-tagged peptides as a reference. D, a pair of Lys11-specific product ions was monitored from HEK293 cell lysate. E, coelution of the light and heavy ion pairs of the Lys11 linkage peptide at a precise retention time during reverse phase LC. Retention time is strictly used to distinguish real native Lys11 peptide signal from noise signals (e.g. the small peak eluted at ∼39 min). The extracted ion currents in the LC-SRM ranged from targeted MS/MS ion monoisotopic m/z −0.5 unit to plus 1.5 unit (see the targeted ions in supplemental Table S2). F, light/heavy ion pairing for Lys29 linkages. G, matched column retention time for the Lys29 peptide pair.
We first set out to analyze total Ub in conjugated form and monomeric form (8 kDa) in HEK293 cells. The cell lysate was resolved on an SDS gel that was divided at 12 kDa into two regions for MS analysis (Fig. 1B). The total Ub per 100 μg of lysate was measured to be 32.2 ± 1.1 pmol, and approximately half of ubiquitin was present as free monomer in cells. This result is largely consistent with two reported immunochemical measurements in mammalian cells (34, 35), suggesting the reliability of the newly developed MS approach for ubiquitin quantification.
We continued to measure the abundance of all possible linkages directly from HEK293 cell lysate using the MS strategy. To reduce complexity of the lysate, we decided to analyze proteins in the gel band above 80 kDa, which includes the vast majority of polyUb species (Fig. 1C), similar to the strategy we used for analyzing yeast lysate (7). According to the absolute amount of all eight linkages (totaling 100%), Lys48 (52%) and Lys63 (38%) are the most abundant, followed by Lys29 (8%), then Lys11 (2%) and Lys6 (0.5%) (Fig. 1, D--G). Although synthetic, labeled linear (i.e. N-terminal), Lys27 and Lys33 linkage peptides were clearly detected, the native peptides were not identified. According to the detection sensitivity of the method, the maximum contribution of each of the three undetected peptides was <0.5% in the HEK293 cells during the steady growing state without perturbation in protein homeostasis.
To study tissue specificity of the polyUb linkages in mammals, we equally mixed fully labeled SILAC mouse tissues (28) with HEK293 cells and examined the relative level of the four main linkages (Lys48, Lys63, Lys29, and Lys11, Fig. 2). In two generations, the mouse tissues were almost completely labeled by heavy isotopes (∼97% labeling efficiency, data not shown). A Coomassie-stained gel of tissue lysates shows different protein patterns. Interestingly, rapidly dividing HEK293 cells have double or more Lys11 linkages than the five differentiated tissues, in agreement with a major role of Lys11 linkages in mammalian cell division (14). The Lys48 linkages and the proteasome subunit Rpn2 are elevated in liver relative to other tissues, suggesting that these Ub chains are important in tissue with a high capacity for protein turnover. Finally, mouse cortex and lung have relatively high Lys63 abundance, consistent with endocytic and secretory functions integral to the function of these tissues. Therefore, Ub profiling may provide information about which Ub signaling pathways predominate in a given sample from mammalian cells or tissues.
FIGURE 2.
PolyUb linkage profiles of mouse tissues. A, Coomassie Blue stained gel shows total protein of five tissue homogenates (15 μg) that were fully labeled by stable isotope. B, relative abundance of polyUb linkages and the proteasome subunit Rpn2, based on the relative intensity of conserved peptide pairs from unlabeled HEK293 lysate and labeled mouse samples by LC-MS analysis. The experiments were repeated once. All data are normalized according to the abundance of each peptide in HEK293 total cell lysate and is shown as mean and S.E. The level of Lys11 linkages in muscle was below the detection limit. The level of unmodified ubiquitin TLS peptide (supplemental Table S2, roughly reflecting the sum of ubiquitin in the region larger than the 80-kDa region) is 52, 59, 48, 69, and 41% in lung, cortex, muscle, liver, and kidney, respectively, compared with HEK293 cells (100%). Statistical significance (asterisk) was calculated between HEK293 cells and different tissues using Student's t test (p < 0.05).
Selective Accumulation of Lys48, Lys63, and Lys11 Linkages in AD and AD/LBD Cases
To better understand the dysregulation of Ub signaling in neurodegenerative disease, we profiled Ub linkages in postmortem frontal cortex from neurodegenerative cases of Alzheimer disease (AD) without or with neocortical Lewy body Disease pathology (termed AD/LBD cases, supplemental Table S1). The cases were confirmed to show moderate to frequent staining for three standard markers of pathology: α-synuclein-positive Lewy bodies, amyloid β plaques, and Tau-positive neurofibrillary tangles (Fig. 3). Western blotting analysis showed a change in overall polyUb accumulation across a number of AD/LBD cases versus control samples (Fig. 3I). More importantly, polyUb linkages were measured in total tissue homogenate in the matched 12 controls, 12 AD cases, and 12 AD/LBD cases by LC-MS using labeled HEK293 cells as internal standard, revealing a significant increase in Lys11, Lys48, and Lys63 in AD samples. However, Lys29 linkages showed little change in the disease specimens (Fig. 3J). The accumulation of Lys48- and Lys63-linked polyUb chains was also verified by tissue immunostaining (Fig. 3, D and H, and supplemental Fig. S1). The MS results suggest a unique polyUb pattern change in Alzheimer disease.
FIGURE 3.
Lys11, Lys48, and Lys63, but not Lys29 linkages accumulate in frontal cortex of AD or AD/LBD specimens. A–H, pathological staining of α-synuclein (Lewy body), amyloid β peptide (plaques), Tau (neurofibrillary tangles), and Lys63-linked polyUb in control and AD/LBD cases, respectively. Wide-field images were captured with a 10× objective, except a 40× objective was used for D and H. Scale bar is 150 μm for all panels but D and H (20 μm). I, elevated level of Ub in AD/LBD cases in Western blotting. J, analysis of four abundant polyUb linkages in total frontal cortex lysate of the matched control (n = 12), pure AD (n = 12), and AD/LBD cases (n = 12) by LC-MS. The asterisk indicates p value <0.05 according to Student's t test.
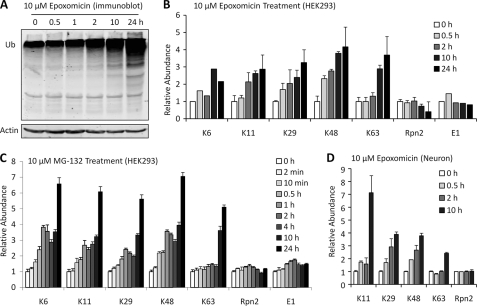
Proteasome Inhibition Increases non-Lys63 Linkages during an Early Response, but Lys63 Linkages also Increase in a Late Response
To understand proteolytic stress underlying polyUb chain accumulation in the AD/LBD cases, we attempted to identify stress conditions in cells that can recapitulate the polyUb pattern in disease and first tested the effect of proteasomal inhibition. As expected, treatment with epoxomicin, a specific proteasome inhibitor, led to a time-dependent accumulation of Ub conjugates (Fig. 4A), and raised the level of all polyUb linkages ∼2–4 fold in 24 h (Fig. 4B). Total Ub level (conjugated and unconjugated) in the cells was also increased ∼15% in 2 h, ∼70% in 4 h, and ∼210% in 24 h, consistent with the idea that ubiquitin itself is also degraded by proteasome (36). A close examination revealed that Lys63 linkages had a delayed response (no significant change in 2 h but a ∼3-fold increase after 10–24 h), whereas other linkages showed a more steady increase with time. This phenomenon was repeated in cells treated with another proteasome inhibitor (MG-132) in a more detailed time course analysis (Fig. 4C). Similarly, Lys63 linkages were stable during the first 4 h and augmented ∼4–5-fold at 10–24 h. Thus, we propose that the change in Lys63 linkages results from a late, indirect response to proteasomal inhibition. Indeed, we observed activation of apoptosis after 10 h of treatment, indicated by the analysis of caspase-3 and -7 activities (supplemental Fig. S2), although the link between apoptosis and Lys63 linkages is not clear. The analysis of polyUb linkages was repeated in mouse primary cortical neurons under proteasomal inhibition and similar results were obtained (Fig. 4D).
FIGURE 4.
Proteasome deficiency increases all detected polyUb linkages, but Lys63 shows a delayed response. A, Western blot of Ub when HEK293 cells were treated with 10 μm epoxomicin. B, Ub linkage profile in a time course during epoxomicin treatment. The signal of Lys6 linkages was weak compared with others and might be subject to more variation. Rpn2 and E1 were also quantified as two internal loading controls. C, Ub linkage profile in cells treated with 10 μm MG-132. D, PolyUb linkage profile using mouse primary neuronal culture.
Recent studies on autophagy suggest that ubiquitinated species, presumably modified by Lys63 linkages, may form inclusions and are then degraded by the autophagy-lysosome system (22, 37). To test whether changes in polyUb occurring at late treatment time points correspond with possible redirection of polyUb conjugates to autophagosomes, we performed immunostaining of MG-132-treated HEK293 cells using recently developed antibodies against Lys48 and Lys63 linkages (27). Upon 4 h of inhibition, both linkages appeared to be located in inclusions in certain cells, and by 10 h of inhibition, nearly every cell had a large inclusion positive for Lys63 and Lys48 chains (Fig. 5). The inclusions were further labeled by the autophagosome marker protein LC3. Our data support the idea that prolonged, severe UPS deficiency induces the localization of an increasing fraction of Lys63 polyUb-modified substrates into LC3-positive inclusions, suggesting a dynamic flow of proteins between the proteasome and lysosome degradation machineries. In addition, other polyUb linkages (e.g. Lys48) may be also involved in this process of directing substrates to the autophagy pathway.
FIGURE 5.
Lys48 and Lys63 linkages accumulate in inclusions (or inclusion-like structures) after proteasome inhibition. A, Lys48 polyUb was imaged by immunofluorescent staining at 0, 4, and 10 h of MG-132 treatment (10 μm). B, Lys63 polyUb (green) was analyzed together with LC3 (red) under the same conditions. The scale bars represent a distance of 10 μm.
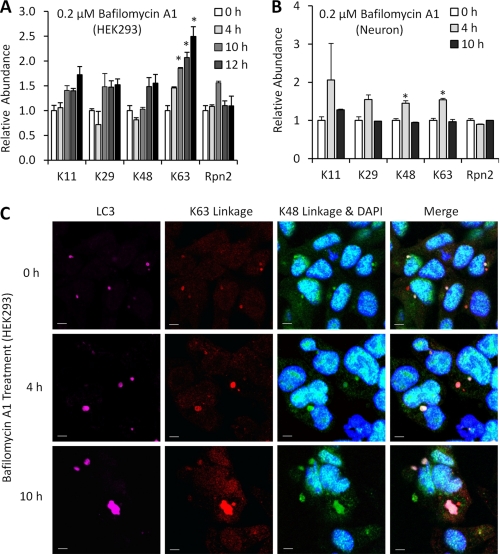
Lysosome Deficiency Leads to an Increase of Lys63 Linkages as well as Modest Augmentation of Lys11, Lys29, and Lys48 Linkages
Since autophagy may play a compensatory role in bulk protein degradation during proteasome impairment, we mapped the changes of polyUb linkages first in HEK293 cells treated by a potent and specific endosomal H+/ATPase inhibitor, bafilomycin A1, which blocks acidification and disturbs the fusion of endosomes and autophagosomes with lysosomes (38). After a 4-h treatment, Lys63 linkages increased 50%, whereas other measured linkages remained unchanged (Fig. 6A). After 10–24 h, Lys63 Ub chains reached the level of ∼2-fold, whereas other linkages (Lys11, Lys29, and Lys48) also increased modestly (∼1.5-fold). The result suggests that Lys63 linkages are the main polyUb chains responsive to the autophagy inhibition in the HEK293 cell model, whereas other linkages may contribute to this event.
FIGURE 6.
Lysosomal deficiency increases Lys63 linkages and other polyUb linkages. A, Ub linkage profile of bafilomycin A1-treated (200 nm) HEK293 cells, shown as mean and S.E. Statistical significance (asterisk) was analyzed between the untreated samples and other samples based on Student's t test (p < 0.05). B, Ub linkage profile of bafilomycin A1-treated primary neurons in a similar time course. C, immunofluorescence of Lys48, Lys63 polyUb chains, and LC3 with varying bafilomycin A1 (200 nm) treatment time. The nuclei are shown by DAPI staining. Lysosome inhibition appeared to increase the size of LC3/Lys48/Lys63-positive structures in HEK293 cells over time. The scale bars represent a distance of 10 μm.
We further attempted the autophagy inhibition treatment in cultured primary embryonic mouse neurons (Fig. 6B), which are thought to support a higher volume of membrane traffic compared with HEK293 cells (Fig. 2). Direct effects of bafilomycin A1 treatment at 4 h are generally consistent with HEK293 trends and include a significant increase in Lys48 and Lys63. After a 10-h treatment, however, conjugated ubiquitin chains decreased to the level at the 0-h time point, suggesting that unlike HEK293 cells, primary neurons cannot maintain ATP-dependent steady state Ub conjugation at 10 h under the bafilomycin A1 concentration of 0.2 μm. Indeed, it was reported that even a 20-fold reduction of this dose may be toxic to neuronal cells treated for longer periods (39).
We further visualized the distribution of Lys48 and Lys63 linkages and LC3 in bafilomycin A1-treated HEK293 cells by triple immunostaining. We noted subtle basal colocalization of Lys63 with LC3-positive structures and more extensive colocalization at 4 and 10 h after bafilomycin A1 treatment, whereas a small fraction of Lys48 linkages were also located in autophagosomes (Fig. 6C). Furthermore the average volume of these stained structures was found to increase from 7.1 μm3 (0 h) to 8.7 μm3 (4 h), then to 10.4 μm3 (10 h) during bafilomycin A1 treatment. In sum, the data suggest that mixed linkages (Lys63 and Lys48) are enriched within autophagosomes in response to a block in lysosomal targeting in HEK293 cells, whereas Lys63 chains may play a major role in mediating the sorting of Ub conjugates to autophagosomes.
Heat Shock Induces PolyUb Substrate Accumulation with Linkage Specificity Similar to AD Samples
The third model of proteolytic stress we considered was a comparison of basal versus expanded protein folding capacity. We examined the level of different polyUb linkages in response to heat shock at 42 °C, and concomitant chemical potentiation of the heat shock response by the drug riluzole or the small molecule 6-aminonicotinamide (6-AN). Both chemicals can robustly induce the heat shock response via riluzole-dependent stabilization of HSF1 (40), or possibly through 6-AN inhibition of glucose 6-phosphate dehydrogenase (41). Combinatorial absence or presence of either compound and/or heat shock was tested through a total of six conditions (Fig. 7A). We first assessed protein aggregation by differential extraction, the overall partitioning of total HEK293 protein into radioimmune precipitation assay soluble and insoluble fractions. Heat shock treatment doubled bulk protein insolubility from 5.5 to 11.1%, regardless of pretreatment with either riluzole or 6-AN (Fig. 7B), consistent with the notion that heat shock enhances protein unfolding and aggregation.
FIGURE 7.
PolyUb linkage profiles during heat shock response in a cellular proteolytic stress model. A, the diagram of three types of treatment in a time course. HEK293 cells were treated by 6-AN, riluzole, or heat shock alone or were pretreated with 6-AN or riluzole prior to heat shock to increase the heat shock response capacity of cells. B, radioimmune precipitation assay buffer soluble and insoluble fractions for the six treatments shown as mean and S.E. Statistical significance (asterisk) was analyzed between the untreated samples and other samples based on Student's t test (p < 0.05). The protein concentrations were quantified to determine the percentage partitioning into the insoluble fraction from HEK293 cells. C, polyUb linkage profile of the total cell lysate. Statistical significance (asterisk) between heat shock treatment and the other two combined treatments (heat shock with 6-AN or riluzole) was based on the analysis of Student's t test (p < 0.05).
We proceeded to profile polyUb linkages in the cell lysate, and found that Lys11, Lys48, and Lys63 linkages were selectively elevated due to heat shock, and this effect was partially attenuated by pretreatment with either riluzole or 6-AN (Fig. 7C). Surprisingly, Lys29 linkages did not change under heat shock, suggesting that the global level of Lys29 was not significantly influenced by heat shock induction. Also in neuronal culture, heat shock treatment augments the level of Lys11, Lys48, and Lys63 linkages but not Lys29 linkages (supplemental Table S3). These results indicate that heat shock results in a unique polyUb linkage pattern, which is more similar to that of AD cases than that associated with the inhibition of proteasome or autophagy pathways.
DISCUSSION
We have developed an MS method for directly measuring polyUb linkages in mammalian cells and tissue and applied this approach toward identifying perturbations in polyUb conjugates in AD. Compared with methods of previous studies in which Ub conjugates were affinity-isolated using Ub binding domains (42) or tagged ubiquitin (43) and then analyzed by MS, this simplified method bypasses the requirement of pre-enrichment of Ub conjugates and thus improves the recovery of Ub conjugates and the sensitivity of detection as well as the analysis turnover time that allows rapid processing of a large number of samples (e.g. >150 samples in this study). This method also reduces variation in measurements of polyUb linkages because pre-enrichment may produce different yields for diverse polyUb chains. Although either heavy stable isotope labeled peptides or proteins can be used as internal standards, the protein standards eliminate error due to variable trypsin digestion efficiency and therefore offer more precise measurement than the peptide standards (7).
In addition to ubiquitin, two Ub-like proteins (Nedd8 and ISG15) also generate GG-tagged peptides on substrates, and thus, our LC-SRM analysis could be confounded. To test this possibility, we analyzed the peptide mixture from the region above 80 kDa on an SDS gel using both shotgun LC-MS/MS and targeted LC-MS/MS. In the targeted analysis, four LC-MS/MS-compatible, “proteotypic” peptides (44) from each of the three proteins (ubiquitin, Nedd8, and ISG15) were selected for MS/MS analysis regardless of their detection by survey MS. This procedure improved the chance to analyze these proteotypic peptides. In both analyses, ISG15 was not identified, and Nedd8 showed a much lower level than ubiquitin (supplemental Table S4). The low amount of Nedd8 relative to ubiquitin may be attributed to modification of cullin proteins by Nedd8 (45), and indeed, four cullin proteins were detected in the shotgun analysis (supplemental Table S4). A critical question is whether Nedd8 could directly modify ubiquitin. Such putative Nedd8-Ub conjugates were not detected in multiple large-scale studies of the ubiquitinated proteome (5, 43, 46), and only trace levels of ubiquitin were found in a study of the Nedd8-associated proteome (45). Therefore, it is unlikely that the eight GG-tagged signature Ub peptides are derived from Nedd8 or ISG15 modification.
The amounts as percentages of polyUb linkages measured in HEK293 cells are slightly different from percentages in yeast: Lys6 (11%), Lys11 (28%), Lys27 (9%), Lys29 (3%), Lys33 (3%), Lys48 (29%), and Lys63 (16%) (7). The mammalian cells have a higher level of Lys48 and Lys63 linkages but a much lower level of Lys6 and Lys11 chains than yeast. The discrepancy could be explained by the species differences in enzymes of Ub conjugation and deubiquitination and relative abundance of chain-specific substrates. It is also possible that the difference could be due to (i) tagging of ubiquitin in yeast and (ii) the analysis procedure, as the yeast analysis was based on affinity captured His-Ub conjugates instead of total cell lysate (7). Our previous study indicated that the effect of ubiquitin tag on the global distribution of polyUb chain linkages is small, less than the effect of genetic background in yeast (7). To test the variation caused by affinity capture, we further compared four abundant linkages (Lys6, Lys11, Lys48, and Lys63) between isolated yeast Ub conjugates and yeast total lysate and found <30% change in any of the linkages (supplemental Fig. S3), suggesting that nickel affinity purification did not have strong bias toward particular chain linkages. Together, the results support that a species difference is the most likely factor underlying the quantitative inconsistency of polyUb linkages between yeast and mammals. Regardless, it should be noted that the total percentage of different linkages cannot predict their functional significance, as the level of chain-specific individual proteins is not known, and critical regulatory proteins are usually of low abundance.
Whether Ub signaling processes may underlie the etiology of Alzheimer disease has remained an open question. Complex polyUb chains indeed have been implicated in neurodegeneration in previous reports. For instance, Lys11, Lys48, and Lys63 linkages are accumulated in affected brain regions in Huntington disease (42), whereas polyUb chains linked through Lys6, Lys11, and Lys48 were observed in Tau aggregates isolated from human Alzheimer disease tissue (47), and immunostaining identified Lys63 chains associated with tangle of pathology of AD cases (48). Our study gave a more systematic survey of polyUb chains and identified the global augmentation of Lys11, Lys48, and Lys63, but not Lys29 chains in total tissue lysate of AD with or without Lewy body disease. Further analysis suggested that the increase in Lys11, Lys48, and Lys63 linkages was also correlated with AD disease stage (data not shown).
To recapitulate the unique polyUb pattern in disease, we proceeded to profile polyUb chains in HEK293 cells and cultured neurons, modeling three distinct causes of failed protein homeostasis: proteasomal inhibition, lysosomal deficiency, and heat shock treatment. It was an unexpected finding that the disease-related polyUb signature is best mimicked in cells subjected to heat shock that forces the cells beyond the limits of their protein folding capacity, suggesting that heat shock-responsive mechanisms (e.g. protein misfolding) may be involved in pathogenesis, but roles for the Ub-proteasome system and lysosomal degradation cannot be excluded. Consistent with the data, the Ub ligase ChIP that functions in alleviating protein unfolding stress, has been shown to synthesize polyubiquitin chains on Hsp70 and Hsp90 by Lys6, Lys11, Lys48, and Lys63, but not Lys29 (49), although the underlying molecular mechanism for this polyUb pattern needs further investigation.
In addition, detailed time course analysis of polyUb linkages in the cellular models supports a dynamic flow of protein degradation between proteasomal and lysosomal systems. To some extent, accumulation of substrates marked by polyUb for delivery to one system may be redirected to the other system to accommodate the burden of proteolysis. The accumulation of polyubiquitinated proteins is consistent with a concomitant loss of function in the UPS or autophagy. During proteasomal inhibition, Lys6, Lys11, Lys27, Lys29, and Lys48 (but not Lys63) linkages increased instantly and the trend continued for 24 h. This result mirrors what was observed in yeast (7), supporting the idea that all non-Lys63 linkages function in proteasomal targeting. Interestingly, at later time points (10 h and after), the level of Lys63 also increased, suggesting the activation of other, late response pathways. It is highly possible that Lys63-linked substrates are targeted through Ub binding proteins (e.g. p62 and NBR1) to autophagy lysosomal degradation (37, 50), corroborated by immunocytochemistry in which Lys63 chains and the autophagy marker LC3 increasingly colocalize after long term inhibition. When the autophagy pathway was inhibited in cells, polyUb chains were changed in an almost reverse pattern: Lys63 increased immediately, and other linkages responded later. Interestingly, the LC3-positive autophagosomes contained both Lys63- and Lys48-linked polyUb chains, implying that Lys48-linked substrates are redirected to the lysosomal pathway. In addition to Lys63 linkages, Lys48 and other non-Lys63 linkages may also act as positive signals in this process. Mixed linkages have also been shown to associate with TDP-43 inclusions in a cellular model of frontotemporal lobar degeneration (51). On the other hand, it is also possible that Lys63 chains are the dominant signal in sorting of substrates with mixed linkages, and the role of other linkages is passive.
One key issue in this study is whether the increase of polyUb linkages in AD brains is caused by Ub signaling dysfunction in neurons, glial cells, or both. As the biochemical analyses revealed only average values of Ub linkages in all cells in the brain samples, we carried out double immunostaining analysis of Lys48 and Lys63 linkages with neuronal and glial markers (NeuN and GFAP proteins, respectively, supplemental Fig. S1). Lys48-positive structures are largely present in neurons and are reminiscent of Lewy bodies and neurofibrillary tangles, and Lys63-stained pathological structures may also represent dystrophic neurites and tangles (Fig. 3, supplemental Fig. S1) as reported previously (48). Moreover, double staining showed highly limited overlap between Lys63 signals and GFAP-positive cells (supplemental Fig. S1). Our results support that dysfunctional neurons contribute to the up-regulation of polyUb chains, but the role of glial cells cannot be ruled out due to a small number of cases used in this pilot study.
In summary, our data indicate a robust specificity of polyUb signals at the global level of the mammalian proteome under different conditions of proteolytic stress, in which a cultured cell model of limited protein refolding capacity under heat shock treatment closely mimics the polyUb profile of late stage AD. It will be of great interest to study different Ub enzymes (e.g. ubiquitin E2s, E3s, and DUBs), substrates and Ub receptors contributing to the unique polyUb patterns under various stress conditions and in various neurodegenerative disorders.
This work was supported, in whole or in part, by National Institutes of Health Grants AG025688, NS055077, and RR025822 and Training Grant NS007480 (to E. B. D. and N. T. S.). This work was also supported by Research Scholar Grant RSG-09-181 from the American Cancer Society.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1–S4 and Figs. S1–S3.
- monoUb
- monoubiquitin
- Ub
- ubiquitin
- polyUb
- polyubiquitin
- SRM
- selected reaction monitoring
- AD
- Alzheimer disease
- 6-AN
- 6-aminonicotinamide.
REFERENCES
- 1. Ciechanover A. (2005) Nat. Rev. Mol. Cell Biol. 6, 79–87 [DOI] [PubMed] [Google Scholar]
- 2. Kerscher O., Felberbaum R., Hochstrasser M. (2006) Annu. Rev. Cell Dev. Biol. 22, 159–180 [DOI] [PubMed] [Google Scholar]
- 3. Hicke L., Schubert H. L., Hill C. P. (2005) Nat. Rev. Mol. Cell Biol. 6, 610–621 [DOI] [PubMed] [Google Scholar]
- 4. Reyes-Turcu F. E., Ventii K. H., Wilkinson K. D. (2009) Annu. Rev. Biochem. 78, 363–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peng J., Schwartz D., Elias J. E., Thoreen C. C., Cheng D., Marsischky G., Roelofs J., Finley D., Gygi S. P. (2003) Nat. Biotechnol. 21, 921–926 [DOI] [PubMed] [Google Scholar]
- 6. Kirisako T., Kamei K., Murata S., Kato M., Fukumoto H., Kanie M., Sano S., Tokunaga F., Tanaka K., Iwai K. (2006) EMBO J. 25, 4877–4887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu P., Duong D. M., Seyfried N. T., Cheng D., Xie Y., Robert J., Rush J., Hochstrasser M., Finley D., Peng J. (2009) Cell 137, 133–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Komander D. (2009) Biochem. Soc. Trans. 37, 937–953 [DOI] [PubMed] [Google Scholar]
- 9. Chau V., Tobias J. W., Bachmair A., Marriott D., Ecker D. J., Gonda D. K., Varshavsky A. (1989) Science 243, 1576–1583 [DOI] [PubMed] [Google Scholar]
- 10. Thrower J. S., Hoffman L., Rechsteiner M., Pickart C. M. (2000) EMBO J. 19, 94–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Finley D., Sadis S., Monia B. P., Boucher P., Ecker D. J., Crooke S. T., Chau V. (1994) Mol. Cell. Biol. 14, 5501–5509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnson E. S., Ma P. C., Ota I. M., Varshavsky A. (1995) J. Biol. Chem. 270, 17442–17456 [DOI] [PubMed] [Google Scholar]
- 13. Kirkpatrick D. S., Hathaway N. A., Hanna J., Elsasser S., Rush J., Finley D., King R. W., Gygi S. P. (2006) Nat. Cell Biol. 8, 700–710 [DOI] [PubMed] [Google Scholar]
- 14. Jin L., Williamson A., Banerjee S., Philipp I., Rape M. (2008) Cell 133, 653–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Williamson A., Wickliffe K. E., Mellone B. G., Song L., Karpen G. H., Rape M. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 18213–18218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu T., Merbl Y., Huo Y., Gallop J. L., Tzur A., Kirschner M. W. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 1355–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hicke L., Dunn R. (2003) Annu. Rev. Cell Dev. Biol. 19, 141–172 [DOI] [PubMed] [Google Scholar]
- 18. Bergink S., Jentsch S. (2009) Nature 458, 461–467 [DOI] [PubMed] [Google Scholar]
- 19. Bhoj V. G., Chen Z. J. (2009) Nature 458, 430–437 [DOI] [PubMed] [Google Scholar]
- 20. Rahighi S., Ikeda F., Kawasaki M., Akutsu M., Suzuki N., Kato R., Kensche T., Uejima T., Bloor S., Komander D., Randow F., Wakatsuki S., Dikic I. (2009) Cell 136, 1098–1109 [DOI] [PubMed] [Google Scholar]
- 21. Zhao S., Ulrich H. D. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 7704–7709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rubinsztein D. C. (2006) Nature 443, 780–786 [DOI] [PubMed] [Google Scholar]
- 23. Sherman M. Y., Goldberg A. L. (2001) Neuron 29, 15–32 [DOI] [PubMed] [Google Scholar]
- 24. Morimoto R. I. (2008) Genes Dev. 22, 1427–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bedford L., Hay D., Devoy A., Paine S., Powe D. G., Seth R., Gray T., Topham I., Fone K., Rezvani N., Mee M., Soane T., Layfield R., Sheppard P. W., Ebendal T., Usoskin D., Lowe J., Mayer R. J. (2008) J. Neurosci. 28, 8189–8198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Komatsu M., Waguri S., Chiba T., Murata S., Iwata J., Tanida I., Ueno T., Koike M., Uchiyama Y., Kominami E., Tanaka K. (2006) Nature 441, 880–884 [DOI] [PubMed] [Google Scholar]
- 27. Newton K., Matsumoto M. L., Wertz I. E., Kirkpatrick D. S., Lill J. R., Tan J., Dugger D., Gordon N., Sidhu S. S., Fellouse F. A., Komuves L., French D. M., Ferrando R. E., Lam C., Compaan D., Yu C., Bosanac I., Hymowitz S. G., Kelley R. F., Dixit V. M. (2008) Cell 134, 668–678 [DOI] [PubMed] [Google Scholar]
- 28. Krüger M., Moser M., Ussar S., Thievessen I., Luber C. A., Forner F., Schmidt S., Zanivan S., Fässler R., Mann M. (2008) Cell 134, 353–364 [DOI] [PubMed] [Google Scholar]
- 29. Bendall S. C., Hughes C., Stewart M. H., Doble B., Bhatia M., Lajoie G. A. (2008) Mol. Cell Proteomics 7, 1587–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liao L., Cheng D., Wang J., Duong D. M., Losik T. G., Gearing M., Rees H. D., Lah J. J., Levey A. I., Peng J. (2004) J. Biol. Chem. 279, 37061–37068 [DOI] [PubMed] [Google Scholar]
- 31. Xu P., Cheng D., Duong D. M., Rush J., Roelofs J., Finley D., Peng J. (2006) Israel J. Chem. 46, 171–182 [Google Scholar]
- 32. Gitcho M. A., Strider J., Carter D., Taylor-Reinwald L., Forman M. S., Goate A. M., Cairns N. J. (2009) J. Biol. Chem. 284, 12384–12398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kirkpatrick D. S., Denison C., Gygi S. P. (2005) Nat. Cell Biol. 7, 750–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Haas A. L., Bright P. M. (1987) J. Biol. Chem. 262, 345–351 [PubMed] [Google Scholar]
- 35. Ryu K. Y., Baker R. T., Kopito R. R. (2006) Anal. Biochem. 353, 153–155 [DOI] [PubMed] [Google Scholar]
- 36. Hanna J., Leggett D. S., Finley D. (2003) Mol. Cell. Biol. 23, 9251–9261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tan J. M., Wong E. S., Kirkpatrick D. S., Pletnikova O., Ko H. S., Tay S. P., Ho M. W., Troncoso J., Gygi S. P., Lee M. K., Dawson V. L., Dawson T. M., Lim K. L. (2008) Hum. Mol. Genet. 17, 431–439 [DOI] [PubMed] [Google Scholar]
- 38. Hammond T. G., Goda F. O., Navar G. L., Campbell W. C., Majewski R. R., Galvan D. L., Pontillon F., Kaysen J. H., Goodwin T. J., Paddock S. W., Verroust P. J. (1998) J. Membr. Biol. 162, 157–167 [DOI] [PubMed] [Google Scholar]
- 39. Pivtoraiko V. N., Harrington A. J., Mader B. J., Luker A. M., Caldwell G. A., Caldwell K. A., Roth K. A., Shacka J. J. (2010) J. Neurochem. 114, 1193–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang J., Bridges K., Chen K. Y., Liu A. Y. (2008) PLoS ONE 3, e2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Finn P. F., Mesires N. T., Vine M., Dice J. F. (2005) Autophagy 1, 141–145 [DOI] [PubMed] [Google Scholar]
- 42. Bennett E. J., Shaler T. A., Woodman B., Ryu K. Y., Zaitseva T. S., Becker C. H., Bates G. P., Schulman H., Kopito R. R. (2007) Nature 448, 704–708 [DOI] [PubMed] [Google Scholar]
- 43. Meierhofer D., Wang X., Huang L., Kaiser P. (2008) J. Proteome Res. 7, 4566–4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mallick P., Schirle M., Chen S. S., Flory M. R., Lee H., Martin D., Ranish J., Raught B., Schmitt R., Werner T., Kuster B., Aebersold R. (2007) Nat. Biotechnol. 25, 125–131 [DOI] [PubMed] [Google Scholar]
- 45. Jones J., Wu K., Yang Y., Guerrero C., Nillegoda N., Pan Z. Q., Huang L. (2008) J. Proteome Res. 7, 1274–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Seyfried N. T., Xu P., Duong D. M., Cheng D., Hanfelt J., Peng J. (2008) Anal. Chem. 80, 4161–4169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cripps D., Thomas S. N., Jeng Y., Yang F., Davies P., Yang A. J. (2006) J. Biol. Chem. 281, 10825–10838 [DOI] [PubMed] [Google Scholar]
- 48. Paine S., Bedford L., Thorpe J. R., Mayer R. J., Cavey J. R., Bajaj N., Sheppard P. W., Lowe J., Layfield R. (2009) Neurosci. Lett. 460, 205–208 [DOI] [PubMed] [Google Scholar]
- 49. Kundrat L., Regan L. (2010) J. Mol. Biol. 395, 587–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kirkin V., McEwan D. G., Novak I., Dikic I. (2009) Mol. Cell 34, 259–269 [DOI] [PubMed] [Google Scholar]
- 51. Seyfried N. T., Gozal Y. M., Dammer E. B., Xia Q., Duong D. M., Cheng D., Lah J. J., Levey A. I., Peng J. (2010) Mol. Cell Proteomics 9, 705–718 [DOI] [PMC free article] [PubMed] [Google Scholar]