Abstract
Pancreatic ductal adenocarcinoma (PDAC) is characterized by pronounced fibrotic reaction composed primarily of type I collagen. Although type I collagen functions as a barrier to invasion, pancreatic cancer cells have been shown to respond to type I collagen by becoming more motile and invasive. Because epithelial-mesenchymal transition is also associated with cancer invasion, we examined the extent to which collagen modulated the expression of Snail, a well known regulator of epithelial-mesenchymal transition. Relative to cells grown on tissue culture plastic, PDAC cells grown in three-dimensional collagen gels induced Snail. Inhibiting the activity or expression of the TGF-β type I receptor abrogated collagen-induced Snail. Downstream of the receptor, we showed that Smad3 and Smad4 were critical for the induction of Snail by collagen. In contrast, Smad2 or ERK1/2 was not involved in collagen-mediated Snail expression. Overexpression of Snail in PDAC cells resulted in a robust membrane type 1-matrix metalloproteinase (MT1-MMP, MMP-14)-dependent invasion through collagen-coated transwell chambers. Snail-expressing PDAC cells also demonstrated MT1-MMP-dependent scattering in three-dimensional collagen gels. Mechanistically, Snail increased the expression of MT1-MMP through activation of ERK-MAPK signaling, and inhibiting ERK signaling in Snail-expressing cells blocked two-dimensional collagen invasion and attenuated scattering in three-dimensional collagen. To provide in vivo support for our findings that Snail can regulate MT1-MMP, we examined the expression of Snail and MT1-MMP in human PDAC tumors and found a statistically significant positive correlation between MT1-MMP and Snail in these tumors. Overall, our data demonstrate that pancreatic cancer cells increase Snail on encountering collagen-rich milieu and suggest that the desmoplastic reaction actively contributes to PDAC progression.
Keywords: Collagen, Matrix Metalloproteinase, Pancreas, SMAD Transcription Factor, Transforming Growth Factor β (TGF-β), MMP-14, Snail, Epithelial-Mesenchymal Transition
Introduction
Epithelial-mesenchymal transition (EMT)2 has been identified as a key step in tumor invasion and metastasis, enabling cells to disrupt normal tissue architecture and invade surrounding structures (1–3). EMT is associated with loss of epithelial markers and up-regulation of mesenchymal markers and is characterized by a change in morphology from cobblestone-like sheets of cells typical of epithelial phenotype to elongated, fusiform cells characteristic of fibroblasts (1–3). One of the key regulators of EMT is the transcription factor Snail (4, 5), which can increase cell invasion and metastases in vivo, in part by increasing expression of matrix metalloproteinases (MMPs) (6, 7). Snail also functions in mesenchymal cells to regulate an MMP-dependent invasion program (8), and a Snail-mediated increase in membrane type 1-MMP (MT1-MMP, MMP-14) in fibroblasts promotes growth and invasion in the collagen-rich tumor microenvironment (8).
Pancreatic cancer, which is associated with a very pronounced type I collagen-rich stroma (9), is an aggressive cancer, with patients often presenting with locally invasive or metastatic disease (10). Although type I collagen is well known to function as a barrier to invasion, analysis of human tumors has shown that increased collagen expression can also be associated with poor prognosis and with increased metastasis (11, 12). Previously, it was shown that pancreatic cancer cells respond to type I collagen by increasing motility and up-regulating mesenchymal markers (13). We had also shown that pancreatic cancer cells respond to type I collagen by inducing MT1-MMP to enhance migration and invasion (14). Collagen-induced MT1-MMP expression was mediated by increased TGF-β signaling (14). TGF-β signaling was also increased in human pancreatic tumors, particularly in areas of fibrosis (14). One way in which TGF-β can signal is through cell-surface serine-threonine kinases to initiate cellular responses (15, 16). Binding of TGF-β1 to its type II receptor (TβRII) promotes TβRII phosphorylation of type I receptor (TβRI), which then phosphorylates the receptor-associated Smads, Smad2 and Smad3 (15, 16). The receptor-associated Smads then bind to Smad4 and translocate to the nucleus, wherein the complex can associate with transcription factors to regulate the expression of target genes (15, 16). In addition to the Smad signaling pathway, TGF-β can also activate MAPK/ERK1/2 signaling to regulate gene expression (17).
Although EMT and the transcription factor Snail have been shown to contribute to cancer progression (1, 2), the role and regulation of Snail in pancreatic cancer has not been well defined. In this study we examined the extent to which collagen modulates Snail and defined the contribution of Snail to collagen invasion. We show that relative to cells grown on tissue culture plastic, pancreatic cancer cells grown in three-dimensional collagen gels induced Snail via increased TGF-β signaling. Collagen-induced Snail in pancreatic cancer cells was mediated by Smad3/4 but did not involve Smad2 or ERK1/2. To further examine the role of Snail in PDAC progression, we generated Snail-inducible pancreatic cancer cells lines. Overexpression of Snail in PDAC cells enhanced MT1-MMP-dependent two-dimensional collagen invasion and promoted MT1-MMP-dependent scattering in three-dimensional collagen. Mechanistically, Snail signaled through ERK1/2 to increase MT1-MMP expression, promote two-dimensional collagen invasion, and induce scattering in three-dimensional collagen. Clinically, human tumor samples with increased Snail also demonstrated increased MT1-MMP expression. Overall, we have found that collagen through TGF-β signaling induces Snail and thereby contributes to pancreatic cancer progression.
EXPERIMENTAL PROCEDURES
Materials
General tissue culture materials were obtained from VWR International (West Chester, PA). Antibodies against MT1-MMP (ab38971) and Smad3 (ab28379) were purchased from Abcam (Cambridge, MA). Antibodies for p-ERK1/2 (9101), Smad4 (9515), Smad2 (3122), and Snail (3879) were purchased from Cell Signaling (Danvers, MA). Antibodies against α-tubulin (sc-8035) and TβRI (sc-398) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). HRP-conjugated secondary antibodies were obtained from Sigma. Type I collagen-coated plates (354400) and type I collagen (354249), which was diluted to 2 mg/ml according to manufacturer's protocol, were purchased from BD Biosciences. TGF-β1 was obtained from Sigma and used at a final concentration of 10 ng/ml. MMP inhibitor GM6001 (561206), TβRI inhibitor SB431542 (1614), and MEK1/2 inhibitor U0126 (9903) were purchased from Calbiochem, Tocris (Ellisville, MO), and Cell Signaling (Danvers, MA), respectively, and used at a final concentration of 10 μm. Nucleofector electroporation kit (VCA-1001) specific for Panc1 cell transfection was purchased from Lonza. Collagenase (4196) was purchased from Worthington Biochemical Co. TIMP-1 (cc1062) and TIMP-2 (cc3327) were purchased from Chemicon International (Temecula CA).
Cell Culture
Premalignant HPDE cells (HPV16-immortalized normal pancreatic ductal epithelium) were generously provided by Dr. M. Tsao (Ontario Cancer Institute), whereas the malignant Panc1, CD18, and AsPC1 cells were purchased from American Type Culture Collection (Manassas, VA). These cells were maintained as previously described (14). To examine the effect of TGF-β1 on Snail expression, equal numbers of Panc1 or HPDE cells were plated in serum containing media, serum-starved overnight, and then treated with 10 ng/ml TGF-β1 for 12 h.
Generation of Pancreatic Cancer Cells Inducibly Expressing Snail
The Snail gene, which was initially cloned into the eukaryotic expression vector pEGFP-C1 (7), was subcloned into pRetroX-Tight-Pur vector, and viral particles were generated as previously published (18). A similar protocol was used to generate viral particles for pTet-On Advanced vector (Clontech). Parental Panc-1, AsPC1, and CD18 cells were first infected with pTet-On, and stable cells resistant to G418 were selected followed by infection with pTight-Snail, or pTight-Luc and stable cell lines resistant to both G418 and puromycin were selected. The stable cell lines were routinely maintained in DMEM with Tet System Approved FBS (Clontech), puromycin, and G418. For Snail induction, doxycycline at a final concentration of 2 μg/ml was added to the growth media.
Down-regulation of TβRI, Smad2, Smad3, Smad4, Snail, MMP-2, and MT1-MMP Expression
TβRI expression was transiently down-regulated using a predesigned and validated Silencer siRNA sequences specific for the receptor from Ambion (siRNA ID 556). Expression of Smad2 and Smad4 was transiently down-regulated using the following duplex siRNAs: Smad4 (50 nmol) forward (5′-UGAAGGACAUUCAAUUCAAdTdT-3′) and reverse (5′-UUGAAUUGAAUGUCCUUCAdTdT-3′) (19); Smad2 (50 nmol) siRNA (S2Si) forward (5′-GUCCCAUGAAAAGACUUAAdTdT-3′) and reverse (5′-UUAAGUCUUUUCAUGGGACdTdT-3′) (20). Smad3 and Snail siRNAs were obtained from Cellogenetics (GLG1108, 100 nmol) and Ambion (siRNA IDs s13186, 50 nmol and s13187, 50 nmol). A validated siRNA for MMP-2 (s8851) and a custom designed siRNA for MT1-MMP targeted against nucleotides 228–248 (5′-AACAGGCAAAGCTGATGCAGA-3′) (21) were purchased from Invitrogen. Panc1 cells were transfected with siRNA for gene of interest or control siRNA using the Nucleofector kit R (Amaxa/Lonza). The transfected cells were allowed to recover on plastic for 48 h then seeded in collagen or on plastic for an additional 24 h. Panc1-Snail growing in collagen gels cells was transfected with siMMP2, siMT1-MMP, or control siRNA using Trans IT-siQUEST transfection reagent (MIR2114) from Mirus (Madison, WI).
Quantitative Real Time-PCR Analysis
Reverse transcription of RNA to cDNA was performed using Taqman Reverse Transcription reagents (N808–0234) from Applied Biosystems. Quantitative gene expression was performed for Snail (Hs00195591_m1) MT1-MMP (Hs00237119_m1) and GAPDH (Hs99999905_m1) with gene-specific Taqman probes, TaqMan Universal PCR Master Mix (4324018), and the 7500 Fast Real-time PCR System from Applied Biosystems. The data were then quantified with the comparative CT method for relative gene expression (14).
Immunoblotting
Immunoblotting was done as described previously (22, 23) and detected by enhanced chemiluminescence Western blotting reagents (Pierce).
Zymographic Analysis of MMP-2 Expression
MMP-2 levels in the conditioned media were determined using SDS-PAGE gelatin zymography as previously described (23).
Analysis of Collagen Invasion
Invasive activity was quantified using a BD Biocoat filter (8-μm pore, from BD Biosciences (354578) coated with 7.5 μg of type I collagen. Panc1-vector or Panc1-Snail cells (2 × 105) were added in 800 μl of serum-free medium in the upper chamber and 1 ml of serum containing medium supplemented with doxycycline. To examine the role for protease or ERK signaling in Snail-mediated invasion, the MMP inhibitor GM6001 or the MEK1/2 inhibitor U0126 was added to the medium. The cells were allowed to invade for 30 h, and the nonmigrating cells were removed from the upper chamber using a cotton swab. The filters were fixed and stained with a Diff-Quik staining kit (B4132-1A, VWR), and migrating cells adherent to the underside of the filter were quantified using an inverted microscope and counting a minimum of five high-powered fields. Data are expressed as relative two-dimensional invasion (number of cells/field).
Embedding and Examination of Cells in Three-dimensional Type I Collagen Gels
A collagen mixture (2 mg/ml) was made by adding the appropriate volumes of sterile water, 10× DMEM, and NaOH and kept on ice until needed (24). 1 × 105 Panc1 or HPDE cells were then suspended in the collagen solution and allowed to gel for 30 min at 37 °C. Regular media was then added on top of the gel and incubated for 24 h. For RNA extraction, the gel-containing cells were processed using an Qiagen RNeasy extraction kit (74106) to extract RNA for qRT-PCR analysis. For protein analyses, cells were extracted out of the gels using collagenase and lysed. For morphological examination of Snail-expressing cells, 2 × 104 cells were suspended in collagen, the resulting cell colonies were examined using a Zeiss Axiovert 40 CFL microscope, and pictures were taken with a Nikon Coolpix 4500 camera. Relative colony scattering was quantified by counting the average number of scattered colonies (loosely arranged, elongated with projections) per field from a minimum of 5 different fields at a 100× magnification.
Human PDAC Tissue Analysis
Pancreatic tissue was obtained from patients with pancreatic adenocarcinoma on an institutional review board-approved protocol. Using de-identified human pancreatic tissue specimens, RNA was isolated from human PDAC tumors and the adjacent matched normal pancreas previously stored in RNAlater and analyzed by qRT-PCR. Snail and MT1-MMP expression in PDAC tumors (n = 11) was normalized to the levels present in the matched adjacent normal pancreas, which was arbitrarily set at 1.0, and analyzed using Spearman's correlation.
Statistical Analysis
All statistical analyses were done using GraphPad Instat 3 (San Diego, CA).
RESULTS
Collagen-induced Snail Expression Is Mediated by TGF-β Signaling
We had previously shown that type I collagen, a major component of human pancreatic tumors (9), can increase the motility and invasion of human pancreatic cancer cells (14). As Snail is a key regulator of EMT that has also been shown to increase motility and invasion (1, 2), we examined the extent to which collagen modulated Snail levels in pancreatic cells. Panc1 and HPDE cells were plated onto tissue culture plastic or in three-dimensional type I collagen gels for 24 h, and mRNA was extracted and analyzed for Snail by quantitative real-time PCR. As shown in Fig. 1A, cells plated in collagen demonstrated a 2–3-fold increase in Snail levels compared with cells plated onto tissue culture plastic. Consistent with the increase in Snail expression, there was repression of E-cadherin and an increase in vimentin in cells grown in three-dimensional collagen (data not shown). Because we had previously published that pancreatic cells demonstrate increased TGF-β1 signaling on encountering type I collagen (14) and because TGF-β1 can induce Snail (Ref. 25, Fig. 1B, and supplemental Fig. S1A), we examined the role of TGF-β signaling in collagen-driven Snail expression by blocking the TGF-β TβRI. Panc1 and HPDE cells were plated onto tissue culture plastic or in three-dimensional type I collagen gels in the presence of DMSO or SB431542, a well characterized inhibitor of TβRI activity (26), and the effect on Snail expression was determined. As shown in Fig. 1C, SB431542 attenuated the effect of collagen on Snail expression. Additionally, Panc1 cells were transfected with control siRNA or siRNA against TβRI, allowed to recover, and then plated on tissue culture plastic or in three-dimensional type I collagen gels. The TβRI siRNA successfully knocked down TβRI protein expression and blocked collagen-induced Snail expression (Fig. 1D). Overall, these experiments demonstrate that collagen-induced Snail expression is mediated by TGF-β signaling.
FIGURE 1.
Collagen-induced Snail expression is mediated by TGF-β signaling. A, Panc1 and HPDE cells were grown on tissue culture plastic or in three-dimensional type I collagen gels (2 mg/ml) for 24 h, and Snail mRNA expression was analyzed by qRT-PCR using the Comparative CT method. The -fold change in Snail expression relative to plastic was determined using GAPDH as normalization control. *, p < 0.05. B, Panc1 cells were plated on tissue culture plastic, serum-starved overnight, then treated with TGF-β1 (10 ng/ml) for 12 h. The cell lysates were analyzed for Snail protein expression by Western blot analysis using α-tubulin as normalization control. C, HPDE and Panc1 cells were grown in three-dimensional collagen in the presence of TβRI kinase inhibitor SB431542 (SB, 10 μm) or DMSO (DM, vehicle control) for 24 h, and then the expression of Snail was analyzed by qRT-PCR. *, p < 0.05. D, Panc1 cells were transfected with a control siRNA (si ctl) or TβRI specific siRNA (si TβRI), allowed to recover, and after 48 h plated on tissue culture plastic or in three-dimensional collagen gels for 24 h. The knockdown of the TβRI protein was analyzed by Western blot analysis using α-tubulin as normalization control (top). The effect of TβRI knockdown on collagen-induced Snail expression was examined using qRT-PCR (bottom). The results are representative of at least three independent experiments.
Smad3, but Not Smad2, Regulates Collagen-induced Snail Expression
We next characterized the signaling downstream of TβRI involved in collagen-induced Snail expression. Because ERK1/2 has been shown to regulate Snail expression (25, 27), we initially evaluated the contribution of ERK1/2 signaling to collagen-induced Snail expression. Panc1 cells were plated onto tissue culture plastic or in three-dimensional type I collagen in the presence of DMSO or the MEK1/2 inhibitor U0126 for 24 h. The cells were then extracted out of the collagen gels using collagenase and analyzed for ERK1/2 phosphorylation and Snail expression. Although U0126 blocked collagen-induced ERK1/2 phosphorylation, it did not affect collagen-induced Snail expression (Fig. 2A), indicating that collagen does not signal through ERK1/2 to regulate Snail in pancreatic cancer cells. As Smads are canonical mediators of TGF-β signaling (15, 16), we next examined their contribution to collagen regulation of Snail in pancreatic cells. Panc1 and HPDE cells were transfected with control siRNA or siRNA against the requisite co-Smad Smad4, allowed to recover, and then plated onto tissue culture plastic or grown in three-dimensional type I collagen gels. As shown in Fig. 2B and supplemental Fig. S1, B and C, knocking down Smad4 attenuated collagen-induced Snail expression in PDAC cells. We also evaluated the relative contribution of Smad2 and Smad3 to collagen-induced Snail expression. Even though siRNA against Smad2 effectively knocked down Smad2 protein expression (Fig. 2C), it failed to block collagen-induced Snail expression. In contrast, the siRNAs against Smad3 blocked collagen-induced Snail expression (Fig. 2D). These results demonstrate that collagen signals through Smad3 to regulate collagen-induced Snail expression in pancreatic cancer cells.
FIGURE 2.
Smad3, but not Smad2, regulates collagen-induced Snail expression. A, Panc1 cells were plated on tissue culture plastic (PL) or in three-dimensional collagen gels (2 mg/ml, COL) for 24 h in the presence of DMSO or U0126 (10 μm). The lysates were then analyzed for ERK1/2 phosphorylation and α-tubulin by Western blot analysis (top). The cell lysates were also analyzed for Snail expression by qRT-PCR using GAPDH as normalization control (bottom). B–D, Panc1 cells were transfected with control siRNA (si ctl), siRNA against Smad4 (si Sd4, B), Smad2 (si Sd2, C) or Smad3 (si Sd3, D) (50 nm). After 48 h the transfected cells were plated on plastic or in three-dimensional collagen for 24 h. The specific down-regulation of each of the Smad proteins was analyzed by Western blot analysis using α-tubulin as normalization control (top). The effect of knockdown of each of the Smad on collagen-induced Snail mRNA expression was analyzed by qRT-PCR using GAPDH as normalization control (bottom). *, p < 0.05. The results are representative of at least three independent experiments.
Snail Promotes MMP-dependent Two-dimensional Collagen Invasion and Induces MMP-dependent Scattering in Three-dimensional Collagen Gels
To understand the role of Snail in pancreatic cancer progression, we generated Panc1 cell lines expressing Snail protein using a tet-on system (18). In the absence of doxycycline there was minimal Snail protein, whereas with the addition of doxycycline there was robust Snail expression (Fig. 3A). To examine the effect of Snail expression on invasion, Panc1-vector and Panc1-Snail cells were plated in Boyden chambers overlaid with type I collagen to create a barrier to invasion. As shown in Fig. 3, B and C, Snail-expressing cells demonstrated a ∼2.5-fold increased two-dimensional invasion through collagen-coated transwell chambers. Importantly, Snail expression in Panc1 cells did not affect proliferation on collagen-coated tissue culture plates. In addition, we examined the effect of Snail on pancreatic cancer cells grown in three-dimensional collagen gels. Compared with control cells, which grew as compact spheroids, Panc1 cells expressing Snail grew as less compact colonies and showed evidence of serpentine growth in three-dimensional collagen gels, which we classified as “scattered colonies” (Fig. 3D). Quantifying these changes, there was a ∼5-fold increase in the number of scattered colonies in the Snail-expressing cells compared with control Panc1 cells (Fig. 3E). To determine whether the increased numbers of scattered colonies were not as a result of Snail promoting proliferation in three-dimensional, Panc1 cells were extracted by collagenase digestion of the gel and counted. Snail did not affect Panc1 cell numbers in three-dimensional, indicating that the Snail-induced phenotypic changes are not due to increased proliferation. Because MMPs mediate growth and invasion in three-dimensional collagen gels (28), we treated the Snail-expressing cells with the broad-spectrum MMP inhibitor GM6001. As shown in Fig. 3, F and G, Snail-induced changes in three-dimensional colonies were blocked by GM6001. Moreover, Snail-induced two-dimensional collagen invasion was also blocked by GM6001 (Fig. 3H), indicating that Snail increases MMP expression or function in pancreatic cancer cells.
FIGURE 3.
Snail promotes MMP-dependent two-dimensional collagen invasion and induces MMP-dependent scattering in three-dimensional collagen gels. A, Panc1 cells were transfected with pTet-On (Clontech) vector and co-transfected with pTight-Luc or pTight-Snail and cell lines resistant to both G418 and puromycin selected (Panc1-vector, Panc1-Snail). Equal number of Panc1-vector and Panc1-Snail cells were plated on plastic and treated with or without doxycycline (2 μg/ml) for 16 h. The expression of Snail was analyzed by Western blot analysis using α-tubulin as normalization control. B and C, Panc1-vector and Panc1-Snail cells (2 × 105) were added in 800 μl of serum-free medium to the upper chamber of BD Biocoat porous polycarbonate filters (8 μm pore) coated with 7.5 μg of type I collagen, and 1 ml of serum-containing medium supplemented with doxycycline was added to the lower chamber. The cells were allowed to invade over 30 h, and the non-invading cells were removed from the upper chamber. The filters were then fixed, stained, and photographed (B), and the relative invasion was quantified (C). D and E, Panc1-vector and Panc1-Snail cells were embedded in three-dimensional collagen gels (2 mg/ml), and doxycycline-containing media was changed every 2 days for 6 days. The effect of Snail on colony morphology was examined by phase contrast microscopy (D), and the number of scattered colonies per field was quantified as detailed under “Experimental Procedures” (E). F and G, Panc1-vector and Panc1-Snail cells were grown in three-dimensional collagen gels (2 mg/ml) and doxycycline with DMSO (vehicle control) or GM6001 (10 μm) added every 2 days for 6 days. The effect on colony morphology in at least five fields was examined by phase contrast microscopy (F), and the average number of scattered colonies per field was quantified (G). H, the effect of GM6001 on invasion of Panc1-vector and Panc1-Snail cells through type I collagen-coated membrane was also determined. *, p < 0.05. The results are representative of at least three independent experiments.
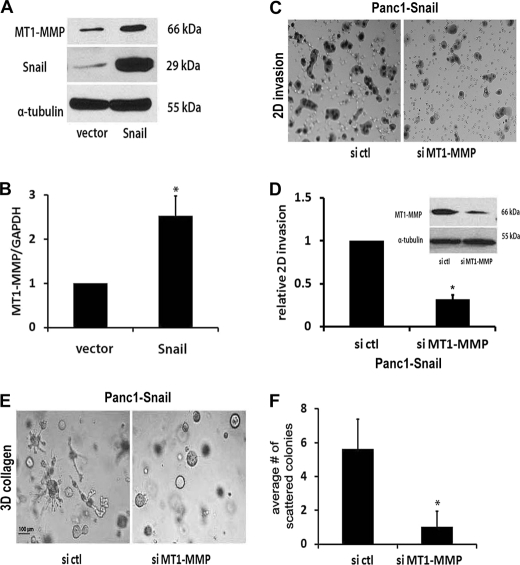
Snail Increases MT1-MMP Expression in Pancreatic Cancer Cells
We had previously shown that collagen invasion by pancreatic cancer cells is mediated by MT1-MMP (14), a key proteinase that also promotes growth in the collagen-rich tumor microenvironment (28). Thus, we examined the effect of Snail on MT1-MMP levels in pancreatic cancer cells. Panc1-vector and Panc1-Snail cells were plated onto tissue culture plastic and treated with doxycycline to induce Snail. As shown in Fig. 4, A and B, expression of Snail in Panc1 cells increased MT1-MMP at both the mRNA and protein levels. Snail also increased MT1-MMP expression in CD18 and AsPC1 pancreatic cancer cell lines (supplemental Fig. S2). We next examined the effect of down-regulating MT1-MMP on Snail-induced two-dimensional collagen invasion and three-dimensional scattering. Panc1-Snail cells were transfected with control siRNA or siRNA against MT1-MMP, allowed to recover, and then plated onto collagen-coated transwell chambers or grown in three-dimensional collagen gels. Cells transfected with siRNA against MT1-MMP, which decreased MT1-MMP protein levels (Fig. 4D, inset), demonstrated significant reduction in two-dimensional collagen invasion (Fig. 4, C and D). The MT1-MMP siRNA also significantly decreased the number of scattered colonies induced by Snail expression in three-dimensional collagen gels (Fig. 4, E and F). We also examined the effect of blocking MT1-MMP function using TIMP-2, a well defined endogenous inhibitor of MT1-MMP activity (29), on Panc1-Snail cells grown in three-dimensional collagen gels. Unlike untreated cells or cells treated with TIMP-1, which does not block MT1-MMP activity (30, 31), TIMP-2 significantly decreased the number of Snail-induced scattered colonies observed in three-dimensional collagen gels (Fig. 5, A and B). Because MMP-2 has also been shown to play a role in invasion of cancer cells and to act in concert with MT1-MMP (32), we examined whether MMP-2 was involved in Snail-induced scattering in three-dimensional using siRNA to silence its expression. The siRNA decreased MMP-2 mRNA by > 90% and decreased MMP-2 protein levels (Fig. 5C). However, the MMP-2 siRNA did not significantly decrease the number of scattered colonies induced by Snail expression in three-dimensional collagen gels (Fig. 5, D and E). Overall, these results indicate that the scattering phenotype induced by Snail in three-dimensional collagen is mediated by MT1-MMP.
FIGURE 4.
Snail increases MT1-MMP expression in pancreatic cancer cells. A and B, Panc1- vector and Panc1-Snail cells were induced with doxycycline (2 μg/ml) for 24 h, and the lysates were analyzed for Snail, MT1-MMP, and α-tubulin protein expression by Western blotting (A) and MT1-MMP and GAPDH mRNA expression by qRT-PCR (B). C and D, doxycycline-treated Panc1-Snail cells were transfected with control siRNA (si ctl) or MT1-MMP-specific siRNA (si MT1-MMP), allowed to recover for 24 h, and added to type I collagen-coated transwell chambers as detailed in Fig. 3 and allowed to invade. The knock down of MT1-MMP was determined after 48 h by Western blotting using α-tubulin as loading control (D, inset). A representative figure of the invading cells after 24 h (C) and the quantification of the relative invasion from 4 independent experiments are shown (D). E and F, the transfected Panc1-Snail cells were also seeded in three-dimensional collagen gels (2 mg/ml), doxycycline-containing media supplemented with control siRNA or MT1-MMP-specific siRNA was changed every 2 days for 6 days, the effect on colony morphology was examined by phase microscopy (E), and the average number of scattered colonies was quantified (F). The results are representative of at least three independent experiments. *, p < 0.05.
FIGURE 5.
TIMP-2 blocks Snail-induced scattering in three-dimensional. A and B, Panc1-Snail cells were grown in three-dimensional collagen gels (2 mg/ml) with TIMP-1 (5 μg/ml) or TIMP-2 (5 μg/ml)-co-polymerized. The doxycycline-containing media supplemented with TIMP-1 or TIMP-2 was changed every 2 days for 6 days, the effect on colony morphology was examined by phase microscopy (A), and the average number of scattered colonies quantified (B). *, p < 0.05. C, Panc1-Snail cells were transfected with control siRNA (si ctl) or MMP-2-specific siRNA (si MMP2) and allowed to recover for 24 h. The cells were then serum-starved for 24 h, and the conditioned media were analyzed for MMP-2 expression by gelatin zymography. D and E, the transfected Panc1-Snail cells were also seeded in three-dimensional collagen gels (2 mg/ml), the doxycycline-containing media supplemented with control siRNA or MMP-2-specific siRNA were changed every 2 days for 6 days, the effect on colony morphology was examined by phase microscopy (D), and the average number of scattered colonies was quantified (E). The results are representative of at least three independent experiments.
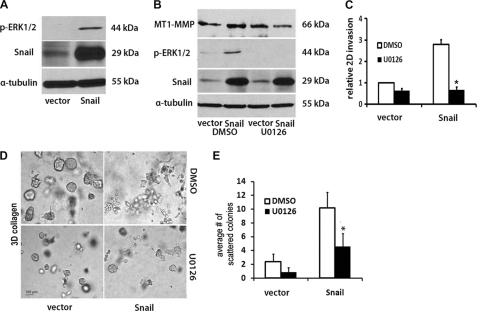
ERK1/2 Mediates Snail-induced MT1-MMP Expression and Collagen Invasion
As Snail was previously shown to increase ERK1/2 phosphorylation (6), we examined the role of ERK1/2 in Snail-mediated MT1-MMP up-regulation and collagen invasion by pancreatic cancer cells. Initially, we examined the effect of Snail on ERK1/2 phosphorylation. As shown in Fig. 6A, induction of Snail enhanced ERK1/2 phosphorylation. We next examined the effect of MEK1/2 inhibitor U0126 on MT1-MMP expression and Snail-mediated collagen invasion. Panc1-vector and Panc1-Snail cells were treated with vehicle control (DMSO) or U0126 and co-treated with doxycycline to induce Snail. As shown in Fig. 6B, Snail-induced ERK1/2 phosphorylation was blocked by U0126. Snail increased MT1-MMP in the presence of DMSO; however, Snail-induced MT1-MMP expression was attenuated in the presence of U0126. We also examined the effect of U0126 on Snail-induced invasion through collagen-coated transwell chambers. Snail promoted two-dimensional collagen invasion by ∼3-fold; however, in the presence of U0126, Snail-induced collagen invasion was abrogated (Fig. 6C). The effect of U0126 on Snail-induced scattering of Panc1 colonies in three-dimensional collagen was also determined. As shown in Fig. 6, D and E, there was significant reduction in the number of scattered colonies after U0126 treatment.
FIGURE 6.
ERK1/2 mediates Snail-induced MT1-MMP expression and collagen invasion. A, Panc1-vector and Panc1-Snail cells were induced with doxycycline (2 μg/ml) for 24 h, and the lysates were analyzed for Snail, p-ERK1/2, and α-tubulin expression by Western blot analysis. B, Panc1-vector and Panc1-Snail cells were induced with doxycycline (2 μg/ml) for 24 h in the presence of DMSO or MEK1/2 inhibitor U0126 (10 μm). The lysates were analyzed for Snail, p-ERK1/2, MT1-MMP, and α-tubulin expression by Western blotting. C, Panc1-vector and Panc1-Snail cells (2 × 105) in 800 μl of serum-free medium were added to the upper chamber of BD Biocoat porous polycarbonate filters (8 μm pore) coated with 7.5 μg of type I collagen and 1 ml of serum containing medium supplemented with doxycycline, and either DMSO or U0126 (10 μm) was added to the lower chamber. The cells were allowed to invade for 30 h, and the non-invading cells were removed from the upper chamber, membranes were fixed, and the relative invasion was quantified. D and E, Panc1-vector or Panc1-Snail cells were suspended in three-dimensional gel (2 mg/ml), and fresh serum-containing medium supplemented with doxycycline and either DMSO or U0126 (10 μm) was added every 2 days for 6 days. The effect on colony morphology was examined by phase contrast microscopy (D), and the average number of scattered colonies per field was quantified (E). The results are representative of at least three independent experiments. *, p < 0.05.
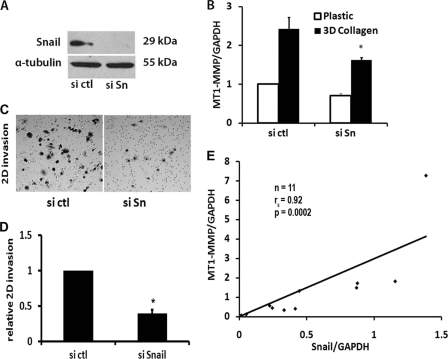
Collagen-induced MT1-MMP Expression Involves Snail
We had previously shown that collagen induces MT1-MMP to regulate pancreatic cancer invasion (14). Given the above findings that Snail can increase MT1-MMP in PDAC cells, we examined the extent to which Snail mediates collagen-induced MT1-MMP expression. Panc1 cells were transfected with control siRNA or siRNA against Snail, allowed to recover, and then plated in three-dimensional type I collagen gels. The Snail-specific siRNA blocked Snail protein levels in Panc1 (Fig. 7A) cells and partially attenuated collagen-induced MT1-MMP expression (Fig. 7B), indicating that Snail contributes to collagen-induced MT1-MMP expression. We also examined the effect of down-regulating Snail on collagen invasion. Panc1 cells transfected with control siRNA or siRNA against Snail were plated onto collagen-coated transwell chambers and allowed to invade over 30 h. Cells transfected with siRNA against Snail demonstrated significant reduction in collagen invasion (Fig. 7, C and D). Finally, we examined whether there was any relationship between expression of MT1-MMP and that of Snail in human PDAC tumor tissue. RNA isolated from de-identified human PDAC tumor samples was analyzed for MT1-MMP and Snail by qRT-PCR and normalized to the adjacent matched normal pancreas. As shown in Fig. 7E, tumor samples with increased Snail demonstrated increased MT1-MMP levels, with a Spearman correlation coefficient of 0.92 (p = 0.0002).
FIGURE 7.
Collagen-induced MT1-MMP expression involves Snail. A and B, Panc1 cells were transfected with control siRNA (si ctl) or a combination of two different Snail specific siRNAs (si Sn), allowed to recover for 48 h, and then plated on tissue culture plastic or in collagen gels for 24 h. The specific knockdown of Snail protein was determined by Western blotting using α-tubulin as normalization control (A), and the effect of Snail siRNA on collagen-induced MT1-MMP was analyzed by qRT-PCR using GAPDH as normalization control (B). The results are representative of at least three independent experiments. *, p < 0.05. C and D, Panc1 cells were transfected with control siRNA or specific siRNAs, allowed to recover for 24 h, added to type I collagen-coated transwell chambers as detailed in Fig. 3, and allowed to invade. A representative figure of the invading cells after 24 h (C) and the quantification of the relative invasion from 4 independent experiments are shown (D). *, p < 0.05. E, using de-identified human pancreatic tissue specimens (n = 11) obtained on an institutional review board-approved protocol, RNA was isolated from human PDAC tumors and the adjacent matched normal pancreas and analyzed by qRT-PCR. Snail and MT1-MMP expression in PDAC tumors was normalized to the levels present in the matched adjacent normal pancreas, which was arbitrarily set at 1.0, and analyzed using Spearman's correlation.
DISCUSSION
The desmoplastic reaction, a hallmark of human pancreatic ductal adenocarcinomas, is composed of collagen-rich extracellular matrix together with proliferating stromal cells (9). Besides functioning as a barrier to invasion type I collagen, the major extracellular matrix in the desmoplastic reaction, has also been shown to enhance tumor progression. The collagen fibers can act as highways for migration and facilitate metastasis by directing cancer cells to the vasculature (33, 34). Increased expression of type I collagen is detected in gene expression signatures associated with increased risk of metastasis (11, 12). Previously, we had shown that the type I collagen, the major extracellular matrix in the desmoplastic reaction, affected PDAC cellular behavior by increasing cellular motility and promoting expression of MT1-MMP (14). In this report we demonstrate that collagen can also increase expression of Snail, a key regulator of EMT (4, 5), in human pancreatic ductal cells and in cancer cells. Previous studies have shown that there is a direct relationship between Snail expression and the invasive phenotype (35, 36). Consistent with those reports, we now show that human PDAC tumors with increased Snail also demonstrate increased MT1-MMP expression. We were also able to show that Snail can increase expression of MT1-MMP in PDAC cells to regulate invasion in the three-dimensional microenvironment. Significantly, only MT1-MMP, the primary regulator of interstitial collagenolysis, confers a three-dimensional growth advantage by removing matrix confines necessary to drive proliferative responses (37).
We show that collagen-induced Snail expression was mediated by TGF-β1 signaling. TGF-β1 is a well defined inducer of Snail in a variety of cell lines, including human pancreatic cancer cells (7, 25, 38). Treatment with the highly specific inhibitor of TβRI or siRNA against TβRI blocked collagen-induced Snail expression. Previously, we had shown that PDAC cells increase TGF-β1 levels and demonstrate increased Smad phosphorylation upon encountering collagen (14). Clinically, human pancreatic tumor samples also demonstrate increased TGF-β1 signaling in areas of fibrosis (14). Recently, it was shown that collagen also signals through the integrin-linked kinase to regulate Snail expression (39, 40). Importantly, integrins and integrin-associated proteins have been shown to regulate various growth factor signaling pathways including TGF-β signaling (41, 42). The collagen integrin receptor α2β1, which associates with integrin-linked kinase (43), has been shown to interact with the TβRI-TβRII heterodimeric complex to modulate Smad signaling (44, 45). It is, therefore, possible that collagen promotes a higher order structure involving the TGF-β receptors, integrins, and integrin-linked kinase to activate Smad signaling and subsequent Snail expression.
Although TGF-β1 can signal via MAPK signaling pathways to regulate Snail expression (25), we found that collagen-induced Snail expression does not involve ERK1/2 but involves Smad signaling. Knocking down Smad4 abrogated collagen-induced Snail expression in our model system. Interestingly, mutations in Smad4 are seen in about 50% of human PDAC tumors (9, 10). Although Smad4 has tumor-suppressive effects early in tumorigenesis, transgenic mouse models have shown that Smad4 in fact can be tumor-promoting in advanced pancreatic tumors (46, 47). For example, loss of Smad4 in combination with activated KrasG12D in mouse pancreas results in tumor growth, but the tumors that develop are well differentiated in nature (46). In contrast, tumors with intact Smad4 show evidence of EMT, have poorly differentiated phenotype, and even show TGF-β-dependent growth in a subset of advanced tumors (46).
Although TGF-β1 can signal through both Smad2 and Smad3 to regulate Snail expression (38), we show a differential role of Smad2 and Smad3 in collagen-induced Snail expression. Collagen-induced Snail expression involves Smad3 but not Smad2 in pancreatic cancer cells. Interestingly, knock-out mouse studies have shown that Smad3 and Smad2 have varying roles in development (48). Smad2-null embryos die in utero (49, 50), although Smad3-null embryos are viable and fertile, although the mice are smaller and demonstrate immune dysfunction compared with wild-type control littermates (51). Gene expression studies have also shown that Smad2 and Smad3 differentially regulate TGF-β1-target genes (17, 52). Studies using mouse-embryonic fibroblasts (MEFs) from Smad2-null and Smad3-null mice show that compared with wild-type MEFs, there was no difference in the number of genes deregulated in Smad2-null MEFS after TGF-β1 treatment (17). In contrast, there was a 17-fold difference in the number of genes affected by TGF-β1 treatment between wild-type MEFs and Smad3-null MEFs (17). In addition to the differential role of Smad2 and Smad3 during development, there is also evidence to suggest the differing roles of Smad2 and Smad3 in adult tissue. Liver specific knock-down of Smad3 or Smad2 in adult mice showed that Smad3, but not Smad2, is required for TGF-β-stimulated EMT and growth arrest (53). Similarly, TGF-β1-mediated repression of E-cadherin in kidney epithelial cells required Smad3 and not Smad2 (54, 55). These latter reports clearly demonstrate that Smad3 may have a more significant role in regulating EMT than Smad2.
Snail expression dramatically increased invasion of pancreatic cancer cells in a two-dimensional microenvironment and induced scattering in a three-dimensional microenvironment. Snail increased ERK1/2 phosphorylation and blocking ERK1/2 activation using U0126 inhibited Snail-induced collagen invasion. Although the mechanism is unclear, work from our laboratory and others has shown that Snail can increase ERK1/2 phosphorylation (6, 7). Snail via ERK1/2 increased expression of MT1-MMP, and blocking MT1-MMP with TIMP-2 or siRNA also attenuated Snail-induced invasion. Previously, it was shown that stable transfection of Snail in liver cancer cells increased MT1-MMP expression (56). Recently, inducible expression of Snail in MCF7 breast cancer cells also increased MT1-MMP levels to promote growth and invasion in a chick chorioallantoic membrane assay (36). Also, conditional knock-out of Snail in fibroblasts resulted in decreased MT1-MMP levels and attenuation of invasion in the collagen microenvironment (8). Consistent with these reports, we also show that Snail siRNA can also attenuate collagen-induced MT1-MMP expression and decrease invasion of pancreatic cancer cells.
In summary, we demonstrate that pancreatic cancer cells on encountering type I collagen, which is the most pronounced extracellular matrix protein in human PDAC tumors, induce Snail expression through increased TGF-β1 signaling. Snail in turn promotes invasion of pancreatic cancer cells through increased MT1-MMP expression. These findings clearly demonstrate that the interplay between Snail and collagen results in tumor progression. Overall, this work increases our understanding of the tumor-promoting activity of the prominent collagen-rich stroma present in pancreatic cancer.
This work was supported, in whole or in part, by National Institutes of Health Grant R01CA126888 (NCI; to H. G. M.). This work was also supported by the Rosenberg Family Foundation (to H. G. M.) and the Baseball Charities Foundation (to M. A. S.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- EMT
- epithelial-mesenchymal transition
- MMP
- matrix metalloproteinase
- MT1-MMP
- membrane type 1-MMP
- PDAC
- pancreatic ductal adenocarcinoma
- TβRI
- TGF-β receptor type I
- TβR
- type II receptor
- MEF
- mouse-embryonic fibroblasts
- qRT
- quantitative real-time.
REFERENCES
- 1. Thiery J. P., Acloque H., Huang R. Y., Nieto M. A. (2009) Cell 139, 871–890 [DOI] [PubMed] [Google Scholar]
- 2. Kalluri R., Weinberg R. A. (2009) J. Clin. Invest. 119, 1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee J. M., Dedhar S., Kalluri R., Thompson E. W. (2006) J. Cell Biol. 172, 973–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Batlle E., Sancho E., Francí C., Domínguez D., Monfar M., Baulida J., García De Herreros A. (2000) Nat. Cell Biol. 2, 84–89 [DOI] [PubMed] [Google Scholar]
- 5. Cano A., Pérez-Moreno M. A., Rodrigo I., Locascio A., Blanco M. J., del Barrio M. G., Portillo F., Nieto M. A. (2000) Nat. Cell Biol. 2, 76–83 [DOI] [PubMed] [Google Scholar]
- 6. Jordà M., Olmeda D., Vinyals A., Valero E., Cubillo E., Llorens A., Cano A., Fabra A. (2005) J. Cell Sci. 118, 3371–3385 [DOI] [PubMed] [Google Scholar]
- 7. Sun L., Diamond M. E., Ottaviano A. J., Joseph M. J., Ananthanarayan V., Munshi H. G. (2008) Mol. Cancer Res. 6, 10–20 [DOI] [PubMed] [Google Scholar]
- 8. Rowe R. G., Li X. Y., Hu Y., Saunders T. L., Virtanen I., Garcia de Herreros A., Becker K. F., Ingvarsen S., Engelholm L. H., Bommer G. T., Fearon E. R., Weiss S. J. (2009) J. Cell Biol. 184, 399–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hezel A. F., Kimmelman A. C., Stanger B. Z., Bardeesy N., Depinho R. A. (2006) Genes Dev. 20, 1218–1249 [DOI] [PubMed] [Google Scholar]
- 10. Hidalgo M. (2010) N. Engl. J. Med. 362, 1605–1617 [DOI] [PubMed] [Google Scholar]
- 11. Tavazoie S. F., Alarcón C., Oskarsson T., Padua D., Wang Q., Bos P. D., Gerald W. L., Massagué J. (2008) Nature 451, 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ramaswamy S., Ross K. N., Lander E. S., Golub T. R. (2003) Nat. Genet. 33, 49–54 [DOI] [PubMed] [Google Scholar]
- 13. Shintani Y., Hollingsworth M. A., Wheelock M. J., Johnson K. R. (2006) Cancer Res. 66, 11745–11753 [DOI] [PubMed] [Google Scholar]
- 14. Ottaviano A. J., Sun L., Ananthanarayanan V., Munshi H. G. (2006) Cancer Res. 66, 7032–7040 [DOI] [PubMed] [Google Scholar]
- 15. Akhurst R. J., Derynck R. (2001) Trends Cell Biol. 11, S44–S51 [DOI] [PubMed] [Google Scholar]
- 16. Shi Y., Massagué J. (2003) Cell 113, 685–700 [DOI] [PubMed] [Google Scholar]
- 17. Yang Y. C., Piek E., Zavadil J., Liang D., Xie D., Heyer J., Pavlidis P., Kucherlapati R., Roberts A. B., Böttinger E. P. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 10269–10274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Joseph M. J., Dangi-Garimella S., Shields M. A., Diamond M. E., Sun L., Koblinski J. E., Munshi H. G. (2009) J. Cell Biochem. 108, 726–736 [DOI] [PubMed] [Google Scholar]
- 19. Deckers M., van Dinther M., Buijs J., Que I., Löwik C., van der Pluijm G., ten Dijke P. (2006) Cancer Res. 66, 2202–2209 [DOI] [PubMed] [Google Scholar]
- 20. Diamond M. E., Sun L., Ottaviano A. J., Joseph M. J., Munshi H. G. (2008) J. Cell Sci. 121, 2197–2207 [DOI] [PubMed] [Google Scholar]
- 21. Chun T. H., Sabeh F., Ota I., Murphy H., McDonagh K. T., Holmbeck K., Birkedal-Hansen H., Allen E. D., Weiss S. J. (2004) J. Cell Biol. 167, 757–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Munshi H. G., Wu Y. I., Ariztia E. V., Stack M. S. (2002) J. Biol. Chem. 277, 41480–41488 [DOI] [PubMed] [Google Scholar]
- 23. Munshi H. G., Wu Y. I., Mukhopadhyay S., Ottaviano A. J., Sassano A., Koblinski J. E., Platanias L. C., Stack M. S. (2004) J. Biol. Chem. 279, 39042–39050 [DOI] [PubMed] [Google Scholar]
- 24. Munshi H. G., Stack M. S. (2002) Methods Cell Biol. 69, 195–205 [DOI] [PubMed] [Google Scholar]
- 25. Peinado H., Quintanilla M., Cano A. (2003) J. Biol. Chem. 278, 21113–21123 [DOI] [PubMed] [Google Scholar]
- 26. Inman G. J., Nicolás F. J., Callahan J. F., Harling J. D., Gaster L. M., Reith A. D., Laping N. J., Hill C. S. (2002) Mol. Pharmacol. 62, 65–74 [DOI] [PubMed] [Google Scholar]
- 27. Grotegut S., von Schweinitz D., Christofori G., Lehembre F. (2006) EMBO J. 25, 3534–3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rowe R. G., Weiss S. J. (2009) Annu. Rev. Cell Dev. Biol. 25, 567–595 [DOI] [PubMed] [Google Scholar]
- 29. Brew K., Dinakarpandian D., Nagase H. (2000) Biochim. Biophys. Acta 1477, 267–283 [DOI] [PubMed] [Google Scholar]
- 30. Strongin A. Y., Collier I., Bannikov G., Marmer B. L., Grant G. A., Goldberg G. I. (1995) J. Biol. Chem. 270, 5331–5338 [DOI] [PubMed] [Google Scholar]
- 31. Will H., Atkinson S. J., Butler G. S., Smith B., Murphy G. (1996) J. Biol. Chem. 271, 17119–17123 [DOI] [PubMed] [Google Scholar]
- 32. Hernandez-Barrantes S., Toth M., Bernardo M. M., Yurkova M., Gervasi D. C., Raz Y., Sang Q. A., Fridman R. (2000) J. Biol. Chem. 275, 12080–12089 [DOI] [PubMed] [Google Scholar]
- 33. Condeelis J., Segall J. E. (2003) Nat. Rev. Cancer 3, 921–930 [DOI] [PubMed] [Google Scholar]
- 34. Provenzano P. P., Eliceiri K. W., Campbell J. M., Inman D. R., White J. G., Keely P. J. (2006) BMC Med. 4, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Olmeda D., Jordá M., Peinado H., Fabra A., Cano A. (2007) Oncogene 26, 1862–1874 [DOI] [PubMed] [Google Scholar]
- 36. Ota I., Li X. Y., Hu Y., Weiss S. J. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 20318–20323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hotary K. B., Allen E. D., Brooks P. C., Datta N. S., Long M. W., Weiss S. J. (2003) Cell 114, 33–45 [DOI] [PubMed] [Google Scholar]
- 38. Horiguchi K., Shirakihara T., Nakano A., Imamura T., Miyazono K., Saitoh M. (2009) J. Biol. Chem. 284, 245–253 [DOI] [PubMed] [Google Scholar]
- 39. Medici D., Nawshad A. (2010) Matrix Biol. 29, 161–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tan C., Costello P., Sanghera J., Dominguez D., Baulida J., de Herreros A. G., Dedhar S. (2001) Oncogene 20, 133–140 [DOI] [PubMed] [Google Scholar]
- 41. Margadant C., Sonnenberg A. (2010) EMBO Rep. 11, 97–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nishimura S. L. (2009) Am. J. Pathol. 175, 1362–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hannigan G., Troussard A. A., Dedhar S. (2005) Nat. Rev. Cancer 5, 51–63 [DOI] [PubMed] [Google Scholar]
- 44. Garamszegi N., Garamszegi S. P., Samavarchi-Tehrani P., Walford E., Schneiderbauer M. M., Wrana J. L., Scully S. P. (2010) Oncogene 29, 2368–2380 [DOI] [PubMed] [Google Scholar]
- 45. Kim K. K., Wei Y., Szekeres C., Kugler M. C., Wolters P. J., Hill M. L., Frank J. A., Brumwell A. N., Wheeler S. E., Kreidberg J. A., Chapman H. A. (2009) J. Clin. Invest. 119, 213–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bardeesy N., Cheng K. H., Berger J. H., Chu G. C., Pahler J., Olson P., Hezel A. F., Horner J., Lauwers G. Y., Hanahan D., DePinho R. A. (2006) Genes Dev. 20, 3130–3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Izeradjene K., Combs C., Best M., Gopinathan A., Wagner A., Grady W. M., Deng C. X., Hruban R. H., Adsay N. V., Tuveson D. A., Hingorani S. R. (2007) Cancer Cell 11, 229–243 [DOI] [PubMed] [Google Scholar]
- 48. Brown K. A., Pietenpol J. A., Moses H. L. (2007) J. Cell Biochem. 101, 9–33 [DOI] [PubMed] [Google Scholar]
- 49. Waldrip W. R., Bikoff E. K., Hoodless P. A., Wrana J. L., Robertson E. J. (1998) Cell 92, 797–808 [DOI] [PubMed] [Google Scholar]
- 50. Heyer J., Escalante-Alcalde D., Lia M., Boettinger E., Edelmann W., Stewart C. L., Kucherlapati R. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 12595–12600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang X., Letterio J. J., Lechleider R. J., Chen L., Hayman R., Gu H., Roberts A. B., Deng C. (1999) EMBO J. 18, 1280–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Piek E., Ju W. J., Heyer J., Escalante-Alcalde D., Stewart C. L., Weinstein M., Deng C., Kucherlapati R., Bottinger E. P., Roberts A. B. (2001) J. Biol. Chem. 276, 19945–19953 [DOI] [PubMed] [Google Scholar]
- 53. Ju W., Ogawa A., Heyer J., Nierhof D., Yu L., Kucherlapati R., Shafritz D. A., Böttinger E. P. (2006) Mol. Cell Biol. 26, 654–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Phanish M. K., Wahab N. A., Colville-Nash P., Hendry B. M., Dockrell M. E. (2006) Biochem. J. 393, 601–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Roberts A. B., Tian F., Byfield S. D., Stuelten C., Ooshima A., Saika S., Flanders K. C. (2006) Cytokine Growth Factor Rev. 17, 19–27 [DOI] [PubMed] [Google Scholar]
- 56. Miyoshi A., Kitajima Y., Sumi K., Sato K., Hagiwara A., Koga Y., Miyazaki K. (2004) Br. J. Cancer 90, 1265–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]