Abstract
The mechanisms behind the loss of epithelial barrier function leading to alveolar flooding in acute lung injury (ALI) are incompletely understood. We hypothesized that the tyrosine kinase receptor human epidermal growth factor receptor-2 (HER2) would be activated in an inflammatory setting and participate in ALI. Interleukin-1β (IL-1β) exposure resulted in HER2 activation in human epithelial cells and markedly increased conductance across a monolayer of airway epithelial cells. Upon HER2 blockade, conductance changes were significantly decreased. Mechanistic studies revealed that HER2 trans-activation by IL-1β required a disintegrin and metalloprotease 17 (ADAM17)-dependent shedding of the ligand neuregulin-1 (NRG-1). In murine models of ALI, NRG-1-HER2 signaling was activated, and ADAM17 blockade resulted in decreased NRG-1 shedding, HER2 activation, and lung injury in vivo. Finally, NRG-1 was detectable and elevated in pulmonary edema fluid from patients with ALI. These results suggest that the ADAM17-NRG-1-HER2 axis modulates the alveolar epithelial barrier and contributes to the pathophysiology of ALI.
Keywords: ADAM ADAMTS, Cell Adhesion, Cell Surface Receptor, Epithelial Cell, Lung, HER2, Acute Lung Injury, Epithelial Permeability, Neuregulin
Introduction
Acute lung injury (ALI)3 is a severe clinical disorder with an annual incidence of ∼200,000 and a mortality of 40% in the United States (1). Most commonly seen in the setting of sepsis (2–4), ALI is marked by disruption of the alveolar barrier, leukocyte activation, release of inflammatory cytokines, and hypercoagulability. The net effect is an increase in alveolar epithelial permeability, resulting in alveolar flooding with protein-rich edema and life-threatening hypoxemia (5). An intact epithelial barrier is essential to maintaining normal pulmonary fluid balance. Indeed, damage to the endothelium alone is insufficient to cause pulmonary edema, whereas epithelial injury results in severe lung injury (6–8). The epithelium provides a greater resistance to proteins and fluid than the capillary endothelium and is responsible for the active ion transport-dependent removal of edema fluid from the distal air spaces of the lung (9–11).
The tyrosine kinase receptor human epidermal growth factor receptor-2 (HER2) is expressed by pulmonary bronchial epithelial cells and is involved in multiple physiologic processes, including cell proliferation and wound repair. The HER receptor family consists of four type 1, membrane-bound tyrosine kinase receptors: HER1 or epidermal growth factor receptor (EGFR), HER2, HER3, and HER4 (12). HER2 has no known ligand and requires partnering with another HER family member for activation. HER2 and 3 are highly expressed in pulmonary bronchial epithelial cells (compared with HER1 and 4) (13). HER3 is the receptor for the ligand neuregulin-1 (NRG-1), but HER3 has no intrinsic signaling properties (14). Upon NRG-1 binding, HER3 heterodimerizes with HER2, resulting in activation of the HER2 tyrosine kinase domain, HER2 autophosphorylation, and initiation of downstream intracellular signaling cascades (13). NRG-1 is expressed in bronchial epithelial cells (13, 15, 16) and is shed from the cell surface to bind HER3 in an auto/paracrine fashion (17), resulting in receptor complex formation and activation. This pathway has been demonstrated to participate in normal lung growth and development (18) as well as in the pathogenesis of epithelial malignancies (14, 19), where HER2 is a therapeutic target in breast cancer.
We have demonstrated previously that this ligand-receptor system participates in recovery from bleomycin-induced fibrotic lung injury in mice. Murine bleomycin lung injury has an early, inflammatory phase marked by epithelial damage and loss of barrier function followed by fibrosis, closely modeling some features of human ALI (20). Inactivation of HER2 signaling in transgenic mice or pharmacologic blockade of the NRG-1-HER2 pathway prior to bleomycin injury attenuated pulmonary fibrosis in mice at 21 days as well as decreased bronchoalveolar lavage (BAL) protein at 3 days, suggesting that HER2 might participate in signaling relevant to late and early phases of injury (21, 22).
The cytokine interleukin-1β (IL-1β) has been identified as a central mediator of epithelial permeability, ALI, and pulmonary fibrosis (23–29). Given that IL-1β and HER2 activation have both been implicated in the pathogenesis of lung injury, an interaction between the two signaling pathways is possible. We hypothesized that IL-1β and HER2 signaling interact in airway and alveolar epithelial cells and that HER2 participates in IL-1β-induced changes in epithelial barrier function. The questions of whether HER2 participates in increased epithelial permeability, a hallmark of ALI, have remained unstudied. Our results confirmed that HER2 is trans-activated by IL-1β in a ligand-dependent fashion through a disintegrin and metalloprotease 17 (ADAM17)-mediated release of NRG-1 in human bronchial and alveolar epithelial cells (type I-like and alveolar type II). In murine models, ALI was associated with increased levels of NRG-1 found in BAL and HER2 activation whereas pharmacologic blockade of ADAM17 attenuated injury and decreased HER2 activation and NRG-1 shedding. Finally, we found that NRG-1 is detectable and elevated in pulmonary edema samples from patients with ALI. These data identify a novel inflammatory signaling pathway in the lung with direct diagnostic and therapeutic implications.
EXPERIMENTAL PROCEDURES
Reagents
Polyclonal rabbit antibodies directed against EGFR (1005), phospho-EGFR (Tyr-1173), HER2 (C-18), phospho-HER2 (Tyr-1248), HER3 (C-17), NRG-1α/β1/2 (C-20), and monoclonal mouse antibodies directed against HER2 (F-11), were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). A monoclonal rabbit antibody to phospho-HER3 (Tyr-1289) was from Cell Signaling (Danvers, MA). The monoclonal antibody directed against β-actin (clone Ac-74) was from Sigma. Recombinant NRG-1 and NRG-1 antibody targeting an extracellular domain (Ab-1, clone 7D4) were from Neomarkers (Fremont, CA). AG825, a selective tyrphostin-type HER2 kinase inhibitor, was from Calbiochem. Human recombinant IL-1β was from BIOSOURCE (Camarillo, CA). Human recombinant neuregulin was from Neomarkers (Freemont, CA). TAPI-2 was from Calbiochem.
Cell Culture and Treatment Conditions
The NuLi-1 cell line, an immortalized, nontransformed cell line derived from human airway epithelium of normal genotype, was a gift from Dr. J. Zabner (University of Iowa) (30). Normal human bronchial epithelial cells were obtained by endobronchial brushes from human subjects under a protocol approved by Case Western Reserve University Institutional Review Board. Immunohistochemical staining of these cells confirmed their epithelial lineage, as >95% of cells expressed cytokeratin and thyroid transcription factor 1, specific markers of pulmonary epithelial cells. A549 cells derived from a human lung adenocarcinoma were obtained from American Type Culture Collection (Manassas, VA). Primary human type I-like and type II alveolar epithelial cells were harvested and maintained as described previously (31).
Electrical Resistance Measurement
Electrical resistance measurements were determined as described previously (16). NuLi-1 cells were seeded onto permeable filters (Costar transwells; Corning, Inc., Corning, NY) and grown in defined medium (Dulbecco's modified Eagle's medium/Ham's F-12 (1:1) containing 2% v/v Ultroser G supplement, 50 units-50 μg/ml penicillin-streptomycin, 50 μg/ml gentamycin, 2 μg/ml fluconazole, 1.25 μg/ml amphotericin B) present at the apical and basal surface of cells for 72 h. Medium above the cells was then removed, and cells were grown at air-liquid interface until confluent. Confluence was confirmed by measuring electrical resistance across the membrane using an ohmmeter (EVOM; World Precision Instruments). Resistance greater than 850 Ω was taken to indicate confluent cell monolayers. Monolayers were treated as described in each experimental protocol. Resistance was measured at each time point indicated to assess response to stimuli and inhibitors. For ease of presentation, data are displayed as “normalized conductance,” which is the inverse of measured resistance normalized for each condition to its base-line value.
Large Molecule Permeability Measurement
Large molecule permeability was assessed in NuLi-1 monolayers using measured passage of FITC-labeled dextran (70 kDa; Sigma). Monolayers were incubated with IL-1β with or without specified inhibitors. One hour before analysis, FITC-labeled dextran (1 mg/ml) was added to the medium on the apical side of the monolayer. At the times indicated, the medium below the monolayer was collected, and fluorescence was measured at excitation 485 nm and emission 510 nm.
Western Blotting
Western blotting was performed as described previously (21, 22). For all experimental conditions, cells were pretreated with specific inhibitors for 2 h before IL-1β stimulation: AG825 (10 μm) and TAPI-2 (100 μm).
Gene Silencing with Small Interfering RNA
Small interfering RNA (siRNA) oligonucleotides targeting NRG-1 and ADAM17 mRNAs were obtained from Ambion (Austin, TX). siRNAs were transfected into NuLi-1 cells with Lipofectamine (Invitrogen) according to the manufacturer's instructions using a ratio of 40 nm siRNA:5 μl Lipofectamine. A non-mammalian sequence siRNA was used as a negative control (scrambled siRNA).
Preparation of Conditioned Medium
NuLi-1 cells were cultured to 90% confluence. The cells were incubated in serum-free medium overnight before treating with the appropriate stimulus for the specified time points. The serum-free medium was aspirated and equal volumes concentrated 10× using Amicon Centriplus filters with a molecular mass cutoff of 3 kDa. NRG-1 was immunoprecipitated from the concentrated medium with an antibody targeting the extracellular domain (Ab-1, clone 7D4). Western blot analysis was performed with an antibody against the C terminus of NRG-1 (C-20; Santa Cruz Biotechnology).
Murine Lung Injury Models
Bleomycin-induced lung injury was performed in mice as described previously (21, 22). Control animals were injected with an equal volume of PBS. The day of bleomycin injury was considered day 0. TAPI-2 (1 mg/kg mouse weight) was given intratracheally in a fashion similar to bleomycin on day 0 (concomitant with bleomycin) and serially every 24 h on days 1 and 2 (total of three doses). Injury was assessed on day 3. Indices of lung injury (BAL, lung collection, and histology) were performed as described previously (21, 22).
Human Edema Fluid Samples
Pulmonary edema fluid was collected from patients at the University of California, San Francisco, as in prior studies (32, 33). NRG-1 was measured via commercially available ELISA (R & D Systems, Minneapolis, MN).
Statistical Analysis
Values in the text and figure legends represent the means ± S.E. of at least three independent experiments. Groups were compared using an ANOVA followed by a Bonferroni post test or a Student's t test where appropriate. A p value <0.05 was considered significant. Representative Western blots displayed represent at least three independent experiments.
RESULTS
IL-1β-induced Epithelial Permeability Is HER2-dependent
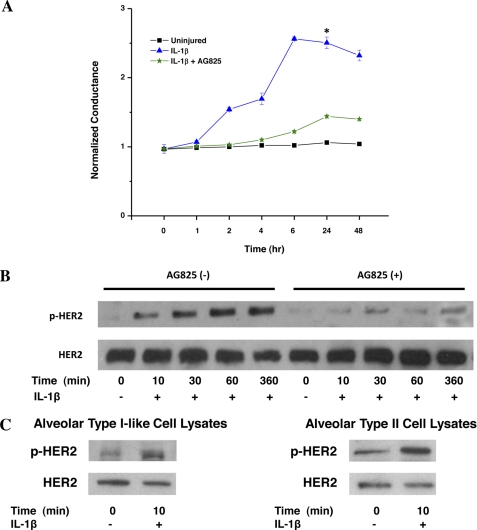
In our initial experiments, we sought to determine whether HER2 signaling participated in IL-1β-induced loss of barrier function across an epithelial monolayer, utilizing an immortalized, nontransformed cell line derived from airway epithelial cells (NuLi-1 cells). NuLi-1 cells were grown at an air-fluid interface to a confluent monolayer, exposed to IL-1β, and conductance was determined. IL-1β (10 ng/ml) induced a significant and sustained loss of barrier function as seen by increased conductance across the monolayer (Fig. 1A). In contrast, HER2 blockade with AG825, a small molecule inhibitor targeting the HER2 ATP binding site which effectively and specifically blocks HER2 kinase activity (supplemental Fig. 1, A and B), attenuated IL-1β-mediated changes in conductance across the monolayer. These conductance experiments were confirmed using a transwell system measuring passage of FITC-labeled dextran (40 kDa). HER2 blockade decreased large molecule permeability across the epithelial monolayer after IL-1β exposure (supplemental Fig. 2).
FIGURE 1.
IL-1β-mediated increased conductance across airway epithelium is HER2-dependent. A, conductance across a NuLi-1 epithelial monolayer after IL-1β exposure with and without AG825 (n > 3). *, p < 0.005. Data are presented as means ± S.E. (error bars). B, NuLi-1 cell lysates analyzed for HER2 and pHER2 following IL-1β exposure with and without AG825 (n > 3). C, HER2 activation assessed in human type I-like and type II alveolar epithelial cell lysates exposed to IL-1β (n = 3).
The requirement for HER2 signaling in IL-1β-mediated loss of airway epithelial barrier function suggests that IL-1β and HER2 interact, resulting in HER2 activation in human pulmonary epithelial cells. To assess this, NuLi-1 cells were incubated with IL-1β, and Western blots of total cell lysates were performed for activated HER2 (tyrosine-phosphorylated HER, pHER2). IL-1β stimulation resulted in a rapid increase in HER2 phosphorylation in NuLi-1 cells with a 2.4-fold increase in pHER2 at 1 h (Fig. 1B). IL-1β-induced HER2 activation was effectively blocked by AG825. To confirm our findings in other pulmonary epithelial cell types, A549 (transformed, human alveolar epithelial cells) and human bronchial epithelial cells were exposed to IL-1β as above, with similar increases in HER2 phosphorylation (data not shown).
We hypothesize that the NRG-1-HER2 axis participates in acute lung injury, a disease characterized by increased alveolar epithelial permeability. However, whether normal (nontransformed) human alveolar cells express HER2 or HER3 is unknown. To test this, we determined the effect of IL-1β on HER2 activation in primary human type I-like cells and primary, human alveolar type II cells. Alveolar type II pneumocytes expressed HER2, HER3, and NRG-1 as determined by gene expression microarray studies (data not shown), but not HER4. To assess HER2 trans-activation, cells were incubated with IL-1β, and Western blots of total cell lysates were performed for pHER2. Similar to our findings in NuLi-1 cells, IL-1β induced HER2 phosphorylation in both type I-like and type II alveolar epithelial cells (Fig. 1C).
HER2 signaling requires heterodimerization, and HER2 can dimerize with HER3, its preferred dimerization partner, as well as EGFR (HER1). To determine which receptor participated in HER2 activation, HER3 and EGFR activation was assessed after exposure to IL-1β. IL-1β stimulated phosphorylation of HER3 with kinetics similar to that seen for HER2; however, EGFR phosphorylation was not observed (supplemental Fig. 3, A and B), suggesting that EGFR does not participate in HER2 activation or signaling after IL-1β exposure. To determine the need for a functional HER2/HER3 receptor complex for HER2 trans-activation, we inhibited HER2 signaling using 2C4, a monoclonal HER2 antibody that sterically inhibits HER2 recruitment into a receptor complex with another HER receptor. 2C4 inhibited both increased HER2 activation by IL-1β as well as conductance across the monolayer (supplemental Fig. 4, A and B).
IL-1β-induced HER2 Activation Is Dependent on NRG-1
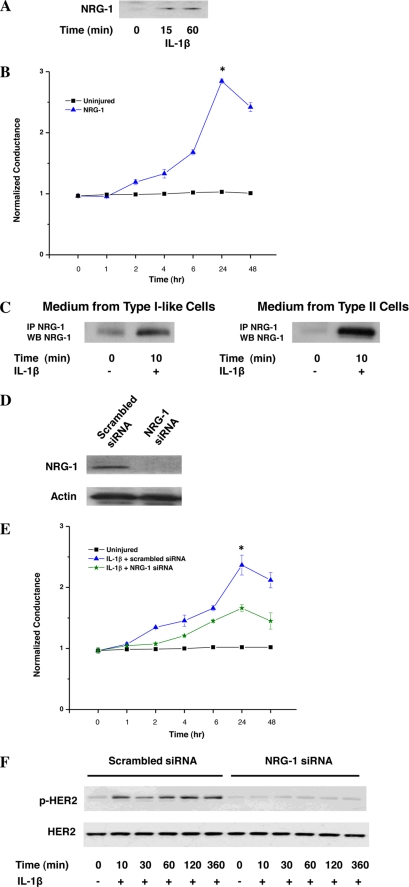
Based on our observation that IL-1β exposure resulted in HER3 phosphorylation at Tyr-1289, a site specific for NRG-1-induced HER3 activation, we hypothesized that HER2 trans-activation and increased trans-epithelium conductance following IL-1β were mediated through NRG-1, the ligand for HER3. To test our hypothesis, we first assessed the effect of exogenous NRG-1 on epithelial permeability. Treatment with NRG-1 mimicked the effect seen with IL-1β (Fig. 2A). We next sought to determine whether IL-1β stimulation resulted in increased shedding and/or release of endogenous NRG-1 from airway epithelial cells. IL-1β stimulation resulted in a rapid increase in NRG-1 in the media of NuLi-1 cells (Fig. 2B) and A549 cells (data not shown). In addition, IL-1β resulted in increased NRG-1 in cultured media from both type I-like and type II alveolar epithelial cells (Fig. 2C).
FIGURE 2.
IL-1β HER2 activation is NRG-1 dependent. A, NuLi-1 cells were exposed to NRG-1, and conductance was followed (n > 3). *, p < 0.001. Data are presented as means ± S.E. (error bars). B, NRG-1 was measured in conditioned media from IL-1β-stimulated NuLi-1 cells (n > 3). C, conditioned media from human type I-like and type II alveolar epithelial cells were analyzed for NRG-1 (n = 3). D, lysates from NuLi-1 cells transduced with NRG-1 siRNA or scrambled sequence were probed for NRG-1 (n > 3). E, conductance in NuLi-1 cells transduced with NRG-1 siRNA or scrambled siRNA was assessed following IL-1β stimulation (n > 3). *, p < 0.03. Data are presented as means ± S.E. F, HER2 activation is shown in NuLi-1 cells transduced with NRG-1 siRNA or scrambled siRNA stimulated with IL-1β (n > 3).
We next blocked NRG-1 production in NuLi-1 cells using siRNA silencing (Fig. 2D). As expected, NRG-1 silencing significantly stunted the effect of IL-1β on airway epithelium barrier function (Fig. 2E), resulting in a 52% diminution in maximal conductance. Importantly, IL-1β-induced HER2 activation was reduced in cells transfected with NRG-1 siRNA compared with scrambled siRNA at all time points (Fig. 2F). The same effect of NRG-1 silencing was observed in A549 cells (data not shown).
IL-1β-mediated Shedding of NRG-1 Is ADAM17-dependent
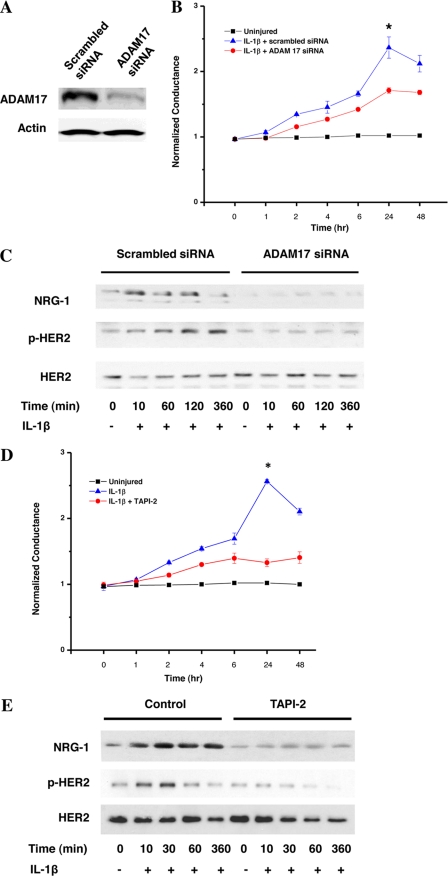
The mechanism by which NRG-1 is released from the epithelial cell monolayer is unknown; however, proteolytic shedding of members of the NRG family (and other HER ligands) has been observed in other systems (34). ADAM17 is known to be important in EGF release and EGFR activation. To determine whether ADAM17 participates in NRG-1 shedding, we screened NuLi-1 cells for all ADAMs using quantitative, real-time PCR and found that ADAM17 was the ADAM with the highest expression with a mean cycle threshold of 26.04 ± 0.50 (other ADAMs cycle threshold range was 29–33). In addition, ADAM17 was expressed in type I-like and type II cells as measured by Western blotting. Utilizing specific siRNA against ADAM17 on NuLi-1 (Fig. 3A), there was a decrease in IL-1β-induced conductance changes (Fig. 3B). ADAM17 silencing in NuLi-1 also resulted in a significant decrease in NRG-1 release and HER2 activation (Fig. 3C). Use of TAPI-2, a pharmacologic ADAM17 blocker, also decreased IL-1β-induced changes in our model of epithelial permeability as measured by electrical conductance (Fig. 3D), large molecule permeability (supplemental Fig. 2), as well as decreased pHER2 (Fig. 3E).
FIGURE 3.
IL-1β-induced NRG-1 shedding and HER2 signaling are ADAM17-dependent. A, ADAM17 protein levels in NuLi-1 cells transduced with ADAM17-specific siRNA or scrambled siRNA. B, conductance in NuLi-1 cells transduced with ADAM17 siRNA or scrambled siRNA following treatment with IL-1β. *, p < 0.03. Data are presented as means ± S.E. (error bars). C, shed NRG-1 and cellular HER2 activation in NuLi-1 cells transduced with ADAM17 siRNA or scrambled siRNA following exposure to IL-1β. D, conductance in NuLi-1 cells after exposure to IL-1β with the ADAM17 inhibitor TAPI-2 (30-min pretreatment). *, p < 0.001. Data are presented as means ± S.E. E, HER2 activation and shed NRG-1 in NuLi-1 cells exposed to IL-1β with and without TAPI-2 (all experiments, n > 3).
ADAM17 Blockade Attenuates ALI in Mice
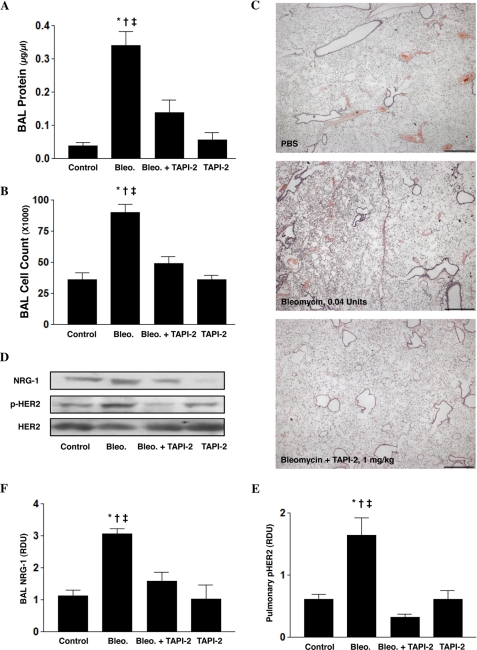
Our in vitro studies led us to hypothesize that intratracheal TAPI-2 treatment targeting the alveolar epithelium might inhibit NRG-1 release and subsequent HER2 activation, thereby reducing alveolar leak in vivo. Using a bleomycin lung injury model, intratracheal bleomycin (0.04 unit) resulted in significant lung injury as measured by BAL alveolar protein and cell count found on day 3 (Fig. 4, A and B). In contrast, IT administration of TAPI-2 (1.0 mg/kg given on days 0, 1, and 2) significantly attenuated bleomycin of lung injury. Histologic examination of the lungs showed that compared with control, bleomycin resulted in marked pulmonary inflammation that was reduced byTAPI-2 (Fig. 4C).
FIGURE 4.
Intratracheal TAPI-2 protects against bleomycin-induced ALI and is associated with decreased NRG-1 and HER2 activation. Mice (C57BL/6J, male, ages 8–12 weeks) were treated with intratracheal bleomycin (Bleo., 0.04 unit, day 0) alone or with TAPI-2 (TAPI, 1.0 mg/kg) on days 0, 1, and 2. A and B, indices of lung injury include BAL protein *, p < 0.001 control (vehicle) versus bleomycin alone; †, p = 0.003 bleomycin alone versus bleomycin + TAPI-2; ‡, p < 0.001 bleomycin alone versus TAPI-2 alone (A); BAL cell count *, p < 0.001 control (vehicle) versus bleomycin alone; †, p < 0.001 bleomycin alone versus bleomycin + TAPI-2; ‡, p < 0.001 bleomycin alone versus TAPI-2 alone (B). Data are presented as means ± S.E. (error bars). C, pulmonary histology assessed on day 3. Scale bars, 100 μm. D, BAL NRG-1 measured via Western blotting. Pulmonary HER2 activation was assessed in whole lung lysates via Western blotting using a specific pHER2 antibody. Total HER2 was measured using a HER2 antibody. E and F, NRG-1 and pHER2 densitometry. *, p = 0.024 control (vehicle) versus bleomycin (Bleo.) alone; †, p = 0.009 bleomycin alone versus bleomycin + TAPI-2; ‡, p = 0.029 bleomycin alone versus TAPI-2 alone (all experiments, n > 3).
We next assessed whether bleomycin lung injury was associated with changes in NRG-1 shedding in vivo. Bleomycin injury resulted in increased BAL NRG-1 as well as increased pulmonary pHER2 at 72 h (Fig. 4, D–F). In contrast, concurrent TAPI-2 dramatically attenuated bleomycin-mediated release of NRG-1 and HER2 phosphorylation.
NRG-1 Is Detectable in Pulmonary Edema Fluid from Patients with ALI
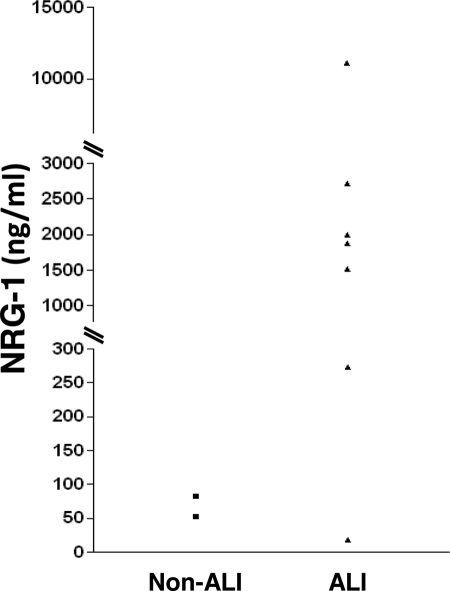
Our in vitro and in vivo data suggested that NRG-1 shedding and HER2 activation participate in epithelial barrier function and ALI. Given this, we hypothesized that NRG-1 would be detectable in pulmonary edema fluid from ALI patients. Previously, edema fluid from ALI patients has been shown to have dramatically elevated levels of IL-1β compared with controls (35). Therefore, we determined whether NRG-1 was present in edema samples from human patients with ALI. Controls (non-ALI) with cardiogenic pulmonary edema (Table 1) were also studied. Similar to our murine ALI model, NRG-1 was detectable in samples from patients with ALI (Fig. 5). In contrast, in samples from patients with cardiogenic pulmonary edema, NRG-1 was dramatically lower.
TABLE 1.
Clinical characteristics of acute pulmonary edema due to acute lung injury or cardiogenic pulmonary edema
| Patient | Type of pulmonary edema | EF/PL ratioa | Age/Sex | Ethnicity | Cause of pulmonary edema | Outcome | NRG-1 |
|---|---|---|---|---|---|---|---|
| ng/ml | |||||||
| 1 | Cardiogenic | 0.66 | 48 F | Asian | Volume overload | Alive | 83 |
| 2 | Cardiogenic | 0.56 | 50 F | Caucasian | Volume overload | Alive | 53 |
| 3 | ALI/ARDSb | 0.69 | 16 F | Unknown | TRALIc | Alive | 18 |
| 4 | ALI/ARDS | 0.82 | 70 | Caucasian | Sepsis | Alive | 1,883 |
| 5 | ALI/ARDS | 1.06 | 60 m | Caucasian | Pneumonia | Dead | 2,003 |
| 6 | ALI/ARDS | 1.27 | 56 m | Caucasian | Sepsis | Dead | 11,113 |
| 7 | ALI/ARDS | 0.77 | 38 F | American Indian | Drug overdose | Alive | 2,723 |
| 8 | ALI/ARDS | 0.70 | 20 m | Hispanic | TRALIc/tumor lysis syndrome | Dead | 273 |
| 9 | ALI/ARDS | 0.94 | 26 F | Caucasian | Severe sepsis | Dead | 1,513 |
a EF/PL ratio, edema fluid-to-plasma protein ratio.
b ARDS, acute respiratory distress syndrome.
c TRALI, transfusion-related acute lung injury.
FIGURE 5.
NRG-1 is elevated in patients with ALI. NRG-1 in pulmonary edema fluid from patients with ALI receiving mechanical ventilation (▴, n = 7) is compared with non-ALI controls (■, congestive heart failure n = 2). Data are presented as individual values.
DISCUSSION
In the current study, we describe a novel signaling pathway whereby the inflammatory cytokine IL-1β induces ADAM17-dependent NRG-1 shedding, resulting in HER2 activation and decreased barrier function in pulmonary epithelia (Fig. 6). Translating this pathway from an in vitro model to an in vivo setting, we found an association between NRG-1-HER2 signaling and ALI in mice and elevated NRG-1 in pulmonary edema fluid from patients with ALI supporting this pathway as an important biological process. Disruption of the epithelial cell barrier is a new role for NRG-1-HER2 signaling and has implications for ALI as well as other pulmonary diseases marked by increased alveolar or airway permeability and tissue edema such as asthma and cystic fibrosis (36, 37). We observed a decrease in permeability of 50% and in previous work noted decreased fibrosis and reduced mortality in murine lung injury models (21), suggesting that this is a major pathway in inflammatory ALI. This is the first report of detection of NRG-1 in edema fluid from patients with ALI. Perhaps most importantly, our studies identify three potential new lung injury therapeutic targets, ADAM17, NRG-1, and HER2, and highlight NRG-1 as a possible biomarker for ALI.
FIGURE 6.
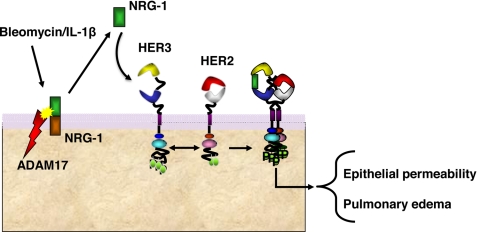
Model of ADAM17-NRG-1-HER2 signaling network in ALI. Our data support a model whereby inflammation results in ADAM17-dependent shedding of NRG-1, subsequent HER2 activation, and initiation of downstream signaling resulting in loss of the alveolar and airway epithelial barrier function and pulmonary edema.
The ligand NRG-1 serves as an essential mediator of IL-1β-mediated HER2 activation. The concept that structurally unrelated receptors can communicate with each other through trans-activation has become increasingly apparent. HER1/EGFR and HER2 can be activated by stimuli that do not interact directly with these receptors, a process that can occur through ligand- dependent or -independent means (12, 38). For example, stimulation of the angiotensin II receptor induces shedding of the EGFR ligand heparin-binding EGF, leading to activation of EGFR in hepatocytes (39). In contrast, agonists of G protein-coupled receptors (such as lysophosphatidic acid) phosphorylate EGFR directly through Src kinase-dependent phosphorylation (40). That IL-6 trans-activation of HER2 is ligand-independent (41) introduces the possibility that different inflammatory stimuli interact with the NRG-1-HER2 pathway in specific ways.
The cytokine IL-1β has been implicated as a central mediator of lung injury, both in animal models and humans (24–29, 42). IL-1β is elevated in BAL from ALI patients and has been considered responsible for the proinflammatory activity found in BAL of ALI patients (35, 42). Pharmacologic and genetic strategies to block IL-1β or its receptor attenuate both bleomycin-induced ALI (24, 25) as well as ventilator-induced lung injury (43). In addition, adenoviral overexpression of IL-1β or exogenous treatment with recombinant IL-1β results in ALI as well as pulmonary fibrosis in animal models (44). Bleomycin injury is most likely multifactorial, although certainly there are data that support that at least part of the injury induced by bleomycin is IL-1β-dependent. Hoshino et al. (26) noted increased expression of IL-1β in lung tissue from patients and mice with bleomycin lung injury. More directly, Gasse et al. (25) demonstrated that bleomycin injury was significantly attenuated in IL-1β receptor knock-out mice and MyD88 knock-out mice and that administration of IL-1β induces an injury similar to bleomycin in mice.
Our previous work identifying HER2 signaling as a required component of bleomycin-induced fibrosis (21, 22), also an IL-1β-dependent process, supports our current findings and highlights NRG-1-HER2 activation as a novel therapeutic target in IL-1β-mediated lung disease. However, our data suggest that the effect of HER2 on IL-1β-mediated epithelial barrier function is only partial. Possibly, non-HER2 effects on the barrier are also at work as well. Notably, the NRG-1-HER2 signaling pathway is present and functional in human bronchial epithelium as well as alveolar epithelial cells. To date, investigations of HER2 signaling in the lung have largely been restricted to bronchogenic carcinoma. The presented data link HER2 to diseases at the alveolar level.
ADAM17 serves a critical function in the release of NRG-1 to result in HER2 activation after IL-1β exposure, and our data suggest that it is a potential target in ALI. ADAM17 is a member of the ADAM family, a group of a multidomain, transmembrane proteins involved in proteolytic shedding of various surface molecules (45). ADAM17, also known as tumor necrosis factor-α-converting enzyme (TACE), was first described as the protease responsible for shedding the membrane-bound form of TNF-α (46). Since then, numerous molecules have been identified as ADAM17 targets, including ligands for the HER family of receptors such as TGF-α, heparin-binding EGF and amphiregulin (47–49).
Insights into ADAM17 function in vivo have been obtained through the analysis of mice lacking functional ADAM17 (TaceΔZn/ΔZn). These mice have a targeted deletion in exon 11 that encodes the catalytic active site of the TACE metalloprotease domain, resulting in a lack of enzymatic activity (50). These animals have substantial perinatal lethality with several phenotypic defects characteristic of loss of signaling through the HER family, specifically loss of EGFR signaling (51–53) including the open-eye phenotype, altered eyelid, hair and whisker development (54, 55), aberrant heart valve development (56, 57), and defects in mammary morphogenesis (58). Based on these observations, ADAM17 is hypothesized to be an essential sheddase for at least three EGFR ligands during development. Notably, we did not see EGFR activation in cell culture system, suggesting that EGFR activation is not a factor in IL-1β-mediated signaling in pulmonary epithelial cells.
Pharmacologic and molecular strategies to block ADAM17-induced shedding successfully attenuated NRG-1 release, HER2 phosphorylation, and IL-1β-mediated changes in the epithelial permeability in vitro. In addition, in vivo treatment with TAPI-2 was associated with decreased NRG-1 shedding and decreased HER2 activation as well as protection in a murine bleomycin ALI model. Notably, use of lower doses of TAPI-2 or one-time dosing showed a trend toward protection which did not reach significance. In addition, lower doses of TAPI-2 were associated with less effective blockade of NRG-1 shedding and HER2 activation (data not shown), suggesting a dose-response relationship. ADAM17 cleaves a number of membrane-bound proteins, most notably TNF-α. How much protection by TAPI-2 is due to inhibiting NRG-1 release and HER2 activation versus other ADAM17 targets remains to be determined. However, our data indicate that ADAM17 is a central and modifiable regulator of NRG-1-HER2 activation during inflammation. The mechanisms governing ADAM17 activation are complex and potentially cell type- and stimulus-specific; however, which pathways lead to ADAM17 activation is a crucial question and requires further investigation.
Our ability to detect elevated NRG-1 in samples from patients with ALI suggests that NRG-1-HER2 signaling is active in patients with lung injury. Although HER2 activation was not directly assessed in humans, given the relatively low dissociation constant of NRG-1 for HER3 (Kd ≈ 100 pm) (59), the presence of free NRG-1 in patient samples and demonstrated HER2/HER3 expression by airway and alveolar cells, receptor activation is likely. The finding of increased NRG-1 and phosphorylated HER2 in murine lung injury models is further in vivo support of pulmonary HER2 activation in patients with ALI. That NRG-1 is measurable in humans raises the possibility that NRG-1 might serve as a biomarker for ALI, reflecting injury as well as HER2 activation. This is particularly intriguing as therapies targeting HER2 currently exist and are used in patients with breast cancer.
ALI results in the death of 90,000 people in the United States each year (1). Currently, there are no available pharmacologic therapies. Our data indicating that the ADAM17-NRG-1-HER2 axis is a central regulator of IL-1β-mediated pulmonary inflammation, epithelial permeability, and lung injury may provide a novel therapeutic target for ALI.
Acknowledgment
We thank Karen Edeen for expertise in alveolar epithelial cell isolation and culture to make these studies possible.
This work was supported, in whole or in part, by National Institutes of Health Grants HL075422 (to J. A. K.), K08 HL086668 (to J. H. F.), HL029891 (to R. S. M.), HL51856 (to M. A. M.), HL51854 (to M. A. M.), HL081332 (to L. B. W.), and HL103836 (to L. B. W.). This work was also supported by Veterans Affairs merit awards (to J. A. K. and R. F. S.) and an American Heart Association established investigator award (to L. B. W.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–4.
- ALI
- acute lung injury
- ADAM17
- a disintegrin and metalloprotease 17
- BAL
- bronchoalveolar lavage
- EGFR
- epidermal growth factor receptor
- HER2
- human epidermal growth factor receptor-2
- NRG-1
- neuregulin-1.
REFERENCES
- 1. Rubenfeld G. D., Caldwell E., Peabody E., Weaver J., Martin D. P., Neff M., Stern E. J., Hudson L. D. (2005) N. Engl. J. Med. 353, 1685–1693 [DOI] [PubMed] [Google Scholar]
- 2. Hudson L. D., Milberg J. A., Anardi D., Maunder R. J. (1995) Am. J. Respir. Crit. Care Med. 151, 293–301 [DOI] [PubMed] [Google Scholar]
- 3. Roupie E., Lepage E., Wysocki M., Fagon J. Y., Chastre J., Dreyfuss D., Mentec H., Carlet J., Brun-Buisson C., Lemaire F., Brochard L. (1999) Intensive Care Med. 25, 920–929 [DOI] [PubMed] [Google Scholar]
- 4. Burden-Gulley S. M., Brady-Kalnay S. M. (1999) J. Cell Biol. 144, 1323–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ware L. B., Matthay M. A. (2000) N. Engl. J. Med. 342, 1334–1349 [DOI] [PubMed] [Google Scholar]
- 6. Wheeler A. P., Bernard G. R. (1999) N. Engl. J. Med. 340, 207–214 [DOI] [PubMed] [Google Scholar]
- 7. Modelska K., Pittet J. F., Folkesson H. G., Courtney Broaddus V., Matthay M. A. (1999) Am. J. Respir. Crit. Care Med. 160, 1450–1456 [DOI] [PubMed] [Google Scholar]
- 8. Pittet J. F., Wiener-Kronish J. P., Serikov V., Matthay M. A. (1995) Am. J. Respir. Crit. Care Med. 151, 1093–1100 [DOI] [PubMed] [Google Scholar]
- 9. Matthay M. A. (1994) Chest 105, 67S–74S [DOI] [PubMed] [Google Scholar]
- 10. Inoue S., Michel R. P., Hogg J. C. (1976) J. Ultrastruct. Res. 56, 215–225 [DOI] [PubMed] [Google Scholar]
- 11. Gorin A. B., Stewart P. A. (1979) J. Appl. Physiol. 47, 1315–1324 [DOI] [PubMed] [Google Scholar]
- 12. Yarden Y., Sliwkowski M. X. (2001) Nat. Rev. Mol. Cell Biol. 2, 127–137 [DOI] [PubMed] [Google Scholar]
- 13. Patel N. V., Acarregui M. J., Snyder J. M., Klein J. M., Sliwkowski M. X., Kern J. A. (2000) Am. J. Respir. Cell Mol. Biol. 22, 432–440 [DOI] [PubMed] [Google Scholar]
- 14. Kern J. A., Robinson R. A., Gazdar A., Torney L., Weiner D. B. (1992) Am. J. Respir. Cell Mol. Biol. 6, 359–363 [DOI] [PubMed] [Google Scholar]
- 15. Holmes W. E., Sliwkowski M. X., Akita R. W., Henzel W. J., Lee J., Park J. W., Yansura D., Abadi N., Raab H., Lewis G. D., et al. (1992) Science 256, 1205–1210 [DOI] [PubMed] [Google Scholar]
- 16. Vermeer P. D., Einwalter L. A., Moninger T. O., Rokhlina T., Kern J. A., Zabner J., Welsh M. J. (2003) Nature 422, 322–326 [DOI] [PubMed] [Google Scholar]
- 17. Gollamudi M., Nethery D., Liu J., Kern J. A. (2004) Lung Cancer 43, 135–143 [DOI] [PubMed] [Google Scholar]
- 18. Patel A. S., Schechter G. L., Wasilenko W. J., Somers K. D. (1998) Oncogene 16, 3227–3232 [DOI] [PubMed] [Google Scholar]
- 19. Slamon D. J., Godolphin W., Jones L. A., Holt J. A., Wong S. G., Keith D. E., Levin W. J., Stuart S. G., Udove J., Ullrich A., et al. (1989) Science 244, 707–712 [DOI] [PubMed] [Google Scholar]
- 20. Shimabukuro D. W., Sawa T., Gropper M. A. (2003) Crit. Care Med. 31, S524–531 [DOI] [PubMed] [Google Scholar]
- 21. Nethery D. E., Moore B. B., Minowada G., Carroll J., Faress J. A., Kern J. A. (2005) J. Appl. Physiol. 99, 298–307 [DOI] [PubMed] [Google Scholar]
- 22. Faress J. A., Nethery D. E., Kern E. F., Eisenberg R., Jacono F. J., Allen C. L., Kern J. A. (2007) J. Appl. Physiol. 103, 2077–2083 [DOI] [PubMed] [Google Scholar]
- 23. Ganter M. T., Roux J., Miyazawa B., Howard M., Frank J. A., Su G., Sheppard D., Violette S. M., Weinreb P. H., Horan G. S., Matthay M. A., Pittet J. F. (2008) Circ. Res. 102, 804–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ortiz L. A., Dutreil M., Fattman C., Pandey A. C., Torres G., Go K., Phinney D. G. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 11002–11007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gasse P., Mary C., Guenon I., Noulin N., Charron S., Schnyder-Candrian S., Schnyder B., Akira S., Quesniaux V. F., Lagente V., Ryffel B., Couillin I. (2007) J. Clin. Invest. 117, 3786–3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoshino T., Okamoto M., Sakazaki Y., Kato S., Young H. A., Aizawa H. (2009) Am. J. Respir. Cell Mol. Biol. 41, 661–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mato N., Fujii M., Hakamata Y., Kobayashi E., Sato A., Hayakawa M., Ohto-Ozaki H., Bando M., Ohno S., Tominaga S., Sugiyama Y. (2009) Eur. Respir. J. 33, 1415–1428 [DOI] [PubMed] [Google Scholar]
- 28. Park W. Y., Goodman R. B., Steinberg K. P., Ruzinski J. T., Radella F., 2nd, Park D. R., Pugin J., Skerrett S. J., Hudson L. D., Martin T. R. (2001) Am. J. Respir. Crit. Care Med. 164, 1896–1903 [DOI] [PubMed] [Google Scholar]
- 29. Siler T. M., Swierkosz J. E., Hyers T. M., Fowler A. A., Webster R. O. (1989) Exp. Lung Res. 15, 881–894 [DOI] [PubMed] [Google Scholar]
- 30. Zabner J., Karp P., Seiler M., Phillips S. L., Mitchell C. J., Saavedra M., Welsh M., Klingelhutz A. J. (2003) Am. J. Physiol. Lung Cell Mol. Physiol. 284, L844–854 [DOI] [PubMed] [Google Scholar]
- 31. Wang J., Edeen K., Manzer R., Chang Y., Wang S., Chen X., Funk C. J., Cosgrove G. P., Fang X., Mason R. J. (2007) Am. J. Respir. Cell Mol. Biol. 36, 661–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ware L. B., Matthay M. A. (2001) Am. J. Respir. Crit. Care Med. 163, 1376–1383 [DOI] [PubMed] [Google Scholar]
- 33. Bhandari V., Choo-Wing R., Lee C. G., Zhu Z., Nedrelow J. H., Chupp G. L., Zhang X., Matthay M. A., Ware L. B., Homer R. J., Lee P. J., Geick A., de Fougerolles A. R., Elias J. A. (2006) Nat. Med. 12, 1286–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Blobel C. P., Carpenter G., Freeman M. (2009) Exp. Cell Res. 315, 671–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Olman M. A., White K. E., Ware L. B., Simmons W. L., Benveniste E. N., Zhu S., Pugin J., Matthay M. A. (2004) J. Immunol. 172, 2668–2677 [DOI] [PubMed] [Google Scholar]
- 36. Goto Y., Uchida Y., Nomura A., Sakamoto T., Ishii Y., Morishima Y., Masuyama K., Sekizawa K. (2000) Am. J. Respir. Cell Mol. Biol. 23, 712–718 [DOI] [PubMed] [Google Scholar]
- 37. Sobonya R. E., Taussig L. M. (1986) Am. Rev. Respir. Dis. 134, 290–295 [DOI] [PubMed] [Google Scholar]
- 38. Carpenter G. (1999) J. Cell Biol. 146, 697–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shah B. H., Yesilkaya A., Olivares-Reyes J. A., Chen H. D., Hunyady L., Catt K. J. (2004) Mol. Endocrinol. 18, 2035–2048 [DOI] [PubMed] [Google Scholar]
- 40. Thomas S. M., Brugge J. S. (1997) Annu. Rev. Cell Dev. Biol. 13, 513–609 [DOI] [PubMed] [Google Scholar]
- 41. Qiu Y., Ravi L., Kung H. J. (1998) Nature 393, 83–85 [DOI] [PubMed] [Google Scholar]
- 42. Pugin J., Ricou B., Steinberg K. P., Suter P. M., Martin T. R. (1996) Am. J. Respir. Crit. Care Med. 153, 1850–1856 [DOI] [PubMed] [Google Scholar]
- 43. Frank J. A., Pittet J. F., Wray C., Matthay M. A. (2008) Thorax 63, 147–153 [DOI] [PubMed] [Google Scholar]
- 44. Kolb M., Margetts P. J., Anthony D. C., Pitossi F., Gauldie J. (2001) J. Clin. Invest. 107, 1529–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Seals D. F., Courtneidge S. A. (2003) Genes Dev. 17, 7–30 [DOI] [PubMed] [Google Scholar]
- 46. Black R. A., Rauch C. T., Kozlosky C. J., Peschon J. J., Slack J. L., Wolfson M. F., Castner B. J., Stocking K. L., Reddy P., Srinivasan S., Nelson N., Boiani N., Schooley K. A., Gerhart M., Davis R., Fitzner J. N., Johnson R. S., Paxton R. J., March C. J., Cerretti D. P. (1997) Nature 385, 729–733 [DOI] [PubMed] [Google Scholar]
- 47. Sahin U., Weskamp G., Kelly K., Zhou H. M., Higashiyama S., Peschon J., Hartmann D., Saftig P., Blobel C. P. (2004) J. Cell Biol. 164, 769–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Blobel C. P. (2005) Nat. Rev. Mol. Cell Biol. 6, 32–43 [DOI] [PubMed] [Google Scholar]
- 49. Ohtsu H., Dempsey P. J., Eguchi S. (2006) Am. J. Physiol. Cell Physiol. 291, C1–10 [DOI] [PubMed] [Google Scholar]
- 50. Peschon J. J., Slack J. L., Reddy P., Stocking K. L., Sunnarborg S. W., Lee D. C., Russell W. E., Castner B. J., Johnson R. S., Fitzner J. N., Boyce R. W., Nelson N., Kozlosky C. J., Wolfson M. F., Rauch C. T., Cerretti D. P., Paxton R. J., March C. J., Black R. A. (1998) Science 282, 1281–1284 [DOI] [PubMed] [Google Scholar]
- 51. Sibilia M., Wagner E. F. (1995) Science 269, 234–238 [DOI] [PubMed] [Google Scholar]
- 52. Miettinen P. J., Berger J. E., Meneses J., Phung Y., Pedersen R. A., Werb Z., Derynck R. (1995) Nature 376, 337–341 [DOI] [PubMed] [Google Scholar]
- 53. Threadgill D. W., Dlugosz A. A., Hansen L. A., Tennenbaum T., Lichti U., Yee D., LaMantia C., Mourton T., Herrup K., Harris R. C., et al. (1995) Science 269, 230–234 [DOI] [PubMed] [Google Scholar]
- 54. Luetteke N. C., Lee D. C., Palmiter R. D., Brinster R. L., Sandgren E. P. (1993) Cell Growth & Differ. 4, 203–213 [PubMed] [Google Scholar]
- 55. Mann G. B., Fowler K. J., Gabriel A., Nice E. C., Williams R. L., Dunn A. R. (1993) Cell 73, 249–261 [DOI] [PubMed] [Google Scholar]
- 56. Jackson L. F., Qiu T. H., Sunnarborg S. W., Chang A., Zhang C., Patterson C., Lee D. C. (2003) EMBO J. 22, 2704–2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Iwamoto R., Yamazaki S., Asakura M., Takashima S., Hasuwa H., Miyado K., Adachi S., Kitakaze M., Hashimoto K., Raab G., Nanba D., Higashiyama S., Hori M., Klagsbrun M., Mekada E. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 3221–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sternlicht M. D., Sunnarborg S. W., Kouros-Mehr H., Yu Y., Lee D. C., Werb Z. (2005) Development 132, 3923–3933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sliwkowski M. X., Schaefer G., Akita R. W., Lofgren J. A., Fitzpatrick V. D., Nuijens A., Fendly B. M., Cerione R. A., Vandlen R. L., Carraway K. L., 3rd (1994) J. Biol. Chem. 269, 14661–14665 [PubMed] [Google Scholar]