Abstract
The double-stranded RNA analog, poly(I:C), extracellularly activates both the endosomal Toll-like receptor (TLR) 3 and the cytoplasmic RNA helicase, melanoma differentiation-associated gene 5, leading to the production of type I interferons (IFNs) and inflammatory cytokines. The mechanism by which extracellular poly(I:C) is delivered to TLR3-positive organelles and the cytoplasm remains to be elucidated. Here, we show that the cytoplasmic lipid raft protein, Raftlin, is essential for poly(I:C) cellular uptake in human myeloid dendritic cells and epithelial cells. When Raftlin was silenced, poly(I:C) failed to enter cells and induction of IFN-β production was inhibited. In addition, cellular uptake of B-type oligodeoxynucleotide that shares its uptake receptor with poly(I:C) was suppressed in Raftlin knockdown cells. Upon poly(I:C) stimulation, Raftlin was translocated from the cytoplasm to the plasma membrane where it colocalized with poly(I:C), and thereafter moved to TLR3-positive endosomes. Thus, Raftlin cooperates with the uptake receptor to mediate cell entry of poly(I:C), which is critical for activation of TLR3.
Keywords: Dendritic Cell, Double-stranded RNA, Endocytosis, Interferon, Toll-like Receptors (TLR), Adjuvant, Poly(I:C)
Introduction
Polyriboinosinic:polyribocytidylic acid (poly(I:C)),2 a synthetic double-stranded RNA (dsRNA), has been used as a potent type I interferon (IFN-α/β) inducer in both in vitro and in vivo studies since the discovery of anti-viral activity of type I IFNs (1–3). Many types of cells including fibroblasts, epithelial cells, and myeloid dendritic cells (DCs), produce IFN-β upon stimulation with poly(I:C). Studies have demonstrated that extracellular poly(I:C) is recognized by Toll-like receptor (TLR) 3 and cytoplasmic RNA helicase, melanoma differentiation-associated gene 5 (MDA5), and induces innate immune responses including the production of type I IFNs and inflammatory cytokines (4–8). More recently, experimental evidence has accumulated that poly(I:C) acts as an adjuvant that enhances antibody production, natural killer cell activation, and cytotoxic T lymphocyte induction through the activation of TLR3 and/or MDA5 (9–15).
Human TLR3 localizes to the endosomal compartments in myeloid DCs, whereas it localizes to both the cell surface and endosomes of fibroblasts, macrophages, and epithelial cells (5, 16, 17). TLR3 signaling arises from an intracellular compartment in both cell types and requires endosomal maturation. After dsRNA recognition, endosomal TLR3 recruits an adaptor molecule, i.e. Toll-IL-1 receptor domain-containing adaptor molecule-1 (TICAM-1, also called TRIF) that activates the NF-κB, IRF-3, and AP-1 transcription factors, leading to IFN-β production (18, 19). Also, extracellular poly(I:C) is sensed by MDA5 in the cytoplasm, resulting in the activation of IRF-3 and NF-κB via the mitochondrial outer membrane protein IPS-1 (also called MAVS, Cardif, or VISA) (20–23). However, the mechanism by which poly(I:C) is delivered from the extracellular fluid to the intracellular dsRNA sensors remains unresolved.
A recent study showed that CD14 directly binds to poly(I:C) and mediates poly(I:C) cellular uptake (24). Bone marrow-derived macrophages from CD14-deficient mice exhibited impaired, but not completely diminished, responses to poly(I:C). Also, a class A scavenger receptor was identified as a cell surface receptor for poly(I:C) in human epithelial cells, although the response of poly(I:C) was only partially impaired in scavenger receptor A-deficient mice (25). These results suggest that an unidentified cell surface molecule mediates cell entry of poly(I:C). Indeed, we and others demonstrated that poly(I:C) is internalized into CD14-negative human myeloid DCs and HEK293 cells via clathrin-dependent endocytosis, and B- and C-type oligodeoxynucleotides (ODNs) share the uptake receptor with poly(I:C) (26–28).
In this study, we isolated poly(I:C)-binding proteins from CD14-negative cell lysates by sequential affinity chromatography with poly(U)- and poly(I:C)-Sepharose and subjected them to mass spectrometric analysis. Among the proteins identified, we selected several proteins that exhibited a transmembrane domain or a membrane-anchoring motif and examined whether they were involved in poly(I:C)-induced TLR3-mediated signaling. We found that Raftlin, a major lipid raft protein expressed by B cells, plays a critical role in poly(I:C) cellular uptake in human myeloid DCs and epithelial cells.
EXPERIMENTAL PROCEDURES
Cell Culture and Reagents
Human B cell lines Raji, BALL-1, and Namalwa were obtained from the Riken Cell Bank (Tukuba, Japan) and maintained in RPMI 1640 supplemented with 10% heat-inactivated FCS (BioSource Intl., Inc.) and antibiotics. HEK293 cells were obtained from Sumitomo Pharmaceuticals Co., Ltd. (Osaka, Japan) and maintained in Dulbecco's modified Eagle's medium low glucose (Invitrogen) supplemented with 10% heat-inactivated FCS and antibiotics. HeLa cells were kindly provided by Dr. T. Fujita (Kyoto University) and maintained in Eagle's minimal essential medium (Nissui, Tokyo, Japan) supplemented with 1% l-glutamine and 10% heat-inactivated FCS. Human monocyte-derived immature DCs (MoDCs) were generated from CD14+ monocytes by culturing for 6 days in the presence of 500 units/ml of granulocyte-macrophage colony-stimulating factor and 100 units/ml of IL-4 (PeproTech). Bone marrow-derived DCs (BMDCs) were prepared as described (10). Polymyxin B, 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI), saponin, and methyl-β-cyclodextrin (MβCD) were purchased from Sigma. Poly(I:C) was from Amersham Biosciences, FITC-labeled ODN2006 was from InvivoGen, Alexa Fluor 488/cholera toxin subunit B (CTXB) and Alexa Fluor 568/transferrin were from Molecular Probes. MALP-2 was obtained from Biosynthesis (Nagoya, Japan). In addition, the following antibodies were used in this study: anti-dsRNA mAb (K1) (BioLink), anti-β actin mAb (Sigma), anti-clathrin heavy chain mAb (TD.1) (Santa Cruz Biotechnology), anti-Rab5 mAb (Abcam), anti-LAMP1 (H4A3) (BioLegend), HRP-conjugated secondary Abs (BIOSOURCE), FITC-labeled goat anti-mouse IgG (American Qualex), and Alexa Fluorp®-conjugated secondary antibodies (Invitrogen). Anti-human Raftlin polyclonal antibody was prepared as described (29). Anti-human TLR3 mAb (clone TLR3.7) was generated in our laboratory (5). Texas Red-labeled poly(I:C) was prepared using the 5′ EndTagTM Nucleic Acid Labeling System (Vector Laboratories, Burlingame, CA) according to the manufacturer's instructions.
Mice
Raftlin−/− mice were provided by Dr. A. Yoshimura (Keio University). Mice were maintained under specific pathogen-free conditions in the animal facility of the Hokkaido University Graduate School of Medicine. Animal experiments were performed according to the guidelines established by the Hokkaido University Animal Care and Use Committee.
Plasmids
The cDNA fragment encoding the ORF of human TLR2 or TLR3 was amplified by RT-PCR from total RNA prepared from MoDCs, and was ligated into the cloning site of the expression vector pEF-BOS, a gift from Dr. S. Nagata (Kyoto University) (5). Complementary DNA for human Raftlin was generated by PCR from cDNA derived from Raji cells using specific primers (forward primer, 5′-CTCGAGGCCGCCACCATGGGTTG-3′; reverse primer, 5′-GGATCCTTGTTTTCTTCAACCGTACCAAGCTC-3′), and was ligated into the cloning site of the expression vector pEYFP-N1 (C-terminal yellow fluorescent protein (YFP) tag, Clontech).
Isolation of Poly(I:C)-binding Proteins
Raji cells (1 × 1010) were washed twice with Dulbecco's phosphate-buffered saline, frozen and thawed three times in Dulbecco's phosphate-buffered saline (5 × 107/ml), and centrifuged at 20,000 × g for 10 min. Cell pellets were lysed in lysis buffer (1% Nonidet P-40 in buffer A (20 mm Tris-HCl, pH 7.4, 140 mm NaCl, 25 mm iodoacetamide, 10 mm EDTA, 2 mm PMSF and protease inhibitor mixture)) for 20 min at room temperature. After centrifugation at 10,000 × g for 10 min, supernatants were filtrated with Minisalt GF (Zartorius stedim, Japan) and sequentially applied to Sepharose, poly(U)-Sepharose, and poly(I:C)-Sepharose equilibrated with binding buffer (0.2% Nonidet P-40 in buffer A). The poly(I:C)-binding molecules were eluted from poly(I:C)-Sepharose with elution buffer (1.4 m NaCl in washing buffer) after being washed with washing buffer (10 mm CHAPS in buffer A). The eluates were mixed with new poly(U)-Sepharose and rotated for 1 h at 4 °C. After centrifugation, supernatants were mixed with new poly(I:C)-Sepharose. The poly(U)- and poly(I:C)-Sepharose were washed three times with 5 volumes of washing buffer and binding molecules were eluted with elution buffer. The eluates were concentrated using YM-50 Microcon (Millipore).
Mass Spectrometry
The poly(U)- or poly(I:C)-binding molecules were separated on a 10% SDS-PAGE gel under reducing conditions, and the region of the gels containing proteins from about 250,000 to 20,000 was cut at about 1–2-mm intervals as described previously (30). After in-gel digestion with modified trypsin, the resulting peptides were analyzed by LC/MS/MS. The ion spectrum data generated by LC/MS/MS were screened against the international protein index human data base (version 3.29) with Mascot (Matrix Science, London, UK) to identify high-scoring proteins.
RNA Interference and Luciferase Reporter Assay
siRNA duplexes (LYRIC, catalog number s40866; 4F2, catalog number s12944; Raftlin, catalog numbers s23219, s23217, and s23218; negative control, catalog number AM4635) were obtained from Ambion-Applied Biosystems. siRNA for TICAM-1 was purchased from Xeragon Inc. (Birmingham, AL) (18). HEK293 cells cultured in 24-well plates were transfected with 20 pmol of each siRNA together with the expression vector for human TLR3 or TLR2 (200 ng), IFN-β promoter or ELAM reporter plasmid (60 ng), and an internal control vector (1.5 ng) using Lipofectamine 2000. Forty-eight hours after transfection, cells were washed once and then stimulated with 10 μg/ml of poly(I:C) or MALP-2 (200 nm) for 6 h. Cells were lysed and dual luciferase activities were measured according to the manufacturer's instructions (Promega). The Firefly luciferase activity was normalized to the Renilla activity and expressed as the fold-induction relative to the activity of unstimulated vector-transfected cells. In the case of HeLa cells, cells in 24-well plates were transfected with 20 pmol of each siRNA using Lipofectamine 2000. Knockdown of Raftlin in human MoDCs was preformed by electroporation as described previously (31). Briefly, MoDCs (1.4 × 106/80 μl) were transfected with control siRNA or siRNA for Raftlin (500 pmol) using a Gene-Pulser (Bio-Rad) and then cultured for 36 h in the presence of 500 milliunits/ml of granulocyte-macrophage colony-stimulating factor. The viability of the cells transfected with control and Raftlin siRNAs was 84 and 87%, respectively. Knockdown of mouse Raftlin-2 in Raftlin−/− BMDCs was performed with shRNA lentiviral particles (Santa Cruz) according to the manufacturer's instructions. Briefly, Raftlin−/− BMDCs in 24-well plates were infected with control shRNA lentiviral particles or mouse Raftlin-2 shRNA lentiviral particles at a multiplicity of infection of 2 and incubated in complete medium containing Polybrene (5 μg/ml). Twenty-four hours after infection, medium was replaced with complete medium and cells were further incubated for 24 h. The viability of the cells infected with control lentivirus and mouse Raftlin-2 shRNA-expressing lentivirus was 82 and 76%, respectively.
Quantitative PCR (qPCR)
Total RNA was extracted with the RNeasy mini kit (Qiagen, Valencia, CA) and 0.5 μg of RNA was reverse-transcribed using the high capacity cDNA Reverse Transcripition kit (Applied Biosystems) with random primers according to the manufacturer's instructions. Quantitative PCR was performed with the indicated primers (supplemental Table S1) using the Step One Real-time PCR system (Applied Biosystems).
Immunoblotting
Cells were lysed in lysis buffer (20 mm Tris-HCl, pH 7.4) containing 150 mm NaCl, 1% Nonidet P-40, 10 mm EDTA, 25 mm iodoacetamide, 2 mm PMSF and a protease inhibitor mixture (Roche Applied Science). Lysates were clarified by centrifugation and subjected to SDS-PAGE (10% gel) under reducing conditions, followed by immunoblotting with anti-Raftlin pAb or anti-β actin mAb.
Immunoprecipitation
HeLa cells were stimulated with 40 μg/ml of poly(I:C) for 30 min at 37 °C. At timed intervals, cells were lysed in lysis buffer for 30 min on ice. Lysates were pre-cleared with protein G-Sepharose (GE Healthcare) and incubated with 1 μg of anti-clathrin heavy chain mAb. Immunocomplexes were recovered by incubation with Protein G-Sepharose, washed once with lysis buffer, and resuspended in denaturing buffer. Samples were analyzed by SDS-PAGE (10% gel) under reducing conditions, followed by immunoblotting with anti-Raftlin pAb (1:1000) and HRP-conjugated secondary Ab. The membrane was re-probed with anti-clathrin heavy chain mAb (1:400).
Confocal Microscopy
HeLa cells (1 × 105 cells/well) were plated onto micro coverglasses (Matsunami Glass) in 12-well plates. The next day, cells were incubated with 40 μg/ml of poly(I:C) for 30 min at 4 °C. Cells were washed once and further incubated for 5–30 min at 37 °C. At timed intervals, cells were fixed with 4% paraformaldehyde for 30 min and permeabilized with PBS containing 0.5% saponin and 1% BSA for 30 min. Fixed cells were blocked in PBS containing 1% BSA and labeled with anti-Raftlin pAb (1:500), anti-human TLR3 mAb (20 μg/ml), or Alexa Fluor 488-CTXB (10 μg/ml) for 60 min at room temperature. Alexa Fluor 488- or Alexa Fluor 568-conjugated secondary Abs (1:400) were used to visualize the primary Abs. Nuclei were stained with DAPI (2 μg/ml) in PBS for 10 min before mounting onto glass slides using PBS containing 2.3% DABCO and 50% glycerol. Cells were visualized at a ×63 magnification with an LSM510 META microscope (Zeiss, Jena, Germany).
For uptake study, HeLa cells or HEK293 cells transfected with control siRNA or siRNA for Raftlin were incubated with 40 μg/ml of Texas Red/poly(I:C), Alexa Fluor 568/transferrin (25 μg/ml), or FITC/ODN2006 (40 μg/ml) for 30 min at 4 °C. After washing, cells were further incubated at 37 °C. At timed intervals, fixed cells were visualized as described above. In the case of HEK293 cells, cells (1 × 105 cells/well) were plated onto poly-l-lysine-coated glass (BD Bioscience) in a 24-well plate and cultured for 12 h.
Control or Raftlin knockdown MoDCs (2 × 105/100 μl) were incubated with 40 μg/ml of Texas Red/poly(I:C) for 30 min at 4 °C, washed once, and then incubated for 5–30 min at 37 °C. At timed intervals, cells were fixed with 4% paraformaldehyde for 15 min and centrifuged by Cytospin3 (Shandon). After mounting with ProLong Gold with DAPI (Molecular Probes), cells were visualized by confocal microscopy. In some experiments, MoDCs were pretreated with 1 mm MβCD for 1 h at 37 °C. Viability of cells treated with MβCD was 93.3%. For staining of endosomes, fixed cells were permeabilized with PBS containing 0.5% saponin and 1% BSA for 30 min (staining of TLR3 and early endosome), or PBS containing 100 μg/ml of digitonin and 1% BSA for 30 min (staining of late endosome). After blocking, cells were labeled with anti-Raftlin pAb (1:500), anti-human TLR3 mAb (20 μg/ml), anti-Rab5 mAb (4 μg/ml), or anti-LAMP1 mAb (H4A3) (1:200) for 60 min at room temperature.
Flow Cytometry
Cells were incubated with the indicated concentrations of poly(I:C) in culture medium for 30 min at 4 °C. After washing, cells were labeled with anti-dsRNA mAb (K1) or mouse IgG2a as a control (1 μg) in the presence of human IgG (10 μg) for 30 min at 4 °C in FACS buffer (Dulbecco's phosphate-buffered saline containing 0.5% BSA and 0.1% sodium azide) and then incubated with FITC-labeled secondary Ab. Cells were analyzed on a FACS Calibur flow cytometer (BD Biosciences).
Statistical Analysis
Statistical significance of differences between groups was determined by the Student's t test.
RESULTS
Raftlin Participates in Poly(I:C)-induced TLR3-mediated Signaling
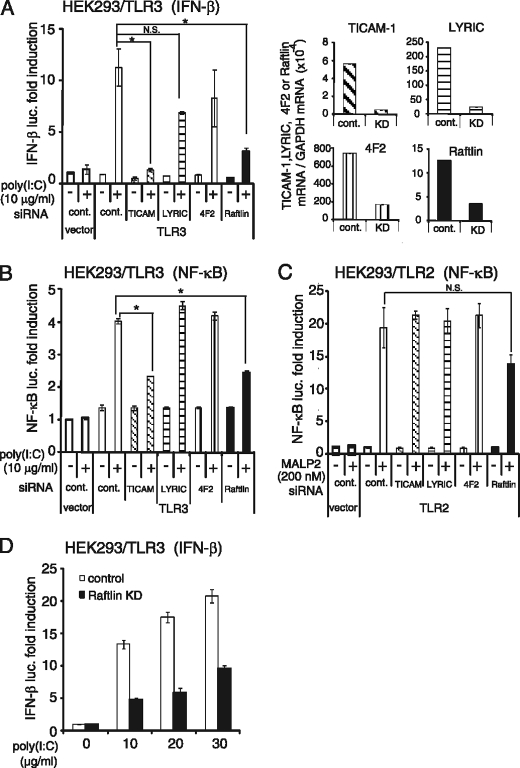
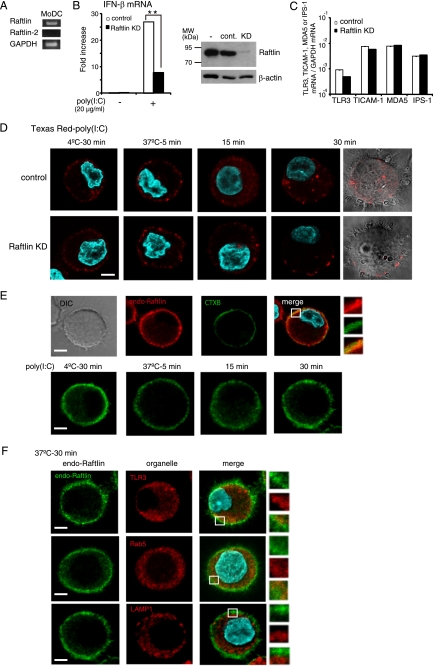
We previously demonstrated that poly(I:C) binds to human MoDCs and HEK293 cells (27). Because poly(I:C) also activates B cells (32), we screened B cell lines capable of binding poly(I:C) and found that Raji cells bound poly(I:C) at an equivalent level to MoDCs (supplemental Fig. S1). To identify the proteins involved in poly(I:C) cellular uptake, we isolated the poly(I:C)-binding proteins from Raji cell lysates by sequential affinity chromatography using Sepharose, poly(U)-Sepharose, and poly(I:C)-Sepharose. The eluate from poly(U)- or poly(I:C)-Sepharose was subjected to SDS-PAGE, followed by mass spectrometric analyses. A total of 127 proteins were identified, which preferentially bound to poly(I:C)-Sepharose rather than to poly(U)-Sepharose (supplemental Table S2). They included several proteins with a dsRNA-binding motif, such as interferon-induced dsRNA-activated protein kinase (supplemental Table 3). Also, clathrin heavy chain 1 and several cytoskeleton molecules, such as tubulin and actinin-1, were identified, suggesting that poly(I:C) uptake machinery might be isolated from the cell lysates as a complex. In the membrane/cytoskeleton group, only four are membrane-associated proteins (supplemental Table S3). We selected transmembrane proteins LYRIC (also called Astrocyte elevated gene 1) and 4F2 cell surface antigen heavy chain (4F2, also named CD98), and a cytoplasmic protein Raftlin that contains a membrane-anchoring motif at the N terminus. Because HEK293 cells express these molecules, we first examined whether they are involved in poly(I:C)-induced TLR3-mediated signaling by gene silencing. As a positive control, knockdown of TICAM-1 was performed. Interestingly, poly(I:C)-induced TLR3-mediated IFN-β promoter activation was greatly reduced when Raftlin was knocked down in HEK293 cells, whereas silencing of the LYRIC or 4F2 genes did not affect poly(I:C) function (Fig. 1A, left-hand panel). Poly(I:C)-induced TLR3-mediated NF-κB activation was also decreased in Raftlin knockdown HEK293 cells, in a similar way to TICAM-1 knockdown cells (Fig. 1B). In contrast, TLR2-mediated NF-κB activation was substantially induced in all cells subjected to gene silencing (Fig. 1C). The failure of IFN-β promoter activation in Raftlin knockdown HEK293 cells was also observed when cells were stimulated with increasing amounts of poly(I:C) (Fig. 1D). These results strongly suggest that Raftlin participates in poly(I:C)-induced TLR3 activation.
FIGURE 1.
Raftlin participates in poly(I:C)-induced TLR3-mediated signaling. HEK293 cells were transfected with the indicated siRNAs (20 pmol) together with the expression vector for human TLR3 (A, B, and D), human TLR2 (C), or empty vector and reporter plasmid. Forty-eight hours after transfection, cells were washed and stimulated with 10–30 μg/ml of poly(I:C) or 200 nm MALP-2. After 6 h, the luciferase reporter activities were measured and expressed as fold-induction relative to the activity of unstimulated vector-transfected cells. Representative data from a minimum of three separate experiments are shown (mean ± S.D.). In each experiment, knockdown (KD) efficiency was assessed 48 h after transfection by qPCR. Expression of each gene was normalized to GAPDH mRNA expression. As shown in the right-hand panels of A, expression of the indicated genes is efficiently silenced (knockdown efficiency: TICAM-1, 91.4%; LYRIC, 89.5%; 4F2, 77.4%; Raftlin, 71.8%). *, p < 0.05 (t test).
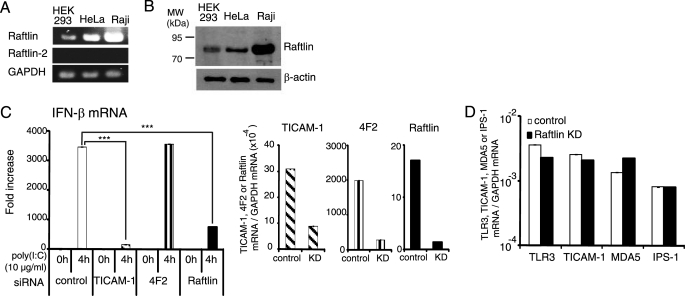
Raftlin Is Essential for Poly(I:C)-induced IFN-β Production in HeLa Cells
Raftlin was originally identified as a major lipid raft protein required for lipid raft integrity, B cell receptor signal transduction, and modulation of T cell receptor signaling (29, 33). We analyzed the expression of Raftlin and Raftlin-2, a homologue of Raftlin, in HEK293, HeLa, and Raji cells by RT-PCR. As shown in Fig. 2A, these cell lines express Raftlin but not Raftlin-2 mRNA. The protein expression level of Raftlin was further examined by immunoblotting with an anti-human Raftlin pAb (29). Raftlin was abundantly expressed in Raji cells, and expressed at lower levels in HEK293 and HeLa cells (Fig. 2B). Poly(I:C)-induced IFN-β mRNA expression was greatly diminished by knockdown of Raftlin in HeLa cells, in a similar way to TICAM-1 knockdown. In contrast, HeLa cells transfected with siRNA for 4F2 or LYRIC efficiently responded to poly(I:C) (Fig. 2C, left-hand panel, and supplemental Fig. S2). The expression of TLR3, TICAM-1, MDA5, and IPS-1 was not affected by knockdown of Raftlin (Fig. 2D). Thus, Raftlin plays a critical role in poly(I:C)-induced IFN-β production.
FIGURE 2.
Raftlin is essential for poly(I:C)-induced IFN-β production in HeLa cells. A, expression of Raftlin and Raftlin-2 mRNAs in human cell lines. B, protein expression level of Raftlin in human cell lines. Cell lysates (3 μg) were separated on 10% SDS-PAGE, followed by immunoblotting with anti-Raftlin pAb or anti-β-actin mAb. C, poly(I:C)-induced IFN-β mRNA expression in HeLa cells. HeLa cells were transfected with the indicated siRNAs (20 pmol) using Lipofectamine 2000. Forty-eight hours after transfection, cells were washed and stimulated with 10 μg/ml of poly(I:C) for 4 h (left-hand panel). Total RNA was extracted and qPCR was performed using primers for the respective genes (C and D). Expression of each gene was normalized to GAPDH mRNA expression. Data are shown as the mean ± S.D., although the values are too small to represent. Representative data from three independent experiments are shown. ***, p < 0.001.
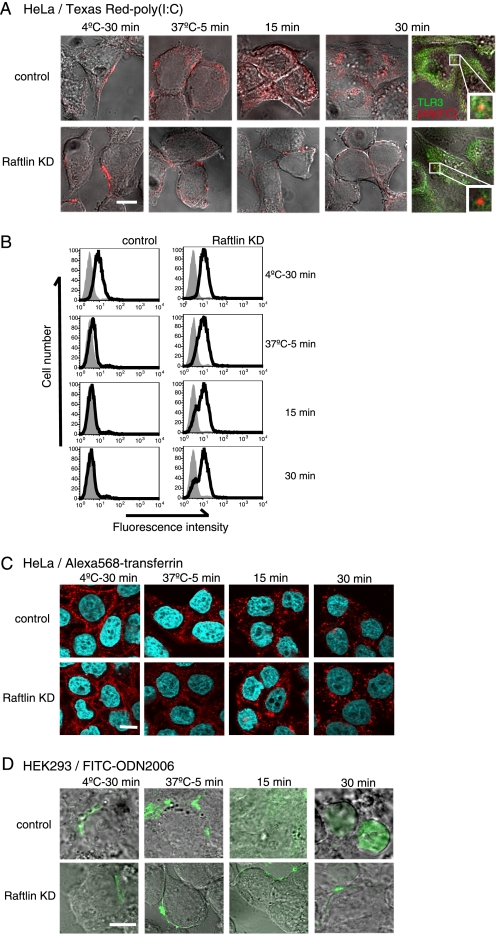
Raftlin Is Indispensable for Poly(I:C) Cellular Uptake
To examine the role of Raftlin in poly(I:C)-induced cellular responses, we analyzed cell entry of poly(I:C) in Raftlin knockdown HeLa cells. Texas Red-labeled poly(I:C), whose biological activity was similar to that of unlabeled poly(I:C) (supplemental Fig. S3), unevenly bound to the cell surface of HeLa cells either transfected with control siRNA or Raftlin siRNA after 30 min incubation at 4 °C (Fig. 3A, left panels). When the incubation condition was changed to 37 °C for 5 min, poly(I:C) was detected as speckles at the cell surface in both cells, although some of the poly(I:C) was internalized in control cells (second set of panels). However, after 15 min, poly(I:C) localized diffusely in the endosomal compartments in control cells, whereas it still resided on the cell surface as speckles in Raftlin knockdown cells (third set of panels). Thus, clustering of the uptake receptor occurs without internalization in Raftlin knockdown cells. After 30 min, poly(I:C) accumulated in the endosomal compartments in control cells, where it colocalized with TLR3 (Fig. 3A, upper right panel). In contrast, Raftlin knockdown HeLa cells did not permit cell entry of poly(I:C). Consistent with these results, flow cytometric analysis showed that surface poly(I:C) disappeared in control but not in Raftlin knockdown HeLa cells (Fig. 3B). After a 30-min incubation at 37 °C, poly(I:C) was detected on the cell surface of ∼80% of HeLa cells transfected with Raftlin-siRNA, which reflects the knockdown efficiency.
FIGURE 3.
Knockdown of Raftlin suppresses cellular uptake of poly(I:C) and B-type ODN but not transferrin. HeLa cells (A and C) and HEK293 cells (D) were transfected with control siRNA (upper panels) or siRNA for Raftlin (lower panels). Forty-eight hours after transfection, cells were washed and incubated with 40 μg/ml of Texas Red/poly(I:C) (A), 25 μg/ml Alexa Fluor 568/transferrin (C), or 40 μg/ml of FITC/ODN2006 (D) for 30 min at 4 °C. After washing, cells were incubated for up to 30 min at 37 °C. At timed intervals, cells were fixed and visualized by confocal microscopy. In some experiments, cells were fixed or permeabilized and stained with anti-TLR3 mAb. A, red, Texas Red-poly(I:C); green, TLR3. C, red, Alexa 568/transferrin; blue, nuclei with DAPI. D, green, FITC/ODN2006. Bar, 10 μm. B, flow cytometric analysis of poly(I:C) uptake. Control and Raftlin knockdown HeLa cells were incubated with 20 μg/ml of poly(I:C) for 30 min at 4 °C. After washing, cells were incubated for up to 30 min at 37 °C. At the indicated time points, cells were labeled with anti-dsRNA mAb (black lines) or mouse IgG2a (shaded histogram) and FITC-labeled secondary Ab. The cells were analyzed on a FACS Calibur.
Because poly(I:C) is internalized into cells by the clathrin-dependent endocytic pathway, we examined whether uptake of transferrin, which occurs in a clathrin-dependent manner, is suppressed by Raftlin knockdown. As shown in Fig. 3C, transferrin was internalized into HeLa cells irrespective of Raftlin knockdown. We previously reported that B- and C-type ODNs share their uptake receptor with poly(I:C) in HEK293 cells and MoDCs and are delivered to TLR3-positive endosomes in MoDCs (27). Indeed, FITC-labeled B-type ODN (ODN 2006) failed to enter cells when Raftlin was silenced in HEK293 cells (Fig. 3D). These results indicate that Raftlin is essential for uptake of poly(I:C) and B- and C-type ODNs via receptor-mediated endocytosis.
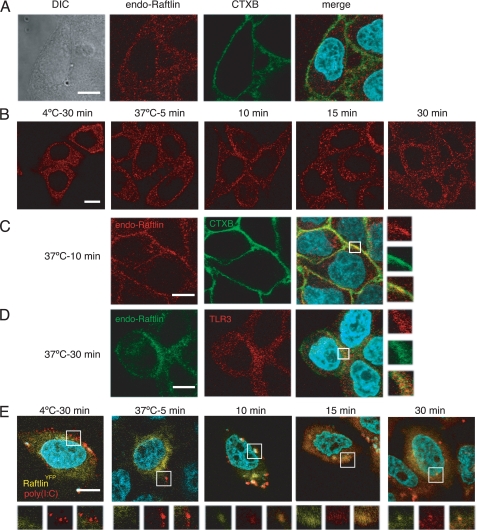
Raftlin Is Involved in the Uptake Machinery for Poly(I:C)
A previous study showed that Raftlin is localized exclusively in lipid rafts by fatty acylation of the N-terminal Gly-2 and Cys-3 residues in human B cells (29). We analyzed the subcellular localization of Raftlin in HeLa cells. Endogenous Raftlin was localized diffusely in the cytoplasm and did not merge with CTXB, which binds to the lipid raft molecule GM1, suggesting the cell type-dependent localization of Raftlin (Fig. 4A). We next examined the translocation of Raftlin in response to poly(I:C). At the poly(I:C) binding step (4 °C, 30 min), Raftlin resided in the cytoplasm (Fig. 4B, left panel). After a 5-min incubation at 37 °C, most of the Raftlin remained localized in the cytoplasm. However, after 10 min, membrane-associated Raftlin was observed, which partially colocalized with CTXB (Fig. 4, B, third panel, and C). Interestingly, Raftlin transferred to the endosomal structures from the plasma membrane within 15 min, and colocalized with TLR3 after 30 min of incubation (Fig. 4D).
FIGURE 4.
Translocation of Raftlin in response to poly(I:C). A, confocal images of endogenous Raftlin in HeLa cells. Fixed and permeabilized cells were stained with anti-Raftlin pAb and Alexa Fluor 488/CTXB. Red, endogenous Raftlin; green, CTXB; blue, nuclei with DAPI. Bar, 10 μm. B–D, spatiotemporal mobilization of endogenous Raftlin in response to poly(I:C). HeLa cells were incubated with 40 μg/ml of poly(I:C) as described in the legend to Fig. 3. At the indicated periods, cells were fixed and stained with anti-Raftlin pAb (B), anti-Raftlin pAb and Alexa Fluor 488/CTXB (C), or anti-Raftlin pAb and anti-TLR3 mAb (D). Representative data from the indicated time points are shown. B and C, red, endogenous Raftlin; green, Alexa 488/CTXB. D, green, endogenous Raftlin; red, TLR3; blue, nuclei with DAPI. Bar, 10 μm. E, association of Raftlin with poly(I:C). Confocal images of poly(I:C) uptake by HeLa cells expressing RaftlinYFP. HeLa cells were transfected with RaftlinYFP and incubated with 40 μg/ml of Texas Red/poly(I:C) as described above. At the indicated periods, cells were fixed and visualized by confocal microscopy. Lower panels show ×2 magnified images of the insets in the upper panels. Yellow, RaftlinYFP; red, Texas Red/poly(I:C); blue, nuclei with DAPI. Bar, 10 μm.
To visualize the spatiotemporal mobilization of Raftlin and poly(I:C), HeLa cells were transfected with the expression vector for RaftlinYFP and incubated with Texas Red-labeled poly(I:C). The subcellular localization and translocation of RaftlinYFP in response to poly(I:C) were almost similar to those observed with endogenous Raftlin (Fig. 4E). Notably, RaftlinYFP co-localized with Texas Red-poly(I:C) at the plasma membrane after 10-min incubation at 37 °C. Thereafter, poly(I:C) was internalized, spread to the endosomal compartments, and then accumulated in the organelles as shown in Fig. 3A. A membrane-associated RaftlinYFP appeared to move along with internalized poly(I:C) (Fig. 4E).
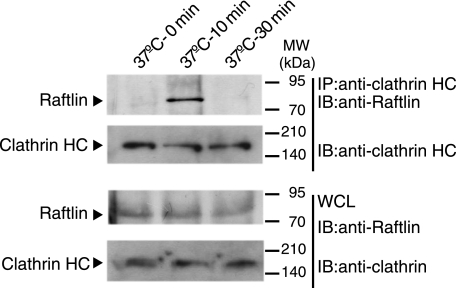
To clarify the function of Raftlin in poly(I:C) internalization mediated by clathrin, we examined physical association of Raftlin with clathrin. As shown in Fig. 5, Raftlin did not interact with clathrin in unstimulated HeLa cells. When cells were stimulated with poly(I:C), Raftlin was co-immunoprecipitated with clathrin after a 10-min stimulation. However, after 30 min, Raftlin did not interact with clathrin any more. These results suggest that after poly(I:C) binding to the uptake receptor, Raftlin was recruited to the plasma membrane and associates with the clathrin complex to modulate cargo sorting and delivery.
FIGURE 5.
Raftlin associates with clathrin in response to poly(I:C). HeLa cells were stimulated with 40 μg/ml of poly(I:C) for 0–30 min at 37 °C. At timed intervals, cells were lysed in lysis buffer and clathrin was immunoprecipitated (IP) using an anti-clathrin heavy chain (HC) mAb. The immunoprecipitants were resolved on SDS-PAGE (10% gel) under reducing conditions followed by immunoblotting (IB) with anti-Raftlin pAb or anti-clathrin HC mAb. Whole cell lysates (WCL) were subjected to immunoblotting with anti-Raftlin pAb or anti-clathrin HC mAb to detect endogenous protein expression. Molecular mass markers are indicated on the right.
Raftlin Is Critical for Poly(I:C)-induced IFN-β Production in Human Myeloid DCs
Human MoDCs expressed Raftlin but not Raftlin-2 mRNA (Fig. 6A). When DCs were electrically transfected with siRNA for Raftlin, Raftlin expression was decreased compared with cells transfected with control siRNA (Fig. 6B, right-hand panel). Poly(I:C)-induced IFN-β mRNA expression was diminished in the Raftlin knockdown DCs (Fig. 6B, left-hand panel). The mRNA expression levels of TICAM-1, MDA5, and IPS-1 in Raftlin knockdown DCs were comparable with those in control DCs, although TLR3 expression was slightly reduced compared with control cells (Fig. 6C). Again, entry of poly(I:C) into Raftlin knockdown DCs was inhibited (Fig. 6D).
FIGURE 6.
Raftlin is critical for poly(I:C)-induced IFN-β production in human myeloid DCs. A, MoDCs express Raftlin but not Raftlin-2 mRNA. B, poly(I:C)-induced IFN-β production was decreased in Raftlin knockdown DCs (left-hand panel). Control and Raftlin knockdown DCs in a 24-well plate (7 × 105/ml) were stimulated with 20 μg/ml of polymyxin B-treated poly(I:C) for 4 h. Total RNA was extracted and subjected to RT qPCR analysis for the expression of IFN-β. Data are representative of three separate experiments with similar results (mean ± S.D.). **, p < 0.01. Protein expression of Raftlin in DCs (4 × 105) before and after siRNA transfection is shown (B, right panel). C, expression of TLR3, TICAM-1, MDA5, and IPS-1 in DCs. Total RNA from control and Raftlin knockdown DCs were extracted and subjected to RT-qPCR analysis for the expression of mRNA. Expression of each gene was normalized to GAPDH mRNA expression. D, uptake of Texas Red/poly(I:C) by MoDCs transfected with control siRNA (upper panels) or siRNA for Raftlin (lower panels). Control or Raftlin knockdown DCs were incubated with 40 μg/ml of Texas Red/poly(I:C) for 30 min at 4 °C. After washing, cells were incubated for up to 30 min at 37 °C. At the indicated periods, cells were fixed and visualized by confocal microscopy. Representative images from 20 fields in the indicated time points are shown. Red, Texas Red/poly(I:C); blue, nuclei with DAPI. Bar, 5 μm. E and F, confocal images of endogenous Raftlin in MoDCs in response to poly(I:C). E, upper panels, DCs were fixed and stained with anti-Raftlin pAb and Alexa 488/CTXB. Red, endogenous Raftlin; green, Alexa 488/CTXB; blue, nuclei with DAPI. E, lower panels, and F, DCs were incubated with 40 μg/ml of poly(I:C) as described in the legend to Fig. 4. At the indicated periods, cells were fixed and stained with anti-Raftlin pAb, anti-TLR3 mAb, anti-Rab5 mAb, or anti-LAMP1 mAb and Alexa Fluor-conjugated secondary Abs. Representative data from the indicated time points are shown. Green, endogenous Raftlin; red, TLR3, Rab5, or LAMP-1; blue, nuclei with DAPI. Bar, 5 μm.
Raftlin was localized to both the plasma membrane and the cytoplasm of DCs (Fig. 6E, upper panels). Although membrane-associated Raftlin partially colocalized with CTXB, lipid raft disruption with MβCD in DCs did not affect poly(I:C) cellular uptake (supplemental Fig. S4). The mobilization of Raftlin in response to poly(I:C) was similar to that observed in HeLa cells (Fig. 6E, lower panels). After 30 min, Raftlin colocalized with TLR3 and Rab5 but not with LAMP1, indicating that Raftlin, together with the poly(I:C) uptake receptor, moves from the plasma membrane to the TLR3-positive early endosomes (Fig. 6F).
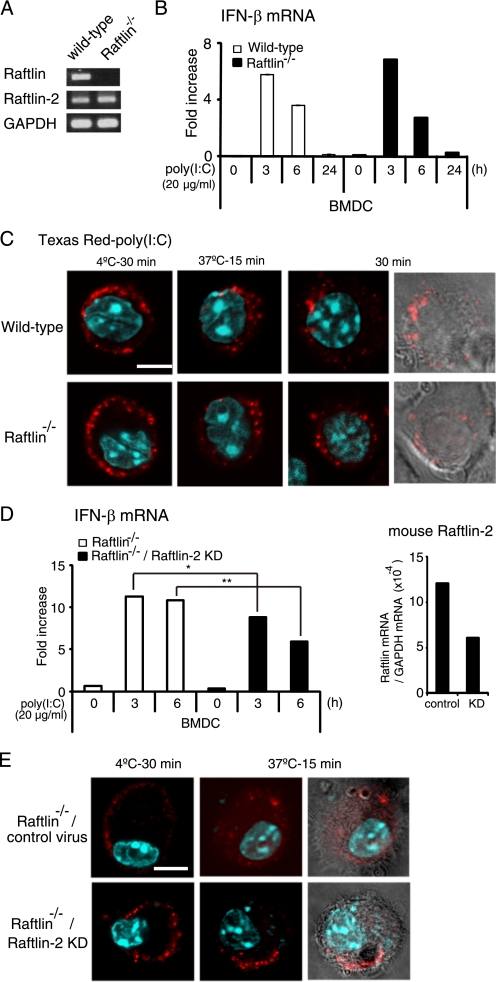
To determine the physiological function of Raftlin, we analyzed poly(I:C)-induced IFN-β production by BMDCs from wild-type or Raftlin−/− mice. Remarkably, wild-type and Raftlin−/− BMDCs expressed mouse Raftlin-2 mRNA at equivalent levels (Fig. 7A). There was no significant difference in poly(I:C)-induced IFN-β production between wild-type and Raftlin−/− BMDCs (Fig. 7B). In addition, cellular uptake of poly(I:C) in Raftlin−/− BMDCs was comparable with that in wild-type BMDCs (Fig. 7C). To test the possibility that the Raftlin function is compensated with Raftlin-2 in Raftlin−/− BMDCs as observed in B cell receptor signaling in Raftlin−/− mouse B cells (33), we knocked down of mouse Raftlin-2 in Raftlin−/− BMDCs by infection with Raftlin-2 shRNA-expressing lentiviral particles and analyzed the cellular response to poly(I:C). Mouse Raftlin-2 expression was partially decreased in Raftlin−/− BMDCs (Fig. 7D, right-hand panel). Poly(I:C)-induced IFN-β mRNA expression was partially but significantly decreased in Raftlin-2 knockdown Raftlin−/− BMDCs (Fig. 7D, left-hand panel). Furthermore, internalization of Texas Red-labeled poly(I:C) was inhibited in ∼40% of Raftlin−/− BMDCs infected with mouse Raftlin-2 shRNA lentiviral particles, reflecting the knockdown efficiency of mouse Raftlin-2 (Fig. 7E). These results suggest that mouse Raftlin-2 participates in poly(I:C) cellular uptake in Raftlin−/− BMDCs.
FIGURE 7.
Poly(I:C) uptake and IFN-β production by Raftlin−/− BMDCs. A, expression of Raftlin and Raftlin-2 mRNAs in BMDCs from wild-type or Raftlin−/− mice. B, IFN-β mRNA expression in BMDCs from wild-type or Raftlin−/− mice in response to poly(I:C). Cells were stimulated with 20 μg/ml of polymyxin B-treated poly(I:C). At the indicated time points, cells were washed and total RNA was extracted. RT qPCR was performed using the primers for mouse IFN-β. Data (mean ± S.D.) are representative of three separate experiments with similar results. C, BMDCs from wild-type (upper panels) or Raftlin−/− mice (lower panels) were incubated with 40 μg/ml of Texas Red/poly(I:C) for 30 min at 4 °C. After washing, cells were incubated for up to 30 min at 37 °C. At timed intervals, cells were fixed and visualized by confocal microscopy. Representative images from 20 fields in the indicated time points are shown. Red, Texas Red/poly(I:C); blue, nuclei with DAPI. Bar, 5 μm. D, poly(I:C)-induced IFN-β mRNA expression in control and Raftlin-2 knockdown Raftlin−/− BMDCs. Left-hand panel, control and Raftlin-2 knockdown Raftlin−/− BMDCs were stimulated with 20 μg/ml of polymyxin B-treated poly(I:C). At the indicated time points, IFN-β mRNA expression was analyzed as described above. Right-hand panel, mouse Raftlin-2 mRNA expression. Representative data from two independent experiments are shown. *, p < 0.05; **, p < 0.01. E, poly(I:C) cellular uptake by control and Raftlin-2 knockdown Raftlin−/− BMDCs. Representative images from 20 fields in the indicated time points are shown. In Raftlin−/− BMDCs infected with mouse Raftlin-2 shRNA lentiviral particles, 40% of cells fail to internalize poly(I:C) after a 15-min incubation at 37 °C. The number of cells lacking Texas Red/poly(I:C) internalization/total number of cells were for control Raftlin−/− BMDCs, 0/137, and Raftlin-2 knockdown Raftlin−/− BMDCs, 106/260. Red, Texas Red/poly(I:C); blue, nuclei with DAPI. Bar, 5 μm.
DISCUSSION
Recent studies using mouse implanted tumor models indicate that poly(I:C) is a promising adjuvant for tumor vaccines because it promotes adaptive anti-tumor responses through the activation of myeloid DCs and induction of type I IFN production by multiple type of cells (10–15). However, it remains unresolved how poly(I:C) is delivered from the extracellular fluid to the intracellular poly(I:C) sensors localized on the endosomal membrane or cytoplasm.
In this study, we demonstrated that Raftlin is essential for poly(I:C)-induced cellular responses in human myeloid DCs and epithelial cells by mediating the cellular uptake of poly(I:C). Raftlin was originally identified as a major raft protein in B cells that co-localized with B cell receptor in the lipid raft before and after B cell receptor stimulation (29). However, subcellular localization of endogenous Raftlin appears to depend on the cell types. We found that in unstimulated HeLa cells, endogenous Raftlin localized diffusely in the cytoplasm and did not co-localize with CTXB (Fig. 4), whereas in MoDCs it localized to both the plasma membrane and the cytoplasm and membrane-associated Raftlin partially merged with CTXB (Fig. 6E). In these cells, poly(I:C) stimulation induced the translocation of Raftlin from the cytoplasm to the plasma membrane where it cooperated with uptake receptor to deliver poly(I:C) to TLR3-positive endosomes (Figs. 4 and 6F). Thus, Raftlin is involved in the nucleocapture complex triggering poly(I:C)-mediated TLR3 activation, and appears to act downstream of immune receptors in a cell type-specific manner.
Notably, Raftlin−/− BMDCs that express Raftlin-2 normally took up poly(I:C) (Fig. 7C). The expression of Raftlin or Raftlin-2 depends on the cell type. Mouse Raftlin-2 is expressed in B cells but not T cells and is thought to function in a similar way to Raftlin in mouse B cells (33). Because poly(I:C)-induced IFN-β mRNA expression and internalization of poly(I:C) were decreased when mouse Raftlin-2 was knocked down in Raftlin−/− BMDCs (Fig. 7, D and E), mouse Raftlin-2 participates in poly(I:C) uptake in Raftlin−/− BMDCs. In humans, MoDCs, HEK293 cells, and HeLa cells did not express Raftlin-2 mRNA. Hence, human Raftlin plays a key role in poly(I:C)-induced cellular responses in the absence of Raftlin-2.
The molecular mechanism by which Raftlin cooperates with the uptake receptor to mediate cell entry of poly(I:C) and B- and C-type ODNs is currently unknown. As shown in Fig. 3A, clustering of the uptake receptor occurs without internalization in the absence of Raftlin. Because disruption of the lipid raft by treatment with MβCD did not affect internalization of poly(I:C) (supplemental Fig. S4), Raftlin may participate in the assembly of the uptake machinery for poly(I:C) and B- and C-type ODNs independently of lipid raft function. We have previously demonstrated that poly(I:C) is internalized via the clathrin-mediated endocytosis (27). Indeed, Raftlin associated with clathrin after poly(I:C) stimulation (Fig. 5). Thus, Raftlin might be involved in the clathrin and clathrin-associated adapter protein complexes at the plasma membrane and participates in cargo sorting and delivery to the TLR3-positive endosome (34).
Intriguingly, A-type ODN that activates plasmacytoid DCs to induce IFN-α was unable to bind to myeloid DCs (27). A-type ODN binds to high mobility group box 1 and augments the binding of high mobility group box 1 to receptor for advanced glycation end products (35). A-type ODN-high mobility group box 1 complex enhances TLR9-mediated IFN-α production by plasmacytoid DCs in a receptor for advanced glycation end product-dependent manner, although receptor for advanced glycation end products is not essential for internalization of A-type ODN or A-type ODN-high mobility group box 1 complexes. An unidentified uptake receptor for A-type ODN must reside on plasmacytoid DCs. Although whether Raftlin participates in the uptake of A-type ODN in human plasmacytoid DCs remains to be examined, we surmise that exogenous nucleic acids are recognized by cell surface receptors that form distinct nucleocapture complexes to deliver nucleic acids to intracellular organelles.
The adjuvant activity of poly(I:C) is derived from the activation of two innate immune sensors, TLR3 and MDA5, in myeloid DCs. No RNA molecule has been reported besides poly(I:C) that extracellulary activates either TLR3 or MDA5. Identification of the uptake receptor for poly(I:C) in DCs is important to elucidate the mechanism by which poly(I:C) is localized to TLR3 and MDA5, as well as to develop a poly(I:C)-related adjuvant that is selectively transferred to TLR3 and/or MDA5.
Acknowledgments
We thank Dr. Y. Miyamoto and K. Shida (Osaka Medical Center for Cancer, Osaka, Japan) for technical support and Drs. H. Oshiumi, T. Ebihara, H. Takaki, H. H. Aly, and J. Kasamatsu for invaluable discussions. We also thank Dr. J. P. Atkinson (Washington University) for reviewing this manuscript.
This work was supported in part by grants-in-aid from the Ministry of Education, Science, and Culture, the Ministry of Health, Labor, and Welfare of Japan, and the Akiyama Foundation, NorthTec Foundation, Yakult Foundation, and the Program of Founding Research Centers for Emerging and Reemerging Infectious Diseases, MEXT.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1–S3 and Figs. S1–S4.
- poly(I:C)
- polyriboinosinic:polyribocytidylic acid
- 4F2
- 4F2 cell-surface antigen heavy chain
- DCs
- dendritic cells
- BMDC
- bone marrow-derived DC
- CTXB
- cholera toxin subunit B
- MDA5
- melanoma differentiation-associated gene 5
- MβCD
- methyl-β-cyclodextrin
- MoDC
- monocyte-derived immature DC
- ODN
- oligodeoxynucleotide
- TICAM-1
- Toll-IL-1 receptor-containing adaptor molecule-1
- TLR
- Toll-like receptor.
REFERENCES
- 1. Nagano Y., Kojima Y. (1954) Compt. Pend. Soc. Biol. 148, 1700–1702 [PubMed] [Google Scholar]
- 2. Lindenmann J., Burke D. C., Isaacs A. (1957) Br. J. Exp. Pathol. 38, 551–562 [PMC free article] [PubMed] [Google Scholar]
- 3. Field A. K., Tytell A. A., Lampson G. P., Hilleman M. R. (1967) Proc. Natl. Acad. Sci. U.S.A. 58, 1004–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alexopoulou L., Holt A. C., Medzhitov R., Flavell R. A. (2001) Nature 413, 732–738 [DOI] [PubMed] [Google Scholar]
- 5. Matsumoto M., Kikkawa S., Kohase M., Miyake K., Seya T. (2002) Biochem. Biophys. Res. Commun. 293, 1364–1369 [DOI] [PubMed] [Google Scholar]
- 6. Yoneyama M., Kikuchi M., Matsumoto K., Imaizumi T., Miyagishi M., Taira K., Foy E., Loo Y. M., Gale M., Jr., Akira S., Yonehara S., Kato A., Fujita T. (2005) J. Immunol. 175, 2851–2858 [DOI] [PubMed] [Google Scholar]
- 7. Kato H., Takeuchi O., Sato S., Yoneyama M., Yamamoto M., Matsui K., Uematsu S., Jung A., Kawai T., Ishii K. J., Yamaguchi O., Otsu K., Tsujimura T., Koh C. S., Reis e Sousa C., Matsuura Y., Fujita T., Akira S. (2006) Nature 441, 101–105 [DOI] [PubMed] [Google Scholar]
- 8. Gitlin L., Barchet W., Gilfillan S., Cella M., Beutler B., Flavell R. A., Diamond M. S., Colonna M. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 8459–8464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schultz O., Diebold S. S., Chen M., Näslund T. I., Nolte M. A., Alexopoulou L., Azuma Y. T., Flavell R. A., Liljeström P., Reis e Sousa C. (2005) Nature 433, 887–892 [DOI] [PubMed] [Google Scholar]
- 10. Matsumoto M., Seya T. (2008) Adv. Drug Deliv. Rev. 60, 805–812 [DOI] [PubMed] [Google Scholar]
- 11. Kumar H., Koyama S., Ishii K. J., Kawai T., Akira S. (2008) J. Immunol. 180, 683–687 [DOI] [PubMed] [Google Scholar]
- 12. McCartney S., Vermi W., Gilfillan S., Cella M., Murphy T. L., Schreiber R. D., Murphy K. M., Colonna M. (2009) J. Exp. Med. 206, 2967–2976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miyake T., Kumagai Y., Kato H., Guo Z., Matsushita K., Satoh T., Kawagoe T., Kumar H., Jang M. H., Kawai T., Tani T., Takeuchi O., Akira S. (2009) J. Immunol. 183, 2522–2528 [DOI] [PubMed] [Google Scholar]
- 14. Longhi M. P., Trumpfheller C., Idoyaga J., Caskey M., Matos I., Kluger C., Salazar A. M., Colonna M., Steinman R. M. (2009) J. Exp. Med. 206, 1589–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ebihara T., Azuma M., Oshiumi H., Kasamatsu J., Iwabuchi K., Matsumoto K., Saito H., Taniguchi T., Matsumoto M., Seya T. (2010) J. Exp. Med. 207, 2675–2687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matsumoto M., Funami K., Tanabe M., Oshiumi H., Shingai M., Seto Y., Yamamoto A., Seya T. (2003) J. Immunol. 171, 3154–3162 [DOI] [PubMed] [Google Scholar]
- 17. Funami K., Sasai M., Ohba Y., Oshiumi H., Seya T., Matsumoto M. (2007) J. Immunol. 179, 6867–6872 [DOI] [PubMed] [Google Scholar]
- 18. Oshiumi H., Matsumoto M., Funami K., Akazawa T., Seya T. (2003) Nat. Immunol. 4, 161–167 [DOI] [PubMed] [Google Scholar]
- 19. Yamamoto M., Sato S., Hemmi H., Hoshino K., Kaisho T., Sanjo H., Takeuchi O., Sugiyama M., Okabe M., Takeda K., Akira S. (2003) Science 301, 640–643 [DOI] [PubMed] [Google Scholar]
- 20. Kawai T., Takahashi K., Sato S., Coban C., Kumar H., Kato H., Ishii K. J., Takeuchi O., Akira S. (2005) Nat. Immunol. 6, 981–988 [DOI] [PubMed] [Google Scholar]
- 21. Seth R. B., Sun L., Ea C. K., Chen Z. J. (2005) Cell 122, 669–682 [DOI] [PubMed] [Google Scholar]
- 22. Meylan E., Curran J., Hofmann K., Moradpour D., Binder M., Bartenschlager R., Tschopp J. (2005) Nature 437, 1167–1172 [DOI] [PubMed] [Google Scholar]
- 23. Xu L. G., Wang Y. Y., Han K. J., Li L. Y., Zhai Z., Shu H. B. (2005) Mol. Cell 19, 727–740 [DOI] [PubMed] [Google Scholar]
- 24. Lee H. K., Dunzendorfer S., Soldau K., Tobias P. S. (2006) Immunity 24, 153–163 [DOI] [PubMed] [Google Scholar]
- 25. Limmon G. V., Arredouani M., McCann K. L., Corn Minor R. A., Kobzik L., Imani F. (2008) FASEB J. 22, 159–167 [DOI] [PubMed] [Google Scholar]
- 26. Krieg A. M. (2002) Annu. Rev. Immunol. 20, 709–760 [DOI] [PubMed] [Google Scholar]
- 27. Itoh K., Watanabe A., Funami K., Seya T., Matsumoto M. (2008) J. Immunol. 181, 5522–5529 [DOI] [PubMed] [Google Scholar]
- 28. Ranjith-Kumar C. T., Duffy K. E., Jordan J. L., Eaton-Bassiri A., Vaughan R., Hoose S. A., Lamb R. J., Sarisky R. T., Kao C. C. (2008) Mol. Cell. Biol. 28, 4507–4519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saeki K., Miura Y., Aki D., Kurosaki T., Yoshimura A. (2003) EMBO J. 22, 3015–3026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Obuse C., Iwasaki O., Kiyomitsu T., Goshima G., Toyoda Y., Yanagida M. (2004) Nat. Cell Biol. 6, 1135–1141 [DOI] [PubMed] [Google Scholar]
- 31. Ebihara T., Shingai M., Matsumoto M., Wakita T., Seya T. (2008) Hepatology 48, 48–58 [DOI] [PubMed] [Google Scholar]
- 32. Ding C., Wang L., Al-Ghawi H., Marroquin J., Mamula M., Yan J. (2006) Eur. J. Immunol. 36, 2013–2024 [DOI] [PubMed] [Google Scholar]
- 33. Saeki K., Fukuyama S., Ayada T., Nakaya M., Aki D., Takaesu G., Hanada T., Matsumura Y., Kobayashi T., Nakagawa R., Yoshimura A. (2009) J. Immunol. 182, 5929–5937 [DOI] [PubMed] [Google Scholar]
- 34. Ohno H. (2006) J. Cell Sci. 119, 3719–3721 [DOI] [PubMed] [Google Scholar]
- 35. Tian J., Avalos A. M., Mao S. Y., Chen B., Senthil K., Wu H., Parroche P., Drabic S., Golenbock D., Sirois C., Hua J., An L. L., Audoly L., La Rosa G., Bierhaus A., Naworth P., Marshak-Rothstein A., Crow M. K., Fitzgerald K. A., Latz E., Kiener P. A., Coyle A. J. (2007) Nat. Immunol. 8, 487–496 [DOI] [PubMed] [Google Scholar]