Abstract
Oxidative stress is common in many clinically important cardiac disorders, including ischemia/reperfusion, diabetes, and hypertensive heart disease. Oxidative stress leads to derangements in pump function due to changes in the expression or function of proteins that regulate intracellular Ca2+ homeostasis. There is growing evidence that the cardiodepressant actions of reactive oxygen species (ROS) also are attributable to ROS-dependent signaling events in the sarcomere. This minireview focuses on myofilament protein post-translational modifications induced by ROS or ROS-activated signaling enzymes that regulate cardiac contractility.
Keywords: Cardiac Muscle, Oxidative Stress, Protein Kinases, Protein Phosphorylation, Signal Transduction, Contractile Proteins, Myofilament Regulation, Protein Oxidation, Sarcomeres
Introduction
Cardiac myofilament activation is tightly regulated by protein-protein interactions that convert the chemical energy of ATP into the mechanical energy of muscle contraction. In the healthy heart, these protein-protein interactions are precisely tuned (largely through a series of orchestrated phosphorylations on myofilament proteins) to accommodate differences in hemodynamic load during rest and exercise. Disease-specific alterations in the post-translational modification of myofilament proteins lead to miscommunication between sarcomeric proteins and to contractile dysfunction.
Direct Reactive Oxygen Species-dependent Modifications of Sarcomeric Proteins
Many models of oxidative stress lead to heart failure syndromes that are not associated with changes in Ca2+ homeostasis and are likely attributable to reactive oxygen species (ROS)2-dependent modifications of sarcomeric proteins (1–6). ROS-dependent protein modifications typically map to reactive cysteines (cysteines flanked by basic or aromatic residues that form thiolate anions and are susceptible to redox modifications at physiological pH).
Studies in detergent-permeabilized cardiac fibers show that ROS reduce Ca2+-activated force, with no immediate effect on fibers in rigor (with inactive cross-bridges) (7), suggesting that ROS act on regions of the myofilaments exposed by Ca2+ activation and inaccessible in attached cross-bridges (and that ROS do not induce nonspecific effects that disrupt the integrity of the sarcomeric lattice). Some studies identify the myosin heavy chain (MHC) as a redox sensor in the sarcomere because redox modifications at Cys697 and Cys707 decrease myosin ATPase activity and lead to myofilament dysfunction (6, 8–10). Other redox modifications of proteins in the thin filament (actin and tropomyosin (Tm)) also lead to defects in actin-myosin cross-bridge formation and thin filament activation by Ca2+ (11). Cys374 in actin (which indirectly interacts with myosin) may be particularly important because Cys374 oxidation results in changes in maximum actomyosin ATPase activity and actin filament sliding velocity (12). Cys374 also is the likely target of a glutathionylation reaction that decreases Tm-actin binding cooperativity and maximum developed force in permeabilized trabeculae (13). Redox modification of Tm (in this case, dimerization due Cys190 oxidation) is detected in ischemic pig hearts and also may contribute to ROS-induced myofilament dysfunction because it alters Tm flexibility and Tm-thin filament interactions (14, 15).
Titin (the giant sarcomeric protein that controls passive tension and functions as a molecular scaffold to recruit signaling proteins that regulate mechanotransduction) also is ROS-sensitive. Oxidative stress leads to the formation of one or more disulfide bonds involving the titin N2-B domain (which contains six Cys residues). This redox modification decreases the extensibility of titin and increases its passive tension (16, 17). Desmin is the main intermediate filament protein that forms a network around sarcomeric Z-discs, links neighboring myofibrils, and connects myofilaments to other cellular structures (nuclei, cytoskeleton, and mitochondria) (11). Redox-modified (oxidized or nitrated) forms of desmin accumulate in insoluble aggregates that disrupt the sarcomeric lattice, have a toxic effect on the proteasome, and may contribute to contractile dysfunction (18, 19).
MHC, cardiac troponin (cTn) T, Tm, actin, cardiac myosin-binding protein C (cMyBP-C), myofibrillar bound creatine kinase, and α-actinin are Tyr-nitrated following treatment with peroxynitrite (20, 21). Actin, cTnC, cTnI, desmin, myosin light chain, and Tm also are Tyr-nitrated in the aging heart in association with a decrease in contractile function (22). With the exception of α-actinin (where Tyr nitration alters longitudinal force transmission between adjacent sarcomeres) (21), the functional consequences of individual sarcomeric protein Tyr nitration remain unclear.
Sarcomeric Protein Phosphorylation
Myofilament activation is modulated through sarcomeric protein phosphorylation. Because several sarcomeric proteins are phosphorylated by ROS-sensitive enzymes, stimuli that alter the intracellular redox state and shift the balance of cellular kinase versus phosphatase activity are predicted to alter cardiac contractility. Sarcomeric proteins cTnI, cTnT, cMyBP-C, and titin are phosphorylated by ROS-sensitive enzymes. cTnI contains functionally important phosphorylation clusters at Ser23/Ser24, Ser43/Ser45, and Thr144 (as well as additional phosphorylation sites at Thr32, Thr52, Ser76/Ser77, Thr130, and Ser150 that are less well characterized) (23–25). cTnI Ser23/Ser24 phosphorylation is traditionally attributed to the β-adrenergic receptor (β-AR)/cAMP pathway involving PKA. cTnI Ser23/Ser24 phosphorylation reduces myofilament Ca2+ sensitivity and is required for the β-AR-dependent lusitropic response. In some settings, cTnI Ser23/Ser24 phosphorylation also is attributable to PKG, various PKC isoforms, or PKC-activated enzymes such as p90RSK and PKD (26–32). Ser43/Ser45 and Thr144 are traditionally viewed as sites for phosphorylation by PKC. Although recent studies show that Thr144 is a good in vitro substrate for PKCβ and Tyr-phosphorylated PKCδ (27, 28), PKCs with cTnI Ser43/Ser45 kinase activity have not been identified. In fact, several laboratories have reported that Ser43/Ser45 is a relatively poor in vitro substrate for many PKC isoforms. Nevertheless, WT cTnI replacement with a cTnI mutant harboring phosphomimetic substitutions at Ser43, Ser45, and Thr144 leads to pronounced changes in contractile function in transgenic mice, suggesting that myofilaments may be very sensitive to PKC-dependent changes in cTnI phosphorylation (33).
cTnT phosphorylation at Thr206 (by PKC or Raf-1 but not by PKA or PKG) (34, 35) results in decreased maximum force and myofilament Ca2+ sensitivity. PKC and ASK-1 also phosphorylate cTnT at other sites (Table 1) (36, 37). Because phosphorylation (or phosphomimetic substitutions) at sites other than Thr206 does not lead to gross changes in mechanical function, some have speculated that other post-translational modifications (PTMs) on cTnT might regulate its scaffolding function (particularly because enzymes such as PKA and PKG phosphorylate cTnI only when anchored to cTnT) (38, 80).
TABLE 1.
ROS-induced modifications of cardiac sarcomeric proteins
| Protein | Modification type | Major target site | Functional effect | Ref. |
|---|---|---|---|---|
| Actin | Oxidation | Cys374 | ↓Myosin ATPase activity | 11 |
| ↓Actin filament sliding velocity | 12 | |||
| ↑F-actin depolymerization | 13 | |||
| ↓Tm-actin binding | 10 | |||
| ↓Maximum force | 13 | |||
| Tyr | ↓Contractile function | 20, 22, 96 | ||
| α-Actinin | Oxidation | Tyr | ↓Longitudinal force transmission | 21 |
| Desmin | Oxidation | Cys | ↓Proteasome degradation | 18, 19 |
| ↑Aggregate formation | 18, 19 | |||
| ↑Myofibrillar disarray | 18, 19 | |||
| Tyr | ↓Contractile function | 22 | ||
| MHC | Oxidation | Cys697/Cys707 | ↑Myosin inhibition | 8, 10 |
| ↓Maximum force | 7, 9, 10 | |||
| Tyr | ↓Contractile function | 20 | ||
| cMyBP-C | Oxidation | Cys, Tyr | ↓Contractile function | 9, 20 |
| Phosphorylation | Ser302 (PKA, PKCϵ, PKCδ, PKD) | ↓Thin-thick filament interactions, ↑force generation | 32, 39, 40 | |
| Titin | Oxidation | N2-B Cys | ↓Extensibility | 16 |
| ↑Passive tension | 16 | |||
| Phosphorylation | Ser469 (PKA, PKG) | ↓Passive tension | 41, 42 | |
| Ser11878/Ser12022 (PKCα) | ↑Passive tension | 43 | ||
| Tm | Oxidation | Cys190 | ↓Contractile function | 11, 14 |
| ↓Flexibility | 13, 15 | |||
| ↓Binding to actin | 14 | |||
| ↓Assembly of actin·Tm complexes | 15 | |||
| Tyr | ↓Contractile function | 20, 22 | ||
| cTnI | Phosphorylation | Ser23/Ser24 (PKA, PKC, PKG, PKD, p90RSK) | ↑Ca2+ dissociation from TnC | 23–32 |
| ↓Ca2+ sensitivity | ||||
| ↑Rate of relaxation | ||||
| Ser43/Ser45 (PKC) | ↓Maximal force | 33 | ||
| Thr144 (PKCβ, tyrosine-phosphorylated PKCδ, Mst1) | ↑Myofilament Ca2+ sensitivity | 28 | ||
| Thr32/Thr52/Thr130 (Mst1) | Altered conformation | 71 | ||
| Ser151 (PAK-3) | ↑Myofilament Ca2+ sensitivity | 23 | ||
| cTnT | Oxidation | Tyr | ↓Contractile function | 22 |
| Phosphorylation | Thr206 (PKC, Raf) | ↓Maximal force | 34, 36 | |
| ↓Myofilament Ca2+ sensitivity | ||||
| Thr197/Ser201 (PKC, ASK-1) | May exacerbate the effect of Thr206 | 34, 37 | ||
| Ser274/Thr287 (PKC) | May exacerbate the effect of Thr206 | 34, 36 | ||
cMyBP-C phosphorylation at Ser273, Ser282, and Ser302 is generally attributed to PKA and viewed as a mechanism that decreases thick-thin filament interactions and increases force generation (39). There is recent evidence that Ser302 (but not Ser273 or Ser282) also is phosphorylated by PKCϵ, PKCδ, and PKD and that Ser302 phosphorylation alone may be sufficient to regulate contractile function (32, 40).
The titin elastic region (consisting of serially linked immunoglobulin-like domains, the N2-B element, and a PEVK domain) is phosphorylated by PKA or PKG. PKA- or PKG-dependent phosphorylation of human titin at Ser469 in the N2-B element decreases the passive tension of titin (41, 42). PKG (but not PKA) also phosphorylates titin at other sites that do not influence its mechanical properties but could in theory control docking interactions on the titin scaffold (42). PKCα phosphorylates titin at two highly conserved sites in the PEVK region (Ser11878 and Ser12022), leading to an increase in the passive tension of titin (an effect opposite to the actions of PKA or PKG) (43).
ROS-dependent Regulation of Sarcomeric Protein Phosphorylation
Oxidative stress typically increases protein phosphorylation by inhibiting protein phosphatases and stimulating protein kinases. The invariant Cys in the active sites of protein-tyrosine phosphatases is highly susceptible to ROS-dependent inactivation (44). ROS-dependent inactivation of protein-tyrosine phosphatases is sufficient to increase protein Tyr phosphorylation. However, ROS-dependent increases in Src activity also are detected in cardiomyocytes and some other cell types (45–47). Changes in protein Tyr phosphorylation typically influence cell growth, survival, and differentiation rather than sarcomeric protein phosphorylation. However, ROS-dependent changes in protein Tyr phosphorylation indirectly influence sarcomeric protein phosphorylation by activating Ser/Thr kinases such as PKC and PKD (see below).
Oxidative stress also inactivates the Ser/Thr phosphatase calcineurin (or protein phosphatase 2B) (48). Redox regulation of calcineurin may impact transcriptional programs that regulate cardiac hypertrophy, but direct effects on the sarcomere are unlikely because myofilament protein phosphorylation is not disordered in transgenic mouse models of altered calcineurin activity (49). Rather, myofilament protein dephosphorylation is generally attributed to PP1 (protein phosphatase 1) or PP2A. Most studies have focused on PP2A, which co-immunoprecipitates with cTnT and cTnI, co-localizes to the Z-disc with ROS-sensitive enzymes (PKCϵ, PKCζ, PAK-1, and p38 MAPK), and contributes to dynamic changes in cTnI and cMyBP-C phosphorylation (50–53). The role of PP1-dependent sarcomeric protein dephosphorylation seems more tenuous because transgenic mouse models of altered PP1 activity display changes in phospholamban (but not cTnI) phosphorylation (54, 55). In fact, the assumption that ROS inactivate PP1 and PP2A is not supported by in vitro biochemical studies or cell-based studies, which show that oxidative stress increases PP1 and/or PP2A activity (56–59). This may be via an indirect mechanism because several ROS-activated kinases (PKCζ, PAK-1, and p38 MAPK) increase PP1 and/or PP2A activity (50, 52, 60). The functional consequences of ROS-dependent changes in PP1 or PP2A activity are difficult to predict because most studies have scrutinized kinase (and not phosphatase)-mediated mechanisms that regulate sarcomeric protein phosphorylation. This minireview focuses on the ROS-dependent mechanisms that regulate the various kinases that phosphorylate sarcomeric proteins.
ASK-1
ASK-1 (apoptosis signal-regulating kinase-1) is a ROS-regulated stress-activated MAPK kinase kinase that is abundant in cardiomyocytes and acts as a redox sensor to activate effector pathways that regulate apoptotic/necrotic cell death (61–65). ASK-1 contains a central kinase domain flanked by N- and C-terminal regulatory domains. In resting cells, ASK-1 activity is maintained at low basal levels as a result of inhibitory interactions between the Ser967-phosphorylated C-terminal regulatory domain and 14-3-3 proteins and between the N-terminal regulatory domain and reduced thioredoxin-1 (Trx1). Oxidation of Trx1 leads to the dissociation of ASK-1·Trx1 complexes. Oxidative stress also leads to the dissociation of the ASK-1·14-3-3 complex due to ASK-1 Ser967 dephosphorylation (presumably due to the activation of a ROS-sensitive phosphatase) and/or 14-3-3 phosphorylation by a ROS-regulated kinase (PKD, Mst (mammalian sterile 20-like kinase) family kinases, or the catalytic fragment of PKCδ) (66–68). Once released from these inhibitory constraints, ASK-1 is activated as a result of oligomerization and activation loop (Ser845) autophosphorylation.
ASK-1 is activated by H2O2 or agonists for G-protein-coupled receptors that increase ROS accumulation in vitro in cardiomyocyte cultures and by pressure overload or myocardial infarction in vivo in the intact heart (37, 68, 69). This increase in ASK-1 activity contributes to ventricular remodeling by activating pathways involving JNK or NF-κB (69). Recent evidence indicates that ASK-1 co-localizes with sarcomeric structures, where it phosphorylates cTnT at Thr197 and Ser201 (37). Overexpression of the constitutively active ASK-1ΔN deletion mutant leads to increased cTnT phosphorylation and decreased fractional shortening in cardiomyocyte cultures (37). However, the role of cTnT phosphorylation in the cardiodepressant actions of ASK-1 remains uncertain because (a) cTnT phosphorylation at Thr206 (not Thr197 or Ser201) has been implicated in the control of thin filament function, and (b) ASK-1ΔN overexpression also decreases the Ca2+ transient amplitude (34, 37).
Mst1
Mst1 is another ROS-activated Ser/Thr kinase that activates p38 MAPK/JNK and caspase-dependent mechanisms that amplify apoptosis (70, 71). Mst1 also phosphorylates cTnI and cTnT; cTnT phosphorylation is detected only when Mst1 is anchored to cTnI. Mst1-dependent cTnI phosphorylation has been mapped to Thr144 as well as novel sites (Thr32, Thr52, and Thr130) that may influence the conformation of cTnI and its binding affinity for cTnT and cTnC (71).
PKA
PKA holoenzyme is a heterotetramer composed of two catalytic (C) subunits kept in an inactive conformation by two cAMP-binding regulatory (R) subunits. PKA activation is generally attributed to the β-AR/cAMP pathway; cAMP binding to the R subunit frees the C subunit to phosphorylate target substrates. However, ROS-dependent mechanisms that regulate PKA also influence myofilament protein phosphorylation.
PKA holoenzymes are classified as type I or II based upon the identity of their R subunit (RI or RII) that targets PKA to different subcellular compartments through interaction with PKA-anchoring proteins. Both PKA RI and RII subunits are ubiquitously expressed in cardiac myocytes and were shown to interact with myofilaments (38). The presence of two distinct PKA isoforms anchored at the sarcomeres could impart a more dynamic modulation of myofilament function in response to varying cAMP levels, and the combined regulation could provide a more refined physiological response.
Oxidative modifications of the RI subunit (at a pair of reactive cysteines not found in the RII subunit) result in the formation of interprotein disulfide dimers that display increased affinity for α-MHC, translocate to the myofibrillar fraction, and phosphorylate cTnI and cMyBP-C (72). This ROS-dependent (cAMP-independent) mechanism involving type I PKA has been linked to an increase in cardiac contractility.
Oxidative modification of the C subunit at Cys199 (one of two highly conserved Cys residues in the active sites of PKA and other Ser/Thr kinases such as PKC, PKG, and AKT) has the opposite effect to decrease PKA activity (73). Mutagenesis studies suggest that Cys199 does not directly influence catalytic activity. Rather, the thiol modification at Cys199 indirectly decreases catalytic activity by rendering the C subunit susceptible to phosphatase-mediated dephosphorylation at Thr197, a stable PTM at an adjacent site in the activation loop that is required for kinase activity (74). Cell-based studies suggest that similar thiol modifications may decrease activation loop phosphorylation and inactivate related kinases (such as PKC) (74).
PKG
PKG is activated by autocrine/paracrine stimuli that increase NO and cGMP. Mammalian PKGs are homodimers of identical subunits; each PKG monomer contains an N-terminal regulatory domain (consisting of an autoinhibitory pseudosubstrate sequence, tandem cGMP-binding cassettes, and a leucine zipper dimerization domain) and a C-terminal catalytic domain. An autoinhibitory interaction between the pseudosubstrate domain and the catalytic pocket maintains PKG in an inactive/resting state; cGMP binding induces a conformational change that relieves autoinhibition and permits activation.
Three molecular forms of mammalian PKG have been identified: PKGIα and PKGIβ arise through alternative mRNA splicing and differ only at their extreme N-terminal ∼100 amino acids (the dimerization domain), whereas PKGII is the product of a different gene locus. Because PKGI is the major isoform in cardiomyocytes, it is the focus of this discussion (75, 76). The distinct N-terminal homodimerization domains of PKGIα and PKGIβ underlie isoform-specific interactions with docking proteins and cell substrates. Elements within the dimerization domain influence the kinetics of cGMP binding and PKG activation; PKGIα and PKGIβ have identical cGMP-binding cassettes, but PKGIα binds cGMP with 10-fold higher affinity than PKGIβ (77, 78). Native PKGI has traditionally been viewed as a constitutive dimer. However, a recent study challenged this assumption and showed that PKGIα dimerization is a ROS-regulated mechanism; this study concluded that the PKGIα dimers identified in previous studies are artifacts of oxidation during sample preparation (79). PKGIα dimerization results from disulfide bond formation between reactive Cys42 residues that abut in the enzyme homodimer; PKGIβ does not contain a reactive Cys at this position and is not ROS-sensitive. The PKGIα dimers that accumulate during oxidative stress display a high level of cGMP-independent catalytic activity (79). Moreover, whereas cGMP activates PKGIα by increasing its maximum velocity (Vmax and not the Km for substrate), ROS-activated (disulfide-linked) PKGIα dimers display a marked (>10-fold) increase in Km for substrate. Some have speculated that this ROS-induced increase in PKGIα affinity for substrate underlies the ROS-induced change in PKGIα subcellular compartmentation in smooth muscle cells; ROS-activated/disulfide-linked PKGIα translocates to membrane and myofilament fractions (which contain functionally important PKG substrates). Future studies that examine the subcellular compartmentation and substrates of ROS-activated PKGIα in cardiomyocytes may be quite revealing, given evidence that a PKGIα-docking interaction (via its homodimerization domain) with cTnT is required for the rapid/efficient phosphorylation of cTnI (80). PKG-targeting mechanisms may be critical for substrate phosphorylation in cardiomyocytes, where PKG expression is quite low (∼10-fold lower compared with PKA expression) (81), and the Vmax for cTnI phosphorylation by PKG is 12-fold lower than that for cTnI phosphorylation by PKA (82).
PKCs
PKCs are Ser/Thr kinases that are activated by growth factor-dependent pathways that mobilize Ca2+ and promote diacylglycerol (DAG) accumulation. PKC isoforms contain a highly conserved C-terminal catalytic domain and are subdivided into three classes based on differences in their N-terminal regulatory domains. The regulatory domains of conventional or Ca2+-sensitive PKCs (α, βI/βII, and γ) and novel PKCs (in cardiomyocytes, PKCδ and PKCϵ) contain a C1 domain (consisting of tandem Cys-rich sequences) that binds lipid cofactors such as DAG and phorbol 12-myristate 13-acetate (PMA). Atypical PKCs (ζ and i/l) contain an abbreviated C1 domain (with only one Cys-rich motif) that binds phosphatidylinositol 1,4,5-trisphosphate or ceramide but not DAG or PMA. PKC activation is generally attributed to stimuli that promote DAG accumulation and anchor the enzyme in its active conformation to membranes. However, ROS-dependent mechanisms that activate PKCs by oxidizing C1 domain Cys residues (which disrupts autoinhibitory intramolecular constraints) also have been identified (83, 84).
A ROS-dependent mechanism involving Tyr phosphorylation by Src specifically activates PKCδ (and not other PKC isoforms). We recently demonstrated that Src phosphorylates PKCδ at Tyr311 in vitro and in H2O2-treated cardiomyocytes and that Tyr311-phosphorylated PKCδ accumulates in the soluble fraction as a constitutively active lipid-independent enzyme; this form of the enzyme is poised to phosphorylate proteins in the sarcomere, not just on lipid membranes (85). We showed that 1) allosterically activated PKCδ phosphorylates cTnI at Ser23/Ser24, 2) Tyr311-phosphorylated PKCδ phosphorylates cTnI at Ser23/Ser24 and Thr144, and 3) a PKCδ mutant harboring a Y311F substitution selectively phosphorylates cTnI at Ser23/Ser24 but not Thr144 (28). Functional studies in detergent-skinned cardiomyocytes show that allosterically activated PKCδ depresses tension at submaximum but not maximum Ca2+, as predicted for cTnI Ser23/Ser24 phosphorylation. Src-phosphorylated PKCδ (which phosphorylates cTnI at both Ser23/Ser24 and Thr144) depresses maximum tension and cross-bridge kinetics; under these conditions, the effect of cTnI Thr144 phosphorylation predominates.
Stimulus-specific differences in PKCδ phosphorylation of sarcomeric proteins have been identified in H2O2- and PMA-treated cardiomyocytes (86). Here, PMA and H2O2 increase cTnI and cMyBP-C phosphorylation via a PKC-dependent mechanism, but only the H2O2-dependent increase in cTnI and cMyBP-C phosphorylation requires Src, presumably reflecting a role for Tyr-phosphorylated PKCδ. PMA and H2O2 elicit distinct cTnI and cMyBP-C phosphorylation patterns in PKCδ-overexpressing cardiomyocytes, providing further evidence that stimulus-specific differences in the PTM of PKCδ impact its enzymology and PKCδ-mediated sarcomeric protein phosphorylation. In a more general sense, these results caution against extrapolations regarding the cellular actions of ROS-activated enzymes based upon studies that examined myofilament protein regulation during signaling by G-protein-coupled receptors. Stimulus-specific differences in the subcellular compartmentalization, binding partners, and enzymology of many signaling enzymes may impact sarcomeric protein phosphorylation.
PKD
PKD consists of a family of Ser/Thr kinases that exert important cardiac actions (87). PKDs contain an N-terminal regulatory C1 domain (which targets the enzyme to DAG- or phorbol ester-enriched membranes), an autoinhibitory pleckstrin homology domain, and a C-terminal kinase domain. Agonists that promote DAG accumulation activate PKD via a novel PKC-dependent pathway that leads to PKD phosphorylation at Ser744/Ser748 in the activation loop. Ser744/Ser748-phosphorylated PKD then autophosphorylates at Ser916, and it displays a high level of activity toward heterologous substrates such as cTnI and cMyBP-C (30, 31). PKD decreases myofilament Ca2+ sensitivity by increasing cTnI Ser23/Ser24 phosphorylation (similar to the actions of PKA). PKD also accelerates cross-bridge cycle kinetics. This effect does not require cTnI Ser23/Ser24 phosphorylation (because it is preserved in cardiomyocytes that express cTnI S23A/S24A in place of WT cTnI); it has been attributed to cMyBP-C phosphorylation at Ser302, the only site in cMyBP-C that is targeted by PKD (32).
PKD is activated during oxidative stress via a mechanism involving the ROS-activated form of PKCδ and the tyrosine kinases Src and c-Abl; Src and c-Abl are not required for growth factor-dependent PKD activation (88, 89). The current model holds that c-Abl phosphorylates PKD at Tyr463 (in the pleckstrin homology domain), leading to a conformational change that permits Src-dependent PKD phosphorylation at Tyr95 (90). This generates a consensus binding motif for the C2 domain of PKCδ, which activates PKD. ROS-activated PKD phosphorylates CREB (cAMP-responsive element-binding protein) in cardiomyocytes (91) and controls ASK-1, NF-κB, and apoptotic cell death in other cell types (66, 92). A potential role for PKD in redox regulation of sarcomeric protein phosphorylation has not been considered.
Conclusions
This minireview has summarized recent studies that identify ROS-induced PTMs of sarcomeric proteins that lead to contractile dysfunction (Fig. 1). Studies to date suggest that direct oxidative modifications of sarcomeric proteins lead to a decrease in force generation, whereas sarcomeric protein phosphorylation by ROS-activated enzymes decrease myofilament Ca2+ sensitivity. However, these conclusions are based largely on experiments that rely on reductionist approaches to resolve the functional consequences of myofilament protein phosphorylation by a single ROS-activated enzyme or ROS-dependent PTMs of a single contractile protein. Extrapolations to the in vivo context must be made with caution for several reasons.
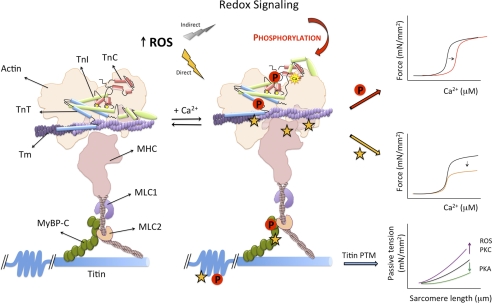
FIGURE 1.
Schematic of Ca2+-dependent cardiac myofilament activation and ROS-induced PTMs that alter this process. At low Ca2+, actin-myosin cross-bridges are inhibited (left). At high Ca2+, Ca2+ binding to the regulatory site of cTnC induces a conformational change in cTnI causing disinhibition of actin. This conformational change is transmitted to cTnT, which moves Tm and exposes a weak myosin-binding site on actin. Myosin binding to actin enhances activation by pushing Tm farther away, leading to strong cross-bridge formation. This activation mechanism is regulated in a highly specific manner by phosphorylation reactions on individual sarcomeric proteins. Myofilament activation and contractile function also are altered during oxidative stress due to direct oxidative modifications (yellow stars) of specific sites on contractile proteins or ROS-induced changes in the activity of kinases or phosphatases that regulate sarcomeric protein phosphorylation (P). MLC, myosin light chain; mN, millinewtons.
An inherent assumption of studies in reductionist models is that kinases act in a stereotypical fashion to phosphorylate a fixed set of sarcomeric proteins (or consensus phosphorylation sites within a given sarcomeric protein). The stimulus-specific differences in cTnI phosphorylation by PKCδ identified in our studies emphasize that standard in vitro approaches may not necessarily incorporate protocols that capture all regulatory phosphorylations that control in vivo enzyme activity (or substrate specificity). This approach also will not detect changes in phosphorylation due to ROS-dependent modifications of myofilament proteins that alter the accessibility of phosphorylation sites on individual substrate proteins.
ROS-dependent modifications of sarcomeric proteins do not occur in isolation. Rather, ROS-activated kinases typically phosphorylate multiple proteins in the sarcomere; many ROS-activated enzymes also sit upstream in signaling cascades that regulate effectors with Ser/Thr kinase activity. For example, PKC isoforms activate PKD, p90RSK, and Raf-1; ROS-activated pathways involving PKD, PKC, or Mst phosphorylate 14-3-3 proteins, leading to decreased inhibitory interactions with ASK-1. Progress in understanding ROS-induced changes in contractile performance in vivo must consider the ensemble actions of multiple ROS-activated enzymes on multiple sarcomeric proteins.
Oxidative stress may induce a spectrum of responses that vary according to the identity of the free radical species, the location of the ROS signal, and/or the level of oxidative stress. This issue has been addressed directly in studies of redox regulation of PKA, where ROS shift the balance of cellular kinase versus phosphatase activity in a dose-dependent manner. Low levels of oxidant stress amplify PKA responses by inactivating phosphatases that counteract PKA-dependent phosphorylations; high levels of oxidative stress inactivate PKA (presumably due to direct Cys oxidation of the C subunit) and decrease substrate phosphorylation (93).
The importance of a particular ROS-dependent protein phosphorylation or oxidative modification may be context-dependent; it may be influenced by PTMs elsewhere in that particular protein or in other proteins in the sarcomere. For example, cTnI Thr144 phosphorylation alone has little effect on force generation or Ca2+ sensitivity, but Thr144 phosphorylation prevents Ca2+ desensitization due to cTnI Ser23/Ser24 phosphorylation (i.e. Thr144 phosphorylation becomes functionally important in a Ser23/Ser24-phosphorylated background) (94). Similarly, the functional consequences of PKC-dependent cTnI Ser43/Ser45/Thr144 phosphorylation are amplified during acidosis (95). PTMs of sarcomeric proteins that are inert under normal physiological conditions and become functionally important only in a pathologic microenvironment may represent novel therapeutic targets.
This work was supported, in whole or in part, by National Institutes of Health Grants AG032009 and HL77860. This is the fourth article in the Thematic Minireview Series on Signaling in Cardiac Sarcomeres in Health and Disease. This minireview will be reprinted in the 2011 Minireview Compendium, which will be available in January, 2012.
- ROS
- reactive oxygen species
- MHC
- myosin heavy chain
- Tm
- tropomyosin
- cTn
- cardiac troponin
- cMyBP-C
- cardiac myosin-binding protein C
- β-AR
- β-adrenergic receptor
- PTM
- post-translational modification
- Trx1
- thioredoxin-1
- C
- catalytic
- R
- regulatory
- DAG
- diacylglycerol
- PMA
- phorbol 12-myristate 13-acetate.
REFERENCES
- 1. Ytrehus K., Myklebust R., Mjøs O. D. (1986) Cardiovasc. Res. 20, 597–603 [DOI] [PubMed] [Google Scholar]
- 2. Vaage J., Antonelli M., Bufi M., Irtun O., DeBlasi R. A., Corbucci G. G., Gasparetto A., Semb A. G. (1997) Free Radic. Biol. Med. 22, 85–92 [DOI] [PubMed] [Google Scholar]
- 3. Wang L., Lopaschuk G. D., Clanachan A. S. (2008) J. Mol. Cell. Cardiol. 45, 787–795 [DOI] [PubMed] [Google Scholar]
- 4. Schulz R., Dodge K. L., Lopaschuk G. D., Clanachan A. S. (1997) Am. J. Physiol. 272, H1212–H1219 [DOI] [PubMed] [Google Scholar]
- 5. Gao W. D., Liu Y., Marban E. (1996) Circulation 94, 2597–2604 [DOI] [PubMed] [Google Scholar]
- 6. Luo J., Xuan Y. T., Gu Y., Prabhu S. D. (2006) J. Mol. Cell. Cardiol. 40, 64–75 [DOI] [PubMed] [Google Scholar]
- 7. MacFarlane N. G., Miller D. J. (1992) Circ. Res. 70, 1217–1224 [DOI] [PubMed] [Google Scholar]
- 8. Passarelli C., Petrini S., Pastore A., Bonetto V., Sale P., Gaeta L. M., Tozzi G., Bertini E., Canepari M., Rossi R., Piemonte F. (2008) J. Muscle Res. Cell Motil. 29, 119–126 [DOI] [PubMed] [Google Scholar]
- 9. Rao V. S., La Bonte L. R., Xu Y., Yang Z., French B. A., Guilford W. H. (2007) Am. J. Physiol. Heart Circ. Physiol. 293, H654–H659 [DOI] [PubMed] [Google Scholar]
- 10. Tiago T., Simão S., Aureliano M., Martín-Romero F. J., Gutiérrez-Merino C. (2006) Biochemistry 45, 3794–3804 [DOI] [PubMed] [Google Scholar]
- 11. Canton M., Neverova I., Menabò R., Van Eyk J., Di Lisa F. (2004) Am. J. Physiol. Heart Circ. Physiol. 286, H870–H877 [DOI] [PubMed] [Google Scholar]
- 12. Crosbie R. H., Miller C., Cheung P., Goodnight T., Muhlrad A., Reisler E. (1994) Biophys. J. 67, 1957–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen F. C., Ogut O. (2006) Am. J. Physiol. Cell Physiol. 290, C719–C727 [DOI] [PubMed] [Google Scholar]
- 14. Canton M., Skyschally A., Menabò R., Boengler K., Gres P., Schulz R., Haude M., Erbel R., Di Lisa F., Heusch G. (2006) Eur. Heart J. 27, 875–881 [DOI] [PubMed] [Google Scholar]
- 15. Williams D. L., Jr., Swenson C. A. (1982) Eur. J. Biochem. 127, 495–499 [PubMed] [Google Scholar]
- 16. Grützner A., Garcia-Manyes S., Kötter S., Badilla C. L., Fernandez J. M., Linke W. A. (2009) Biophys. J. 97, 825–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Singal P. K., Khaper N., Palace V., Kumar D. (1998) Cardiovasc. Res. 40, 426–432 [DOI] [PubMed] [Google Scholar]
- 18. Janué A., Odena M. A., Oliveira E., Olivé M., Ferrer I. (2007) J. Neuropath. Exp. Neur. 66, 711–723 [DOI] [PubMed] [Google Scholar]
- 19. Maloyan A., Osinska H., Lammerding J., Lee R. T., Cingolani O. H., Kass D. A., Lorenz J. N., Robbins J. (2009) Circ. Res. 104, 1021–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Snook J. H., Li J., Helmke B. P., Guilford W. H. (2008) Free Radic. Biol. Med. 44, 14–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Borbély A., Tóth A., Edes I., Virág L., Papp J. G., Varró A., Paulus W. J., van der Velden J., Stienen G. J., Papp Z. (2005) Cardiovasc. Res. 67, 225–233 [DOI] [PubMed] [Google Scholar]
- 22. Kanski J., Behring A., Pelling J., Schöneich C. (2005) Am. J. Physiol. Heart Circ. Physiol. 288, H371–H381 [DOI] [PubMed] [Google Scholar]
- 23. Buscemi N., Foster D. B., Neverova I., Van Eyk J. E. (2002) Circ. Res. 91, 509–516 [DOI] [PubMed] [Google Scholar]
- 24. Zabrouskov V., Ge Y., Schwartz J., Walker J. W. (2008) Mol. Cell. Proteomics 7, 1838–1849 [DOI] [PubMed] [Google Scholar]
- 25. Noland T. A., Jr., Raynor R. L., Kuo J. F. (1989) J. Biol. Chem. 264, 20778–20785 [PubMed] [Google Scholar]
- 26. Lee D. I., Vahebi S., Tocchetti C. G., Barouch L. A., Solaro R. J., Takimoto E., Kass D. A. (2010) Basic Res. Cardiol. 105, 337–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang H., Grant J. E., Doede C. M., Sadayappan S., Robbins J., Walker J. W. (2006) J. Mol. Cell. Cardiol. 41, 823–833 [DOI] [PubMed] [Google Scholar]
- 28. Sumandea M. P., Rybin V. O., Hinken A. C., Wang C., Kobayashi T., Harleton E., Sievert G., Balke C. W., Feinmark S. J., Solaro R. J., Steinberg S. F. (2008) J. Biol. Chem. 283, 22680–22689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Itoh S., Ding B., Bains C. P., Wang N., Takeishi Y., Jalili T., King G. L., Walsh R. A., Yan C., Abe J. (2005) J. Biol. Chem. 280, 24135–24142 [DOI] [PubMed] [Google Scholar]
- 30. Haworth R. S., Cuello F., Herron T. J., Franzen G., Kentish J. C., Gautel M., Avkiran M. (2004) Circ. Res. 95, 1091–1099 [DOI] [PubMed] [Google Scholar]
- 31. Cuello F., Bardswell S. C., Haworth R. S., Yin X., Lutz S., Wieland T., Mayr M., Kentish J. C., Avkiran M. (2007) Circ. Res. 100, 864–873 [DOI] [PubMed] [Google Scholar]
- 32. Bardswell S. C., Cuello F., Rowland A. J., Sadayappan S., Robbins J., Gautel M., Walker J. W., Kentish J. C., Avkiran M. (2010) J. Biol. Chem. 285, 5674–5682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kirk J. A., MacGowan G. A., Evans C., Smith S. H., Warren C. M., Mamidi R., Chandra M., Stewart A. F., Solaro R. J., Shroff S. G. (2009) Circ. Res. 105, 1232–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sumandea M. P., Pyle W. G., Kobayashi T., de Tombe P. P., Solaro R. J. (2003) J. Biol. Chem. 278, 35135–35144 [DOI] [PubMed] [Google Scholar]
- 35. Pfleiderer P., Sumandea M. P., Rybin V. O., Wang C., Steinberg S. F. (2009) J. Muscle Res. Cell Motil. 30, 67–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sumandea M. P., Vahebi S., Sumandea C. A., Garcia-Cazarin M. L., Staidle J., Homsher E. (2009) Biochemistry 48, 7722–7731 [DOI] [PubMed] [Google Scholar]
- 37. He X., Liu Y., Sharma V., Dirksen R. T., Waugh R., Sheu S. S., Min W. (2003) Am. J. Pathol. 163, 243–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sumandea C. A., Garcia-Cazarin M. L., Bozio C. H., Sievert G. A., Balke C. W., Sumandea M. P. (2011) J. Biol. Chem. 286, 530–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barefield D., Sadayappan S. (2010) J. Mol. Cell. Cardiol. 48, 866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xiao L., Zhao Q., Du Y., Yuan C., Solaro R. J., Buttrick P. M. (2007) Biochemistry 46, 7054–7061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yamasaki R., Wu Y., McNabb M., Greaser M., Labeit S., Granzier H. (2002) Circ. Res. 90, 1181–1188 [DOI] [PubMed] [Google Scholar]
- 42. Krüger M., Kötter S., Grützner A., Lang P., Andresen C., Redfield M. M., Butt E., dos Remedios C. G., Linke W. A. (2009) Circ. Res. 104, 87–94 [DOI] [PubMed] [Google Scholar]
- 43. Hidalgo C., Hudson B., Bogomolovas J., Zhu Y., Anderson B., Greaser M., Labeit S., Granzier H. (2009) Circ. Res. 105, 631–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Meng T. C., Fukada T., Tonks N. K. (2002) Mol. Cell 9, 387–399 [DOI] [PubMed] [Google Scholar]
- 45. Aikawa R., Komuro I., Yamazaki T., Zou Y., Kudoh S., Tanaka M., Shiojima I., Hiroi Y., Yazaki Y. (1997) J. Clin. Invest. 100, 1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Giannoni E., Buricchi F., Raugei G., Ramponi G., Chiarugi P. (2005) Mol. Cell. Biol. 25, 6391–6403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kemble D. J., Sun G. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 5070–5075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Namgaladze D., Hofer H. W., Ullrich V. (2002) J. Biol. Chem. 277, 5962–5969 [DOI] [PubMed] [Google Scholar]
- 49. Wilkins B. J., Molkentin J. D. (2002) J. Physiol. 541, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ke Y., Wang L., Pyle W. G., de Tombe P. P., Solaro R. J. (2004) Circ. Res. 94, 194–200 [DOI] [PubMed] [Google Scholar]
- 51. Robia S. L., Kang M., Walker J. W. (2005) Am. J. Physiol. Heart Circ. Physiol. 289, H1941–HH1950 [DOI] [PubMed] [Google Scholar]
- 52. Wu S. C., Solaro R. J. (2007) J. Biol. Chem. 282, 30691–30698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vahebi S., Ota A., Li M., Warren C. M., de Tombe P. P., Wang Y., Solaro R. J. (2007) Circ. Res. 100, 408–415 [DOI] [PubMed] [Google Scholar]
- 54. Yang F., Aiello D. L., Pyle W. G. (2008) Biochem. Cell Biol. 86, 70–78 [DOI] [PubMed] [Google Scholar]
- 55. Nicolaou P., Hajjar R. J., Kranias E. G. (2009) J. Mol. Cell. Cardiol. 47, 365–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sommer D., Coleman S., Swanson S. A., Stemmer P. M. (2002) Arch. Biochem. Biophys. 404, 271–278 [DOI] [PubMed] [Google Scholar]
- 57. Cicchillitti L., Fasanaro P., Biglioli P., Capogrossi M. C., Martelli F. (2003) J. Biol. Chem. 278, 19509–19517 [DOI] [PubMed] [Google Scholar]
- 58. Pham F. H., Sugden P. H., Clerk A. (2000) Circ. Res. 86, 1252–1258 [DOI] [PubMed] [Google Scholar]
- 59. Deshmukh P. A., Blunt B. C., Hofmann P. A. (2007) Am. J. Physiol. Heart Circ. Physiol. 292, H792–H799 [DOI] [PubMed] [Google Scholar]
- 60. Liu Q., Hofmann P. A. (2003) Am. J. Physiol. Heart Circ. Physiol. 285, H97–H103 [DOI] [PubMed] [Google Scholar]
- 61. Tobiume K., Inage T., Takeda K., Enomoto S., Miyazono K., Ichijo H. (1997) Biochem. Biophys. Res. Commun. 239, 905–910 [DOI] [PubMed] [Google Scholar]
- 62. Tobiume K., Matsuzawa A., Takahashi T., Nishitoh H., Morita K., Takeda K., Minowa O., Miyazono K., Noda T., Ichijo H. (2001) EMBO Rep. 2, 222–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yamaguchi O., Watanabe T., Nishida K., Kashiwase K., Higuchi Y., Takeda T., Hikoso S., Hirotani S., Asahi M., Taniike M., Nakai A., Tsujimoto I., Matsumura Y., Miyazaki J., Chien K. R., Matsuzawa A., Sadamitsu C., Ichijo H., Baccarini M., Hori M., Otsu K. (2004) J. Clin. Invest. 114, 937–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Liu Q., Wilkins B. J., Lee Y. J., Ichijo H., Molkentin J. D. (2006) Mol. Cell. Biol. 26, 3785–3797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liu Q., Sargent M. A., York A. J., Molkentin J. D. (2009) Circ. Res. 105, 1110–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang W., Zheng S., Storz P., Min W. (2005) J. Biol. Chem. 280, 19036–19044 [DOI] [PubMed] [Google Scholar]
- 67. Zhou J., Shao Z., Kerkela R., Ichijo H., Muslin A. J., Pombo C., Force T. (2009) Mol. Cell. Biol. 29, 4167–4176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hirotani S., Otsu K., Nishida K., Higuchi Y., Morita T., Nakayama H., Yamaguchi O., Mano T., Matsumura Y., Ueno H., Tada M., Hori M. (2002) Circulation 105, 509–515 [DOI] [PubMed] [Google Scholar]
- 69. Yamaguchi O., Higuchi Y., Hirotani S., Kashiwase K., Nakayama H., Hikoso S., Takeda T., Watanabe T., Asahi M., Taniike M., Matsumura Y., Tsujimoto I., Hongo K., Kusakari Y., Kurihara S., Nishida K., Ichijo H., Hori M., Otsu K. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 15883–15888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yamamoto S., Yang G., Zablocki D., Liu J., Hong C., Kim S. J., Soler S., Odashima M., Thaisz J., Yehia G., Molina C. A., Yatani A., Vatner D. E., Vatner S. F., Sadoshima J. (2003) J. Clin. Invest. 111, 1463–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. You B., Yan G., Zhang Z., Yan L., Li J., Ge Q., Jin J. P., Sun J. (2009) Biochem. J. 418, 93–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Brennan J. P., Bardswell S. C., Burgoyne J. R., Fuller W., Schröder E., Wait R., Begum S., Kentish J. C., Eaton P. (2006) J. Biol. Chem. 281, 21827–21836 [DOI] [PubMed] [Google Scholar]
- 73. Humphries K. M., Juliano C., Taylor S. S. (2002) J. Biol. Chem. 277, 43505–43511 [DOI] [PubMed] [Google Scholar]
- 74. Humphries K. M., Deal M. S., Taylor S. S. (2005) J. Biol. Chem. 280, 2750–2758 [DOI] [PubMed] [Google Scholar]
- 75. Feil R., Lohmann S. M., de Jonge H., Walter U., Hofmann F. (2003) Circ. Res. 93, 907–916 [DOI] [PubMed] [Google Scholar]
- 76. Lukowski R., Rybalkin S. D., Loga F., Leiss V., Beavo J. A., Hofmann F. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 5646–5651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ammendola A., Geiselhöringer A., Hofmann F., Schlossmann J. (2001) J. Biol. Chem. 276, 24153–24159 [DOI] [PubMed] [Google Scholar]
- 78. Surks H. K., Mochizuki N., Kasai Y., Georgescu S. P., Tang K. M., Ito M., Lincoln T. M., Mendelsohn M. E. (1999) Science 286, 1583–1587 [DOI] [PubMed] [Google Scholar]
- 79. Burgoyne J. R., Madhani M., Cuello F., Charles R. L., Brennan J. P., Schröder E., Browning D. D., Eaton P. (2007) Science 317, 1393–1397 [DOI] [PubMed] [Google Scholar]
- 80. Yuasa K., Michibata H., Omori K., Yanaka N. (1999) J. Biol. Chem. 274, 37429–37434 [DOI] [PubMed] [Google Scholar]
- 81. Lincoln T. M., Hall C. L., Park C. R., Corbin J. D. (1976) Proc. Natl. Acad. Sci. U.S.A. 73, 2559–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Blumenthal D. K., Stull J. T., Gill G. N. (1978) J. Biol. Chem. 253, 324–326 [PubMed] [Google Scholar]
- 83. Gopalakrishna R., Jaken S. (2000) Free Radic. Biol. Med. 28, 1349–1361 [DOI] [PubMed] [Google Scholar]
- 84. Korichneva I., Hoyos B., Chua R., Levi E., Hammerling U. (2002) J. Biol. Chem. 277, 44327–44331 [DOI] [PubMed] [Google Scholar]
- 85. Rybin V. O., Guo J., Sabri A., Elouardighi H., Schaefer E., Steinberg S. F. (2004) J. Biol. Chem. 279, 19350–19361 [DOI] [PubMed] [Google Scholar]
- 86. Avner B. S., Hinken A. C., Yuan C., Solaro R. J. (2010) Am. J. Physiol. Heart Circ. Physiol. 299, H723–H730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Avkiran M., Rowland A. J., Cuello F., Haworth R. S. (2008) Circ. Res. 102, 157–163 [DOI] [PubMed] [Google Scholar]
- 88. Waldron R. T., Rozengurt E. (2000) J. Biol. Chem. 275, 17114–17121 [DOI] [PubMed] [Google Scholar]
- 89. Waldron R. T., Rey O., Zhukova E., Rozengurt E. (2004) J. Biol. Chem. 279, 27482–27493 [DOI] [PubMed] [Google Scholar]
- 90. Döppler H., Storz P. (2007) J. Biol. Chem. 282, 31873–31881 [DOI] [PubMed] [Google Scholar]
- 91. Ozgen N., Guo J., Gertsberg Z., Danilo P., Jr., Rosen M. R., Steinberg S. F. (2009) Mol. Pharmacol. 76, 896–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Storz P., Toker A. (2003) EMBO J. 22, 109–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Humphries K. M., Pennypacker J. K., Taylor S. S. (2007) J. Biol. Chem. 282, 22072–22079 [DOI] [PubMed] [Google Scholar]
- 94. Lu Q. W., Hinken A. C., Patrick S. E., Solaro R. J., Kobayashi T. (2010) J. Biol. Chem. 285, 11810–11817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Engel P. L., Hinken A., Solaro R. J. (2009) J. Mol. Cell. Cardiol. 47, 359–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mihm M. J., Yu F., Reiser P. J., Bauer J. A. (2003) Biochimie 85, 587–596 [DOI] [PubMed] [Google Scholar]