Abstract
Angiotensin II type 2 receptor (AT2R) counteracts most effects of angiotensin II type 1 receptor (AT1R). We hypothesized that direct AT2R stimulation reduces renal production of the inflammatory cytokines tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and transforming growth factor-β1 (TGF-β1) and enhances the production of nitric oxide (NO) and cyclic guanosine 3′,5′-monophosphate (cGMP) in the clipped kidney of 2-kidney, 1-clip (2K1C) hypertension rat model. We used Sprague-Dawley rats to evaluate changes in renal interstitial fluid recovery levels of TNF-α, IL-6, NO, and cGMP; renal expression of AT1R, AT2R, TGF-β1, TNF-α, and IL-6 in sham and 2K1C rats treated for 4 days with vehicle, AT2R agonist compound 21 (C21), or AT2R antagonist PD123319 (PD), alone and combined (n=6, each group). Systolic blood pressure increased significantly in 2K1C and was not influenced by any treatment. Clipped kidneys showed significant increases in renal expression of AT1R, AT2R, TNF-α, IL-6, TGF-β1 and decreases in NO and cGMP levels. These factors were not influenced by PD treatment. In contrast, C21 caused significant decrease in renal TNF-α, IL-6, TGF-β1 and an increase in NO and cGMP levels. Combined C21 and PD treatment partially reversed the observed C21 effects. Compared to sham, there were no significant changes in TNF-α, IL-6, TGF-β1, NO, or cGMP in the nonclipped kidneys of 2K1C animals. We conclude that direct AT2R stimulation reduces early renal inflammatory responses and improves production of NO and cGMP in renovascular hypertension independent of blood pressure reduction.
Keywords: angiotensin receptor, cytokines, inflammation, kidney, renin-angiotensin system, renovascular hypertension
Renal production of angiotensin (Ang) II is increased in renovascular hypertension. The majority of the patho-physiologic effects of Ang II are mediated by the Ang II subtype 1 receptor (AT1R). Activation of Ang II subtype 2 receptor (AT2R) is believed to counteract the effects of AT1R through inhibiting cellular proliferation and differentiation, enhancing vasodilation and natriuresis.1,2 In the kidney, AT2R is localized to renal vessels, glomeruli, and tubules.3,4 However, the role AT2R plays in the kidney and cardiovascular diseases is not fully elucidated.
Previous studies demonstrated increased tissue inflammation with activation of the renin-angiotensin system.5–6 Activation of AT1R stimulated the production of interleukin (IL)-6, tumor necrosis factor (TNF)-α, transforming growth factor (TGF)-β1,6–7 and induction of oxidative stress.8 Blockade of AT2R reduced renal production of nitric oxide (NO) and cyclic guanosine 3′,5′-monophosphate (cGMP).9–11 These events suggested a cross-talk between AT1R and AT2R that could influence development of organ disease.
Elucidating the beneficial effects of the AT2R activity was limited because of lack of available specific agonists for this receptor. Previous studies evaluated the role of AT2R by manipulating its expression or blockade.12–14 Recently, a novel AT2R agonist, compound 21 (C21), was developed. C21 is a nonpeptide, orally active, specific, and highly selective agonist for AT2R.15 This compound was recently tested in vivo and initial studies suggested that it could reduce tissue inflammation and fibrosis.16–18
The present study was conducted to examine the role of direct AT2R stimulation on early changes in systolic blood pressure (SBP), renal inflammation, and production of NO and cGMP in 2-kidney, 1-clip (2K1C) Goldblatt hypertension rat model.
Subjects and Methods
Animal Preparation
Experiments were approved by the University of Virginia Animal Care and Use Committee and conducted in male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) weighing 230 to 260 grams. Animals were fed a normal-sodium diet and tap water ad libitum for the whole experiment and a minimum of 1 week was allowed to adjust to our animal care facility. Rats were randomly allocated to different groups, sham (n=6) and 2K1C-treated for 4 days (n=6 each treatment) with vehicle (5% dextrose in water, intraperitoneal), C21 (0.3 mg/kg/d, intraperitoneal; Vicore), PD123319 (PD), or C21 combined with PD. Treatments were started at the same time and administered throughout the 4-day duration of the study. PD (Sigma Aldrich) was infused at a dose of 10 mg/kg per day using an osmotic minipump (model 2001; Alzet). Details of surgical procedures are available in the online Data Supplement (available online at http://hyper.ahajournals.org).
Body Weight and SBP Monitoring
Body weight and SBP were obtained before surgery and at the end of the study. SBP was assessed by tail-cuff plethysmography (model SC-1000; Hatteras Instruments).
In Vivo Renal Interstitial Fluid Collections
The in vivo renal interstitial fluid (RIF) recovery levels of TNF-α, IL-6, NO, and cGMP were determined as previously described.9,10 Please see the online Data Supplement.
RIF Storage and Assays
RIF collections were stored at −80°C until assayed. Please see the online Data Supplement.
Determination of mRNA Expression
The procedures for mRNA measurements of TNF-α, IL-6, TGF-β1, AT1R, and AT2R were performed as previously described. 19 Please see the online Data Supplement.
Western Blot Analysis
Preparations of kidney tissue lysate and protein quantitation of AT1R, AT2R, and TGF-β 1 were performed as previously described.19 Please see the online Data Supplement.
Renal Morphology
Renal tissues obtained from sham and 2K1C rats were fixed in 10% neutral buffered formalin, dehydrated, and embedded in paraffin per standard techniques. Sections were cut at a thickness of 4 μm and stained with hematoxylin and eosin. Renal tissue was examined under light microscopy.
Statistical Analysis
Comparisons among different treatment groups were assessed by ANOVA followed by a Tukey test for post hoc comparisons. Data are expressed as mean±SE. P<0.05 is considered statistically significant.
Results
Body and Kidney Weights
As shown in Supplemental Table SI (available online at http://hyper.ahajournals.org), body weights were not different between groups. Clipped kidney weight was significantly greater compared to that of contralateral kidneys of vehicle-treated 2K1C group or kidneys of the sham group (P < 0.05). C21 treatment reduced the increase in clipped kidney weight (P < 0.05). Compared to vehicle treatment, PD treatment did not influence clipped kidney weight. Combined C21 and PD treatment reversed the C21 effect on clipped kidney weight (P < 0.05). There were no significant differences between sham and nonclipped kidney weights of the 2K1C groups.
SBP
There were no significant differences in baseline SBP between all groups (Table SI). SBP was significantly elevated in vehicle-treated 2K1C rats compared to sham control (P<0.05). C21 or PD alone or combined did not influence the elevated SBP.
Renal Expressions of AT1 and AT2 Receptors
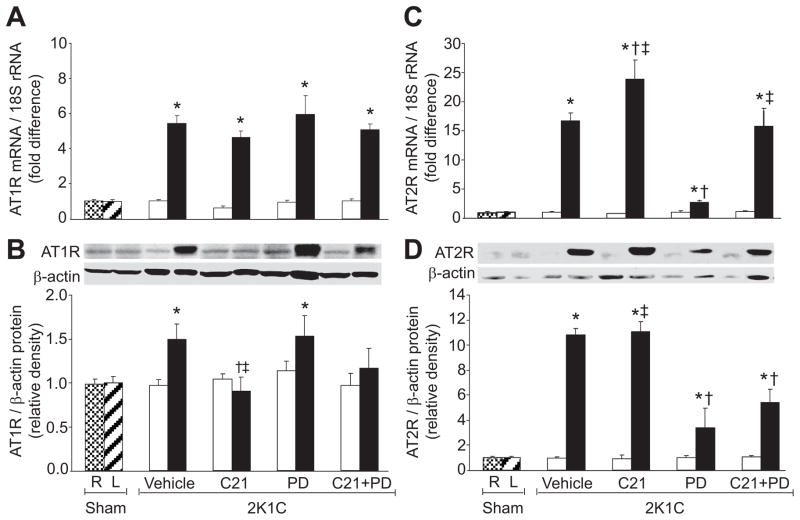
Renal AT1R mRNA and protein expressions (Figure 1A, B) were significantly increased (P<0.05) in clipped kidneys of vehicle-treated and PD-treated groups. C21 treatment significantly (P<0.05) reduced AT1R protein but not mRNA expression in the clipped kidneys. In contrast, PD treatment did not cause significant changes in this receptor mRNA or protein expression. Combined C21 and PD reversed the reduction in AT1R protein expression that was seen with C21 treatment alone in the clipped kidneys. AT2R mRNA and protein expressions (Figure 1C, D) were significantly increased (P<0.05) in the vehicle-treated clipped kidneys group. AT2R mRNA increased further (P<0.05) in clipped kidneys of rats treated with C21. In contrast, PD treatment caused significant reduction in AT2R mRNA and protein (P<0.05) in clipped kidneys. Similarly, PD reversed the effects of C21 treatment on AT2R mRNA and protein expression (P<0.05). In nonclipped kidneys, there were no significant changes in AT1R or AT2R mRNA and protein expressions.
Figure 1.
Renal angiotensin II type 1 (AT1R) and type 2 (AT2R) receptor expressions in right (R) and left (L) kidneys of sham and non-clipped (open bars) and clipped (solid bars) kidneys of 2-kidney, 1-clip (2K1C) hypertension rats treated with vehicle, compound 21 (C21), PD123319 (PD), or C21 plus PD. A and C, mRNA levels of AT1R and AT2R, respectively. B and D, Western blot analyzes of AT1R and AT2R. Top, Representative blots. Bottom, Quantitative results normalized to β-actin. n=5, each group. Data are mean±SEM. *P<0.05 vs sham and corresponding nonclipped kidneys. †P<0.05 vs 2K1C vehicle-treated. ‡P<0.05 vs 2K1C treated with PD.
Markers of Inflammation
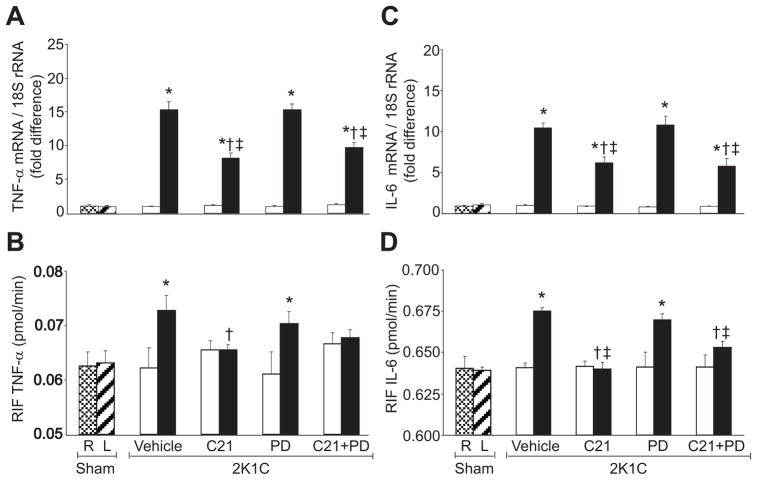
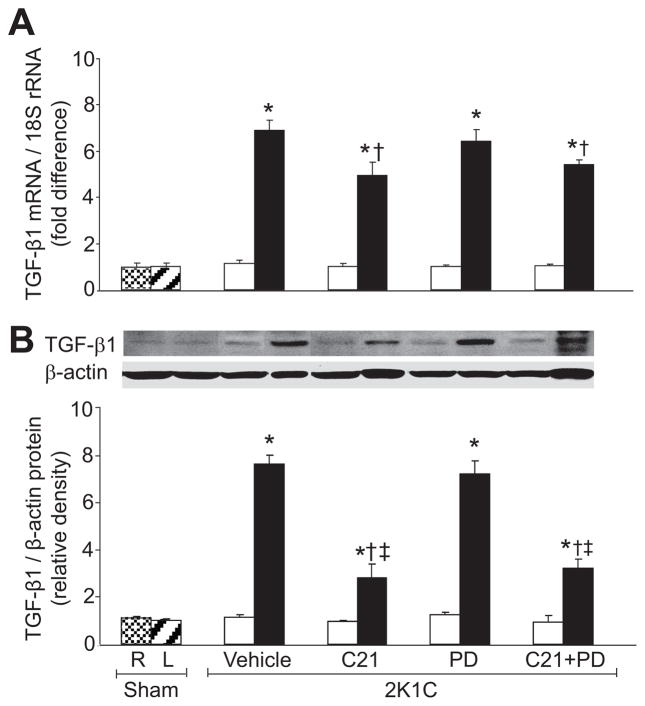
There were no significant differences in TNF-α mRNA, IL-6 mRNA, and their RIF protein recovery rates between sham group and nonclipped kidneys of 2K1C groups with any treatment (Figure 2). Compared to sham group, TNF-α and IL-6 mRNA expressions and their RIF recovery rates were significantly increased (P<0.05) in clipped kidneys of the vehicle-treated group (Figure 2). In clipped kidneys, C21 treatment caused significant reduction (P<0.05) in TNF-α and IL-6 mRNA expressions and their RIF recovery rates. PD treatment did not influence these inflammatory factors. Compared to vehicle-treated rats, TNF-α mRNA, IL-6 mRNA, and IL-6 RIF protein recovery rates were reduced (P<0.05) in the clipped kidneys of the combined C21 and PD treatment group. Similarly, renal mRNA and protein expressions of TGF-β1 (Figure 3) were significantly increased (P<0.05) in the clipped kidneys of 2K1C vehicle-treated and PD-treated rats. In the clipped kidneys, renal TGF-β1 mRNA and protein expressions were significantly reduced (P<0.05) in animals treated with C21 alone and C21 combined with PD.
Figure 2.
Renal mRNA expression and renal interstitial fluid (RIF) levels of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) in right (R) and left (L) kidneys of sham and nonclipped (open bars) and clipped (solid bars) kidneys of 2-kidney, 1-clip (2K1C) hypertension rats treated with vehicle, compound 21 (C21), PD123319 (PD), or C21 plus PD. A and C, mRNA levels of TNF-α and IL-6, respectively (n=5). B and D, RIF levels of TNF-α and IL-6 (n=6). Data are mean±SEM. *P<0.05 vs sham and corresponding nonclipped kidneys. †P<0.05 vs 2K1C vehicle-treated. ‡P<0.05 vs 2K1C treated with PD.
Figure 3.
Renal expression of transforming growth factor-β1 (TGF-β1) in right (R) and left (L) kidneys of sham and nonclipped (open bars) and clipped (solid bars) kidneys of 2-kidney, 1-clip (2K1C) hypertension rats treated with vehicle, compound 21 (C21), PD123319 (PD), or C21 plus PD. A, mRNA levels of TGF-β1. B, Western blot analyzes of TGF-β1. Top, Representative blots. Bottom, Quantitative results normalized to β-actin. n=5, each group. Data are mean±SEM. *P<0.05 vs sham and corresponding nonclipped kidneys. †P<0.05 vs 2K1C vehicle-treated. ‡P<0.05 vs 2K1C treated with PD.
NO and cGMP
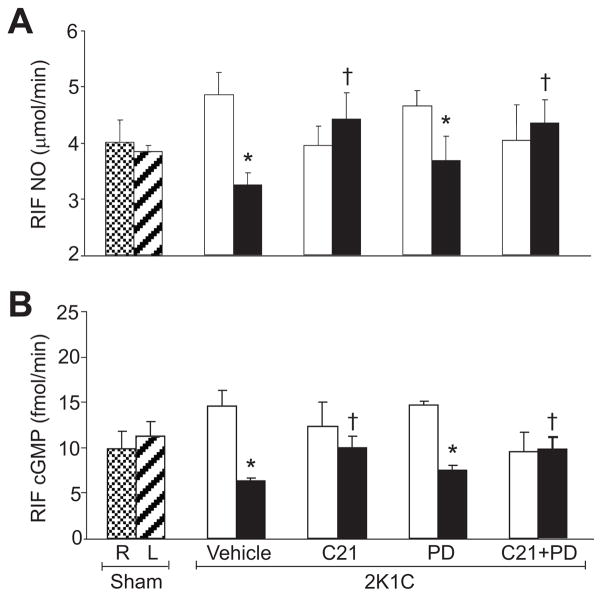
There were no significant differences in RIF NO (Figure 4A) and cGMP (Figure 4B) recovery rates between sham and nonclipped kidneys of 2K1C-treated groups. Compared to sham, these recovery rates were significantly reduced in clipped kidneys of vehicle-treated rats (P<0.05). In clipped kidneys, C21 treatment caused significant increase in RIF NO and cGMP recovery rates (P<0.05). In contrast, PD treatment did not cause significant changes in NO or cGMP of clipped kidneys. The recovery rates of NO and cGMP were significantly higher (P<0.05) in clipped kidneys of combined C21-treated and PD-treated rats.
Figure 4.
Renal interstitial fluid (RIF) levels of nitric oxide (NO; A) and cGMP (B) in right (R) and left (L) kidneys of sham and non-clipped (open bars) and clipped (solid bars) kidneys of 2-kidney, 1-clip (2K1C) hypertension rats treated with vehicle, compound 21 (C21), PD123319 (PD), or C21 plus PD. n=6, each group. Data are mean±SEM. *P<0.05 vs sham and corresponding nonclipped kidneys. †P<0.05 vs 2K1C vehicle-treated. ‡P<0.05 vs 2K1C treated with PD.
Renal Morphological Changes
Figure 5 shows hematoxylin and eosin staining of renal cortex (upper panel) and medulla (lower panel) of sham control and 2K1C groups at the end of study. Glomerular, tubular, and interstitium morphology were normal in sham group (Figure 5A). Inflammatory cell infiltration was markedly increased in clipped kidney of vehicle-treated rats (Figure 5B). C21 treatment (Figure 5C) significantly reduced the inflammatory cell infiltration in renal cortex and medulla. Renal inflammatory cells infiltration was not affected by PD treatment (Figure 5D). Combined and PD treatment caused slight reduction in the cell infiltrate of the renal tissue (Figure 5E).
Figure 5.
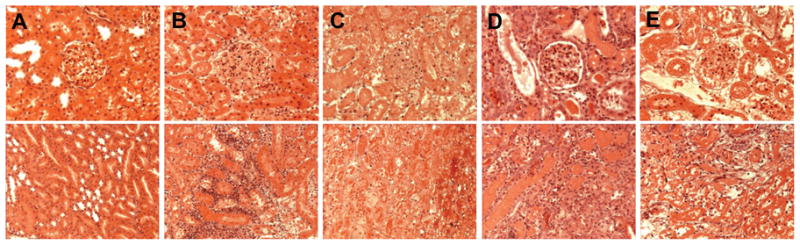
Hematoxylin and eosin of renal cortex (upper panel) and medulla (lower panel) of sham (A) and 2-kidney, 1-clip hypertension rats treated with vehicle (B), compound 21 (C21; C), PD123319 (PD; D), or combined C21 and PD (E). Inflammatory cell (blue) infiltration was observed in renal cortex and medulla of the vehicle-treated clipped kidney group (B) that was not influenced by PD treatment (D). Cellular infiltration was reduced significantly in rats treated with C21 (C) and slightly decreased in the combined C21 and PD treatment group (×200 magnification).
Discussion
Renovascular hypertension is associated with increased activity of the renin-angiotensin system and elevated blood pressure.20 In the experimental model of this disease, 2K1C Goldblatt hypertension, development of inflammation, and generation of reactive oxygen species are prominent in the ischemic kidney, followed by progressive kidney fibrosis.5,21 Because most studies in this hypertension model were conducted several weeks after renal artery clipping, the initial mechanisms that are involved in the pathological process and lead to renal loss are not well-elucidated. In this study, we aimed at evaluating early renal inflammatory process after renal clipping and its response to AT2R stimulation. Our study demonstrated an increase in the production of the inflammatory factors TNF-α, IL-6, and TGF-β1 and reduction of NO and cGMP in the ischemic kidneys 4 days after clipping of the renal artery while nonclipped kidneys were not affected. Direct pharmacological AT2R stimulation reversed this process and promoted reduction of the inflammatory markers TNF-α, IL-6, and TGF-β1 and enhanced production of NO and cGMP. These effects were independent of changes in blood pressure.
In the present study, AT1R and AT2R expressions increased in the clipped kidneys within 4 days of induction of renal ischemia. This finding suggests that in the clipped kidneys, the increased production of Ang II and the associated inflammatory process most likely contributed to the observed increase in renal AT1R and AT2R expressions. Previous studies demonstrated an increase22–25 or a decrease26 in the expression of these receptors in response to injury. This controversial information was probably influenced by the differences in the duration of injury, animal model, and/or the involved tissue. AT2R expression was shown to be upregulated in experimental models of overt renal damage27 and its activation was associated with inhibition of tissue fibrosis.27,28 Thus, our observation of early increase in renal AT2R expression after renal ischemia suggests a role for this receptor in the injury-healing process.
In our study, AT2R stimulation with C21 led to an increase in AT2R expression in the clipped kidneys. Previous studies demonstrated increased AT2R expression during the stimulation of this receptor with Ang II.29,30 This effect was at the AT2R gene promoter activity level and inhibited by blockade with PD.31 Our data are consistent with these studies and confirm that AT2R stimulation enhances its gene activity. Thus, AT2R stimulation may have positive feedback on its expression. It is not clear why C21 treatment did not affect AT2R expression in the nonclipped kidneys. It is possible that exposure of this kidney to systemic hypertension may have prevented the upregulation of AT2R.
In the current study, we observed a trend for reduction of AT1R mRNA, although it did not reach statistical significance or a significant reduction in this receptor protein with AT2R stimulation. A previous study22 demonstrated increased AT1R expression in absence of AT2R expression and activity. Our results confirm this principal of regulation of AT1R expression by AT2R. Our report of more reduction in AT1R protein than its mRNA possibly reflects the influence of AT2R on AT1R protein synthesis and degradation.
In the present study, we report increased production of IL-6, TNF-α, and TGF-β1 and inflammatory cell infiltration in the clipped kidney. Increased intrarenal Ang II is linked to renal inflammatory cell infiltration,32 and activation of AT1R is known to stimulate the renal production of inflammatory factors, including IL-6, TNF-α, and TGF-β1.6,7 Enhanced renal inflammation is associated with increased tissue fibrosis and loss of kidney function.33 Pharmacological blockade of AT1R is well-documented to reduce renal inflammation and to be renoprotective in clinical and experimental settings.34,35 In contrast, activation of AT2R was reported to stimulate vasodilation,9–11 whereas its blockade was associated with reduction of renal function and production of the vasodilatory factors NO and cGMP.8–10 However, the effects of direct AT2R stimulation without influencing the AT1R in the kidney are unknown. Recently, the in vivo effects of direct AT2R stimulation by C21 on tissue inflammation were reported. In these studies, C21 infusion reduced cardiac tissue inflammation in rats after the development of myocardial infarction,16 prevented the development of inflammation and fibrosis in kidneys of spontaneously hypertensive stroke-prone rats,17 and diminished dermal toxic inflammation in mice.18 In the present study, we demonstrated reduction of renal inflammation in ischemic kidneys of 2K1C rats in response to direct AT2R stimulation. Interestingly, concomitant administration of C21 and PD treatments did not completely reverse the C21 effects on the monitored inflammatory factors. It is possible that the different affinity of these drugs for AT2R and the route of their administration could have influenced their combined effects. C21 has 25 000-times and PD has 3500-times higher affinity for AT2R than for AT1R.15 In addition, the C21 dose was administered daily as a single injection intraperitoneally, whereas the PD dose was administered over the course of 24 hours via osmotic minipump. Thus, it is possible that the AT2 receptor was exposed to higher concentrations of C21 before PD.
The lack of the effect of C21 on blood pressure despite increased production of NO and cGMP is consistent with previous reports.16,17 The hypertension model used in the current study is characterized by increased Ang II production20 and increased activation of AT1R in the kidney, and systemic circulation contributed to the observed elevation of blood pressure. Another possible mechanism that could contribute to elevated blood pressure in this model is related to inactivation of NO with increased oxidative stress.36 Taken together, our results suggest that the beneficial effects of AT2R stimulation are most likely related to direct reduction in renal inflammation. Previous studies demonstrated that AT2R blockade resulted in reduction of renal NO and cGMP.10,37 However, the effects of direct AT2R stimulation on renal production of these factors as demonstrated in the current study have not been previously reported. Our current data confirm our previous findings suggesting that AT2R activation directly mediates renal vasodilatory mechanisms by stimulating renal production of NO and cGMP signaling pathways.
We conclude that direct pharmacological stimulation of AT2R by C21 reduces inflammation and stimulates renal production of NO and cGMP in ischemic kidneys of 2K1C hypertensive rats. These effects were independent of blood pressure changes. Our findings suggest a role of AT2R in reducing renovascular inflammatory responses in the 2K1C rat model.
Perspectives
The results of the present study support the concept that AT2R stimulation could be an important therapeutic tool in the management of pathological conditions associated with increased production of inflammatory factors. The novel AT2R agonist, C21, is a promising therapeutic agent that could help the treatment of a variety of renal and cardiovascular diseases.
Supplementary Material
Acknowledgments
Sources of Funding
This study was supported by grants DK-078757 and HL091535 from the National Institutes of Health (H.M.S.).
Footnotes
Disclosures
None.
References
- 1.Siragy HM. The angiotensin II type 2 receptor and the kidney. J Renin Angiotensin Syst. 2010;11:33–36. doi: 10.1177/1470320309347786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrissey JJ, Klahr S. Effect of AT2 receptor blockade on the pathogenesis of renal fibrosis. Am J Physiol Renal Physiol. 1999;276:F39–F45. doi: 10.1152/ajprenal.1999.276.1.F39. [DOI] [PubMed] [Google Scholar]
- 3.Ozono R, Wang ZQ, Moore AF, Inagami T, Siragy HM, Carey RM. Expression of the subtype 2 angiotensin (AT2) receptor protein in rat kidney. Hypertension. 1997;30:1238–1246. doi: 10.1161/01.hyp.30.5.1238. [DOI] [PubMed] [Google Scholar]
- 4.Miyata N, Park F, Li XF, Cowley AW., Jr Distribution of angiotensin AT1 and AT2 receptor subtypes in the rat kidney. Am J Physiol Renal Physiol. 1999;277:F437–F446. doi: 10.1152/ajprenal.1999.277.3.F437. [DOI] [PubMed] [Google Scholar]
- 5.Lerman LO, Textor SC, Grande JP. Mechanisms of tissue injury in renal artery stenosis: ischemia and beyond. Prog Cardiovasc Dis. 2009;52:196–203. doi: 10.1016/j.pcad.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferreri NR, Escalante BA, Zhao Y, An SJ, McGiff JC. Angiotensin II induces TNF production by the thick ascending limb: functional implications. Am J Physiol Renal Physiol. 1998;274:F148–F155. doi: 10.1152/ajprenal.1998.274.1.F148. [DOI] [PubMed] [Google Scholar]
- 7.Wolf G, Schneider A, Wenzel U, Helmchen U, Stahl RA. Regulation of glomerular TGF-beta expression in the contralateral kidney of two-kidney, one-clip hypertensive rats. J Am Soc Nephrol. 1998;9:763–772. doi: 10.1681/ASN.V95763. [DOI] [PubMed] [Google Scholar]
- 8.Chabrashvili T, Kitiyakara C, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Effects of ANG II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase, and SOD expression. Am J Physiol Regul Integr Comp Physiol. 2003;285:R117–R124. doi: 10.1152/ajpregu.00476.2002. [DOI] [PubMed] [Google Scholar]
- 9.Siragy HM, Inagami T, Carey RM. NO and cGMP mediate angiotensin AT2 receptor-induced renal renin inhibition in young rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1461–R1467. doi: 10.1152/ajpregu.00014.2007. [DOI] [PubMed] [Google Scholar]
- 10.Siragy HM, Carey RM. The subtype 2 (AT2) angiotensin receptor mediates renal production of nitric oxide in conscious rats. J Clin Invest. 1997;100:264–269. doi: 10.1172/JCI119531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abadir PM, Carey RM, Siragy HM. Angiotensin AT2 receptors directly stimulate renal nitric oxide in bradykinin B2-receptor-null mice. Hypertension. 2003;42:600 – 604. doi: 10.1161/01.HYP.0000090323.58122.5C. [DOI] [PubMed] [Google Scholar]
- 12.Wu L, Iwai M, Nakagami H, Li Z, Chen R, Suzuki J, Akishita M, de Gasparo M, Horiuchi M. Roles of angiotensin II type 2 receptor stimulation associated with selective angiotensin II type 1 receptor blockade with valsartan in the improvement of inflammation-induced vascular injury. Circulation. 2001;104:2716–2721. doi: 10.1161/hc4601.099404. [DOI] [PubMed] [Google Scholar]
- 13.Siragy HM, Inagami T, Ichiki T, Carey RM. Sustained hypersensitivity to angiotensin II and its mechanism in mice lacking the subtype-2 (AT2) angiotensin receptor. Proc Natl Acad Sci U S A. 1999;96:6506–6510. doi: 10.1073/pnas.96.11.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu C, Dandapat A, Chen J, Liu Y, Hermonat PL, Carey RM, Mehta JL. Over-expression of angiotensin II type 2 receptor (agtr2) reduces athero-genesis and modulates LOX-1, endothelial nitric oxide synthase and heme-oxygenase-1 expression. Atherosclerosis. 2008;199:288–294. doi: 10.1016/j.atherosclerosis.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Wan Y, Wallinder C, Plouffe B, Beaudry H, Mahalingam AK, Wu X, Johansson B, Holm M, Botoros M, Karlén A, Pettersson A, Nyberg F, Fändriks L, Gallo-Payet N, Hallberg A, Alterman M. Design, synthesis, and biological evaluation of the first selective nonpeptide AT2 receptor agonist. J Med Chem. 2004;47:5995–6008. doi: 10.1021/jm049715t. [DOI] [PubMed] [Google Scholar]
- 16.Kaschina E, Grzesiak A, Li J, Foryst-Ludwig A, Timm M, Rompe F, Sommerfeld M, Kemnitz UR, Curato C, Namsolleck P, Tschöpe C, Hallberg A, Alterman M, Hucko T, Paetsch I, Dietrich T, Schnackenburg B, Graf K, Dahlöf B, Kintscher U, Unger T, Steckelings UM. Angiotensin II type 2 receptor stimulation: a novel option of therapeutic interference with the renin-angiotensin system in myocardial infarction? Circulation. 2008;118:2523–2532. doi: 10.1161/CIRCULATIONAHA.108.784868. [DOI] [PubMed] [Google Scholar]
- 17.Gelosa P, Pignieri A, Fändriks L, de Gasparo M, Hallberg A, Banfi C, Castiglioni L, Turolo L, Guerrini U, Tremoli E, Sironi L. Stimulation of AT2 receptor exerts beneficial effects in stroke-prone rats: focus on renal damage. J Hypertens. 2009;27:2444–2451. doi: 10.1097/HJH.0b013e3283311ba1. [DOI] [PubMed] [Google Scholar]
- 18.Rompe F, Artuc M, Hallberg A, Alterman M, Ströder K, Thöne-Reineke C, Reichenbach A, Schacherl J, Dahlöf B, Bader M, Alenina N, Schwaninger M, Zuberbier T, Funke-Kaiser H, Schmidt C, Schunck WH, Unger T, Steckelings UM. Direct angiotensin II type 2 receptor stimulation acts anti-inflammatory through epoxyeicosatrienoic acid and inhibition of nuclear factor kappaB. Hypertension. 2010;55:924–931. doi: 10.1161/HYPERTENSIONAHA.109.147843. [DOI] [PubMed] [Google Scholar]
- 19.Huang J, Siragy HM. Glucose promotes the production of interleukine-1beta and cyclooxygenase-2 in mesangial cells via enhanced (Pro)renin receptor expression. Endocrinology. 2009;150:5557–5565. doi: 10.1210/en.2009-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Navar LG, Zou L, Von Thun A, Tarng Wang C, Imig JD, Mitchell KD. Unraveling the mystery of Goldblatt hypertension. News Physiol Sci. 1998;13:170–176. doi: 10.1152/physiologyonline.1998.13.4.170. [DOI] [PubMed] [Google Scholar]
- 21.Chade AR, Rodriguez-Porcel M, Grande JP, Krier JD, Lerman A, Romero JC, Napoli C, Lerman LO. Distinct renal injury in early atherosclerosis and renovascular disease. Circulation. 2002;106:1165–1171. doi: 10.1161/01.cir.0000027105.02327.48. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka M, Tsuchida S, Imai T, Fujii N, Miyazaki H, Ichiki T, Naruse M, Inagami T. Vascular response to angiotensin II is exaggerated through an upregulation of AT1 receptor in AT2 knockout mice. Biochem Biophys Res Commun. 1999;258:194–198. doi: 10.1006/bbrc.1999.0500. [DOI] [PubMed] [Google Scholar]
- 23.Modrall JG, Quinones MJ, Frankhouse JH, Hsueh WA, Weaver FA, Kedes L. Upregulation of angiotensin II type 1 receptor gene expression in chronic renovascular hypertension. J Surg Res. 1995;59:135–140. doi: 10.1006/jsre.1995.1144. [DOI] [PubMed] [Google Scholar]
- 24.Hiyoshi H, Yayama K, Takano M, Okamoto H. Stimulation of cyclic GMP production via AT2 and B2 receptors in the pressure-overloaded aorta after banding. Hypertension. 2004;43:1258–1263. doi: 10.1161/01.HYP.0000128022.24598.4f. [DOI] [PubMed] [Google Scholar]
- 25.Hiyoshi H, Yayama K, Takano M, Okamoto H. Angiotensin type 2 receptor-mediated phosphorylation of eNOS in the aortas of mice with 2-kidney, 1-clip hypertension. Hypertension. 2005;45:967–973. doi: 10.1161/01.HYP.0000164571.77710.19. [DOI] [PubMed] [Google Scholar]
- 26.Wang ZQ, Millatt LJ, Heiderstadt NT, Siragy HM, Johns RA, Carey RM. Differential regulation of renal angiotensin subtype AT1A and AT2 receptor protein in rats with angiotensin-dependent hypertension. Hypertension. 1999;33:96–101. doi: 10.1161/01.hyp.33.1.96. [DOI] [PubMed] [Google Scholar]
- 27.Jones ES, Vinh A, McCarthy CA, Gaspari TA, Widdop RE. AT2 receptors: functional relevance in cardiovascular disease. Pharmacol Ther. 2008;120:292–316. doi: 10.1016/j.pharmthera.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruiz-Ortega M, Esteban V, Suzuki Y, Ruperez M, Mezzano S, Ardiles L, Justo P, Ortiz A, Egido J. Renal expression of angiotensin type 2 (AT2) receptors during kidney damage. Kidney Int Suppl. 2003;86:S21–S26. doi: 10.1046/j.1523-1755.64.s86.5.x. [DOI] [PubMed] [Google Scholar]
- 29.Shibata K, Makino I, Shibaguchi H, Niwa M, Katsuragi T, Furukawa T. Up-regulation of angiotensin type 2 receptor mRNA by angiotensin II in rat cortical cells. Biochem Biophys Res Commun. 1997;239:633–637. doi: 10.1006/bbrc.1997.7521. [DOI] [PubMed] [Google Scholar]
- 30.Zahradka P, Yau L, Lalonde C, Buchko J, Thomas S, Werner J, Nguyen M, Saward L. Modulation of the vascular smooth muscle angiotensin subtype 2 (AT2) receptor by angiotensin II. Biochem Biophys Res Commun. 1998;252:476–480. doi: 10.1006/bbrc.1998.9669. [DOI] [PubMed] [Google Scholar]
- 31.De Paolis P, Porcellini A, Gigante B, Giliberti R, Lombardi A, Savoia C, Rubattu S, Volpe M. Modulation of the AT2 subtype receptor gene activation and expression by the AT1 receptor in endothelial cells. J Hypertens. 1999;17:1873–1877. doi: 10.1097/00004872-199917121-00015. [DOI] [PubMed] [Google Scholar]
- 32.Ozawa Y, Kobori H, Suzaki Y, Navar LG. Sustained renal interstitial macrophage infiltration following chronic angiotensin II infusions. Am J Physiol Renal Physiol. 2007;292:F330–F339. doi: 10.1152/ajprenal.00059.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Remuzzi G, Benigni A, Remuzzi A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest. 2006;116:288–296. doi: 10.1172/JCI27699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naito T, Ma LJ, Yang H, Zuo Y, Tang Y, Han JY, Kon V, Fogo AB. Angiotensin type 2 receptor actions contribute to angiotensin type 1 receptor blocker effects on kidney fibrosis. Am J Physiol Renal Physiol. 2010;298:F683–F691. doi: 10.1152/ajprenal.00503.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 36.Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? Hypertension. 2004;44:248–252. doi: 10.1161/01.HYP.0000138070.47616.9d. [DOI] [PubMed] [Google Scholar]
- 37.Siragy HM, Carey RM. The subtype-2 (AT2) angiotensin receptor regulates renal cyclic guanosine 3′, 5′-monophosphate and AT1 receptor-mediated prostaglandin E2 production in conscious rats. J Clin Invest. 1996;97:1978–1982. doi: 10.1172/JCI118630. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.