Abstract
Objectives
Abnormalities in auditory steady state response (ASSR) at gamma range frequencies have been found in bipolar disorder, but the relationship of these neurophysiological disturbances to clinical factors has not been well characterized. We therefore evaluated the ASSR in bipolar disorder and examined its sensitivity to clinical symptoms, cognitive function, and pharmacological treatment.
Methods
A total of 68 patients with bipolar disorder and 77 control participants were evaluated. Click trains presented at 20, 30, 40, and 50 Hz evoked ASSRs. Mean trial power (MTP) and phase locking factor (PLF) measured response magnitude and phase synchronization of the ASSR at each stimulation frequency. Clinical state, pharmacological treatment, and neuropsychological performance were assessed, and their respective relationships with ASSR measures were evaluated.
Results
Patients with bipolar disorder showed reduced MTP and PLF compared to control participants. Bipolar disorder patients taking psychotropic medications had decreased PLF relative to patients withdrawn from medications. Control participants performed better on neuropsychological tests than bipolar disorder patients; however, test scores did not correlate with ASSR measures.
Conclusions
Deficits in the generation and maintenance of ASSR are present in bipolar disorder, implicating disturbances in auditory pathways. ASSR may be sensitive to medication status. Other clinical features, including mood state, psychotic features, cognitive performance, smoking, or history of substance use disorder, were unrelated to MTP or PLF.
Keywords: audition, bipolar disorder, electroencephalography, steady state potentials
Periodic auditory stimulation entrains the electroencephalogram (EEG) to produce an auditory steady state response (ASSR) that synchronizes to the phase and frequency of the external stimulus (1). The ASSR can therefore probe frequency response characteristics of sensory neural circuits with respect to phase synchronization and response magnitude (1, 2). The ASSR is thought to reflect the activity of neurons in the brainstem, thalamocortical projections, and auditory cortex (1, 3–7). In humans, the ASSR is largest for clicks or modulated tones presented at 40 Hz (1, 8). Deficits of ASSR have been demonstrated in schizophrenia and early-onset or first-episode psychosis at 30 Hz (2, 9) and 40 Hz (2, 9–14).
Whereas the ASSR deficit appears to be sensitive to schizophrenia, it may not be specific to the disorder. O’Donnell and colleagues (15) measured ASSR in unmedicated patients during manic or mixed episodes of bipolar disorder and found reduced EEG signal power at stimulation frequencies of 20, 30, 40, and 50 Hz and reduced EEG phase synchronization at 20, 40, and 50 Hz (15). Furthermore, Spencer and colleagues (9) measured ASSR in a mixed affective disorder group of first-episode psychosis patients with primary diagnosis of bipolar disorder (n = 13) or major depressive disorder (n = 3) and found reduced EEG signal power at 30 Hz and reduced EEG phase synchronization at 30 Hz and 40 Hz. Finally, magnetoencephalography (MEG) recordings in euthymic bipolar disorder patients showed reduced power and phase locking for transient gamma band response (20–70 ms) and ASSR (200–500 ms) during 40 Hz stimulation (16).
ASSR deficits in bipolar disorder may index disturbances of GABAergic neurotransmission within auditory pathways and cortex. In terms of cellular mechanisms, decreased GABA transmission, glutamate receptor expression, and glutamic acid decarboxylase, an enzyme involved in GABA synthesis and regulation, have been implicated in bipolar disorder (17–19). Uhlhaas and Singer (20) induced oscillations in hippocampal slices to investigate the effects of spontaneous, pharmacological, and tetanic stimulation and concluded that GABA circuits modulate the generation and synchronization of beta and gamma range oscillations, whereas glutamate determines strength, duration, and range of oscillations. Consequently, GABAergic dysregulation in bipolar disorder may alter the ASSR at these frequencies.
Clinical features may be associated with variations in the ASSR. Deficits in auditory processing and perception in both schizophrenia and bipolar disorder are suggested by clinical symptoms (e.g., auditory hallucinations) and abnormal auditory event-related potentials (ERPs) (see 21 for review), including P50 gating measures (22–25) and P300 ERPs (25–29). Psychotic features, often associated with schizophrenia, are present in 58–80% of bipolar disorder patients and may influence ERP responses (22, 30). Acute (i.e., symptoms of mania or depression) and euthymic (asymptomatic) illness phases in bipolar disorder are associated with behavioral, cognitive, and EEG differences (31). ASSR deficits could be linked to cognitive deficits, which are evident across depressive, hypomanic, and euthymic phases (32, 33).
EEG measures including ASSR may also be affected by medications and illicit drug use (11, 34). Acute administration of alcohol suppresses 40 Hz ASSRs (35, 36). The cholinergic and GABA agonist nicotine can influence EEG waveforms and ERPs (see 37, 38 for review) because cholinergic circuits modulate oscillatory facilitation and response (20, 39). Prescribed medications (e.g., mood stabilizers, antipsychotics, antidepressants) could affect the ASSR (17, 40–44).
The present study assessed ASSRs in bipolar disorder, and tested the relationship of ASSR measures to mood state, history of psychosis, medication status, drug history, and cognitive functioning of bipolar disorder patients. Age and gender effects were also investigated as potential covariates. Mean trial power (MTP), or event-related spectral perturbation, and phase locking factor (PLF), or intertrial coherence, were used as measures of ASSR. MTP quantifies baseline-normalized EEG activity at specific frequencies during stimulation. Because MTP captures the change in power relative to background activity in the baseline period, it reflects both phase-locked and non-phase-locked activity across trials. PLF measures phase synchronization of EEG activity across trials at particular temporal intervals and frequencies (45). PLF values can range from 0 (absence of synchronization) to 1 (perfect synchronization, or phase reproducibility across trials at a given latency). We expected to find decreased power and phase locking of ASSR in bipolar disorder patients compared to control participants and tested whether clinical variables modulated the ASSR.
Methods
Participants
Two groups of participants were evaluated: 68 patients with a diagnosis of type I bipolar disorder and 77 nonpsychiatric comparison participants (see Table 1 for characteristics). Exclusion criteria for all participants included a history of neurological or cardiovascular disease, failure to complete a grade-school education, clinically documented hearing loss, head injury that resulted in loss of consciousness, current substance or alcohol dependence and electroconvulsive therapy. Participants were paid for participation. The protocol was approved by the Indiana University–Purdue University Indianapolis Human Subjects Review Committee. All participants received detailed information about the study protocol and gave written and oral informed consent.
Table 1.
Participant demographic information
| Control (n = 77) | Bipolar disorder (n = 68)a | ||
|---|---|---|---|
| Euthymic (n = 22) | Acute (n = 43) | ||
| Sex (M:F) | 30:47 | 12:10 | 11:32 |
| Age, mean (SD) | 41.0 (10.3) | 43.6 (10.5) | 42.6 (10.3) |
| Years of education, mean (SD) | 14.8 (2.4) | 14.4 (2.3) | 13.1 (3.3) |
| Age at onset, mean (SD) | – | 25.0 (7.1) | 24.6 (8.9) |
| Illness duration, years, mean (SD) | – | 18.6 (11.4) | 18.1 (12.0) |
| YMRS score, mean (SD) | – | 3.9 (3.9) | 16.0 (9.9) |
| MADRS score, mean (SD) | – | 3.6 (2.6) | 15.3 (9.9) |
| Psychotic features present, n | – | 12 | 15 |
| Medication (on:off) | – | 21:1 | 30:13 |
| Smokes > 1 pack cigarettes/week, n | 12 | 10 | 13 |
| Substance abuse or dependence history, n | – | 10 | 15 |
Young Mania Rating Scale (YMRS) and Montgomery-Åsberg Depression Rating Scale (MADRS) measures were not completed proximal to the electroencephalograph date for three bipolar disorder patients, of whom two were off medications. Therefore, these three patients were not included in either illness phase category.
Bipolar disorder patients were diagnosed using the research modules of the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) (46), clinical observations, and chart review. Bipolar disorder patients, described in Table 1, were referred by physicians and recruited at the Indiana University School of Medicine Neuroscience Clinical Research Center (Indianapolis, IN, USA). Sixteen patients had discontinued anticonvulsant mood stabilizers, lithium, antipsychotic, and antidepressant medications for at least three days prior to the EEG (range: 3–730, median = 8, SD = 196); however, 7 patients were prescribed benzodiazepines or similar sedative medications if required and therefore were not completely unmedicated. Table 2 describes the prescribed medication types among bipolar disorder patients. Three months of remission from substance dependence were required for inclusion.
Table 2.
Medication types among bipolar disorder groups
| Euthymic (n) | Acute (n) | |
|---|---|---|
| Atypical antipsychotic | 11 | 21 |
| Conventional antipsychotic | 3 | 4 |
| Lithium | 7 | 8 |
| Benzodiazepine | 1 | 11 |
| Antidepressant | 6 | 16 |
| Anticonvulsant | 8 | 14 |
Patients taking psychotropic medications typically used multiple medications. Anticonvulsant medications included Depakote (valproic acid). Of unclassified patients (n = 3), two were not taking medications and one was taking an atypical antipsychotic, benzodiazepine, and antidepressant.
Nonpsychiatric control participants were recruited through newspaper advertisements. Participants were excluded from participation if they had a current or past substance abuse or dependence, diagnosis of any current or past Axis I psychiatric illness, or first-degree relatives with schizophrenia or bipolar disorder. The SCID-I was used to rule out Axis I disorders in healthy participants. Control participants had completed more years of education than bipolar disorder patients [t(143) = 3.011, p < 0.01], but did not differ in age or gender distribution (χ2 = 0.008).
Clinical and neuropsychological assessment
The Young Mania Rating Scale (YMRS) (47) and Montgomery-Åsberg Depression Rating Scale (MADRS) (48) were used to characterize the phase of illness in patients within a week of EEG testing (Table 2). A manic episode was characterized by a score of > 12 on the YMRS and a depressed episode by a score of > 8 on the MADRS according to empirically derived criteria where the two scales were calibrated with the Clinical Global Impression Scale–Bipolar Version (49). Participants in the euthymic phase did not meet criteria for mania or depression.
Subtests from the Wechsler Adult Intelligence Scale (WAIS-III) (50) were used to assess abstract verbal reasoning (Similarities task), short-term auditory memory and working memory (Digit Span task), visuospatial perception (Picture Completion task), and working memory and psychomotor speed (Digit Symbol Coding task). The Trail Making Test A assessed psychomotor speed, and Trails B assessed the ability to shift between sets within working memory (51). The word-list learning test form of Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) (52) was used to assess episodic memory. Table 3 shows performance on these measures.
Table 3.
Significant effects from the analyses of mean trial power (MTP) and phase locking factor (PLF)
| ASSR analysis | Factor | Omnibus ANOVA |
|---|---|---|
| Control versus BD | ||
| MTP | F | F(2.56, 363.12) = 7.36*** |
| F × G | F(2.56, 363.12) = 3.22* | |
| PLF | F | F(2.60,368.19) = 77.91*** |
| F × G | F(2.60,368.19) = 2.82* | |
| G | F(1,143) = 5.12* | |
| Medication effect | ||
| MTP | F | F(2.54,367.75) = 3.25* |
| F × G | F(5.07,367.75) = 2.35* | |
| PLF | F | F(2.59,365.55) = 47.71*** |
| F × G | F(5.185,365.55) = 1.96, p = 0.081 | |
| G | F(2,141) = 5.15** | |
| Bipolar disorder analysis: PLF | ||
| Mood state | F | F(2.68,165.96) = 16.29*** |
| Psychotic symptoms | F | F(2.62,170.39) = 22.08*** |
| Substance use history | F | F(2.61,169.65) = 20.61*** |
| Smoking | F | F(2.57,138.56) = 21.35*** |
| Follow-up analysis | Condition | Post-hoc | Direction | |
|---|---|---|---|---|
| Control versus BD | t-test | |||
| MTP | 40 Hz | t(143) = 2.79** | C > BD | |
| 40 Hz | t(143) = 2.31* | C > BD | ||
| PLF | 50 Hz | t(143) = 2.15* | C > BD | |
| Medication effect | ANOVA | Tukey HSD | ||
| MTP | 40 Hz | F(2,142) = 4.1* | C > BD− | 0.076 |
| C > BD+ | 0.053 | |||
| 50 Hz | F(2,142) = 4.2* | C > BD+ | 0.052 | |
| BP– > BD+ | * | |||
| PLF | 40 Hz | F(2,142) = 4.0* | C > BD+ | * |
| 50 Hz | F(2,142) = 5.0* | BP– > BD+ | 0.060 | |
| C > BD+ | * | |||
ASSR = auditory steady state response; BD = bipolar disorder; F = frequency; G = group; C = control; BD+ = on medication; BD− = off medication; HSD = honestly significant difference.
p < 0.05,
p < 0.01,
p < 0.001.
Electrophysiological assessment
During the evaluation, participants were seated comfortably with eyes open while listening to trains of clicks presented through Etymotic insert earphones (Etymotic Research, Inc., Elk Grove Village, IL, USA). The individual stimuli were 1 ms duration clicks (80 dB SPL), presented in trains which varied in rate of presentation (20, 30, 40, and 50 Hz) in each of four blocks. The duration of the click train was 450 ms for 20 Hz, 467 ms for 30 Hz, 475 ms for 40 Hz, and 480 ms for 50 Hz. Each block had 80 trains of clicks with a 700 ms intertrain interval. Order of conditions was randomized across participants.
The EEG was continuously recorded (band pass 0.1–200 Hz, sampling rate 1000 Hz) and digitized (Neuroscan SynAmps, Compumedics Neuroscan, Charlotte, NC, USA) from the scalp, using a 29-channel electrode cap. Recordings were referenced to the nose. Electrode impedances were maintained at < 10 kOhm. For each stimulus condition, the EEG was segmented into epochs with a 350 ms baseline before stimulus onset to 350 ms post-stimulus padding after the end of each 512 ms click train using Brain Vision Analyzer software (Brain Products, GmbH, Gilching, Germany). Epochs were corrected for ocular artifacts using the Gratton, Coles, and Donchin algorithm (53). Epochs with voltage exceeding ± 150 μV at any site were automatically excluded from further analyses. Participants with fewer than 20 remaining segments in a frequency condition (20, 30, 40, or 50 Hz) were excluded from analysis in that condition. A mean number of 67 (SD = 14), 67 (SD = 12), 67 (SD = 14), and 66 (SD = 14) trials were included in the 20, 30, 40, and 50 Hz conditions, respectively. The number of segments accepted for signal processing did not differ significantly between groups.
Time-frequency analyses
Time-frequency analyses were applied to single-trial EEG epochs using MATLAB (The MathWorks, Inc., Natick, MA, USA) and EEGLAB software (45). After applying a 100% Hanning window to the data, a time-frequency spectrogram using short time windowed fast fourier transform (FFT) was computed for a segment of EEG for the different component frequencies. The spectrogram was used to measure change in power from baseline (MTP) and intertrial coherence (PLF). A sliding window of 128 ms duration with 10 ms time steps was used for the spectrogram analysis. The frequency resolution was 1.953 Hz.
MTP was obtained by averaging the power from individual trials after subtracting the mean from the baseline period (350 ms before stimulus onset). PLF was obtained by first averaging the normalized complex output from spectrogram for every time period and frequency, then taking its absolute value (or complex norm) (54). For statistical analysis, mean values were obtained for the 100–500 ms interval after stimulus onset for the frequencies corresponding to 5 Hz below and above the stimulation frequency.
Statistical analysis
Analyses are reported for the FCz electrode where the signal power was largest. Differences in EEG measures were analyzed for 20, 30, 40, and 50 Hz stimulation frequencies using repeated-measures analysis of variance (ANOVA) with Greenhouse-Geisser epsilon adjustments when appropriate, performed separately for MTP and PLF analyses. The within-subject factor was frequency (4: 20, 30, 40, 50 Hz). The between-subject factor was group (2: control, bipolar). Adding age and gender as covariates did not change results for the reported MTP and PLF analyses. A repeated-measures ANOVA for between-subject factor of group (3: control, bipolar off medication, bipolar on medication) by frequency (4: 20, 30, 40, 50 Hz) was used to determine the effect of medication on ASSR. To assess the relationship between cognitive test scores and EEG response, a single average value was calculated for MTP and PLF at the frequency of interest for the 100–500 ms interval after stimulus onset. For all analyses, post-hoc t-tests were used to clarify main effects or interactions revealed by ANOVA.
Separate analyses on bipolar disorder patients were used to assess the between-subject factors of phase of illness (2: acute, euthymic), psychotic symptoms (2: present, absent), history of substance use disorder (2: present, absent), and smoking status (2: smoker, nonsmoker). Participants smoking less than one pack of cigarettes per week were excluded from analysis of smoking effects. Independent samples t-tests assessed differences of bipolar disorder patients with differing illness phase or medication states on factors of age, years of education, age of onset, illness duration, and clinical measures of mood. Chi-square testing assessed differences of bipolar disorder patients with differing illness phase or medication states on the factors of gender, psychotic features, and drug abuse/dependence history. Multiple regression analysis was used to explore potential relationships between neuropsychological measures and EEG measures of MTP and PLF. A two-tailed p-value of ≤ 0.05 was used for significance testing.
Results
Clinical measures
Phase of illness
Euthymic patients scored lower than acute patients on the YMRS [t(63) = 5.491, p < 0.001] and MADRS [t(63) = 5.430, p < 0.001], but did not differ on age, age of onset, illness duration, or years of education. Acute patients included more females (χ2 = 5.340, p = 0.021) and more participants taking medication (1 acute versus 13 euthymic) compared to the euthymic group; however, no differences were found between patient groups for presence of psychotic features, drug abuse/dependence history, and smoking status.
Electrophysiological measures
Mean trial power
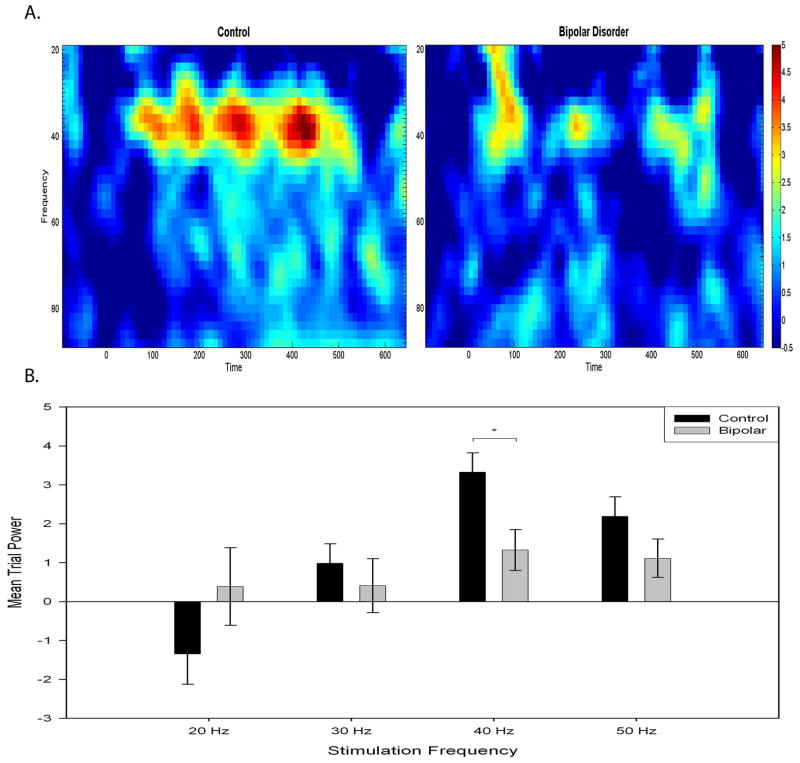
Bipolar disorder patients had reduced MTP response at 40 Hz (Fig. 1). Results from a (group = 2) × (frequency = 4) repeated-measures ANOVA can be found in Table 3. A main effect for frequency indicated that both groups showed a stronger response at 40 Hz than the other frequencies, and a group × frequency interaction supports a differential response between groups (see Fig. 2). Post-hoc t-tests revealed decreased 40 Hz MTP for bipolar disorder patients. MTP response was not affected by phase of illness, psychotic symptoms, smoking, or substance use history.
Fig. 1.

Averaged auditory steady state response waveforms for the 40 Hz stimulation frequency of control and bipolar disorder participants.
Fig. 2.
A. Mean trial power spectrogram at 40 Hz stimulation for control participants and bipolar disorder patients showing magnitude of response across time. B. Mean trial power as a function of stimulation frequency for control participants and bipolar disorder patients recorded at site FCz. Error bars represent ± 1 standard error. *p < 0.05.
Phase locking factor
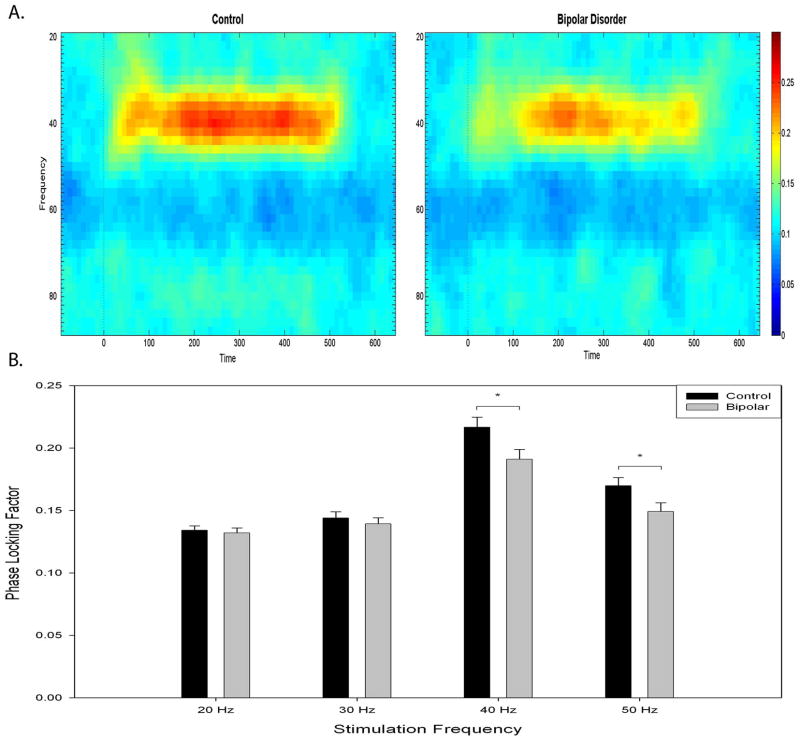
Bipolar disorder patients had reduced PLF response at 40 Hz (Fig. 1) and 50 Hz. Results from a (group = 2) × (frequency = 4) repeated-measures ANOVA can be found in Table 3. A main effect for group, frequency, and a group × frequency interaction indicated a larger overall response for control participants, a larger response at 40 Hz than other frequencies, and a differential response between groups (see Fig. 3). Post-hoc t-tests revealed decreased 40 Hz and 50 Hz PLF for bipolar disorder patients (Table 3).
Fig. 3.
A. Phase locking factor spectrogram at 40 Hz stimulation for control and bipolar disorder participants showing synchrony across time. B. Phase locking factor as a function of stimulation frequency for control and bipolar disorder participants recorded at site FCz. Error bars represent ± 1 standard error. *p < 0.05.
For bipolar disorder patients, separate repeated-measures ANOVA for between-subject factors of the investigated variables (i.e., phase of illness, psychotic symptoms, smoking, and substance use history) at each frequency showed main effects of frequency, with greatest response at 40 Hz.
Medication status
Patients on medications had lower YMRS [t(63) = 3.290, p = 0.002) and MADRS [t(63) = 2.393, p = 0.020) scores, but did not differ on age, age of onset, illness duration, or years of education. Medicated patients were marginally more likely to have a history of drug abuse or dependence (χ2 = 3.838, p = 0.050) than patients off medications; however, no differences were found between patient groups for gender, presence of psychotic features, and smoking status.
Medication status influenced PLF and MTP response at 40 Hz and 50 Hz. Results from a (group = 3) × (frequency = 4) repeated-measures ANOVA can be found in Table 3. One-way ANOVAs for each stimulation frequency were used to interpret the interaction effects (Table 3). For MTP, patients off medication had greater response than those on medication at 50 Hz, and a trend for greater response of control participants than bipolar disorder patients was found for 40 Hz and 50 Hz. For PLF, control participants had greater responses than patients on medications at 40 Hz and 50 Hz, but control participants did not differ from unmedicated patients (Fig. 4). There was a trend for unmedicated participants to have a greater response compared to those on medication at 50 Hz. These differences persisted when the ANOVA included only patients (n = 9) who discontinued all medications, including benzodiazepines and sedatives.
Fig. 4.
Phase locking factor as a function of stimulation frequency for bipolar disorder (BD) patients taking medication and patients that discontinued medication with comparison to control participant response. Error bars represent ± 1 standard error. *Control participants differ from medicated patients at 40 Hz and 50 Hz (p < 0.05).
Neuropsychological measures
WAIS-III subtest performance scores were age scaled and analyzed for Picture Completion, Digit Symbol Coding, Similarities, and Digit Span measures. One-way ANOVA for group (control, bipolar) on each measure showed that control participants performed better than bipolar disorder patients on Picture Completion, Digit Symbol, and Digit Span (Table 4). Trail Making Test performance was analyzed for completion time of Trails A and Trails B. One-way ANOVA for group (control, bipolar) on each measure showed that control participants performed better than bipolar disorder patients on Trails A and Trails B completion time (Table 4). CERAD performance was analyzed for grand total retention and delay retention scores. One-way ANOVA for group (control, bipolar) on each measure showed that control participants performed better than bipolar disorder patients did overall and after a 20-minute delay (Table 4).
Table 4.
Performance on neuropsychological measures
| Control | Euthymic BD | Acute BD | Control versus all BD | N (Control, BD) | |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |||
| WAIS-III score | |||||
| Picture Completion | 11.0 (3.5) | 10.0 (3.6) | 8.4 (3.4) | < 0.01 | 73, 67 |
| Digit Symbol | 10.9 (2.8) | 8.8 (2.9) | 7.9 (3.0) | < 0.001 | 74, 68 |
| Similaritiesa | 10.0 (3.2) | 11.0 (2.7) | 8.5 (2.8) | n.s. | 73, 67 |
| Digit Span | 11.0 (2.9) | 10.7 (3.1) | 9.5 (3.5) | < 0.05 | 73, 68 |
| Trails A (sec) | 25.9 (10.3) | 30.9 (10.0) | 36.5 (14.1) | < 0.001 | 46, 62 |
| Trails B (sec) | 55.4 (21.8) | 74.3 (45.1) | 90.9 (40.8) | < 0.001 | 46, 62 |
| CERAD grand total | 24.5 (2.9) | 21.7 (3.6) | 22.1 (3.3) | < 0.001 | 46, 62 |
| CERAD delay total | 7.8 (2.0) | 6.4 (1.8) | 7.0 (1.9) | < 0.01 | 46, 62 |
BD = bipolar disorder; WAIS = Wechsler Adult Intelligence Scale; Trails = Trail Making Test; CERAD = Consortium to Establish a Registry for Alzheimer’s Disease.
Euthymic patients scored higher than acute patients on WAIS-III Similarities, t(62) = 3.5, p < 0.05.
Hierarchical linear regression was used to examine whether cognitive measures predicted ASSR response among control participants and bipolar disorder patients. In the first step, analysis was based on group membership (bipolar = 1, control = 2). In the second step, the model was tested using eight predictors, including scores on the WAIS-III (Picture Completion, Digit Symbol Coding, Similarities, Digit Span), Trails A and B, and CERAD total and delay. The dependent variables were 40 Hz MTP and 40 Hz PLF. For 40 Hz MTP, the initial group model analysis was significant [R2 = 0.07, F(1,104) = 8.198, p = 0.005], with group membership as a significant predictor (β = −0.270, t = −2.863, p = 0.005). The cognitive measures model analysis approached significance [R2 = 0.15, F(9,96) = 1.938, p = 0.055]; however, no cognitive predictor was significant. For 40 Hz PLF, the initial group model approached significance [R2 = 0.03, F(1,104) = 3.173, p = 0.078], and group membership approached significance (β = −0.172, t = −1.781, p = 0.078).
Discussion
Auditory steady state response
The present study replicated a gamma range ASSR deficit in bipolar disorder patients using a larger sample size (9, 55) and suggested that this deficit was not related to clinical factors with the exception of medication status. Patients had reduced power (MTP) at 40 Hz and reduced synchronization (PLF) at 40 Hz and 50 Hz. Medication use was related to ASSR response, whereas other clinical factors, including phase of illness, history of psychotic episodes, cognitive function, smoking, and history of substance abuse and dependence, were unrelated to ASSR measures.
Dysregulated GABA function in bipolar disorder may play a role in the ASSR deficit at gamma frequencies of stimulation. Gamma range oscillatory activity appears to be dependent on GABAergic modulation, and both preclinical and clinical evidence suggests that GABAergic neurotransmission is altered in bipolar disorder. Postmortem brains of bipolar disorder patients have shown increased gamma-2 subunits of the GABAA receptors that may influence GABA neurotransmission and benzodiazepine binding, leading to changes in ASSR (56). Anticonvulsant mood stabilizers as well as lithium typically upregulate GABAergic levels or activity in animal models, and cerebrospinal fluid, plasma, and imaging studies suggest similar effects in patients with bipolar disorder (17–19).
Patients who had discontinued mood stabilizers and antipsychotic medication showed gamma range ASSR PLF values comparable to those of control participants, in contrast to decreased PLF response of patients currently receiving these medications. MTP at 50 Hz, but not at 40 Hz, was also larger in unmedicated compared to medicated patients. Medication may therefore have an effect on GABA function and ultimately the ASSR in bipolar disorder. To our knowledge, no previous studies have compared medicated and unmedicated bipolar disorder patients for ASSR measures. In a sample of schizophrenic patients, Hong and colleagues (11) found that those taking atypical antipsychotic medications had greater PLF at 40 Hz than both healthy controls and patients taking conventional antipsychotic medications. However, it is important to note that patients in neither the present study nor the Hong et al. study experimentally manipulated pharmacological treatment, and it is therefore possible that differences between groups were due to individual differences unrelated to medication. Additionally, in the present sample benzodiazepines or similar sedative medications were provided as required to otherwise unmedicated patients. Sedatives could affect gamma range ASSRs, since these are GABA agonists. In the neonatal ventral hippocampal rodent model of schizophrenia, ASSR magnitude and synchronization were decreased by applying GABA agonist muscimol, and the effect was reversed when a GABAA receptor antagonist was given prior to muscimol administration (57). Clarification of the role of pharmacological effects on the ASSR in bipolar disorder awaits experimental studies, ideally with patients tested both on and off medication.
Neurophysiological and imaging evidence indicate that the ASSR is primarily generated by the auditory cortex across a wide range of frequencies, although thalamic, brainstem and cochlear processing may also affect the response (58). Consequently, abnormalities in these pathways could play a role in ASSR deficits. Maharajh and colleagues (16) proposed that a reduction in the neuronal population or the inability of neurons to synchronize consistently to the stimulus between trials may underlie PLF reduction in bipolar disorder. Meta-analyses of structural magnetic resonance imaging (MRI) findings indicated that temporal lobe volume is not affected in bipolar disorder (59, 60). The few studies that quantified superior temporal gyrus (STG) or auditory cortex in bipolar disorder suggest an interaction with stage of illness. STG volumetric reductions have been reported in children and adolescents with bipolar disorder (61) and adult bipolar disorder patients (62), while studies of primarily bipolar disorder patients at first episode have found no such abnormalities (63, 64). Frey and colleagues (65) found that length of bipolar illness was inversely correlated with total gray matter volume. There is scant cellular neuropathology of the STG in bipolar disorder, but a recent cytaoarchitectural assessment of the planum temporale reported reduced neuronal clustering, reduced neural size in layer 3, and glial cell reduction in layer 6 (66). Moreover, neuroanatomical location estimates using MEG revealed diminished interhemispheric asymmetry of the primary auditory cortex in bipolar disorder patients, suggesting cortical disorganization at the STG (67). Reite and colleagues (67) suggest that neurodevelopmental abnormalities underlie functional disorganization of the primary auditory cortex and may contribute to the disturbed function of GABAergic interneurons in layer 3 that modulate the ASSR. In conjunction with the present data, these findings suggest that more anatomically specific measures of auditory cortical regions and cellular features may reveal abnormalities in these regions in bipolar disorder, although they may not be evident at first episode.
Cognitive function
Consistent with many previous studies, cognitive performance was impaired in bipolar disorder patients, particularly on tests of psychomotor performance and verbal learning (32, 33). In the present data set, ASSR measures had little relationship with neuropsychological measures, suggesting that ASSR deficits do not index cellular disturbances which have a general relationship to cognitive function in bipolar disorder.
Future directions
Gamma range ASSR deficits may be a neurophysiological commonality shared by bipolar disorder and schizophrenia, perhaps associated with common genetic risk factors or neuropathological processes. Dysfunctional GABA and glutamate signaling may underlie auditory EEG abnormalities present in both disorders, interacting with psychopharmacological medications common to both patient groups. The interpretation of the gamma range abnormalities in bipolar disorder depends on delineation of the relationship of these deficits to the course of the illness and to cellular mechanisms that affect auditory responses. Understanding the neuropathology of GABA and glutamate in bipolar disorder could explain their role in disturbance of the ASSR. Animal models of bipolar disorder and pharmacological manipulations would help to experimentally characterize mechanisms responsible for deficiencies in the ASSR and could provide a cross-species measure to assess pharmacological interventions. Findings of gray matter STG volumetric reduction in chronic patients and cellular abnormalities in postmortem auditory cortex suggest that the illness may have direct effects on auditory processing. Investigating the relationship of the ASSR with structural MRI of auditory pathways may reveal anatomical contributions to abnormal response in bipolar disorder.
Acknowledgments
This research was supported by National Institute of Mental Health (NIMH) Grants R21 MH071876 (BFO), RO1 MH62150 (BFO), and R01 MH07498 (WPH), and by a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression (WPH). We thank Ashley Steffen, Misty Bodkins, Jennifer Boggs, and Colleen Merrill for their assistance with collecting the data presented in this report.
Footnotes
There are no conflicts of interest for any of the authors of this article. No author has any possible financial gain from the findings presented here.
References
- 1.Picton TW, John MS, Dimitrijevic A, Purcell D. Human auditory steady-state responses. Int J Audiol. 2003;42:177–219. doi: 10.3109/14992020309101316. [DOI] [PubMed] [Google Scholar]
- 2.Light GA, Hsu JL, Hsieh MH, et al. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol Psychiatry. 2006;60:1231–1240. doi: 10.1016/j.biopsych.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 3.Pastor MA, Artieda J, Arbizu J, Marti-Climent JM, Penuelas I, Masdeu JC. Activation of human cerebral and cerebellar cortex by auditory stimulation at 40 Hz. J Neurosci. 2002;22:10501–10506. doi: 10.1523/JNEUROSCI.22-23-10501.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pastor MA, Thut G, Pascual-Leone A. Modulation of steady-state auditory evoked potentials by cerebellar rTMS. Exp Brain Res. 2006;175:702–709. doi: 10.1007/s00221-006-0588-2. [DOI] [PubMed] [Google Scholar]
- 5.Makela JP, Hari R. Evidence for cortical origin of the 40 Hz auditory evoked response in man. Electroencephalogr Clin Neurophysiol. 1987;66:539–546. doi: 10.1016/0013-4694(87)90101-5. [DOI] [PubMed] [Google Scholar]
- 6.Pantev C, Roberts LE, Elbert T, Ross B, Wienbruch C. Tonotopic organization of the sources of human auditory steady-state responses. Hear Res. 1996;101:62–74. doi: 10.1016/s0378-5955(96)00133-5. [DOI] [PubMed] [Google Scholar]
- 7.Hari R, Hamalainen M, Joutsiniemi SL. Neuromagnetic steady-state responses to auditory stimuli. J Acoust Soc Am. 1989;86:1033–1039. doi: 10.1121/1.398093. [DOI] [PubMed] [Google Scholar]
- 8.Galambos R, Makeig S, Talmachoff PJ. A 40-Hz auditory potential recorded from the human scalp. Proc Natl Acad Sci U S A. 1981;78:2643–2647. doi: 10.1073/pnas.78.4.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spencer KM, Salisbury DF, Shenton ME, McCarley RW. Gamma-band auditory steady-state responses are impaired in first episode psychosis. Biol Psychiatry. 2008;64:369–375. doi: 10.1016/j.biopsych.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brenner CA, Sporns O, Lysaker PH, O’Donnell BF. EEG synchronization to modulated auditory tones in schizophrenia, schizoaffective disorder, and schizotypal personality disorder. Am J Psychiatry. 2003;160:2238–2240. doi: 10.1176/appi.ajp.160.12.2238. [DOI] [PubMed] [Google Scholar]
- 11.Hong LE, Summerfelt A, McMahon R, et al. Evoked gamma band synchronization and the liability for schizophrenia. Schizophr Res. 2004;70:293–302. doi: 10.1016/j.schres.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Kwon JS, O’Donnell BF, Wallenstein GV, et al. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teale P, Collins D, Maharajh K, Rojas DC, Kronberg E, Reite M. Cortical source estimates of gamma band amplitude and phase are different in schizophrenia. Neuroimage. 2008;42:1481–1489. doi: 10.1016/j.neuroimage.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson TW, Hernandez OO, Asherin RM, Teale PD, Reite ML, Rojas DC. Cortical gamma generators suggest abnormal auditory circuitry in early-onset psychosis. Cereb Cortex. 2008;18:371–378. doi: 10.1093/cercor/bhm062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Donnell BF, Hetrick WP, Vohs JL, Krishnan GP, Carroll CA, Shekhar A. Neural synchronization deficits to auditory stimulation in bipolar disorder. Neuroreport. 2004;15:1369–1372. doi: 10.1097/01.wnr.0000127348.64681.b2. [DOI] [PubMed] [Google Scholar]
- 16.Maharajh K, Abrams D, Rojas DC, Teale P, Reite ML. Auditory steady state and transient gamma band activity in bipolar disorder. Int Congr Ser. 2007;1300:707–710. [Google Scholar]
- 17.Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 18.Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology. 2007;32:1888–1902. doi: 10.1038/sj.npp.1301312. [DOI] [PubMed] [Google Scholar]
- 19.Brambilla P, Perez J, Barale F, Schettini G, Soares JC. GABAergic dysfunction in mood disorders. Mol Psychiatry. 2003;8:721–737. doi: 10.1038/sj.mp.4001362. [DOI] [PubMed] [Google Scholar]
- 20.Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 21.Thaker GK. Neurophysiological endophenotypes across bipolar and schizophrenia psychosis. Schizophr Bull. 2008;34:760–773. doi: 10.1093/schbul/sbn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olincy A, Martin L. Diminished suppression of the P50 auditory evoked potential in bipolar disorder subjects with a history of psychosis. Am J Psychiatry. 2005;162:43–49. doi: 10.1176/appi.ajp.162.1.43. [DOI] [PubMed] [Google Scholar]
- 23.Schulze KK, Hall MH, McDonald C, et al. P50 auditory evoked potential suppression in bipolar disorder patients with psychotic features and their unaffected relatives. Biol Psychiatry. 2007;62:121–128. doi: 10.1016/j.biopsych.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Carroll CA, Kieffaber PD, Vohs JL, O’Donnell BF, Shekhar A, Hetrick WP. Contributions of spectral frequency analyses to the study of P50 ERP amplitude and suppression in bipolar disorder with or without a history of psychosis. Bipolar Disord. 2008;10:776–787. doi: 10.1111/j.1399-5618.2008.00622.x. [DOI] [PubMed] [Google Scholar]
- 25.Hall MH, Rijsdijk F, Kalidindi S, et al. Genetic overlap between bipolar illness and event-related potentials. Psychol Med. 2007;37:667–678. doi: 10.1017/S003329170600972X. [DOI] [PubMed] [Google Scholar]
- 26.O’Donnell BF, Vohs JL, Hetrick WP, Carroll CA, Shekhar A. Auditory event-related potential abnormalities in bipolar disorder and schizophrenia. Int J Psychophysiol. 2004;53:45–55. doi: 10.1016/j.ijpsycho.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Souza VB, Muir WJ, Walker MT, et al. Auditory P300 event-related potentials and neuropsychological performance in schizophrenia and bipolar affective disorder. Biol Psychiatry. 1995;37:300–310. doi: 10.1016/0006-3223(94)00131-L. [DOI] [PubMed] [Google Scholar]
- 28.Schulze KK, Hall MH, McDonald C, et al. Auditory P300 in patients with bipolar disorder and their unaffected relatives. Bipolar Disord. 2008;10:377–386. doi: 10.1111/j.1399-5618.2007.00527.x. [DOI] [PubMed] [Google Scholar]
- 29.Muir WJ, St Clair DM, Blackwood DH. Long-latency auditory event-related potentials in schizophrenia and in bipolar and unipolar affective disorder. Psychol Med. 1991;21:867–879. doi: 10.1017/s003329170002986x. [DOI] [PubMed] [Google Scholar]
- 30.Carlson GA, Meyer SE. Phenomenology and diagnosis of bipolar disorder in children, adolescents, and adults: complexities and developmental issues. Dev Psychopathol. 2006;18:939–969. doi: 10.1017/S0954579406060470. [DOI] [PubMed] [Google Scholar]
- 31.Hayden EP, Bodkins M, Brenner C, et al. A multimethod investigation of the behavioral activation system in bipolar disorder. J Abnorm Psychol. 2008;117:164–170. doi: 10.1037/0021-843X.117.1.164. [DOI] [PubMed] [Google Scholar]
- 32.Malhi GS, Ivanovski B, Hadzi-Pavlovic D, Mitchell PB, Vieta E, Sachdev P. Neuropsychological deficits and functional impairment in bipolar depression, hypomania and euthymia. Bipolar Disord. 2007;9:114–125. doi: 10.1111/j.1399-5618.2007.00324.x. [DOI] [PubMed] [Google Scholar]
- 33.Martinez-Aran A, Vieta E, Reinares M, et al. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry. 2004;161:262–270. doi: 10.1176/appi.ajp.161.2.262. [DOI] [PubMed] [Google Scholar]
- 34.Mucci A, Volpe U, Merlotti E, Bucci P, Galderisi S. Pharmaco-EEG in psychiatry. Clin EEG Neurosci. 2006;37:81–98. doi: 10.1177/155005940603700206. [DOI] [PubMed] [Google Scholar]
- 35.Jaaskelainen IP, Hirvonen J, Saher M, et al. Dose-dependent suppression by ethanol of transient auditory 40-Hz response. Psychopharmacology. 2000;148:132–135. doi: 10.1007/s002130050034. [DOI] [PubMed] [Google Scholar]
- 36.Jaaskelainen IP, Naatanen R, Sillanaukee P. Effect of acute ethanol on auditory and visual event-related potentials: a review and reinterpretation. Biol Psychiatry. 1996;40:284–291. doi: 10.1016/0006-3223(95)00385-1. [DOI] [PubMed] [Google Scholar]
- 37.Pritchard W, Sokhadze E, Houlihan M. Effects of nicotine and smoking on event-related potentials: a review. Nicotine Tob Res. 2004;6:961–984. doi: 10.1080/14622200412331324848. [DOI] [PubMed] [Google Scholar]
- 38.Sacco KA, Bannon KL, George TP. Nicotinic receptor mechanisms and cognition in normal states and neuropsychiatric disorders. J Psychopharmacol. 2004;18:457–474. doi: 10.1177/0269881104047273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez R, Kallenbach U, Singer W, Munk MH. Short- and long-term effects of cholinergic modulation on gamma oscillations and response synchronization in the visual cortex. J Neurosci. 2004;24:10369–10378. doi: 10.1523/JNEUROSCI.1839-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thase ME. Bipolar depression: issues in diagnosis and treatment. Harv Rev Psychiatry. 2005;13:257–271. doi: 10.1080/10673220500326425. [DOI] [PubMed] [Google Scholar]
- 41.Miklowitz DJ, Johnson SL. The psychopathology and treatment of bipolar disorder. Annu Rev Clin Psychol. 2006;2:199–235. doi: 10.1146/annurev.clinpsy.2.022305.095332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- 43.Buckley PF. Substance abuse in schizophrenia: a review. J Clin Psychiatry. 1998;59 (Suppl 3):26–30. [PubMed] [Google Scholar]
- 44.Leonard S, Adler LE, Benhammou K, et al. Smoking and mental illness. Pharmacol Biochem Behav. 2001;70:561–570. doi: 10.1016/s0091-3057(01)00677-3. [DOI] [PubMed] [Google Scholar]
- 45.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 46.First MB, Spitzer RL, Miriam G, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen (SCID-I/P W/PSY SCREEN) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 47.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 48.Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 49.Berk M, Ng F, Wang WV, et al. The empirical redefinition of the psychometric criteria for remission in bipolar disorder. J Affect Disord. 2008;106:153–158. doi: 10.1016/j.jad.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 50.Wechsler D. WAIS-III: Administration and Scoring Manual: Wechsler Adult Intelligence Scale. 3. San Antonio: Psychological Corp; 1997. [Google Scholar]
- 51.Reitan R, Wolfson D. The Halstead-Reitan neuropsychological test battery: theory and clinical interpretation. Tucson: Neuropsychology Press; 1993. [Google Scholar]
- 52.Welsh KA, Butters N, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology. 1994;44:609–614. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]
- 53.Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- 54.Tallon-Baudry C, Bertrand O, Delpuech C, Pernier J. Stimulus specificity of phase-locked and non-phase-locked 40 Hz visual responses in human. J Neurosci. 1996;16:4240–4249. doi: 10.1523/JNEUROSCI.16-13-04240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Donnell BF, Hetrick WP, Bodkins M, et al. Event-related potential abnormalities in bipolar disorder: relationship to symptoms, medication and substance disorders. In: Kotlar MB, editor. New Developments in Mania Research. Hauppauge: Nova Science Publishers; 2006. pp. 115–133. [Google Scholar]
- 56.Dean B. The neurobiology of bipolar disorder: findings using human postmortem central nervous system tissue. Aust N Z J Psychiatry. 2004;38:135–140. doi: 10.1080/j.1440-1614.2004.01319.x. [DOI] [PubMed] [Google Scholar]
- 57.Vohs JL, Chambers RA, Krishnan GP, O’Donnell BF, Berg S, Morzorati SL. GABAergic modulation of the 40 Hz auditory steady-state response in a rat model of schizophrenia. Int J Neuropsychopharmacol. 2010;13:487–497. doi: 10.1017/S1461145709990307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brenner CA, Krishnan GP, Vohs JL, et al. Steady state responses: electrophysiological assessment of sensory function in schizophrenia. Schizophr Bull. 2009;35:1065–1077. doi: 10.1093/schbul/sbp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. Br J Psychiatry. 2009;195:194–201. doi: 10.1192/bjp.bp.108.059717. [DOI] [PubMed] [Google Scholar]
- 60.Kempton MJ, Geddes JR, Ettinger U, Williams SC, Grasby PM. Meta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Arch Gen Psychiatry. 2008;65:1017–1032. doi: 10.1001/archpsyc.65.9.1017. [DOI] [PubMed] [Google Scholar]
- 61.Chen HH, Nicoletti MA, Hatch JP, et al. Abnormal left superior temporal gyrus volumes in children and adolescents with bipolar disorder: a magnetic resonance imaging study. Neurosci Lett. 2004;363:65–68. doi: 10.1016/j.neulet.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 62.Takahashi T, Malhi GS, Wood SJ, et al. Gray matter reduction of the superior temporal gyrus in patients with established bipolar I disorder. J Affect Disord. 2009;123:276–282. doi: 10.1016/j.jad.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 63.Takahashi T, Wood SJ, Soulsby B, et al. An MRI study of the superior temporal subregions in first-episode patients with various psychotic disorders. Schizophr Res. 2009;113:158–166. doi: 10.1016/j.schres.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 64.Hirayasu Y, Shenton ME, Salisbury DF, McCarley RW. Hippocampal and superior temporal gyrus volume in first-episode schizophrenia. Arch Gen Psychiatry. 2000;57:618–619. doi: 10.1001/archpsyc.57.6.618. [DOI] [PubMed] [Google Scholar]
- 65.Frey BN, Zunta-Soares GB, Caetano SC, et al. Illness duration and total brain gray matter in bipolar disorder: evidence for neurodegeneration? Eur Neuropsychopharmacol. 2008;18:717–722. doi: 10.1016/j.euroneuro.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 66.Beasley CL, Chana G, Honavar M, Landau S, Everall IP, Cotter D. Evidence for altered neuronal organisation within the planum temporale in major psychiatric disorders. Schizophr Res. 2005;73:69–78. doi: 10.1016/j.schres.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 67.Reite M, Teale P, Rojas DC, Reite E, Asherin R, Hernandez O. MEG auditory evoked fields suggest altered structural/functional asymmetry in primary but not secondary auditory cortex in bipolar disorder. Bipolar Disord. 2009;11:371–381. doi: 10.1111/j.1399-5618.2009.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]