Abstract
Mammalian diacylglycerol kinases (DGK) are a family of enzymes that catalyze the phosphorylation of diacylglycerol to produce phosphatidic acid. The extent of interaction of these enzymes with monoacylglycerols is the focus of the present study. Because of the structural relationship between mono- and diacylglycerols, one might expect the monoacylglycerols to be either substrates or inhibitors of DGK. This would have some consequence to lipid metabolism. One of the lipid metabolites that would be affected is 2-arachidonoyl glycerol (2-AG), which is an endogenous ligand for the CB1 cannabinoid receptor. We determined if the monoglycerides 2-AG or 2-oleoyl glycerol (2-OG) affected diacylglycerol kinase (DGK) activity. We found that 2-AG is a very poor substrate for either the epsilon or the zeta isoforms of DGK. Moreover, 2-AG is an inhibitor for both of these DGK isoforms. 2-OG is also a poor substrate for these two isoforms of DGK. As an inhibitor, 2-OG inhibits DGKε less than does 2-AG, while for DGKζ these two monoglycerides have similar inhibitory potency. These results have implications for the known role of DGKε in neuronal function and in epilepsy since the action of this enzyme will remove 1-stearoyl-2-arachidonoylglycerol, the precursor of the endocannabinoid 2-AG.
Keywords: diacylglycerol kinase, 2-arachidonoyl glycerol, 2-oleoyl glycerol, endocannabinoids
1. Introduction
Diacylglycerol kinase (DGK) from different species exhibits different specificities. Thus bacterial forms of DGK can phosphorylate ceramide as well as diacylglycerol (DAG), while DGK from yeast utilizes CTP, rather than ATP as the source of phosphate [1]. Mammalian DGKs are a family of enzymes comprised of at least 10 isoforms [2]. We undertook this study to evaluate the interactions of 2-acyl-glycerols with isoforms of DGK in order to assess the possible role of this enzyme family in affecting the concentration of these signaling lipids in cells as well as to further understand the nature of substrate and lipid interactions with binding sites on DGKs. Mammalian isoforms of DGK have only been shown to catalyze the phosphorylation of one class of lipid substrates, DAG, using only ATP as the source of phosphate. In the present study we determined if structurally related monoacylglycerols are either substrates or inhibitors of mammalian DGKs.
A particularly important acyl chain for monoacyl- and diacyl-glycerols is arachidonic acid. DAG having arachidonic acid at the sn-2 position is an intermediate in phosphatidylinositol cycling. Arachidonoyl-DAG is preferentially phosphorylated by the isoform DGKε [2]. The monoglyceride with arachidonic acid at the sn-2 position is 2-arachidonoyl glycerol (2-AG). This monoglyceride is an important ligand for the CB1 cannabinoid receptor [3]. 2-AG is known to be generated in the brain by the enzyme diacylglycerol (DAG) lipase [4] and is one of the most abundant molecular species of monoacylglycerols in the brain [5]. The concentration of DAG in brain synaptosomes is at least an order of magnitude higher than that of 2-AG [6]. Interestingly, even in organisms lacking known cannabinoid receptors, such as nematodes, 2-AG has been identified [7]. This suggests that 2-AG, in addition to being a cannabanoid receptor ligand, is also an intermediate in lipid metabolism in organisms without developed endocannabinoid systems. The expression of DAG lipase, that converts DAG to a monoglyceride, is required for axonal growth during development and for retrograde synaptic signaling at mature synapses. Endocannabinoid signaling is a key regulator of synaptic communication throughout the central nervous system [8]. The lysolipid 2-Arachidonoyl-sn-glycero-3-phosphate, an arachidonic acid-containing lysophosphatidic acid is found in rat brain and can be rapidly converted to 2-AG. However, the metabolic fate of 2-AG is not known. A principle metabolic fate of this lipid is its hydrolysis by monoacylglycerol lipase. However, an additional possibility is that 2-AG is also a substrate for diacylglycerol kinase (DGK) to reform lysophosphatidic acid that is also a signaling lipid [9–12]. This possibility was tested in the present study.
2. Materials and methods
2.1. Materials
Lipids were purchased from Avanti Polar Lipids (Alabaster, AL) and were dissolved in 2:1 CHCl3/CH3OH or in pure CHCl3. 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) was stored in 2:1 CHCl3/CH3OH and 0.1% (w/v) butylated hydroxytoluene (BHT). 2-AG and 2-OG were stored in C2H5OH. [γ-32P]ATP (50 µCi/mL) was purchased from Perkin Elmer Life Sciences. All other chemicals and reagents were purchased from Sigma or BioShop Canada.
2.2. Enzyme Preparation for DGK Enzymatic Activity Assay
Human DGKε with a C-terminal hexahistidine tag fusion or DGKζ with a C-terminal FLAG epitope tag fusion were overexpressed in baculovirus-infected Sf21 cells. The cell pellets were resuspended in cold lysis buffer (20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1mM EDTA, 2.4 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1mM sodium orthovanadate and 1:1000 dilution of protease inhibitor (Sigma)) and detergent 1% (v/v) Nonidet P-40 and kept on ice for 10 minutes to lyse. The lysates were centrifuged at 100,000g for 30 minutes at 4°C to solubilize DGK and the supernatant was utilized in the mixed micelle activity assays.
2.3. Detergent-Phospholipid-Mixed Micelle-based DGK Enzymatic Activity Assay
Inhibition of DGKε and DGKζ by 2-AG or 2-OG was studied using enzymatic activity assays following a previously established protocol [13–15]. Lipid films were prepared by evaporation of organic solvent of lipid solutions of the substrate 1-stearoyl-2-arachidonoyl glycerol (SAG) for DGKε or 1,2-dioleoyl glycerol (DOG) for DGKζ. In addition to the substrate, the phospholipid DOPC was added for DGKε or 1,2-dioleoyl-sn-glycero-3-[phospho-L-serine] (DOPS) for DGKζ. The monoglycerides, 2-AG and 2-OG, were tested alone as substrates; or tested as inhibitors with SAG or DOG as substrates. When 2-AG and 2-OG were tested as substrates, SAG and DOG were not included in the lipid films. DOPC or DOPS were added in addition to other lipids so as to maintain the total concentration of all lipids at 24.1 mol% (19 mM). The lipids were dried under stream of nitrogen gas to remove the organic solvent and then further dried in a vacuum dessicator for 2 hours. All lipids and lipid films were covered with Argon gas to avoid oxidation by air. The films were hydrated with 50 µL of 4x assay buffer (200 mM Tris-HCl (pH 7.5), 400 mM NaCl, 20mM MgCl2, 4mM EGTA, 30mM Triton X-100 and 30mM Triton X-114) and vortexed for 2 minutes, followed by addition of 105 µL ddH2O, 20 µL of 10 mM DTT and 5 µL of Sf21 insect cell lysates expressing either DGKε or DGKζ or empty vector controls, to obtain a final volume of 180 µL. The reaction was initiated with 20 µL of 1 mM [γ-32P]ATP (50 µCi/mL) and was stopped after 10 minutes at 25°C with the addition of 2 mL of stop solution (1:1 CHCl3/CH3OH and 0.25 mg/ml dihexadecyl phosphate). The organic layer was washed three times. To allow for maximal separation of the organic and aqueous phases, the mixture was allowed to stand for 2 hours, 20 minutes and 5 minutes, after the first, second and third wash, respectively, with 2 mL of wash solution (7:1 H2O/CH3OH, 1% HClO4, 0.1% H3PO4) used for each wash. The aqueous layer was removed after each wash. 400 µL of the organic layer was collected in a scintillation vial and incorporation of radioactive phosphate into the organic phase was measured by Cerenkov counting using a scintillation counter (Beckman Coulter). The counts were corrected for a blank reaction in which no enzyme was added. The activity is presented as % relative activity taking the control with no inhibitor as 100% activity. The assays were performed in triplicates and the results are presented as the mean ± the standard deviation of the mean. Lysates from mock transfected insect cells were used as negative controls.
3. Results
Because of the specific binding of DGKε to an arachidonoyl group, there was a particular interest to evaluate the behaviour of 2-AG with this isoform of DGK. The substrate specificity and kinetic constants for DGKε has been recently reported [13]. Using the preferred substrate of DGKε, SAG, as a positive control, the rate of phosphorylation of 2-AG was only 6.35 ± 0.15 % that of SAG. Thus, 2-AG essentially is a very poor substrate for DGKε. We also evaluated 2-OG as a substrate of this isoform of DGK, but the rate of phosphorylation was even lower than for 2-AG, reflecting the specificity of DGKε for arachidonoyl containing lipids.
A possible contribution to the slow rate of phosphorylation of these monoglycerides is that their partitioning between aqueous and micellar phases favors water solubility. However, this is not a major factor. 2-OG is a weaker substrate than 2-AG, yet 2-AG should be the more water soluble of the two monoglycerides. In addition, in pure form these monoglycerides are insoluble in water but readily soluble in organic solvent. Lastly, although they are poor substrates, we show below that these monoglycerides can inhibit various isoforms of DGK and can therefore partition into the membrane.
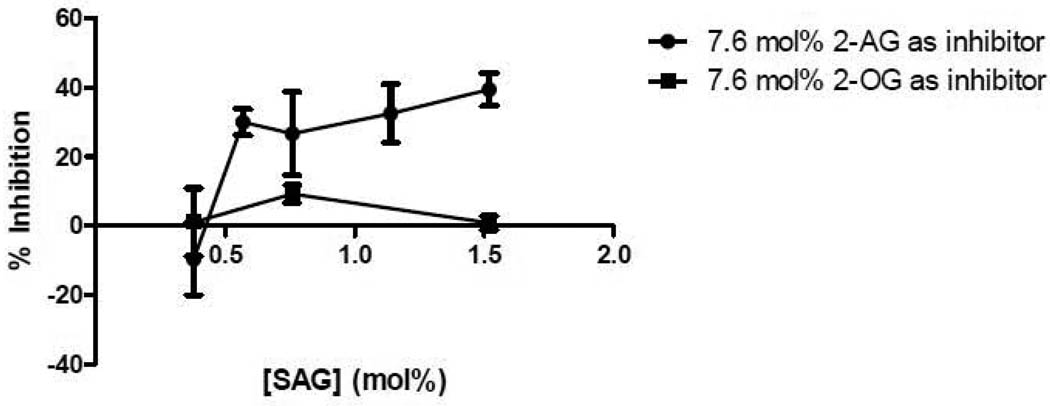
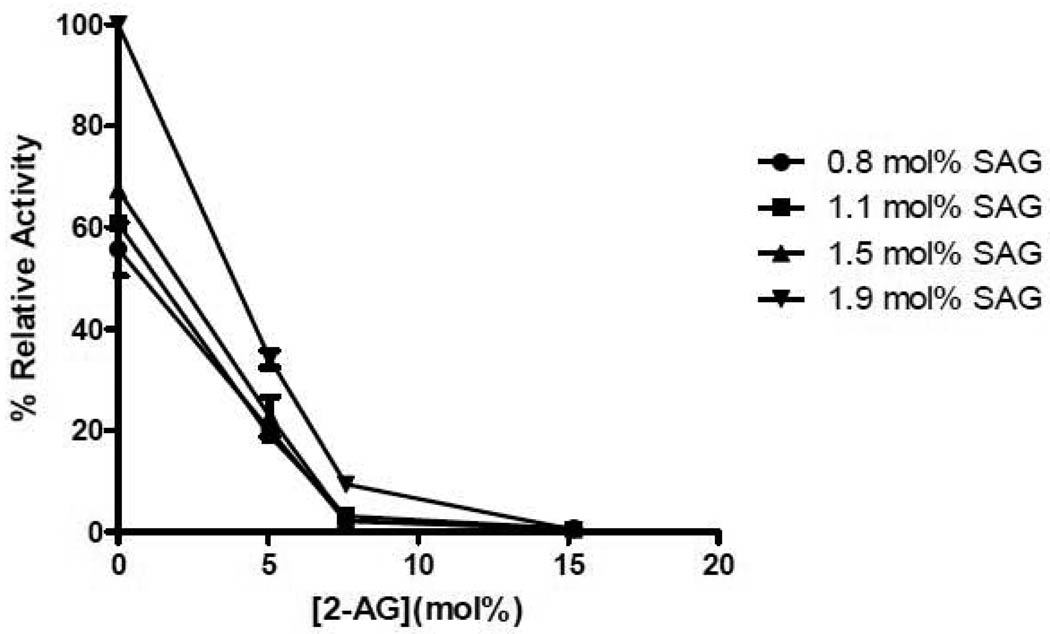
The monoglyceride, 2-AG, was also tested as an inhibitor of DGKε. 7.6 mol% 2-AG was added to micelles containing different amounts of SAG (Fig. 1). The concentration dependence of the inhibition is complex and could not be analyzed quantitatively with any simple kinetic model. At low concentration of SAG there is no inhibition by 2-AG, likely because the 2-AG is a weak substrate. At higher concentrations of SAG, however, there is significant inhibition by 2-AG. At sufficiently high concentrations of 2-AG, there is essentially complete inhibition of the activity of DGKε in phosphorylating SAG (Fig. 2). The error bars given for this graph represent the precision of replicates in the assay performed under identical experimental conditions. There is somewhat greater experiment-to-experiment variation, likely resulting from factors such as different cell lysates being used as the source of enzyme for different experiments as well as the purity of the monoglyceride that is susceptible to both acyl chain migration as well as oxidation of the 2-AG. The extent of inhibition shown in Fig. 2 is somewhat greater than that presented in Fig. 1. Nevertheless, it is clear that the inhibition by 2-AG is much greater than that by 2-OG (Fig. 1). 2-OG has essentially no activity as either a substrate or as an inhibitor for the epsilon isoform of DGK.
Figure 1. Inhibition of DGKε by 2-AG and 2-OG.
Lipid films were created with 0.37, 0.57, 0.76, 1.14 and 1.52 mol% SAG as substrate and 7.6 mol% 2-AG or 2-OG as inhibitor and used in a mixed micelle activity assay. The data is presented as percent inhibition calculated by taking the ratio of the difference in activity due to inhibition over the activity without the inhibitor. The data points are mean ± S.E.M., n=3.
Figure 2. Effect of Varying Concentration of 2-AG on Inhibition of DGKε.
Lipid films were created with 0.76, 1.14, 1.52 and 1.90 mol% SAG as substrate and 0, 5.1, 7.6 and 15.2 mol% 2-AG as inhibitor and used in a mixed micelle activity assay. The data is presented as percent relative activity taking the activity of 1.9 mol% SAG with 0 mol% 2-AG as 100%. The data points are mean ± S.E.M., n=3.
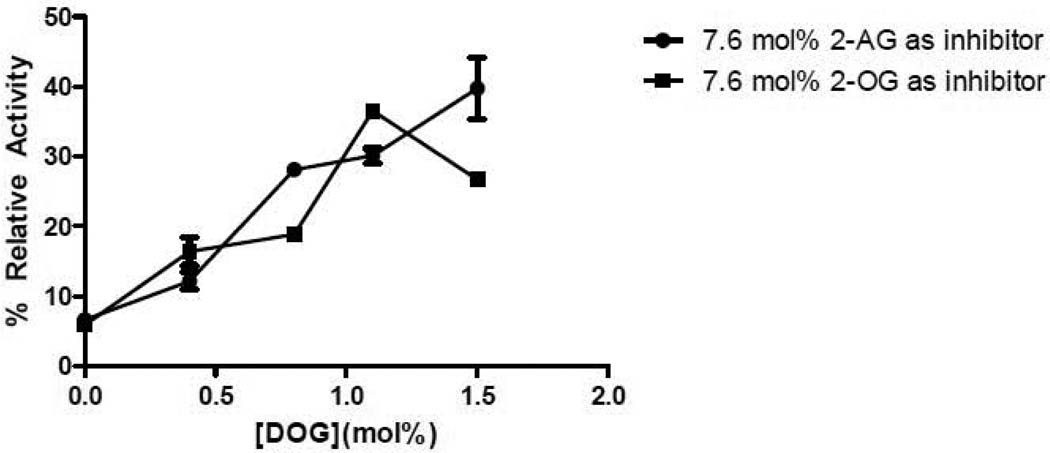
In contrast with DGKε, the isoform DGKζ exhibits similar inhibition with 2-OG and 2-AG (Fig. 3). A determination of the Michaelis-Menten constants of DGKζ has been recently studied in comparison with other isoforms [16].
Figure 3. Inhibition of DGKζ by 2-AG and 2-OG.
Lipid films were created with 0.37, 0.57, 0.76, 1.14 and 1.52 mol% DOG as substrate and 7.6 mol% 2-AG or 2-OG as inhibitor and used in a mixed micelle activity assay. The data is presented as percent relative activity taking the activity of 1.52 mol% DOG without any inhibitor as 100%. The data points are mean ± S.E.M., n=3.
4. Discussion
The finding that neither 2-AG nor 2-OG is a substrate for DGKε or DGKζ shows the specificity of DGKs for diacylglycerols. This is the case even for DGKε that has been shown to have a particularly strong specificity for an arachidonoyl group on the substrate [2]. We have previously shown that DGKε also has specificity for the acyl chain at the sn-1 position with 18:0 being the most favourable acyl chain at that position [13]. The diacylglycerol becomes a poorer substrate as the acyl chain becomes shorter than 18 carbons, but the effect is modest for fatty acids. However, when the acyl chain is completely absent, as with 2-AG, the lipid is essentially no longer a substrate.
We have previously demonstrated that the enantiomer of the natural stereoisomer 1,2-dioleoylglycerol, i.e. 2,3-dioleoylglycerol, exhibits greater inhibition of DGKζ than of DGKε [16]. Similarly, we have determined that 2-AG and 2-OG have a very low potency of inhibition against DGKε compared with the inhibition of these monoglycerides with DGKζ. This behaviour is analogous to the relative inhibitory effects of 2,3-dioleoylglycerol [16]. Since DGKε is more selective in substrate binding than other mammalian DGK isoforms, it is less inhibited by either 2,3-dioleoylglycerol or by 2-OG, than other mammalian DGK isoforms. In addition, although not a potent inhibitor, 2-AG is a better inhibitor of DGKε than is 2-OG. These results can be explained by the fact that DGKε binds arachidonoyl-containing lipids more specifically, as is also indicated by the arachidonoyl substrate-specificity of this isoform.
In addition to furthering our understanding of the properties of diacylglycerol kinases, there may be relevance of these findings to the role of endocannabinoids in neuronal function. 2-AG is an endocannabinoid that can arise from the DAG lipase catalyzed cleavage of SAG, the preferred substrate of DGKε. Another route of metabolism of SAG is by DGKε-catalyzed phosphorylation to generate SAPA. In DGKε knockout mice the conversion of this particular species of diacylglycerol to phosphatidic acid is reduced [17]. Consequently, an alternative path for the SAG metabolism would be its conversion to the endocannabinoid, 2-AG by the DAG lipase. Based on our findings on the inhibitory property of 2-AG on DGKε, there could be a weak feed-forward effect of 2-AG on its own formation as a result of its inhibition of DGKε.
This pathway appears to have importance in epilepsy. DGKε(−/−) mice had significantly fewer motor seizure and epileptic events compared with DGKε(+/+) mice [18]. This could be explained by the fact that in the knockout mice a greater fraction of the SAG would be converted to 2-AG. 2-AG itself is known to have anticonvulsive effects through activation of cannabanoid receptors [19]. Pharmacological studies have shown that it is the type 1 cannabanoid receptor that is linked to epileptic events [20]. The natural resistance of certain species to epileptic seizures has been suggested to be a consequence of their high level of expression of type 1 cannabanoid receptors [21]. The formation of 2-AG resulting in the activation of type 1 cannabanoid receptors will be affected by the activity of DGKε that reduces the fraction of SAG converted to 2-AG. The present study describes the relationship between 2-AG and DGKs that could impinge on neuronal function.
Acknowledgements
This work was supported in part by a grant from the Natural Sciences and Engineering Research Council of Canada, grant 9848 (to R.M.E.) and from the National Institutes of Health Grants R01-CA95463 (to M.K.T.).
Abbreviations used
- DGK
diacylglycerol kinase
- 2-AG
2-arachidonoyl glycerol
- 2-OG
2-oleoyl glycerol
- DOPC
1,2-dioleoyl-sn-glycero-3-phosphocholine
- DOPS
1,2-dioleoyl-sn-glycero-3-[phospho-L-serine]
- SAPA
1-stearoyl-2-arachidonoyl phosphatidic acid
- DOG
1,2-dioleoylglycerol
- SAG
1-stearoyl-2-arachidonoylglycerol
- DAG
diacylglycerol
- DTT
dithiothreitol
- BHT
butylated hydroxytoluene
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References List
- 1.Han GS, O'Hara L, Siniossoglou S, Carman GM. Characterization of the Yeast DGK1-encoded CTP-dependent Diacylglycerol Kinase. J. Biol. Chem. 2008;283:20443–20453. doi: 10.1074/jbc.M802866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topham MK, Epand RM. Mammalian diacylglycerol kinases: molecular interactions and biological functions of selected isoforms. Biochim. Biophys. Acta. 2009;1790:416–424. doi: 10.1016/j.bbagen.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Marzo V. The endocannabinoid system: Its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacological Research. 2009;60:77–84. doi: 10.1016/j.phrs.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, M.Di V, Doherty P. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kondo S, Kondo H, Nakane S, Kodaka T, Tokumura A, Waku K, Sugiura T. 2-Arachidonoylglycerol, an endogenous cannabinoid receptor agonist: identification as one of the major species of monoacylglycerols in various rat tissues, and evidence for its generation through Ca2+-dependent and -independent mechanisms. FEBS Letters. 1998;429:152–156. doi: 10.1016/s0014-5793(98)00581-x. [DOI] [PubMed] [Google Scholar]
- 6.Oka S, Arai S, Waku K, Tokumura A, Sugiura T. Depolarization-induced rapid generation of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand, in rat brain synaptosomes. J Biochem. 2007;141:687–697. doi: 10.1093/jb/mvm070. [DOI] [PubMed] [Google Scholar]
- 7.Lehtonen M, Reisner K, Auriola S, Wong G, Callaway JC. Mass-spectrometric identification of anandamide and 2-arachidonoylglycerol in nematodes. Chem. Biodivers. 2008;5:2431–2441. doi: 10.1002/cbdv.200890208. [DOI] [PubMed] [Google Scholar]
- 8.Katona I, Freund TF. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat. Med. 2008;14:923–930. doi: 10.1038/nm.f.1869. [DOI] [PubMed] [Google Scholar]
- 9.Parrill AL. Lysophospholipid interactions with protein targets. Biochim. Biophys. Acta. 2008;1781:540–546. doi: 10.1016/j.bbalip.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakane S, Oka S, Arai S, Waku K, Ishima Y, Tokumura A, Sugiura T. 2-Arachidonoyl-sn-glycero-3-phosphate, an arachidonic acid-containing lysophosphatidic acid: occurrence and rapid enzymatic conversion to 2-arachidonoyl-sn-glycerol, a cannabinoid receptor ligand, in rat brain. Archives of Biochemistry and Biophysics. 2002;402:51–58. doi: 10.1016/S0003-9861(02)00038-3. [DOI] [PubMed] [Google Scholar]
- 11.Oka S, Toshida T, Maruyama K, Nakajima K, Yamashita A, Sugiura T. 2-Arachidonoyl-sn-glycero-3-phosphoinositol: A Possible Natural Ligand for GPR55. J Biochem. 2009;145:13–20. doi: 10.1093/jb/mvn136. [DOI] [PubMed] [Google Scholar]
- 12.Tomar A, George SP, Mathew S, Khurana S. Differential Effects of Lysophosphatidic Acid and Phosphatidylinositol 4,5-Bisphosphate on Actin Dynamics by Direct Association with the Actin-binding Protein Villin. Journal of Biological Chemistry. 2009;284:35278–35282. doi: 10.1074/jbc.C109.060830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lung M, Shulga YV, Ivanova PT, Myers DS, Milne SB, Brown HA, Topham MK, Epand RM. Diacylglycerol kinase epsilon is selective for both acyl chains of phosphatidic acid or diacylglycerol. J. Biol. Chem. 2009;284:31062–31073. doi: 10.1074/jbc.M109.050617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsh JP, Suen R, Lemaitre RN, Glomset JA. Arachidonoyl-diacylglycerol kinase from bovine testis. Purification and properties. J. Biol. Chem. 1994;269:21155–21164. [PubMed] [Google Scholar]
- 15.Thirugnanam S, Topham MK, Epand RM. Physiological implications of the contrasting Modulation of the Activities of the e and z Isoforms of Diacylglycerol Kinase. Biochemistry. 2001;40:10607–10613. doi: 10.1021/bi010609s. [DOI] [PubMed] [Google Scholar]
- 16.Epand RM, Shulga YV, Timmons HC, Perri AL, Belani JD, Perinpanathan K, Johnson-McIntire LB, Bajjalieh S, Dicu AO, Elias C, Rychnovsky SD, Topham MK. Substrate chirality and specificity of diacylglycerol kinases and the multisubstrate lipid kinase. Biochemistry. 2007;46:14225–14231. doi: 10.1021/bi701584v. [DOI] [PubMed] [Google Scholar]
- 17.Milne SB, Ivanova PT, Armstrong MD, Myers DS, Lubarda J, Shulga YV, Topham MK, Brown HA, Epand RM. Dramatic Differences in the Roles in Lipid Metabolism of Two Isoforms of Diacylglycerol Kinase. Biochemistry. 2008;47:9372–9379. doi: 10.1021/bi800492c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Musto A, Bazan NG. Diacylglycerol kinase epsilon modulates rapid kindling epileptogenesis. Epilepsia. 2006;47:267–276. doi: 10.1111/j.1528-1167.2006.00418.x. [DOI] [PubMed] [Google Scholar]
- 19.Bhaskaran MD, Smith BN. Cannabinoid-mediated inhibition of recurrent excitatory circuitry in the dentate gyrus in a mouse model of temporal lobe epilepsy. PLoS. One. 2010;5:e10683. doi: 10.1371/journal.pone.0010683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rizzo V, Ferraro G, Carletti F, Lonobile G, Cannizzaro C, Sardo P. Evidences of cannabinoids-induced modulation of paroxysmal events in an experimental model of partial epilepsy in the rat. Neurosci. Lett. 2009;462:135–139. doi: 10.1016/j.neulet.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Araujo BH, Torres LB, Cossa AC, Naffah-Mazzacoratti MG, Cavalheiro EA. Hippocampal expression and distribution of CB1 receptors in the Amazonian rodent Proechimys: an animal model of resistance to epilepsy. Brain Res. 2010;1335:35–40. doi: 10.1016/j.brainres.2010.03.031. [DOI] [PubMed] [Google Scholar]