Abstract
In this paper we raise ‘ten questions’ broadly related to ‘omics’, the term systems biology, and why the new biology has failed to deliver major therapeutic advances for many common diseases, especially diabetes and cardiovascular disease. We argue that a fundamentally narrow and reductionist perspective about the contribution of genes and genetic variants to disease is a key reason ‘omics’ has failed to deliver the anticipated breakthroughs. We then point out the critical utility of key concepts from physiology like homeostasis, regulated systems and redundancy as major intellectual tools to understand how whole animals adapt to the real world. We argue that a lack of fluency in these concepts is a major stumbling block for what has been narrowly defined as ‘systems biology’ by some of its leading advocates. We also point out that it is a failure of regulation at multiple levels that causes many common diseases. Finally, we attempt to integrate our critique of reductionism into a broader social framework about so-called translational research in specific and the root causes of common diseases in general. Throughout we offer ideas and suggestions that might be incorporated into the current biomedical environment to advance the understanding of disease through the perspective of physiology in conjunction with epidemiology as opposed to bottom-up reductionism alone.

Michael J. Joyner (left), MD, is the Frank R. and Shari Caywood Professor of Anesthesiology at Mayo Clinic where he was named Distinguished Investigator in 2010. His interests include: cardiovascular regulation in conscious humans, the physiology of world records, and the carotid chemoreceptors as sensors of blood glucose. His undergraduate (1981) and medical (1987) degrees are from the University of Arizona with residency and research training at Mayo. His lab has been continuously funded by the NIH since the early 1990s, and former fellows have established independent research programs at leading institutions in North America, Europe and Japan. Bente Klarlund Pedersen (right) is Professor of Integrative Medicine at Copenhagen University and a specialist in infectious diseases and internal medicine. She is the Director of the Danish National Research Foundation's Centre of Inflammation and Metabolism (CIM) at Rigshospitalet/University Hospital. Her research group has identified skeletal muscle as an endocrine organ that produces and releases signal peptides, socalled ‘myokines’. She has served as President of the International Society of Exercise and Immunology, coordinator of the Muscle Research Cluster at the Faculty of Health Sciences, Copenhagen University and as President of the National Council for Public Health in Denmark.
What does the term ‘systems biology’ mean to physiologists? This question is especially interesting for integrative physiologists who study conscious humans both with and without co-existing disease. To begin to answer this question, we first looked for a clear description of what systems biology is, what problems it hopes to solve, and how it differs from other more ‘traditional’ disciplines in the life sciences. In general we could find no clear definition of what exactly systems biology is and how it differs fundamentally from physiology. However, as part of our search, we found several interesting statements on the systemsbiology.org website. Here are three examples.
‘Systems biology is the study of an organism, viewed as an integrated and interacting network of genes, proteins and biochemical reactions which give rise to life. Instead of analyzing individual components or aspects of the organism, such as sugar metabolism or a cell nucleus, systems biologists focus on all the components and the interactions among them, all as part of one system. These interactions are ultimately responsible for an organism's form and functions.’
‘Traditional biology – the kind most of us studied in high school and college, and that many generations of scientists before us have pursued – has focused on identifying individual genes, proteins and cells, and studying their specific functions. But that kind of biology can yield relatively limited insights about the human body.’
‘As an analogy, if you wanted to study an automobile, and focused on identifying the engine, seat belts, and tail lights, and studied their specific functions, you would have no real understanding of how an automobile operates. More important, you would have no understanding of how to effectively service the vehicle when something malfunctions. So too, a traditional approach to studying biology and human health has left us with a limited understanding of how the human body operates, and how we can best predict, prevent, or remedy potential health problems. Biologists, geneticists, and doctors have had limited success in curing complex diseases such as cancer, HIV, and diabetes because traditional biology generally looks at only a few aspects of an organism at a time.’
The statements above, whatever their merits, provide food for thought and can be used to frame a series of intentionally provocative questions. Along these lines, we have generated ‘10 questions’ in an attempt to critically evaluate the origin of systems biology and what it is or is not and how it does or does not differ from more traditional views and disciplines in the life sciences including integrative physiology. For some of our questions the goal is not to reach a definitive conclusion but to raise issues for discussion and debate. Additionally, some of the questions and answers have significant overlap. Along these lines, our fundamental position is that for at least several centuries, physiology in general and integrative physiology in specific has in fact been doing what systems biology seeks to do. Moreover, we see physiology as hypothesis driven and framed by high-level concepts about integration and regulation of systems versus a more hypothesis-neutral approach for some versions of systems biology. Finally, we also want to discuss whether systems biology has made or is likely to make significant contributions to major global health challenges, an area where physiology has clearly contributed.
Question 1: Why the need for something called systems biology?
There is no clear answer about why the ‘need’ for systems biology arose. However, it seems reasonable to speculate about a potential chain of events, which led to its emergence. During the 1980s and 90s there was vast intellectual and technical progress in the areas of genetics and molecular biology. This progress was exemplified by the identification of the cystic fibrosis gene in 1989 and the associated optimism for gene therapy stemming from it (Collins, 1992; Pearson, 2009). A second key example was the sequencing of the entire human genome announced in 2001 and the idea that a limited number of genetic variants would emerge and explain common diseases like cancer, hypertension, atherosclerosis, diabetes, etc. (Collins, 1992, 1999, 2001; Manolio et al. 2009). The optimism generated by these events may have been informed in part by a mentality that might be described as biological ‘orthopedic surgery’. The idea is that a gene is broken, fix the broken gene and cure disease (Noble, 2008).
This vision has clearly fallen short despite the vast resources devoted to these paradigms by funding agencies and commercial interests. With one or two exceptions gene therapy has failed to deliver (Pearson, 2009). Likewise, simple genetic answers have not emerged for common diseases (Paynter et al. 2010; Pedersen, 2010; Seshadri et al. 2010; Talmud et al. 2010). Figure 1 is an example of how ‘omics’ has failed to provide much predictive insight beyond traditional risk factors for type II diabetes in humans. A genetic risk score based on 20 gene variants associated with type II diabetes has only slightly more predictive power than chance and is much worse than ‘traditional’ risk factor scores based on epidemiological and phenotypic date.
Figure 1. Receiver operating characteristic comparing the so-called gene count score based on 20 independently inherited diabetes risk alleles versus the Framingham Offspring score which considers several measures of blood lipids, age, sex, family history of diabetes, body mass index, blood pressure, and fasting glucose.
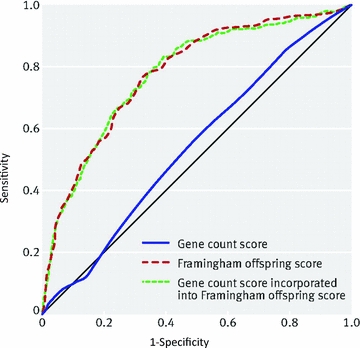
Note that the gene count score did only slightly better than the ‘no discrimination’ line and was much worse than the simple Framingham Offspring score. This figure shows the limited predictive power of common genetic variants that have been linked to disease risk. (Reproduced from Br Med J340, b4838 (Talmud et al. 2010) with permission from BMJ Publishing Group Ltd).
Numerous large-scale genome-wide association studies have also emerged as powerful approaches to identify genetic polymorphisms that are associated with the risk of developing cancer and other complex diseases or to develop risk factors for chronic diseases, e.g. abdominal adiposity. Microarrays permit interrogation of more than one million single-nucleotide polymorphisms (SNPs) at the same time. Novel loci and genetic variants have been identified as markers of disease risk. The number of genetic variants identified is in the range of a handful to approximately 30 (Van der Vegt et al. 2009; Sorensen & Orntoft, 2010; Zhang et al. 2010).
While findings from large-scale genome-wide association studies can provide new insights into the pathogenesis of various diseases, sometimes the enthusiasm expressed for their clinical relevance seems out of proportion to their effect size on phenotypes of interest. Our skepticism is supported by several critical reviews (Dupuy & Simon, 2007; Koscielny, 2008).
It is also interesting to note that drugs targeting channels and other traditional pathways may have dramatic disease-modifying effects in cystic fibrosis (CF) (Donaldson & Boucher, 2007; Verkman & Galietta, 2009). Given the many successes of drugs that target receptors, transporters and channels over the last 40 or so years, was the search for a genetic ‘holy grail’ in CF misguided (Black 1989; Ma & Zemmel, 2002; Rajagopal et al. 2010)? Genetic causes of disease are one thing, suitable drug targets another.
So, perhaps systems biology emerged with the recognition by the molecular and genetic reductionists that their basic intellectual assumptions were failing to deliver the broad-based diagnostic and therapeutic advances they were so confident of (Pearson, 2009). Things were much more complicated than originally envisioned. The idea now is that generating large volumes of ‘hypothesis-neutral’ molecular data in combination with vast computing power will reveal so-called emergent properties and generate the insight missing from the approaches outlined above (Kell & Oliver, 2004; Golub, 2010; Weinberg, 2010).
From the perspective of ‘physiological thinking’, both the challenges associated with targeting single genes for therapy and the lack of simple genetic explanations for common disease is not particularly surprising. Cardinal principles that flow from physiology include the concepts of homeostasis, regulated systems and redundancy. In this context, the most obvious explanation for the failure of molecular biology to deliver vast predictive or curative insights is perhaps general failure to understand the concept of redundancy operating in the context of homeostasis and regulated systems. In general, if there is a central set point for a key ‘homeostatic’ variable like whole body temperature, blood pressure or pH then a number of regulated systems operate to keep these values in a narrow range. This means that if one element of the regulatory landscape is eliminated then other redundant elements can pick up the slack so that the variable of interest (for example, blood pressure) does not change much.
Over the years, classical physiology has shown that the performance of tissues, organs, systems and whole animals can in fact be quite normal when one or more key component is eliminated via denervation, high-dose pharmacological blockade, or more recently genetic knock-out. Classic examples include demonstrations that: (1) the control of breathing during exercise is only minimally altered by chemoreceptor denervation, (2) long-term blood pressure regulation remains remarkably normal during various perturbations directed at the autonomic nervous system, (3) exercise capacity is well maintained in either animals with denervated hearts or humans subjected to high doses of beta-blockade, and (4) the inability to clearly identify a single mechanism or mechanisms that account for more than about 20% of the vasodilator responses in the coronary circulation during exercise (Donald et al. 1964; Cowley et al. 1973; Joyner et al. 1986; Mitchell & Babb, 2006; Duncker & Bache, 2008). Interestingly, ∼80% of yeast genes can be knocked out with little impact on survival in an unstressed environment (Hillenmeyer et al. 2008).
Thus, the key idea in physiology that a single mechanism frequently explains ‘100% of nothing’ has clearly been missing in the reductionist perspective outlined above. Figure 2 is an example of how the coronary circulation continues to dilate normally in exercising dogs when three major dilating pathways are blocked (Tune et al. 2001). Our own work on the redundant nature of how endocrine-like substances and blood-borne signals from exercising muscle operate to regulate metabolism and how the autonomic nervous system contributes to blood pressure regulation reinforce the role of the principles outlined above (Pedersen & Febbraio, 2008; Joyner et al. 2010).
Figure 2. Coronary blood flow plotted against myocardial oxygen consumption in exercising dogs.
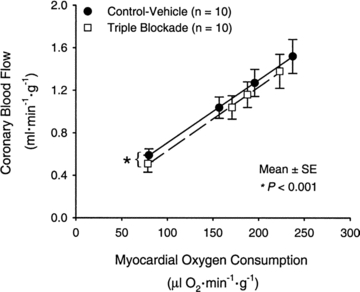
Note the linear relationship between the rise in coronary blood flow and myocardial oxygen consumption. Blockade of nitric oxide synthase, KATP channels, and adenosine receptors had no influence on the magnitude of coronary vasodilatation during exercise. The fact that all three of these powerful vasodilating mechanisms could be blocked and yet there was a normal hyperaemic response to exercise is a classic example of redundancy in physiology. If a physiological response, such as a rise in coronary blood flow during exercise, is critical to survival, typically multiple mechanisms operate to ensure a normal or nearly normal physiological response when one or more mechanism is blocked or inhibited in some way. (Reproduced with permission from Tune et al. 2001; for further discussion see Duncker & Bache, 2008.)
In summary, systems biology begins to recognize the limitations of the reductionist approach to biology. However, recognition of what might be called mega-concepts like homeostasis, regulated systems and redundancy in any proposed new paradigm will be of critical importance.
Question 2: Are the goals of systems biology fundamentally different from the goals of physiology?
In the systemsbiology.org quotes above, the analogy is made between various component parts of a car and the overall function of a car. This type of analogy has been used repeatedly by physiologists over the years to distinguish ‘us from them.’ The implicit assumption underlying the analogy is that if we just have enough parts a car will emerge. However, in a car, a variety of what could be called local and systemic features interact to regulate the overall performance of the car. These include a variety of temperature controls, other systems that regulate the car's ‘body fluids’, and (at least in modern cars) a number electronic sensors and central integrators that operate to some extent like neural and hormonal control mechanisms to preserve the homeostasis of the car under a variety of circumstances (Cannon, 1941: Csete & Doyle, 2002).
So, while systems biology should be applauded for recognizing the limits of reductionism that underpinned molecular biology and genetics, it continues to fail to recognize that a variety of integrating functions between cells, organs, systems, the entire organism and the environment are required to generate a fully functional and highly adaptive animal. This is clearly one area that distinguishes integrative physiology from systems biology. It should also be pointed out that this criticism of reductionism is not new (Csete & Doyle, 2002). A particularly good example comes from the Pickering lecture on blood pressure regulation given by Bjorn Folkow in 1994 (Folkow, 1995).
Question 3: How have traditional life sciences really failed to provide insight into disease?
The introductory statements on the systemsbiology.org website state that new approaches are needed because traditional biology ‘can yield relatively limited insights about the human body’: thus, the ‘limited success in curing complex diseases such as cancer, HIV, and diabetes’. At some level this assertion is dependent on what the boundaries of traditional biology are. If traditional biology as a discipline stops at the cell membrane (or wall) this is potentially correct; if it includes whole organisms and even populations it is an extremely narrow perspective. Since our view of biology is inclusive, we reject these assertions and provide a few examples of how physiology has provided insight into disease. In some cases there are physiologically driven potential cures for certain chronic diseases that we will discuss. In other cases physiologically driven interventions can dramatically alter the course of many chronic diseases. However, as we point out at the end of this paper, patterns of disease have far more to do with culture and environment than discrete biological mechanisms at any level, and it should be noted that the only disease that has ever been eradicated is Smallpox.
First, HIV, while not curable, can certainly be prevented and controlled by a combination of public health measures and drug therapy. Second, the most common recognized killer in the developed world is atherosclerosis (in reality if physical inactivity were seen as a disease state it would be number one) (Chakravarthy & Booth, 2004). However, there has been vast progress against coronary artery disease in the last ∼50 years, including a 50–70% reduction in age-specific death rates in many countries (Heron et al. 2009). Additionally, there is a simple physiological narrative about the balance between oxygen supply and oxygen demand in the heart that underpins our basic understanding of coronary artery disease and its complications (Nelson et al. 1974). When this balance is reduced acutely or chronically, pathophysiological responses occur and various disease syndromes with coherent physiological stories (like congestive heart failure) emerge (Felder et al. 2002).
Importantly, there are multiple physiologically based therapies directed at shifting the balance between oxygen supply and oxygen demand in the heart in a favourable way. Beta-blockers and other antihypertensive therapy reduce myocardial oxygen demand and limit ventricular hypertrophy. Interventional procedures, exercise training, diet and drugs can promote or maintain improved oxygen delivery to the heart (Ehsani et al. 1981; Ornish et al. 1998). Anti-coagulants and thrombolytic therapy stop or reverse acute obstructions of diseased arteries and restore blood flow to the heart (Libby, 2001).
Additionally, physiologically based diagnostic procedures as simple as the electrocardiogram, or exercise stress test, or as complicated as nuclear medicine studies have led to improved and early diagnosis of coronary disease. These physiologically based approaches have also gone hand-in-glove with insights from epidemiological and population-based studies, interventional clinical trials, and public health measures designed at preventing smoking, treating blood pressure, altering dietary fat intake and promoting physical activity.
A similar story can be told about type II diabetes. Whole body tracer studies have revealed great insight into the defects associated with endogenous glucose production, glucose uptake by various tissues, and defects in insulin secretion and action in type II diabetes (Bock et al. 2007; Rizza, 2010). Studies from isolated tissues have explored how insulin receptors and related glucose transport mechanisms are altered in the diabetic state (Henriksen, 2002). Interventional studies have shown that weight loss or increased physical activity can prevent, reverse and even ‘cure’ many of these defects (Rogers et al. 1988; Lindström et al. 2006). There are also simple physiologically based tests like the oral glucose tolerance test to clearly detect who has and who is at risk of the development of type II diabetes. So, physiology in conjunction with epidemiology and interventional studies has provided great insight into diabetes and offers profoundly effective treatment strategies. These observations clearly rebut the assertion that traditional biology has yielded relatively limited insights about the human body. Additionally, depending on the definition of ‘cure’, they also rebut the assertion that traditional biology has had limited success in curing complex diseases.
Question 4: Is systems biology a new discipline, an approach or a collection of tools?
From the perspective of integrative physiology, systems biology appears to be a collection of tools in search of questions versus a collection of hypothesis-driven questions searching for an answer (Kohl et al. 2010). As demonstrated in the companion papers of Drs Hester (Hester et al. 2011) and Secomb, mathematical modelling and so-called computational biology have a long history in physiology. As Denis Noble has pointed out, this dates to at least Harvey's early ideas about cardiac output (Auffray & Noble, 2009). Also, physiology as a discipline has been marked by the targeted use of reductionist tools in the pursuit of hypotheses based on big overriding ideas such as homeostasis, feedback control and redundancy. A classic example is the sequential incorporation of all sorts of ideas ranging from social science and interventional studies in humans to the most reductionistic tools to study something as complicated as blood pressure regulation (Folkow, 1995; Gleiberman, 2009). Models now exist that include thinking about how things like genetic variations in renal sodium handling might interact with social stress to increase the risk of hypertension in some ethnic groups (Gleiberman, 2009).
So, perhaps systems biology is a tool kit in search of questions. It also seems like an indiscriminant effort (see also the comments below about hypothesis neutral science below) to throw more and different combinations of technology at the idea that if we only understood ‘what is wrong with the genes’ we would gain vast new insights into disease. In this context, it is interesting to think about the discovery of endothelium-derived relaxing factor (EDRF) and subsequently nitric oxide (Furchgott, 1996). EDRF was discovered using a standard blood vessel preparation, and the discovery may have been facilitated when a technician failed to remove the endothelium and/or add α-adrenergic blockers. The main EDRF was subsequently shown to be nitric oxide and these twin discoveries have had broad-based implications in every field of the life sciences and led to insights like gas-transmitter signalling and new therapeutic thinking about a number of diseases. In a rhetorical context, it is tempting to ask if insights following from this discovery have done more for biomedical research and patient care than all of the information from the genome so far. Would the genome project have led to the simple fact that this is a legitimate question is something to think about and re-raises a number of questions posed by the great physiologist Julius Comroe in the 1970s about goal-directed research and big science versus curiosity-driven research and smaller teams of investigators (Comroe & Dripps, 1974).
Question 5: Is systems biology still too ‘cell centric’?
If our speculation about the genesis of systems biology stemming from frustrations associated with the inability of reductionist approaches to live up to their (self-generated) hype are correct, the statements about systems biology in specific and the definition of what is or is not ‘traditional’ earlier in this paper still seem too cell centric. For a contrasting example one can argue that hypertension is fundamentally: (1) a failure of several (redundant) mechanisms that sense arterial blood pressure and inform the brain about what is happening in the periphery, (2) failure of the brain to integrate this information and make the appropriate neuro-humoral adjustments, and (3) failure of the peripheral tissues to respond to the neuro-humoral adjustments (Folkow, 1995; DiBona & Esler, 2010; Joyner et al. 2010). Again, similar points can be made for diabetes, congestive heart failure, obesity and other common diseases. These examples argue that a failure to regulate at a systemic level is just as important as the discrete alteration of a cellular function for many diseases. Claude Bernard, Walter Cannon, August Krogh and L. J. Henderson all recognized that a failure of systemic regulation is a key when things go wrong with a ‘whole animal’ (Krogh, 1939; Cannon, 1941; Henderson, 1941; Noble, 2007).
Question 6: What is hypothesis neutral?
One puzzling feature of the ‘new biology’ to physiologists has also been the emergence of so-called ‘hypothesis-neutral’ or ‘discovery science’ (Kell & Oliver, 2004; Golub, 2010; Weinberg, 2010). There are plenty of facets of these terms that can be discussed, but as outsiders looking in, the general idea appears to be that with large enough data sets and enough high-throughput omic technology, certain associations (causal linkages?) between genetic markers, expression profiles, protein synthesis etc. and a phenotype of interest might emerge. Has the use of the word ‘discovery’ emerged because of the negative connotations associated with the term ‘descriptive’ and concerns about funding ‘fishing expeditions’? This is particularly frustrating to physiologists who have heard their discipline described as ‘descriptive’ by the reductionists.
Along these lines, it is interesting to note that pharmacogenomics is one of two areas of ‘omics’ that has generated a number of findings with clear relevance to the treatment of human disease (Pereira & Weinshilboum, 2009; Schroth et al. 2009). In general, those interested in pharmacogenomics are interested in (among other things) understanding why there are responders and non-responders to drug therapy in certain conditions or why certain side effects emerge is some patients but not others. These general questions seem to have generated a much more hypothesis-driven approach informed by key concepts about drug metabolism and action that have parallels to the physiological principles highlighted earlier.
Another area where ‘omics’ is generating striking answers to old questions is in anthropology. Two examples include the emergence of lactase persistence in various populations and the genetic adaptations of populations to unique environmental challenges like extreme altitude (Gibson, 2007; Enattah et al. 2008; Ingram et al. 2009; Simonson et al. 2010; Yi et al. 2010). In both cases these pursuits seem to have been driven by clear prospective questions and hypotheses. Along these lines, would the powerful high-throughput and computational technologies that form the basis of systems biology be better served if they were applied to testing clearly stated hypotheses?
Question 7: Does environment override genetics?
Another potential problem with the cell, genetic and ‘omic’-focused systems biology paradigm outlined above is that it will fail to provide vast insights into the human condition, especially disease, because so much of who gets what disease is fundamentally driven by culture, environment and lifestyle (Fraser & Shavlik, 2001; Marmot, 2006; Murray et al. 2006). There are a number of lines of evidence that favour this argument.
First, as humans in developed countries have become progressively less physically active and exposed to progressively more food, the incidence of obesity and type II diabetes has skyrocketed (Chakravarthy & Booth, 2004; James, 2008; Heron et al. 2009). This has happened in only a few generations and is happening too fast to be attributable to some fundamental change in the human genome (Bassett et al. 2004; Chakravarthy & Booth, 2004; Krebs, 2009). One interpretation of the genome-wide association studies is that variants associated with human traits are only of clinical significance when exposed to a certain environment, e.g. high-fat food, big portion sizes and inactivity. This idea is certainly supported by several knock-out animal models of obesity, where the target gene results in a phenotype only when stressed with a high fat–high sucrose diet. This has been found for many obesity-related genes. One example is the recently identified adipokine Sfrp5 (Ouchi et al. 2010). When Sfrp5-deficient mice were fed a high-calorie diet, they developed severe glucose intolerance and hepatic steatosis, and their adipose tissue showed an accumulation of activated macrophages that was associated with activation of the c-Jun N-terminal kinase signalling pathway. However, when the animals were fed chow, they did not demonstrate the disease phenotype. Thus, genes matter, but typically it is the gene–environment interaction that demonstrates a phenotype.
The observations about obesity and type II diabetes are backed by the more general observation that as humans migrate from one culture to another, their patterns of disease reflect the extent to which they have either retained their ‘original’ culture or adopted the culture of their new country (Marmot et al. 1975; Marmot & Syme, 1976; Curb & Marcus, 1991). This is an old story in epidemiology but one that seems to have been forgotten in the current age of ‘omics.’
Second, as noted above, phenotypic and family history-based methods are far more informative about the risk for future type II diabetes than currently available genetic risk scores (Talmud et al. 2010). Likewise, for cardiovascular disease, the distribution of genetic variants thought to be associated with coronary artery disease is essentially identical in individuals both with and without coronary disease (Paynter et al. 2010). So at least for type II diabetes, coronary artery disease and also Alzheimer's disease, the results so far suggest that the ability of genetic markers to be particularly informative about the risk of these diseases is in fact limited (Pedersen, 2010; Seshadri et al. 2010). In this context, it is interesting to think about human height. Height is a phenotype that is easy to measure and, based on traditional assessment techniques, is 80% heritable. However, genome wide association studies have indicated that only about 5% of this heritability can be attributed to known variants and the most powerful variant that influences height only influences about 0.3% of the overall variation (Weedon & Frayling, 2008). Additionally, no strong linkage to any chromosomal region has been found, suggesting that the finding of some new site that explains a large fraction of the genetics of height is unlikely.
Third, even short-term changes in the environment or behaviour might be more important in the generation of disease phenotypes than previously thought. Normally, environmental influences are thought to take years to exert their influence. However, recent studies clearly demonstrate that just a few days of physical inactivity can be associated with phenotypic changes that would predispose the physically inactive to cardiovascular disease and diabetes (Olsen et al. 2008).
Thus, in contrast to ‘omics’, who gets what disease when seems driven largely by lifestyle, culture and environment (Fraser & Shavlik 2001; Diabetes Prevention Program Research Group, 2002; Bassett et al. 2004; Chakravarthy & Booth, 2004; Kyle & Pichard, 2006; Marmot, 2006; Blair & Morris, 2009; Talmud et al. 2010). We expand on these ideas next.
Question 8. Is ‘bench to bedside’ in reality a one-way street that has failed?
Translational research (a term with a slippery definition), with its mantra ‘bench to bedside and back’, emerged as a top priority for the NIH and other funding agencies in the early 2000s (Dougherty, 2009). This priority was coupled with the NIH Roadmap targeting the re-invigoration of clinical research as a major goal. The topic has been a hot one, featured on the cover of Nature on June 12, 2008. Systems biology, with its stated goal of translating basic research into effective new diagnostic tests and therapies can be viewed as either an appropriate next step or an opportunistic rebranding of reductionism.
The big question is, however, whether the concept of ‘translational research’ on the basis of ‘systems biology’ was new, innovative and radical. In fact, the combination of clinical research and basic research is not new. What has happened during that past decade is that the new ‘omic’ technology was branded as revolutionary, funding and prestige followed the hype, and this lured many promising scientists and physicians to choose a life at the bench. The idea, as outlined in the opening quotes of this paper, was that screening and then modelling the transcriptome, the proteome, the metabolome or any other ‘omes’ would result in a revolution in individualized medicine. However, the fact is that we are still waiting for a strategy whereby these techniques can be successfully applied in the clinic for the benefit of patients and populations.
One fruitful example linking genomics with physiology was recently published by the Vaag group (Alibegovic et al. 2010). The aim of their study was to determine whether the type II diabetes-associated T-allele of transcription factor 7-like 2 (TCF7L2) rs7903146 was associated with impaired insulin secretion to compensate for insulin resistance induced by bed rest. They performed a classical integrative physiological study. A total of 38 healthy young Caucasian men were studied before and after bed rest using the hyperinsulinaemic–euglycaemic clamp technique combined with indirect calorimetry preceded by an intravenous glucose tolerance test. They were able to show that healthy carriers of the T-allele of TCF7L2 rs7903146 exhibited a diminished increase of insulin secretion in response to intravenous glucose to compensate for insulin resistance as induced by bed rest (Fig. 3).
Figure 3. Plasma insulin in C peptide levels in subjects who are carriers of the higher risk T-allele (filled symbols) versus low-risk CC genotype (open symbols) of TCF7L2 rs7903146 in response to an i.v. glucose tolerance test before and after bed rest.
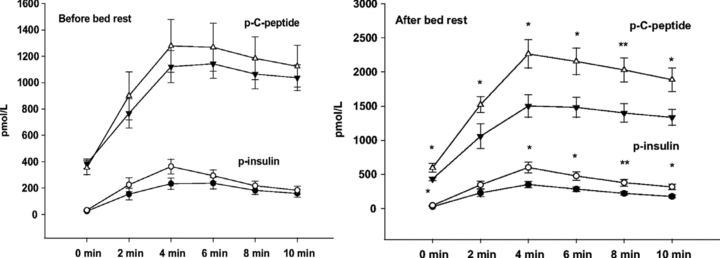
Bed rest is associated with the relative insulin resistance in patients with the low-risk CC allele but they can increase their insulin release to compensate for peripheral insulin resistance. By contrast, this response is blunted in high risk individuals who are carriers of the T-allele. This result is an example of the potential power of small and integrative physiology studies to understand how the selected common genetic variants might contribute to the emergence of disease phenotypes in humans. More importantly, these data emphasize that physical inactivity and lifestyle can unmask genetic risks (Figure from Alibegovic et al. 2010. Copyright 2010 American Diabetes Association. From Diabetes, Vol. 59, 2010; 836–843. Reproduced by permission of The American Diabetes Association.)
Thus, whereas initial classical genome-wide association studies often need more than 100,000 participants in order to identify a gene variant of potential interest, the biological role of such a gene may be identified through advanced physiological experiments and what might be termed ‘high-resolution phenotypoing’ in a limited number of humans. Except for a few examples, we have been waiting for a more fruitful exchange between the basic and clinical research environments. In the meantime, scientists have moved from the bed to the bench. Since the 1970s, the number of clinical investigators at NIH and across the US has steadily dwindled while the total number of researchers has grown. According to Varki & Rosenberg, in their 2002 Nature paper, there were 14,479 US-based physician–scientists in 1998, down from 18,535 in 1983, a 22% decline. In addition, the percentage of physicians engaged in research in the US shrank from 4.6% in 1985 to 1.8% in 2003, according to the 2005 JAMA article by Ley and Rosenberg. Rosenberg, also documented that the number of NIH grants awarded to PhDs, to MDs and to MD–PhDs were basically comparable in 1970. However, by 1997 ∼70% of funded investigators were basic scientists (Rosenberg, 1999). There has also been a long history of major contributions of PhD trained investigators in integrative physiology. There are no data to clearly show a reduction in PhD translational investigators, but we suspect this is also the case.
While there are less definitive data on other countries, it appears that these trends are probably world wide. It is also interesting to note that as the workforce and funding has shifted, the time devoted to teaching medical students and graduate students integrative or systems physiology has been reduced and many traditional physiology departments have either redirected their efforts or vanished (Bunton, 2006). The noted Harvard biologist Daniel Lieberman (personal communication) has commented that it is possible to get a PhD in biology from Harvard without ever studying anything ‘larger than a cell.’
The criticism is that translational research has not delivered new therapies to a degree consistent with the resources devoted to them. The fact is that systems biology dressed as translational medicine is presently at a stage where only the optimist would say the strategy is ‘working’. Whether the lack of progress for strategies that might be called systems biology in a clinical context is due to simply a ‘workforce’ imbalance, or whether they reflect more fundamental issues with the ‘balance of power’ (or at least people and resources) between basic and clinical research can be discussed. However, we fear that this is a real possibility. In this context, we previously pointed out that pharmacogenomics, with its focus on clinical responders and non-responders to drug therapy in patients, seems to be one of several areas where the bench-to-bedside paradigm has been turned on its head and working.
Question 9: Do we need a ‘From man to molecule’ strategy?
As noted in our ‘answer’ to question 8, we believe that translational medicine got it wrong, when the needed two-way approach turned out to be a one-way street. The idea is that translational medicine begins at ‘the bench’ with basic research and then progresses to the clinical level or the patient's ‘bedside’. In this scheme basic scientists provide clinicians with new tools and insights, while clinical researchers make novel observations about human physiology as well as the nature and progression of disease, which could stimulate basic investigations. However, perhaps the important fact to face is that the implications of this bottom-up model of translational research on scientific culture and ultimately health may have significant limitations (Brook, 2010).
Along these lines, we believe that translational medicine can begin at the bedside (or perhaps population), as well as at the bench. In the bedside (or population)-to-bench version, the clinical researchers and/or integrative physiologists need to identify the clinical problem to be solved, and the new techniques should be brought to bear in an integrative setting to identify new biological mechanisms. We suggest that a ‘from-man-to-molecule’ strategy, which includes an integrative physiological approach, with a focus on homeostasis and principles of physiological control will more successfully lead to the development of new diagnostics and treatments. In this context, it should be remembered that the best predictor of future mortality in many population-based studies is a simple exercise test (Blair & Morris 2009).
Question 10: Do we really need an expanded strategy: ‘From culture/environment to man to molecule?’
Systems biology is typically identified as a biology-based inter-disciplinary study field that focuses on complex interactions between and among biological systems operating primarily at the cellular level, claiming that this is a fundamentally new perspective. However, the claimed holistic approach is far from being holistic in the sense of what is necessary to affect major global health challenges.
In this context, history clearly demonstrates that structural changes in a society have more impact on health than any group interventions or sophisticated discovery strategies. This was recently illustrated in Cuba's economic crisis of 1989–2000 which occurred after the collapse of the Soviet Union. This time frame has also been referred to with the Orwellian title of the ‘Special Period’. The crisis resulted in reduced energy intake, increased physical activity and sustained population-wide weight loss. The crisis reduced per capita daily energy intake from 2,899 calories to 1,863 calories. During the crisis period, the proportion of physically active adults increased from 30% to 67%, and a 1.5-unit shift in the body mass index distribution was observed, along with a change in the distribution of body mass index categories. The prevalence of obesity declined from 14% to 7%. During 1997–2002, there were declines in deaths attributed to diabetes (51%), coronary heart disease (35%), stroke (20%) and all causes (18%). These results suggest that population-wide measures designed to reduce energy stores and/or increase physical activity level, may lead to declines in diabetes and cardiovascular disease prevalence and mortality to an extent that has not been seen with any other strategy (Rodríguez-Ojea et al. 2002; Esquível & González 2010).
Another example of the importance of structural changes comes from studies on mortality patterns in East and West Germany (Nolte et al. 2000). In the years after the Second World War, the average life span was 5 years shorter for the population in Germany compared to Sweden. However, from the mid-seventies the life span increased dramatically in West Germany, while East Germany was left behind (Fig. 4). When the Berlin wall fell in 1989, a dramatic increase occurred in the life span of the former East Germany population. By the year 2000, there were no longer any noticeable differences between the life span in Sweden and Germany or between the former West and East Germany. This observation further highlights how much influence political systems have on health conditions.
Figure 4. Life expectancy in East and West Germany, Poland and Sweden from 1950.
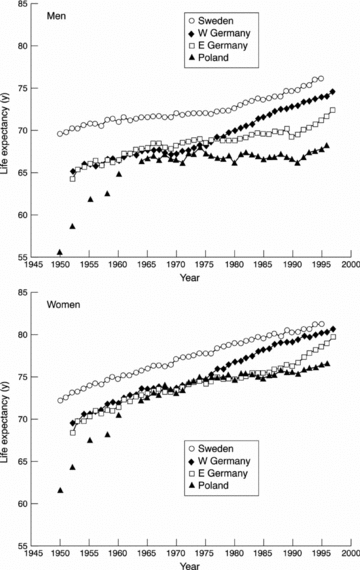
Of note is the rise in life expectancy in East Germany after the fall of the Berlin Wall. There are complex reasons for the differences in these values and how they changed over time, but they demonstrate the power of political systems to influence health outcomes in relatively short periods of time. (Reproduced from J Epidemiol Community Health54, 890–898 (Nolte et al. 2000) with permission from BMJ Publishing Group Ltd.)
To take an even longer view, the major killers during the past two centuries in what is now called the developed world were infectious diseases and it is important to learn lessons about the history of how they were conquered. Cholera was not controlled by campaigns directed towards the individual, but by structural changes including civil engineering and the development of clean water and sewage systems. Tuberculosis was not successfully controlled by campaigns, individual treatments with antibiotics or vaccines, but through improvements of sanitation and the general hygiene of the population as a whole (Wishnow & Steinfeld, 1976). More recently, traffic mortality has declined dramatically in most of the developed world. In the US, traffic fatalities per million vehicle miles travelled have fallen from ∼10 to just over 1 from 1945 to 2008 (NHTSA, 2010). In Denmark, traffic deaths have fallen from 1200 to 300 during the past three decades (http://www.dst.dk). This again is due to numerous structural changes and initiatives including regulations with regard to seat belts, air bags, speed, alcohol, improved roads and technical improvements in cars.
When the success of structural approaches are considered along with what systems biology is unlikely to do, population-wide changes with regard to e.g. obesity and physical inactivity will need to be made at all social and political levels, including provincial, territorial and municipal governments; schools, workplaces and households. Additionally, obesity and physical inactivity affect minorities and lower income populations disproportionately, and the needed structural changes have to include those populations to reduce the already existing inequalities. In general, high income populations typically follow official recommendations with regard to health, whereas lower income populations do not. The typical commuting cyclists are not people who can't afford cars, but those who are well-educated, conscious about the environment and also the health consequences of physical inactivity (Murray et al. 2006; Dill, 2009). With respect to food consumption and physical activity, lower income communities are more resistant to campaigns, but sensitive to availability. Therefore, structural changes that make healthy lifestyles more accessible to these communities will reduce health disparities (Brook, 2010). In the future, systems biology may be used to identify individuals or subpopulations that are more susceptible to certain environmental risk factors, but the risk factors themselves are likely to remain the same even though the extent to which they are present in an environment might change with time. Thus, is it better to reduce or eliminate whole categories of risk factors or work to stratify individuals on the basis of genetic risk and hope they can choose wisely?
Around 1900, there was great interest in what was called ‘physiological hygiene’ and perhaps it is time to go back to the future and focus on interventions that could directly affect total energy intake and physical activity of whole populations thereby promoting health (Krogh, 1939). Population-wide interventions related to energy intake that promote low-energy, nutritious foods, and make fruits and vegetables more readily available and less expensive are one key step. Limiting the availability and increasing the prices of high-energy foods is another. In many developed countries agricultural policies emerged in the first half of the last century when not getting enough calories was the problem (Fields, 2004; Powell et al. 2010). Interventions related to physical activity should include promoting walking and bicycling as means of transportation. In addition, urban planners, schools and workplace designers should prioritize physical activity in their plans. When these measures have been tried, they work.
Summary and conclusions
Systems biology should be lauded for its efforts to move beyond biological reductionism. However, we have argued that the definition and view of systems biology originating from (or perhaps in response to) the reductionist perspective still has a number of limitations including a narrow view of what constitutes ‘traditional biology’. We have also expressed concerns about the utility of hypothesis-neutral approaches to science and perhaps and overly cell centric view of pathophysiology. These limitations seem to be caused by the persistence of a more deterministic view of genes, gene expression, and other flavours of omics as influencing various phenotypes, and a lack of appreciation of the key principles of physiological regulation. Additionally, there also seems to be a general failure to recognize that for humans, both population and individual patterns of disease may have more to do with culture, environment and behaviour than genetic variability (Fig. 5). In this context, we speculate that for a given individual, a low physical activity, high calorie, high social stress lifestyle determines the extent that various ‘risk’ gene variants for metabolic, cardiovascular and other diseases become expressed, translated and or otherwise activated. These over-arching issues can either be rediscovered by the reductionists, or learned from physiologists and others with comprehensive perspectives on human health and disease. The challenge is to use integrative physiology to incorporate the findings from ‘omics’ into models with relevance to patient groups and/or populations, to facilitate the study of gene-physiology, environmental and cultural interactions.
Figure 5. Conceptual model showing the contribution of optimal individual behaviour and the risk of premature death or disability from any cause and the contribution of genetics to this increased risk.
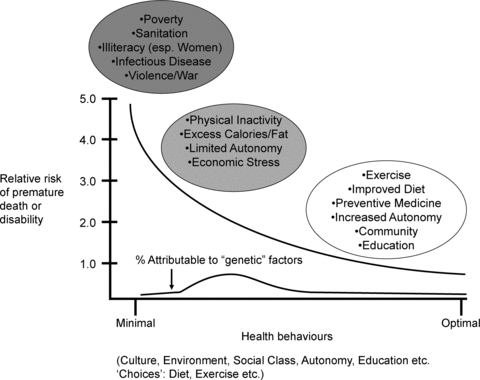
Individual behaviour is influenced by complex interactions among culture, environment, social class, education, and ‘choices’ about things like diet, exercise and seat belt use. In chaotic societies with uncertain food supplies, poor sanitation, no social safety net and warfare or other forms of social displacement, premature death and disability is vastly greater than in more stable environments. Additionally, under these desperate conditions we speculate that genetic variation in individual humans contributes minimally to the pattern of death and disability. At the other end of the spectrum are educated, well-off individuals of high social status who typically engage positive health behaviours. We speculate that these individuals are largely buffered from potentially adverse genetic variants. By contrast are the inactive, obese citizens of many industrialized nations who have limited social autonomy and consistently engage in negative health behaviours. These behaviours and social factors may then interact with certain genetic variants to facilitate the emergence of many diseases such as diabetes, hypertension, cancer and vascular disease. Thus, we speculate that for a given individual a low physical activity, high calorie, high social stress lifestyle determines the extent that various ‘risk’ gene variants for metabolic, cardiovascular and other diseases become expressed or activated. This concept is also demonstrated in Fig. 3. As these interactions are likely to be complex and involve a large number of genetic variants, as shown in Fig. 1, the utility of predictive models based on genetic information is likely to be limited.
Acknowledgments
M.J.J. is supported by National Institutes of Health grants HL 83947, HL 46493, RR 24150, and the Caywood Professorship via the Mayo Foundation. B.K.P. is supported by the Danish National Research Foundation (no. 02-512-55); the Danish Center for Strategic Research in Type 2 Diabetes (grant no. 09-067009 and 09-075724) and the Commission of the European Communities (Grant Agreement no. 223576 – MYOAGE).
Glossary
Abbreviations
- SNP
single-nucleotide polymorphisms
References
- Alibegovic AC, Sonne MP, Højbjerre L, Hansen T, Pedersen O, van Hall G, et al. The T-allele of TCF7L2 rs7903146 associates with a reduced compensation of insulin secretion for insulin resistance induced by 9 days of bed rest. Diabetes. 2010;59:836–843. doi: 10.2337/db09-0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C, Noble D. Origins of systems biology in William Harvey's masterpiece on the movement of the heart and the blood in animals. Int J Mol Sci. 2009;10:1658–1669. doi: 10.3390/ijms10041658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DR, Jr, Schneider PL, Huntington GE. Physical activity in an old order Amish community. Med Sci Sports Exerc. 2004;36:79–85. doi: 10.1249/01.MSS.0000106184.71258.32. [DOI] [PubMed] [Google Scholar]
- Black J. Drugs from emasculated hormones: the principle of syntopic antagonism. Science. 1989;245:486–493. doi: 10.1126/science.2569237. [DOI] [PubMed] [Google Scholar]
- Blair SN, Morris JN. Healthy hearts – and the universal benefits of being physically active: physical activity and health. Ann Epidemiol. 2009;19:253–256. doi: 10.1016/j.annepidem.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Bock G, Chittilapilly E, Basu R, Toffolo G, Cobelli C, Chandramouli V, et al. Contribution of hepatic and extrahepatic insulin resistance to the pathogenesis of impaired fasting glucose. Role of increased rates of gluconeogenesis. Diabetes. 2007;56:1703–1711. doi: 10.2337/db06-1776. [DOI] [PubMed] [Google Scholar]
- Brook RH. Medical leadership in an increasingly complex world. JAMA. 2010;304:465–466. doi: 10.1001/jama.2010.1049. [DOI] [PubMed] [Google Scholar]
- Bunton SA. Recent trends in basic science department reorganizations. Analysis in Brief. 2006;6(1) AAMC Available at http://www.aamc.org/data/aib. [Google Scholar]
- Cannon WB. The body physiologic and the body politic. Science. 1941;93:1–10. doi: 10.1126/science.93.2401.1. [DOI] [PubMed] [Google Scholar]
- Chakravarthy MV, Booth FW. Eating, exercise, and “thrifty” genotypes: connecting the dots toward an evolutionary understanding of modern chronic diseases. J Appl Physiol. 2004;96:3–10. doi: 10.1152/japplphysiol.00757.2003. [DOI] [PubMed] [Google Scholar]
- Collins FS. Contemplating the end of the beginning. Genome Res. 2001;11:641–643. doi: 10.1101/gr.189801. [DOI] [PubMed] [Google Scholar]
- Collins FS. Cystic fibrosis: molecular biology and therapeutic implications. Science. 1992;256:774–779. doi: 10.1126/science.1375392. [DOI] [PubMed] [Google Scholar]
- Collins FS. The human genome project and the future of medicine. Ann NY Acad Sci. 1999;882:42–55. doi: 10.1111/j.1749-6632.1999.tb08532.x. [DOI] [PubMed] [Google Scholar]
- Comroe JH, Jr, Dripps RD. Ben Franklin and open heart surgery. Circ Res. 1974;35:661–669. doi: 10.1161/01.res.35.5.661. [DOI] [PubMed] [Google Scholar]
- Cowley AW, Jr, Liard JF, Guyton AC. Role of the baroreceptor reflex in daily control of arterial blood pressure and other variables in dogs. Circ Res. 1973;32:564–576. doi: 10.1161/01.res.32.5.564. [DOI] [PubMed] [Google Scholar]
- Csete ME, Doyle JC. Reverse engineering of biological complexity. Science. 2002;295:1664–1669. doi: 10.1126/science.1069981. [DOI] [PubMed] [Google Scholar]
- Curb JD, Marcus EB. Body fat and obesity in Japanese Americans. Am J Clin Nutr. 1991;53:1552S–1555S. doi: 10.1093/ajcn/53.6.1552S. [DOI] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBona GF, Esler M. Translational medicine: the antihypertensive effect of renal denervation. Am J Physiol Regul Integr Comp Physiol. 2010;298:R245–R253. doi: 10.1152/ajpregu.00647.2009. [DOI] [PubMed] [Google Scholar]
- Dill J. Bicycling for transportation and health: the role of infrastructure. J Public Health Policy. 2009;30:S95–S110. doi: 10.1057/jphp.2008.56. [DOI] [PubMed] [Google Scholar]
- Donald DE, Milburn SE, Shepherd JT. Effect of cardiac denervation on the maximal capacity for exercise in the racing greyhound. J Appl Physiol. 1964;19:849–852. doi: 10.1152/jappl.1964.19.5.849. [DOI] [PubMed] [Google Scholar]
- Donaldson SH, Boucher RC. Sodium channels and cystic fibrosis. Chest. 2007;132:1631–1636. doi: 10.1378/chest.07-0288. [DOI] [PubMed] [Google Scholar]
- Dougherty ER. Translational science: epistemology and the investigative process. Curr Genomics. 2009;10:102–109. doi: 10.2174/138920209787847005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev. 2008;88:1009–1086. doi: 10.1152/physrev.00045.2006. [DOI] [PubMed] [Google Scholar]
- Dupuy A, Simon RM. Critical review of published microarray studies for cancer outcome and guidelines on statistical analysis and reporting. J Natl Cancer Inst. 2007;99:147–157. doi: 10.1093/jnci/djk018. [DOI] [PubMed] [Google Scholar]
- Ehsani AA, Heath GW, Hagberg JM, Sobel BE, Holloszy JO. Effects of 12 months of intense exercise training on ischemic ST-segment depression in patients with coronary artery disease. Circulation. 1981;64:1116–1124. doi: 10.1161/01.cir.64.6.1116. [DOI] [PubMed] [Google Scholar]
- Enattah NS, Jensen TGK, Nielsen M, Lewinski R, Kuokkanen M, Rasinpera H, et al. Independent introduction of two lactase-persistence alleles into human populations reflects different history of adaptation to milk culture. Am J Hum Genet. 2008;82:57–72. doi: 10.1016/j.ajhg.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquível M, González C. Excess weight and adiposity in children and adolescents in Havana, Cuba: prevalence and trends, 1972 to 2005. MEDICC Rev. 2010;12:13–18. doi: 10.37757/MR2010.V12.N2.5. [DOI] [PubMed] [Google Scholar]
- Felder RB, Francis J, Weiss RM, Zhang Z-H, Wei S-G, Johnson AK. Neurohumoral regulation in ischemia-induced heart failure. Role of the forebrain. Ann NY Acad Sci USA. 2002;940:444–453. doi: 10.1111/j.1749-6632.2001.tb03697.x. [DOI] [PubMed] [Google Scholar]
- Fields S. The fat of the land: do agricultural subsidies foster poor health? Environ Health Perspect. 2004;112:A820–A823. doi: 10.1289/ehp.112-a820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkow B. Integration of hypertension research in the era of molecular biology: G.W. Pickering Memorial Lecture (Dublin 1994) J Hypertens. 1995;13:5–18. [PubMed] [Google Scholar]
- Fraser GE, Shavlik DJ. Ten years of life. Is it a matter of choice? Arch Intern Med. 2001;161:1645–1652. doi: 10.1001/archinte.161.13.1645. [DOI] [PubMed] [Google Scholar]
- Furchgott RF. The discovery of endothelium-derived relaxing factor and its importance in the identification of nitric oxide. JAMA. 1996;276:1186–1188. [PubMed] [Google Scholar]
- Gibson G. Human evolution: thrifty genes and the Dairy Queen. Curr Biol. 2007;17:R295–R296. doi: 10.1016/j.cub.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Gleiberman L. Sodium, blood pressure, and ethnicity: what have we learned? Am J Human Biol. 2009;21:679–686. doi: 10.1002/ajhb.20921. [DOI] [PubMed] [Google Scholar]
- Golub TR. Counterpoint: Data first. Nature. 2010;464:679. doi: 10.1038/464679a. [DOI] [PubMed] [Google Scholar]
- Henderson LJ. The study of man. Science. 1941;94:1–10. doi: 10.1126/science.94.2427.1. [DOI] [PubMed] [Google Scholar]
- Henriksen EJ. Invited review: Effects of acute exercise and exercise training on insulin resistance. J Appl Physiol. 2002;93:788–796. doi: 10.1152/japplphysiol.01219.2001. [DOI] [PubMed] [Google Scholar]
- Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD, Tejada-Vera B. Deaths: Final data for 2006. Hyattsville, MD: National Center for Health Statistics; 2009. pp. 1–117. National vital statistics reports; vol. 57, no. 14. [PubMed] [Google Scholar]
- Hester RL, Iliescu R, Summers R, Coleman TG. Systems biology and integrative physiological modelling. J Physiol. 2011;589:1053–1060. doi: 10.1113/jphysiol.2010.201558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillenmeyer ME, Fung E, Wildenhain J, Pierce SE, Hoon S, Lee W, et al. The chemical genomic portrait of yeast: uncovering a phenotype for all genes. Science. 2008;320:362–365. doi: 10.1126/science.1150021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram CJE, Mulcare CA, Itan Y, Thomas MG, Swallow DM. Lactose digestion and the evolutionary genetics of lactase persistence. Hum Genet. 2009;124:579–591. doi: 10.1007/s00439-008-0593-6. [DOI] [PubMed] [Google Scholar]
- James WPT. The fundamental driers of the obesity epidemic. Obesity Rev. 2008;9:6–13. doi: 10.1111/j.1467-789X.2007.00432.x. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Charkoudian N, Wallin BG. Sympathetic nervous system and blood pressure in humans. Individualized patterns of regulation and their implications. Hypertension. 2010;56:10–16. doi: 10.1161/HYPERTENSIONAHA.109.140186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner MJ, Freund BJ, Jilka SM, Hetrick GA, Martinez E, Ewy GA, Wilmore JH. Effects of beta-blockade on exercise capacity of trained and untrained men: a hemodynamic comparison. J Appl Physiol. 1986;60:1429–1434. doi: 10.1152/jappl.1986.60.4.1429. [DOI] [PubMed] [Google Scholar]
- Kell DB, Oliver SG. Here is the evidence, now what is the hypothesis? The complementary roles of inductive and hypothesis-driven science in the post-genomic era. BioEssays. 2004;26:99–105. doi: 10.1002/bies.10385. [DOI] [PubMed] [Google Scholar]
- Kohl P, Crampin EJ, Quinn TA, Noble D. Systems biology: an approach. Clin Pharmacol Ther. 2010;88:25–33. doi: 10.1038/clpt.2010.92. [DOI] [PubMed] [Google Scholar]
- Koscielny S. Critical review of microarray-based prognostic tests and trials in breast cancer. Curr Opin Obstet Gynecol. 2008;20:47–50. doi: 10.1097/GCO.0b013e3282f39d9e. [DOI] [PubMed] [Google Scholar]
- Krebs JR. The gourmet ape: evolution and human food preferences. Am J Clin Nutr. 2009;90:707S–711S. doi: 10.3945/ajcn.2009.27462B. [DOI] [PubMed] [Google Scholar]
- Krogh A. The teaching of physiology. Science. 1939;89:545–547. doi: 10.1126/science.89.2320.545. [DOI] [PubMed] [Google Scholar]
- Kyle UG, Pichard C. The Dutch famine of 1944–1945: a pathophysiological model of long-term consequences of wasting disease. Curr Opin Clin Nutr Metab Care. 2006;9:388–394. doi: 10.1097/01.mco.0000232898.74415.42. [DOI] [PubMed] [Google Scholar]
- Ley TJ, Rosenberg LE. The physician-scientist career pipeline in 2005: build it, and they will come. JAMA. 2005;294:1343–1351. doi: 10.1001/jama.294.11.1343. [DOI] [PubMed] [Google Scholar]
- Libby P. What have we learned about the biology of atherosclerosis? The role of inflammation. Am J Cardiol. 2001;88:3J–6J. doi: 10.1016/s0002-9149(01)01879-3. [DOI] [PubMed] [Google Scholar]
- Lindström J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemiö K, et al. Sustained reduction in the incidence of type 2 diabetes by ifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006;368:1673–1679. doi: 10.1016/S0140-6736(06)69701-8. [DOI] [PubMed] [Google Scholar]
- Ma P, Zemmel R. Value of novelty? Nat Rev. 2002;1:571–572. doi: 10.1038/nrd884. [DOI] [PubMed] [Google Scholar]
- Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmot MG. Status syndrome: a challenge to medicine. JAMA. 2006;295:1304–1307. doi: 10.1001/jama.295.11.1304. [DOI] [PubMed] [Google Scholar]
- Marmot MG, Syme SL. Acculturation and coronary heart disease in Japanese-Americans. Am J Epidemiol. 1976;104:225–247. doi: 10.1093/oxfordjournals.aje.a112296. [DOI] [PubMed] [Google Scholar]
- Marmot MG, Syme SL, Kagan A, Kato H, Cohen JB, Belsky J. Epidemiologic studies of coronary heart disease and stroke in Japanese men living in Japan, Hawaii and California: prevalence of coronary and hypertensive heart disease and associated risk factors. Am J Epidemiol. 1975;102:514–525. doi: 10.1093/oxfordjournals.aje.a112189. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Babb TG. Layers of exercise hyperpnea: modulation and plasticity. Respir Physiol Neurobiol. 2006;151:251–266. doi: 10.1016/j.resp.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Murray CJL, Kulkarni SC, Michaud C, Tomijima N, Bulzaccheli MT, Iandiorio TJ, Ezzati M. Eight Americas: investigating mortality disparities across races, counties, and race-counties in the United States. PLoS Med. 2006;3:e260. doi: 10.1371/journal.pmed.0030260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RR, Gobel FL, Jorgensen CR, Wang K, Wang Y, Taylor HL. Hemodynamic predictors of myocardial oxygen consumption during static and dynamic exercise. Circulation. 1974;50:1179–1189. doi: 10.1161/01.cir.50.6.1179. [DOI] [PubMed] [Google Scholar]
- NHTSA. An Analysis of the Significant Decline in Motor Vehicle Traffic Fatalities in 2008. Washington, DC: National Highway Traffic Safety Administration; 2010. [DOI] [PubMed] [Google Scholar]
- Noble D. Clade Bernard, the first systems biologist, and the future of physiology. Exp Physiol. 2007;93:16–26. doi: 10.1113/expphysiol.2007.038695. [DOI] [PubMed] [Google Scholar]
- Noble D. Genes and causation. Phil Trans R Soc A. 2008;366:3001–3015. doi: 10.1098/rsta.2008.0086. [DOI] [PubMed] [Google Scholar]
- Nolte E, Shkolnikov V, McKee M. Changing mortality patterns in East and West Germany and Poland. I: long term trends (1960–1997) J Epidemiol Community Health. 2000;54:890–898. doi: 10.1136/jech.54.12.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RH, Krogh-Madsen R, Thomsen C, Booth FW, Pedersen BK. Metabolic responses to reduced daily steps in healthy nonexercising men. JAMA. 2008;299:1261–1263. doi: 10.1001/jama.299.11.1259. [DOI] [PubMed] [Google Scholar]
- Ornish D, Schwerwitz LW, Billings JH, Brown SE, Gould KL, Merritt TA, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998;280:2001–2007. doi: 10.1001/jama.280.23.2001. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Higuchi A, Ohashi K, Oshima Y, Gokce N, Shibata R, et al. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science. 2010;329:454–457. doi: 10.1126/science.1188280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paynter NP, Chasman DI, Pare G, Buring JE, Cook NR, Miletich JP, Ridker PM. Association between a literature-based genetic risk score and cardiovascular events in women. JAMA. 2010;303:631–637. doi: 10.1001/jama.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson H. One gene, twenty years. Nature. 2009;460:165–169. doi: 10.1038/460164a. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- Pedersen NL. Reaching the limits of genome-wide significance in Alzheimer disease: back to the environment. JAMA. 2010;303:1864–1865. doi: 10.1001/jama.2010.609. [DOI] [PubMed] [Google Scholar]
- Pereira NL, Weinshilboum RM. Cardiovascular pharmacogenomics and individualized drug therapy. Nat Rev Cardiol. 2009;6:632–638. doi: 10.1038/nrcardio.2009.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell LM, Han E, Chaloupka FJ. Economic contextual factors, food consumption, and obesity among U.S. adolescents. J Nutr. 2010;140:1175–1180. doi: 10.3945/jn.109.111526. [DOI] [PubMed] [Google Scholar]
- Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev. 2010;9:373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza RA. Pathogenesis of fasting and postprandial hyperglycemia in type 2 diabetes: implications for therapy. Diabetes. 2010;59:2697–2707. doi: 10.2337/db10-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Ojea A, Berdasco JS, Esquível M. The nutrition transition in Cuba in the nineties: an overview. Public Health Nutr. 2002;5:129–133. doi: 10.1079/PHN2001284. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Yamamoto C, King DS, Hagberg JM, Ehsani AA, Holloszy JO. Improvement in glucose tolerance after 1 wk of exercise in patients with mild NIDDM. Diabetes Care. 1988;11:613–618. doi: 10.2337/diacare.11.8.613. [DOI] [PubMed] [Google Scholar]
- Rosenberg LE. The physician-scientist: an essential-and fragile-link in the medical research chain. J Clin Invest. 1999;103:1621–1626. doi: 10.1172/JCI7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroth W, Goetz MP, Hamann U, Fasching PA, Schmidt M, Winter S, et al. Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. JAMA. 2009;302:1429–1436. doi: 10.1001/jama.2009.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri S, Fitzpatrick AL, Ikram MA, DeStefano AL, Gudnason V, Boada M, et al. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA. 2010;303:1832–1840. doi: 10.1001/jama.2010.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson TS, Yang Y, Huff CD, Yun H, Qin G, Witherspoon DJ, et al. Genetic evidence for high-altitude adaptation in Tibet. Science. 2010;329:72–75. doi: 10.1126/science.1189406. [DOI] [PubMed] [Google Scholar]
- Sorensen KD, Orntoft TF. Discovery of prostate cancer biomarkers by microarray gene expression profiling. Expert Rev Mol Diagn. 2010;10:49–64. doi: 10.1586/erm.09.74. [DOI] [PubMed] [Google Scholar]
- Talmud PJ, Hingorani AD, Cooper JA, Marmot MG, Brunner EJ, Kumari M, et al. Utility of genetic and non-genetic risk factors in prediction of type 2 diabetes: Whitehall II prospective cohort study. Br Med J. 2010;340:b4838. doi: 10.1136/bmj.b4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tune JD, Richmond KN, Gorman MW, Feigl EO. KATP+ channels, nitric oxide, and adenosine are not required for local metabolic coronary vasodilation. Am J Physiol Heart Circ Physiol. 2001;280:H868–H875. doi: 10.1152/ajpheart.2001.280.2.H868. [DOI] [PubMed] [Google Scholar]
- Van Der Vegt, de Bock GH, Hollema H, Wesseling J. Microarray methods to identify factors determining breast cancer progression: potentials, limitations, and challenges. Crit Rev Oncol Hematol. 2009;70:1–11. doi: 10.1016/j.critrevonc.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Varki A, Rosenberg LE. Emerging opportunities and career paths for the young physician-scientist. Nat Med. 2002;8:437–439. doi: 10.1038/nm0502-437. [DOI] [PubMed] [Google Scholar]
- Verkman AS, Galietta LJV. Chloride channels as drug targets. Nat Rev. 2009;8:153–171. doi: 10.1038/nrd2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weedon MN, Frayling TM. Reaching new heights: insights into the genetics of human stature. Trends Genet. 2008;24:595–603. doi: 10.1016/j.tig.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Weinberg RA. Point: Hypotheses first. Nature. 2010;464:678. doi: 10.1038/464678a. [DOI] [PubMed] [Google Scholar]
- Wishnow RM, Steinfeld JL. The conquest of the major infectious diseases in the United States: A bicentennial retrospect. Ann Rev Microbiol. 1976;30:427–450. doi: 10.1146/annurev.mi.30.100176.002235. [DOI] [PubMed] [Google Scholar]
- Yi X, Liang Y, Huerta-Sanchez E, Jin X, Cuo ZXP, Pool JE, et al. Sequencing of 50 human exomes reveals adaptation to high altitude. Science. 2010;329:75–78. doi: 10.1126/science.1190371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang W, Chen K. Search for cancer risk factors with microarray-based genome-wide association studies. Technol Cancer Res Treat. 2010;9:107–121. doi: 10.1177/153303461000900201. [DOI] [PubMed] [Google Scholar]


