Abstract
Light is the most potent stimulus for synchronizing the endogenous circadian timing system to the 24 h day. The timing, intensity, duration, pattern and wavelength of light are known to modulate photic resetting of the circadian system and acute suppression of melatonin secretion. The effect of prior photic history on these processes, however, is not well understood. Although previous studies have shown that light history affects the suppression of melatonin in response to a subsequent light exposure, here we show for the first time that a very dim light history, as opposed to a typical indoor room illuminance, amplifies the phase-shifting response to a subsequent sub-saturating light stimulus by 60–70%. This greater efficacy provides evidence for dynamic adaptive changes in the sensitivity of circadian ocular photoreception. This plasticity has important implications for the optimization of light therapy for the treatment of circadian rhythm sleep disorders.
Non-technical summary
The human biological clock organizes and regulates the timing of many biochemical and physiological processes, including the timing of sleep, on a daily basis. Light is the strongest time cue to the circadian clock that keeps these rhythms entrained to the 24 h day. Light exposure at night results in ‘resetting’ of the clock (phase shifting). In the current study, we examined the effects of exposing subjects to two different light levels (very dim light vs. typical room light) before exposure to a 6.5 h light exposure at night. Results showed that the very dim light level, compared to the typical room light level, prior to the light exposure at night caused a substantially greater phase shift of the melatonin rhythm and substantially greater acute melatonin suppression. Thus, prior dim light history sensitizes the human biological clock to the effect of a subsequent light exposure.
Introduction
In mammals, the circadian pacemaker, located in the suprachiasmatic nucleus (SCN) of the anterior hypothalamus, generates circadian (approximately 24 h) rhythms in physiology and behaviour (Moore & Eichler, 1972; Stephan & Zucker, 1972; Ralph et al. 1990). These endogenous circadian rhythms are driven by transcription–translation feedback loops of clock genes within SCN neurons (Siepka et al. 2007). Light is the most important stimulus to adjust the circadian timing and to suppress nighttime melatonin production (Brainard et al. 1997; Czeisler & Gooley, 2007). Although the effects of various properties of light exposures (timing, intensity, duration, pattern and wavelength) have been studied in great detail, the effect of prior photic history has been the subject of few investigations. Animal experiments have revealed that the circadian resetting response to a light stimulus can be reduced by a preceding non-saturating stimulus (Nelson & Takahashi, 1999). More recently, it has been demonstrated that prior photic history affects the sensitivity of the intrinsically photosensitive retinal ganglion cells that mediate circadian photoreception in mammals (Wong et al. 2005). Specifically, these neurons exhibit light and dark adaptation, becoming ‘desensitized’ by exposure to a brief light flash and ‘re-sensitized’ by time spent in darkness.
In humans, there has also been evidence of modulation of the sensitivity of the melatonin-suppressing response by prior lighting conditions (Hébert et al. 2002; Smith et al. 2004; Jasser et al. 2006). The first study to demonstrate photic adaptation in humans (Hébert et al. 2002) compared the amount of melatonin suppression caused by a nighttime 3 h light stimulus (500 lux) following 1 week of exposure to daytime bright light (5000–7000 lux ambient) vs. 1 week of daytime dim light (<200 lux ambient). Results of this field study showed significantly greater suppression after the week of dim light than the week of bright light. Previous work in our laboratory demonstrated that prior photic history over 3 days altered the magnitude of melatonin suppression in response to a subsequent light stimulus (Smith et al. 2004). Greater melatonin suppression was caused by a moderately bright light exposure (200 lux) during the subjective night following 3 days of very dim light (1 lux) as compared to following 3 days of moderately bright light exposure (200 lux). More recently, dim white light (∼18 lux) adaptation was shown to dampen melatonin suppression by subsequent exposure to 460 nm monochromatic light (7.0 μW cm−2 or 3.1 μW cm−2) compared with dark adaptation (Jasser et al. 2006). None of these studies, however, could assess whether prior light history also affects the phase-resetting capacity of light in humans, the property most clinically relevant for the treatment of circadian rhythm sleep disorders, including delayed sleep phase syndrome, advanced sleep phase syndrome, non-24 h sleep/wake disorder, shift work sleep disorder, and jet lag (Sack et al. 2007a,b;). In the current study we present results that support previous findings on the adaptation of melatonin suppression and further expand upon these studies by showing adaptation of the phase-shifting response. Here, we demonstrate that prior photic history modulates (i) the phase resetting capacity and (ii) the magnitude of the melatonin suppression in response to a subsequent sub-saturating light exposure.
Methods
Overview
The research study consisted of a 32 day in-patient protocol. The controlled, randomized, single-blinded, crossover study was designed to compare the effects of two prior light conditions on the melatonin suppression and phase resetting responses to a subsequent light exposure (LE). A total of four LEs were administered to each subject: two experimental LEs and two control LEs. Each LE lasted 6.5 h in duration and was scheduled to occur during the beginning of the subjective night in order to induce maximal phase delays and melatonin suppression by the experimental LEs. The purpose of the control LEs was to insure that any phase shift measured following the experimental LEs was due to the light and not other non-photic factors of the protocol itself. The illuminance of the control LEs was very dim (1 lux) and we expected no significant suppression or phase shift of plasma melatonin levels during these two sessions. The illuminance of the experimental LEs (90 lux), a typical indoor room light level, was selected to provide sufficient stimulus for the suppression and phase shifting of melatonin but not to saturate these circadian responses thereby preventing the effects of the different prior light conditions to emerge. Furthermore, 90 lux results in approximately half of the maximum achievable effect of light on melatonin suppression and circadian phase following very dim illumination and is positioned at the steepest slope of the illuminance–response curve, thereby maximizing sensitivity of the system to slight changes in stimulus strength (Zeitzer et al. 2000). Each control and experimental LE was preceded by 3 days of either very dim or typical room light (1 lux vs. 90 lux) as a prior light history. The very dim light level (1 lux) was selected to provide a subthreshold light level, yet not complete darkness, which would allow for maximum effect in comparison to the brighter 90 lux prior light condition. Furthermore, the 1 lux prior history condition was used in the previous study (Smith et al. 2004) and therefore provided a useful comparison of results between protocols. More detail about the protocol is given below.
Ethical approval
The clinical research studies were conducted according to the principals established by the latest revision of the Declaration of Helsinki. Informed, written consent was obtained from all study participants prior to enrollment and they were paid for their participation in the study. The protocol was approved by the institutional review board of the Brigham and Women's Hospital.
Subject selection
Seventeen healthy young adults between the ages of 18 and 30 years (7 females and 10 males; 23.82 ± 2.77 years, mean ±s.d.) completed a 32 day in-patient protocol. The screening process included medical and psychological assessment via questionnaires, physical examination, comprehensive blood/urine tests, and psychological interview. Subjects were required to refrain from use of caffeine, nicotine and alcohol for 3 weeks prior to admission to the laboratory, which was verified by toxicological blood/urine tests performed during the screening and in-patient protocol. Subjects were excluded from study for any medical, psychological, or sleep disorders, use of any medication (except approved oral contraceptives), recent night-shift work (in the prior 3 years), recent travel across more than one time zone (in the prior 3 months), or if their habitual sleep schedule was extreme in either timing (bedtime earlier than 9 pm or later than 2 am) or duration (<7 h or >9 h per night). Additionally, subjects were asked to maintain a regular 8 h sleep schedule for 3 weeks prior to entering the in-patient laboratory study, during which time subjects were required to call-in to a voicemail system at each bedtime and wake time, to complete daily sleep diaries, and to wear an actigraph (Actiwatch-L) measuring wrist activity (Actiwatch and Actiware systems; Mini Mitter/Respironics, Bend, OR, USA). These data were reviewed prior to admission to the laboratory in order to verify adherence to the study procedures.
Experimental protocol
Following admission to the General Clinical Research Centre of the Brigham and Women's Hospital, subjects lived in a personal suite with no external time cues (no windows or timepieces) for 32 days. The protocol design is shown in Fig. 1. Subjects were in darkness (<0.00006 W cm−2: <0.02 lux) during scheduled sleep episodes. The alignment/re-alignment segment consisted of 3 days of moderately bright ambient room light (1.18 W cm−2: ∼445 lux) at the beginning (days 2–4) and in the middle (days 17–19) of the protocol, to align the circadian phase at the onset of the study and to re-align it following the first experimental LE when a phase shift was expected. The prior light history conditions consisted of 3 days of either the very dim (1 lux) or typical room light (90 lux) throughout the waking episode, followed by a control or experimental LE. Subjects were randomly assigned to the order of the prior light history condition, either 1 lux or 90 lux, for the first half of the study. Within each prior light history condition, however, the order of the LEs (control followed by experimental) was kept the same for all subjects to prevent the phase shifts caused by the experimental LEs affecting subsequent LE timing.
Figure 1. Double-plotted raster of the 32 day protocol.

This raster represents one of the two study designs, i.e. with the sequence of the prior light history being 1 lux for the first half and 90 lux for the second half of the protocol, as an example for a subject with a habitual bedtime of 24.00 h. Different light levels are represented by the following coloured bars: white (450 lux on days 2–4 and 17–19), light grey (90 lux on days 1, 20–23, and 26–28), dark grey (1 lux on days 5–7 and 11–14), and black (sleep episodes in 0 lux). Constant posture conditions are denoted by bars with horizontal stripes. Four 6.5 h LE sessions are represented by bars with a sun symbol: 2 control LE (days 8 and 23) and 2 experimental LE (days 14 and 29).
Four 6.5 h LEs were administered: two experimental LEs with 90 lux illuminance (irradiance of ∼0.23 W m−2 at 137 cm from the floor in the horizontal angle with a maximum of 0.48 W m−2 (∼150 lux) at 187 cm from the floor in the vertical angle) and two control LEs with 1 lux illuminance (∼0.001 W m−2 at 137 cm from the floor in the horizontal angle with a maximum of 0.010 W m−2 (∼3 lux) at 187 cm from the floor in the vertical angle). Figure 1 shows the timing of the control LEs (grey sun symbol) on days 8 and 23, and of the experimental LEs (white sun symbol) on days 14 and 29. All LEs were administered during the subjective night, beginning approximately 1 h before the subject's habitual bedtime, with the goal that the midpoint of the exposure would be 18–24 h after the melatonin midpoint of the previous night and would occur within the 6 h window prior to the peak of melatonin. Subjects were required to remain in constant posture conditions, semi-recumbent in bed, for a total of 14 h: 3 h prior to the LEs, 6.5 h during the LEs, and 4.5 h following the LEs. During the 6.5 h LEs, subjects were asked to alternate their gaze between a fixed target on the wall directly in front of them and a free gaze every 5 min. They were not allowed to close their eyes during any portion of the LEs and light readings were taken from the corneal level in the direction of gaze at each change of gaze (i.e. every 5 min).
Blood was sampled every 30–60 min via an indwelling intravenous catheter during constant posture conditions for 3 consecutive days: the day prior to, during and following each of the four LEs. Constant posture conditions included maintenance of a semi-recumbent posture with the head of the bed at a 45 deg angle; minimal activity (e.g. reading, talking and taking computer tests); constant room temperature; and very dim light (1 lux) except for experimental LEs when light levels were brighter (90 lux). Plasma melatonin was assayed by radioimmunoassay (Pharmasan Labs Inc., Osceola, WI, USA) with an assay sensitivity of 0.7 pg ml−1. The intra-assay and inter-assay coefficient of variation are 5.7–12.1% and 8.4–13.2%, respectively.
Data and statistical analysis
Melatonin suppression for each of the four 6.5 h LEs was calculated as the percentage change in plasma melatonin area under the curve (AUC; trapezoidal method) during the LE as compared to the AUC during the equivalent 6.5 h time window 24 h earlier. The suppression for each control 1 lux LE was then subtracted from the corresponding suppression for each 90 lux LE in the same light history condition. The midpoint of the melatonin curve was used as the circadian phase marker to assess phase shifts and was determined as the average between the dim light melatonin onset and offset (using the 25% of the 3-harmonic cosinor-fitted peak level). Phase shifts (delays) were calculated for each LE by subtracting the melatonin midpoint on the day before LE from the melatonin midpoint on the day after LE. Phase shifts from each control LE (1 lux) was then subtracted from the phase shift of the corresponding experimental LE (90 lux) in the same light history condition. Based on the inclusion criteria for the timing of the experimental LE from the phase response curve, four subjects were excluded from any further analysis if the midpoint of any of the LEs occurred outside of the range of 18–24 h after melatonin midpoint of the previous night. In three of these individuals, the experimental LE following the very dim light history occurred too early, and in the fourth subject, the experimental LE following the very dim light history began too late. Of the 13 subjects included in this analysis, six were randomized to the 1 lux prior light condition first and seven received the 90 lux prior light condition first. Paired Student's t test (two-tailed) was used to compare the melatonin suppression and phase shift results between the very dim (<1 lux) and brighter (90 lux) prior light history conditions. For all comparisons, n= 13 and P < 0.05 were considered statistically significant.
Results
Individual melatonin profiles from three consecutive days, preceding, during and following each LE are shown in Fig. 2. Melatonin suppression and phase-shift results are listed in Table 1. Melatonin suppression caused by the 90 lux LE, and corrected for the control 1 lux LE, was significantly greater (P= 0.0001) following 1 lux prior light history (mean ±s.d.; 86.1%± 13.9%) than following 90 lux prior light history (51.3%± 25.2%). This difference represented a 68% greater suppression of melatonin concentration following very dim light history as compared to following prior room light. In fact, each of the individuals showed greater suppression of melatonin with the 1 lux prior light history (Fig. 3A).
Figure 2. Melatonin profiles under different light conditions.
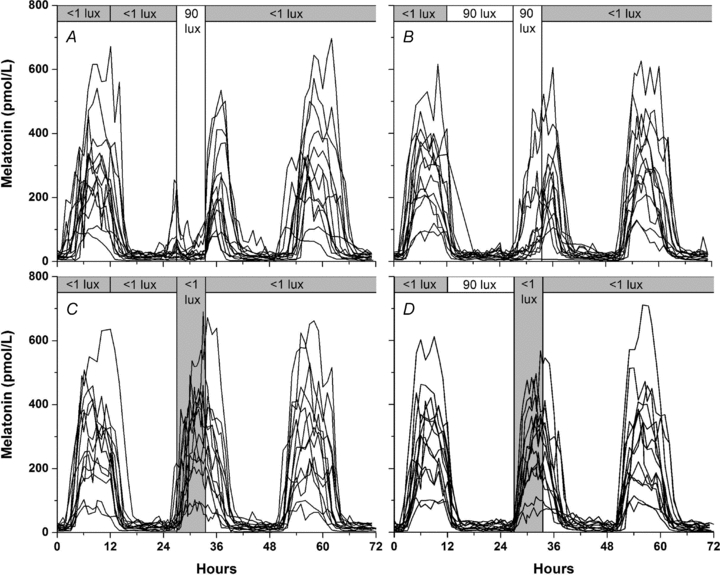
Individual melatonin profiles from 13 subjects during 4 different light conditions: 1 lux prior light history and 90 lux LE (A), 90 lux prior light history and 90 lux LE (B), 1 lux prior light history and 1 lux LE (C), and 90 lux prior light history and 1 lux LE (D). Hourly plasma melatonin levels are shown for 72 h (3 consecutive days) beginning on the day prior to LE (hour 0) in each condition. The vertical bar from 27 to 33.5 h on each panel shows the 6.5 h LE and the top horizontal bar shows the light history and the light conditions prior to and following the LE. Panel C shows the control condition for panel A, and panel D is the control condition for panel B. Note the strong suppression in panel A, compared with weak suppression in panel B.
Table 1.
Melatonin suppression and phase delays in different light conditions
| 1 lux prior light history | 90 lux prior light history | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 lux LE | 90 lux LE | Δ by LE | 1 lux LE | 90 lux LE | Δ by LE | Δ by prior light history | ||||||||
| Subject | Supp | Shift | Supp | Shift | ΔSupp | ΔShift | Supp | Shift | Supp | Shift | ΔSupp | ΔShift | ΔSupp | Δ Shift |
| 23D6 | 14 | 0.01 | 87 | 1.28 | 73 | 1.26 | 29 | −0.61 | 87 | 0.63 | 58 | 1.24 | 15 | 0.03 |
| 25A7 | 1 | 0.11 | 83 | 0.70 | 82 | 0.59 | 44 | 0.40 | 93 | 1.89 | 49 | 1.49 | 33 | −0.91 |
| 25E4 | −17 | 0.04 | 88 | 1.73 | 105 | 1.69 | 1 | 0.05 | 17 | 0.78 | 15 | 0.74 | 90 | 0.96 |
| 25H7 | −3 | 0.57 | 92 | 2.51 | 95 | 1.94 | 22 | 0.88 | 89 | 2.00 | 67 | 1.11 | 28 | 0.82 |
| 25M9 | −10 | 0.48 | 90 | 2.23 | 100 | 1.75 | 2 | 0.81 | 66 | 1.93 | 64 | 1.12 | 36 | 0.64 |
| 25Q5 | −13 | −0.26 | 74 | 1.12 | 88 | 1.38 | 3 | −0.13 | 67 | 1.40 | 64 | 1.53 | 23 | −0.15 |
| 25R7 | 1 | −0.43 | 60 | 1.22 | 59 | 1.65 | 2 | −0.04 | 15 | 1.41 | 13 | 1.45 | 46 | 0.20 |
| 2602 | −13 | 0.77 | 88 | 2.38 | 101 | 1.61 | 4 | 0.66 | 74 | 1.58 | 70 | 0.92 | 31 | 0.69 |
| 2690 | −4 | 0.02 | 78 | 2.07 | 81 | 2.05 | 0 | 0.70 | 16 | 1.51 | 16 | 0.81 | 65 | 1.24 |
| 26C2 | 17 | −0.58 | 86 | 1.80 | 70 | 2.39 | 24 | 0.27 | 49 | 1.43 | 25 | 1.16 | 45 | 1.23 |
| 26E3 | −17 | −0.08 | 78 | 1.73 | 95 | 1.81 | 14 | 0.31 | 82 | 1.12 | 68 | 0.80 | 27 | 1.01 |
| 26F9 | −1 | 0.45 | 94 | 2.45 | 95 | 2.01 | 1 | 0.99 | 90 | 0.72 | 89 | −0.27 | 6 | 2.27 |
| 26P9 | 20 | 0.28 | 96 | 1.91 | 76 | 1.63 | 22 | 0.14 | 91 | 1.38 | 68 | 1.23 | 8 | 0.40 |
| Mean | −2 | 0.11 | 84 | 1.78 | 86 | 1.67 | 13 | 0.34 | 64 | 1.37 | 51 | 1.03 | 35 | 0.65 |
| s.d. | 12 | 0.40 | 10 | 0.56 | 14 | 0.44 | 14 | 0.46 | 30 | 0.45 | 25 | 0.47 | 23 | 0.78 |
Melatonin suppression (Supp; in %) and phase shift (Shift; in hours) under 4 different light conditions: 1 lux prior light history and 1 lux LE, 1 lux prior light history and 90 lux LE, 90 lux prior light history and 1 lux LE, and 90 lux prior light history and 90 lux LE. The differences (Δ) for both melatonin suppression and phase shift are shown as a function of LE (1 lux vs. 90 lux) and as a function of prior light history (1 lux vs. 90 lux). Group mean and standard deviation (s.d.) values for suppression and phase shifts are shown at the bottom. Positive numbers under Shift indicate phase delays.
Figure 3. Adaptation of the response of melatonin suppression and phase shifts by prior light history.
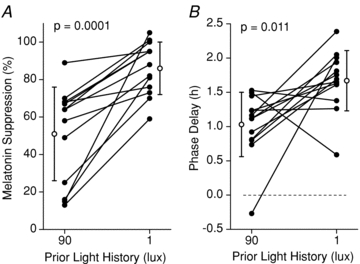
The effect of different prior light history conditions on individual melatonin suppression (A), and phase shifts (B), induced by a 90 lux LE. Data from 13 individuals are shown in the black filled symbols and the group means ±s.d. are represented by the open symbols. The melatonin suppression and phase shift data were corrected for the corresponding control LE.
Similarly, phase delays induced by the experimental 90 lux light stimuli, and corrected for the control 1 lux LE, were significantly larger (P= 0.011) for the group following the very dim prior light history (1.67 h ± 0.44 h) than following the prior room light history (1.03 h ± 0.47 h). Phase delays were 38 min larger following the very dim prior light conditions (1 lux), representing an increased efficacy of 62% (Fig. 3B). There was no order effect for the prior light history conditions (i.e. no significant difference between groups that received the 1 lux vs. 90 lux prior light history in the first half of the study) for either melatonin suppression or the phase shift of melatonin rhythm. This result suggests that the effect of the prior light history on the response of the circadian pacemaker to a subsequent light exposure is ‘washed out’ by the 3 re-entrainment days of moderately bright illuminance (450 lux; days 17–19 Fig. 1).
Discussion
These findings demonstrate that very dim light history sensitizes the circadian timing system to the phase-shifting (63% increase) and melatonin-suppressing (68% increase) effects of a subsaturating light exposure as compared to typical room light history. Similarly, the results show that this moderately dim room illuminance of 90 lux is sufficient to significantly blunt the efficacy of a subsaturating light exposure on the circadian system. Our findings are the first to show the impact of prior photic history on the circadian phase-resetting response to light in humans. In the current study, the pure effect of the experimental LE could be studied by correcting the LE-induced phase shift with any phase shift observed during the control LE. With this particular study design, we could correct for any phase-shifting effects of the light history itself (i.e. due to the daytime light exposure), any phase-shifting due to the protocol design, and any drift of the circadian system from the day before to the day after the LE as a result of individual differences in the endogenous circadian period (τ).
To investigate the effect of prior light history on the phase-resetting response, we targeted the 6.5 h LE to induce the largest phase delay according to the human phase response curve (PRC) (Van Cauter et al. 1994; Khalsa et al. 2003). Based on the published PRC to a single 6.7 h bright light pulse (∼10,000 lux) (Khalsa et al. 2003), we scheduled the LE to begin 15 h after habitual wake time so that the centre of the LE would occur 18–24 h after the melatonin midpoint of the previous night. This placed the stimulus such that it would achieve the maximal phase delay. The mean phase delay induced by the 6.5 h 90 lux LE following very dim light history was 1.67 h, more than 60% of the previously reported maximal delay of 2.7 h by Khalsa et al. using a similar duration (6.7 h) stimulus that was approximately 100-fold brighter (∼10,000 lux) than in the current study (Khalsa et al. 2003). This is in accordance with the published illuminance–response curves for melatonin phase shifts showing that the half-maximal response for phase shifts is achieved by ∼100 lux (Zeitzer et al. 2000). In contrast, the phase delay induced by the 90 lux LE following a 90 lux prior light history was only 1.03 h, less than 40% of the maximal response reported by Khalsa and colleagues.
We found inter-individual differences in adaptation of the phase-resetting and melatonin-suppressing responses to light. One potential factor that could account for the variability is the endogenous circadian period or tau (τ) of the subjects resulting in variability in the timing of the experimental LE following the very dim light history. The current protocol, employing well-controlled conditions, allows for an extended duration (up to 10 days) in the very dim light (1 lux). The timing of the experimental LE did not meet the inclusion criteria (midpoint of the experimental LE occurring between 18 and 24 h after melatonin midpoint of the previous night) in four subjects. This was caused by the drift in the circadian phase of melatonin during the 10 days in this very dim light history, likely to be due to differences in τ, although τ could not be assessed in this protocol. This is supported by the wider temporal spread of melatonin profiles from subjects in the dim prior light condition compared with the 90 lux prior light condition (Fig. 2A and C vs. B and D). Even a modest change in the timing of the LE, which may show slight changes in melatonin suppression, may result in dramatic changes of phase shifts (Khalsa et al. 2003). This further highlights the importance of measuring the phase-resetting response directly rather than rely on melatonin suppression as a proxy of overall circadian photic response. It cannot be assumed that the adaptation effects of prior light history on melatonin suppression would automatically apply to phase shifts. The observed effect of prior light history on both the melatonin suppression and phase-shifting effect of a subsequent light exposure suggests a common underlying mechanism. A potential underlying mechanism for adaptation of the circadian timing system to prior light history is adaptation of the intrinsically photosensitive retinal ganglion cells, which drive circadian phase-shifting and melatonin suppression via the suprachiasmatic nucleus of the hypothalamus, although it is currently unknown whether they share the same time constants for sensitization and desensitization (Wong et al. 2005).
While our results of adaptation of the circadian pacemaker by prior photic history are striking, there is much still unknown regarding the effect of prior light exposure. The time course for sensitization and desensitization of the circadian system is not precisely known and warrants further investigation. If, for example, the time course for sensitization is short, on the order of hours, this could greatly increase the efficacy of light therapy but also have unintended consequences, such as inadvertent phase shifting following a brief period of time in a sensitizing environment (e.g. waking up in the middle of the night after a few hours of sleeping in the dark and turning on a light). Subsequent studies are required to determine whether shorter durations and/or different intensities of light (for prior history and/or light stimulus) achieve the same effect. Whether other non-image forming functions of light, such as light-induced changes in pupil diameter, heart rate, cortisol, alertness and cortical activity are also affected by prior light history and whether the time course (in the ranges of minutes, hours and days) of sensitization and desensitization are similar requires further studies (Scheer et al. 1999; Scheer & Buijs, 1999; Lucas et al. 2001; Lockley et al. 2006). Defining the influence of prior photic history on the timing of the human circadian system has significant implications for application in both scientific and clinical settings. Prior light history conditions should be taken into account in experimental settings and may explain differences between studies. Potentiating the photic sensitivity of the circadian pacemaker would amplify phase shifts in response to light stimuli and may allow the use of lower intensity and/or shorter duration of light stimuli to achieve a similar effect. Ultimately, the clinical implication of such findings may enable the optimization of light therapy in the treatment of circadian phase misalignment. Application for these and other results of studies examining the role of photic history in sensitizing the human circadian system may be most useful in the treatment of shift work sleep disorder with light and/or sleep schedules and thus should be considered in treatment plans. These findings also provide further evidence that both melatonin suppression and circadian phase resetting are under the same photoreceptor and/or transduction pathway.
Acknowledgments
We thank the subjects for their participation in the study and the staff of the Brigham & Women's General Clinical Research Centre and the Chronobiology Core of the Division of Sleep Medicine for their contributions in carrying out the study protocol. We also thank A. Crugnale, D. McCarthy, N. McCarthy and E. Reid for their recruitment of subjects. This work was supported by NIH grant R01 HL077453 and was carried out in a General Clinical Research Centre supported by NIH grant M01-02635. A.-M.C. was supported in part by NIH grant F32 HL078360. F.A.J.L.S. is supported in part by National Institutes of Health Grant P30-HL101299. C.A.C. is supported in part by the National Space and Biomedical Research Institute Grant HFP01601.
Glossary
Abbreviations
- AUC
area under the curve
- LE
light exposure
- PRC
phase response curve
Author contributions
All authors contributed extensively to the work presented and have given final approval for its publication. A-M.C. conducted the inpatient studies, analysed data and wrote the paper. F.A.J.L.S. contributed to the concept of the experiment and designed the study, assisted with the conduct of the inpatient studies and the data analysis, and edited the paper. C.A.C. conceived and designed the study and edited the paper, and the experimental protocols were conducted in his laboratory.
References
- Brainard GC, Rollag MD, Hanifin JP. Photic regulation of melatonin in humans: Ocular and neural signal transduction. J Biol Rhythms. 1997;12:537–546. doi: 10.1177/074873049701200608. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Gooley JJ. Sleep and circadian rhythms in humans. Cold Spring Harb Symp Quant Biol. 2007;72:579–597. doi: 10.1101/sqb.2007.72.064. [DOI] [PubMed] [Google Scholar]
- Hébert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 2002;33:198–203. doi: 10.1034/j.1600-079x.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasser SA, Hanifin JP, Rollag MD, Brainard GC. Dim light adaptation attenuates acute melatonin suppression in humans. J Biol Rhythms. 2006;21:394–404. doi: 10.1177/0748730406292391. [DOI] [PubMed] [Google Scholar]
- Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549:945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockley SW, Evans EE, Scheer FAJL, Brainard GC, Czeisler CA, Aeschbach D. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29:161–168. [PubMed] [Google Scholar]
- Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci. 2001;4:621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Nelson DE, Takahashi JS. Integration and saturation within the circadian photic entrainment pathway of hamsters. Am J Physiol Regul Integr Comp Physiol. 1999;277:R1351–R1361. doi: 10.1152/ajpregu.1999.277.5.R1351. [DOI] [PubMed] [Google Scholar]
- Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Sack RL, Auckley D, Auger RR, Carskadon MA, Wright KP, Jr, Vitiello MV, Zhdanova IV. Circadian rhythm sleep disorders: part I, basic principles, shift work and jet lag disorders. An American Academy of Sleep Medicine review. Sleep. 2007a;30:1460–1483. doi: 10.1093/sleep/30.11.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack RL, Auckley D, Auger RR, Carskadon MA, Wright KP, Jr, Vitiello MV, Zhdanova IV. Circadian rhythm sleep disorders: part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. An American Academy of Sleep Medicine review. Sleep. 2007b;30:1484–1501. doi: 10.1093/sleep/30.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer FAJL, Buijs RM. Light affects morning salivary cortisol in humans. J Clin Endocrinol Metab. 1999;84:3395–3398. doi: 10.1210/jcem.84.9.6102. [DOI] [PubMed] [Google Scholar]
- Scheer FAJL, van Doornen LJP, Buijs RM. Light and diurnal cycle affect human heart rate: possible role for the circadian pacemaker. J Biol Rhythms. 1999;14:202–212. doi: 10.1177/074873099129000614. [DOI] [PubMed] [Google Scholar]
- Siepka SM, Yoo SH, Park J, Lee C, Takahashi JS. Genetics and neurobiology of circadian clocks in mammals. Cold Spring Harb Symp Quant Biol. 2007;72:251–259. doi: 10.1101/sqb.2007.72.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KA, Schoen MW, Czeisler CA. Adaptation of human pineal melatonin suppression by recent photic history. J Clin Endocrinol Metab. 2004;89:3610–3614. doi: 10.1210/jc.2003-032100. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauter E, Sturis J, Byrne MM, Blackman JD, Leproult R, Ofek G, L’Hermite-Balériaux M, Refetoff S, Turek FW, Van Reeth O. Demonstration of rapid light-induced advances and delays of the human circadian clock using hormonal phase markers. Am J Physiol Endocrinol Metab. 1994;266:E953–E963. doi: 10.1152/ajpendo.1994.266.6.E953. [DOI] [PubMed] [Google Scholar]
- Wong KY, Dunn FA, Berson DM. Photoreceptor adaptation in intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48:1001–1010. doi: 10.1016/j.neuron.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


