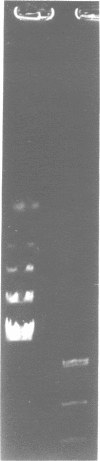
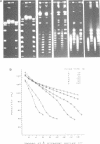
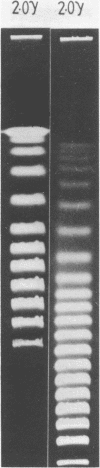
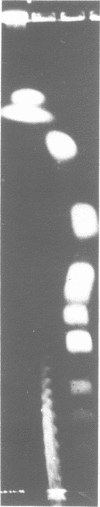
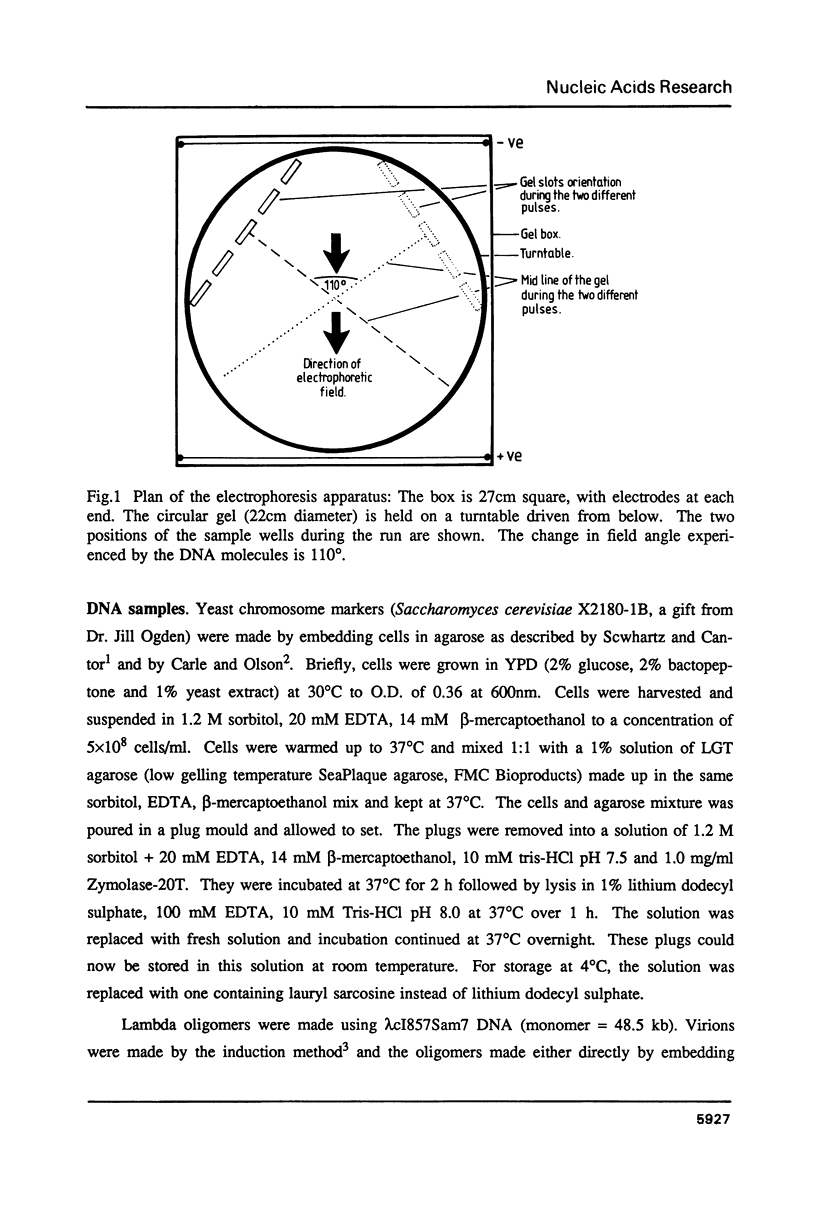
Abstract
The idea that large DNA molecules adopt a stretched conformation as they pass through gels suggests a simple mechanism for the separation of DNA by crossed field electrophoresis: at each change in field direction a DNA molecule takes off in the new direction of the field by a movement which is led by what was formerly its back end. The effect of this ratcheting motion is to subtract from the DNA molecule's forward movement, at each step, an amount which is proportional to its length. We find that this model explains most of the features of the separation, and we describe experiments, using a novel electrophoresis apparatus, which support the model. The apparatus turns the gel between two preset orientations in a uniform electric field at preset time intervals. This separation method has the practical advantage over some others that the DNA molecules follow straight tracks. A further advantage is that the parameters which determine the separation are readily predicted from the simple theory describing their motion.
Full text
PDF









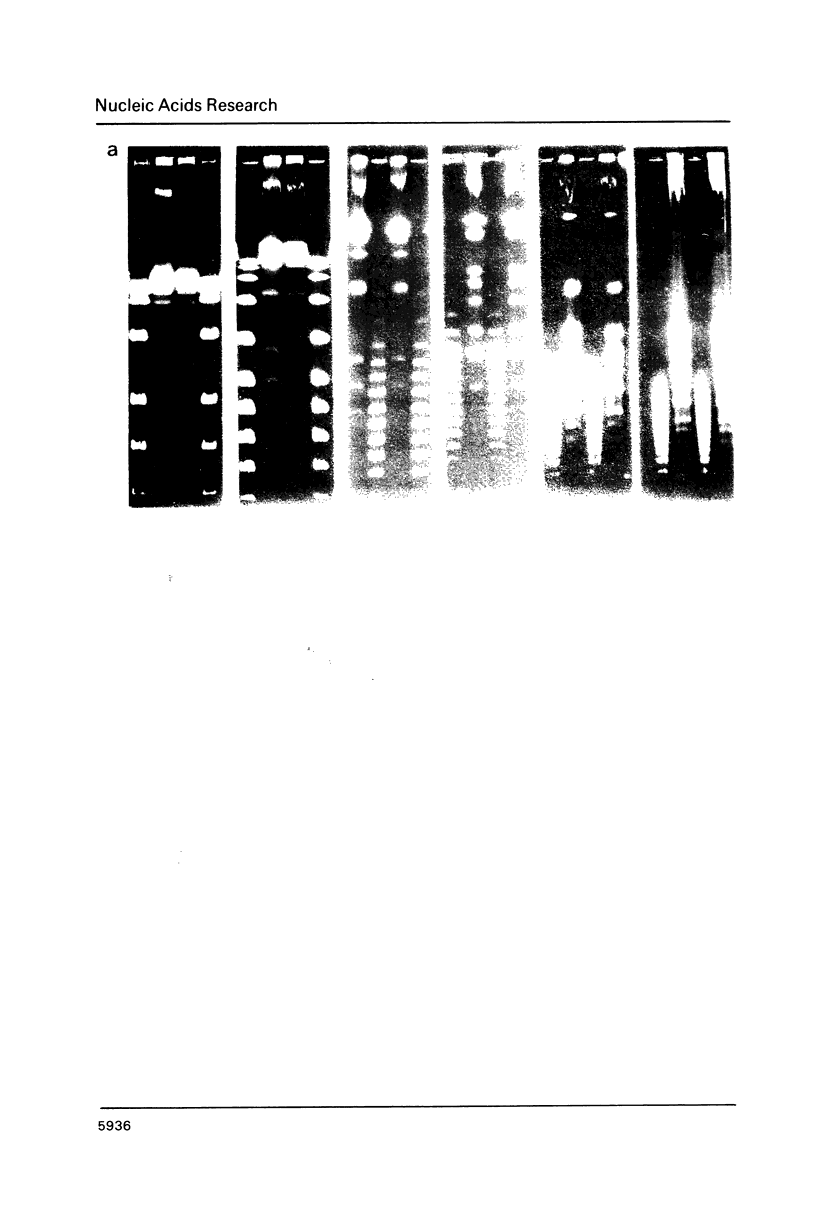
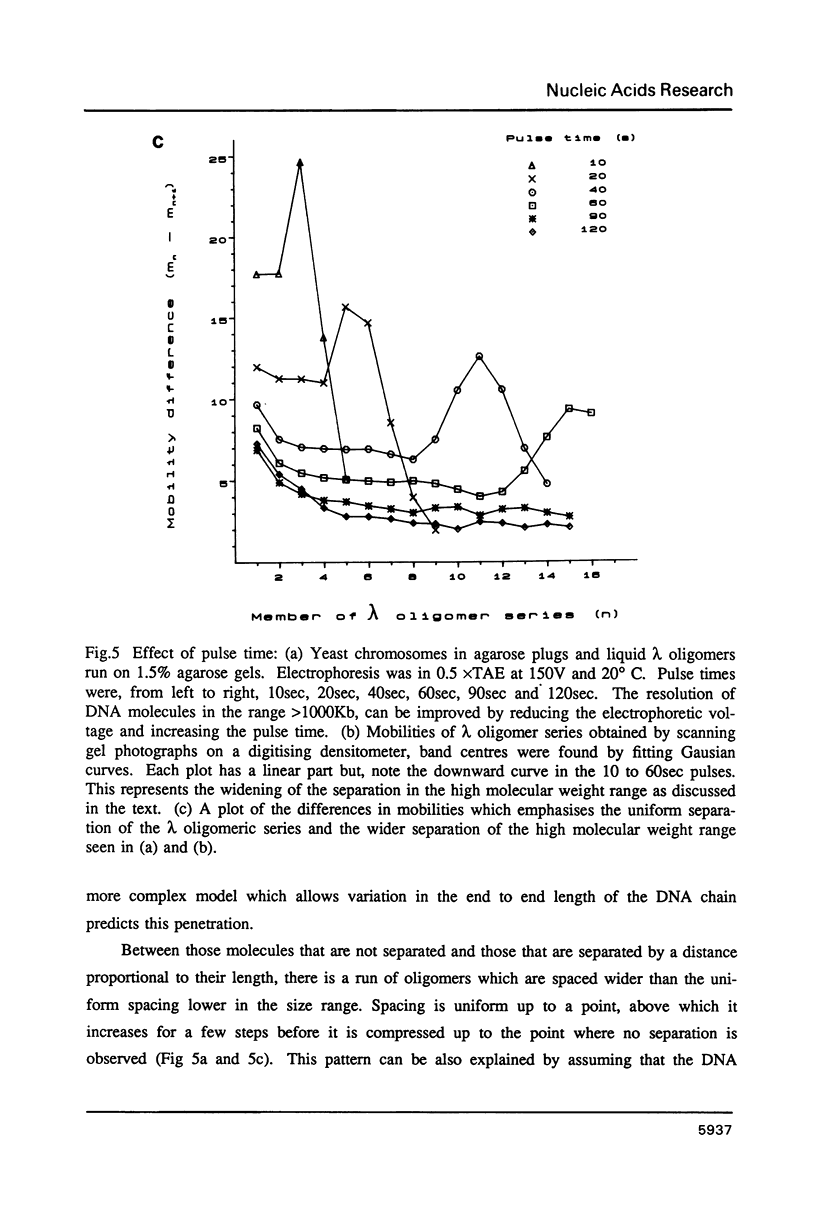




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carle G. F., Frank M., Olson M. V. Electrophoretic separations of large DNA molecules by periodic inversion of the electric field. Science. 1986 Apr 4;232(4746):65–68. doi: 10.1126/science.3952500. [DOI] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. An electrophoretic karyotype for yeast. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3756–3760. doi: 10.1073/pnas.82.11.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Fisher M. P., Dingman C. W. Role of molecular conformation in determining the electrophoretic properties of polynucleotides in agarose-acrylamide composite gels. Biochemistry. 1971 May 11;10(10):1895–1899. doi: 10.1021/bi00786a026. [DOI] [PubMed] [Google Scholar]
- LERMAN L. S. Structural considerations in the interaction of DNA and acridines. J Mol Biol. 1961 Feb;3:18–30. doi: 10.1016/s0022-2836(61)80004-1. [DOI] [PubMed] [Google Scholar]
- Lerman L. S., Frisch H. L. Why does the electrophoretic mobility of DNA in gels vary with the length of the molecule? Biopolymers. 1982 May;21(5):995–997. doi: 10.1002/bip.360210511. [DOI] [PubMed] [Google Scholar]
- Lumpkin O. J., Déjardin P., Zimm B. H. Theory of gel electrophoresis of DNA. Biopolymers. 1985 Aug;24(8):1573–1593. doi: 10.1002/bip.360240812. [DOI] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Serwer P., Hayes S. J. Exclusion of spheres by agarose gels during agarose gel electrophoresis: dependence on the sphere's radius and the gel's concentration. Anal Biochem. 1986 Oct;158(1):72–78. doi: 10.1016/0003-2697(86)90591-9. [DOI] [PubMed] [Google Scholar]
- Yanagida M., Hiraoka Y., Katsura I. Dynamic behaviors of DNA molecules in solution studied by fluorescence microscopy. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):177–187. doi: 10.1101/sqb.1983.047.01.023. [DOI] [PubMed] [Google Scholar]