Abstract
Environmental illumination profoundly influences mammalian physiology and behaviour through actions on a master circadian oscillator in the suprachiasmatic nuclei (SCN) and other hypothalamic nuclei. The retinal and central mechanisms that shape daily patterns of light-evoked and spontaneous activity in this network of hypothalamic cells are still largely unclear. Similarly, the exact nature of the sensory information conveyed by such cells is unresolved. Here we set out to address these issues, through multielectrode recordings from the hypothalamus of red cone knockin mice (Opn1mwR). With this powerful mouse model, the photoreceptive origins of any response can be readily identified on the basis of their relative sensitivity to short and long wavelength light. Our experiments revealed that the firing pattern of many hypothalamic cells was influenced by changes in light levels and/or according to the steady state level of illumination. These ‘contrast’ and ‘irradiance’ responses were driven primarily by cone and melanopsin photoreceptors respectively, with rods exhibiting a much more subtle influence. Individual hypothalamic neurons differentially sampled from these information streams, giving rise to four distinct response types. The most common response phenotype in the SCN itself was sustained activation. Cells with this behaviour responded to all three photoreceptor classes in a manner consistent with their distinct contributions to circadian photoentrainment. These ‘sustained’ cells were also unique in our sample in expressing circadian firing patterns with highest activity during the mid projected day. Surprisingly, we also found a minority of SCN neurons that lacked the melanopsin-derived irradiance signal and responded only to light transitions, allowing for the possibility that rod–cone contrast signals may be routed to SCN output targets without influencing neighbouring circadian oscillators. Finally, an array of cells extending throughout the periventricular hypothalamus and ventral thalamus were excited or inhibited solely according to the activity of melanopsin. These cells appeared to convey a filtered version of the visual signal, suitable for modulating physiology/behaviour purely according to environmental irradiance. In summary, these findings reveal unexpectedly widespread hypothalamic cell populations encoding distinct qualities of visual information.
Non-technical summary
A retinal projection to a brain ‘clock’ in the suprachiasmatic nuclei (SCN) and various other hypothalamic regions allows light to regulate many aspects of physiology and behaviour. The exact nature of light-evoked responses in these hypothalamic regions and the degree to which they rely upon the various classes of retinal photoreceptor is incompletely understood. Multielectrode recordings in the mouse hypothalamus reveal four classes of visually responsive neuron whose activity is altered by changes in illumination (contrast) and/or according to steady-state light levels (irradiance). These cells appear in different locations and vary in their daily profile of electrical activity. The properties of one class suggest a direct association with regulating the SCN clock, while the others appear suited to provide more direct modulations in physiology and behaviour. Together, these findings establish a framework for understanding how light regulates such a diverse array of body systems.
Introduction
Circadian variations in mammalian physiology and behaviour are driven by neural and hormonal outputs from a master clock in the suprachiasmatic nuclei (SCN; Kalsbeek et al. 2006; Brown & Piggins, 2007). This clock generates near-24 h cycles of activity by an endogenous mechanism but is precisely synchronized (entrained) to the local time by external inputs (Challet, 2007). The most reliable environmental signal of time of day is the light–dark cycle and the SCN receives a dense retinal innervation conveying this information (Abrahamson & Moore, 2001).
A significant evolution in our understanding of how the SCN functions has been the realization that it is composed of thousands of cellular oscillators, each capable of sustaining ∼24 h rhythms in electrical activity and/or gene expression in isolation (Welsh et al. 1995; Webb et al. 2009). In parallel, it has become clear that there is considerable variation in the circadian pattern of electrical activity in these neurones. Thus, while the population activity of the SCN is characterized by a single diurnal peak, some SCN neurons exhibit bimodal patterns of activity, or lack obvious rhythmicity, while others show peak activity at other times of day (Jobst & Allen, 2002; Quintero et al. 2003; Saeb-Parsy & Dyball, 2003; Schaap et al. 2003; Karatsoreos et al. 2004; Hughes et al. 2008; Belle et al. 2009; Brown & Piggins, 2009).
An important unanswered question is how this diversity in circadian physiology correlates with the appearance of entraining signals from the retina. One body of work suggests that the SCN cells that exhibit light evoked gene-expression do not themselves show rhythms in electrical activity (Hamada et al. 2001; Jobst & Allen, 2002; Antle et al. 2003; Karatsoreos et al. 2004). However, the relationship between basal firing patterns and electrophysiological light responses remains unexplored. Published work suggests that not all SCN neurons are light responsive, and those that are can be either excited or inhibited (Groos & Mason, 1978, 1980; Meijer et al. 1986; Ying & Rusak, 1994; Aggelopoulos & Meissl, 2000; Drouyer et al. 2007; Mure et al. 2007). At present we do not know if these different light responses occur in neurons with different basal firing patterns, or how the various endogenous patterns of electrical activity in the SCN are altered by light.
Another aspect of the SCN light response that remains incompletely explained is its photoreceptive origin. Entraining signals reach the SCN primarily via a specialised class of retinal ganglion cell that expresses the photopigment melanopsin (melanopsin expressing retinal ganglion cells, mRGCs; Hattar et al. 2006; Goz et al. 2008; Guler et al. 2008; Hatori et al. 2008). The presence of melanopsin allows these cells to be intrinsically photosensitive, but they also receive input from outer retinal rod/cone photoreceptors (Belenky et al. 2003; Dacey et al. 2005; Perez-Leon et al. 2006; Viney et al. 2007; Wong et al. 2007). Accordingly, clock entrainment is at least partially intact in mice lacking either rods+cones (Freedman et al. 1999; Semo et al. 2003) or melanopsin (Panda et al. 2002; Ruby et al. 2002). Similarly, electrophysiological light responses in the SCN show evidence of input from rod and cone, as well as melanopsin photoreceptors (Aggelopoulos & Meissl, 2000; Drouyer et al. 2007; Mure et al. 2007). Data from melanopsin knockout (Opn4−/−) mice support the idea that at least one aspect of the SCN light response (sustained excitation) is especially reliant upon melanopsin. However, the degree to which rods and/or cones also contribute to sustained excitation remains unclear, as does the relative contribution of each photoreceptor class to other features of the SCN light response. Similarly, the possibility that these contributions change as a function of circadian phase remains unexplored, even though rhythms in both photoreceptor function and the SCN response have been reported (Meijer et al. 1996; Barnard et al. 2006; Cameron et al. 2008; Ribelayga et al. 2008; Cameron & Lucas, 2009; Gonzalez-Menendez et al. 2009; Weng et al. 2009).
A further question regarding the electrophysiological response of the SCN to light is its relationship with circadian entrainment. Published work suggests that excitatory responses in the rodent SCN encode irradiance and are enhanced at night. Both of these characteristics are consistent with those of circadian light resetting. However, the reported low sensitivity of SCN light responses (Groos & Mason, 1978, 1980; Meijer et al. 1986, 1992) has been hard to reconcile with the relatively high sensitivity of the circadian clock to light as assessed in behavioural studies (Altimus et al. 2010; Lall et al. 2010). Along a similar line, while cone signals apparently contribute to mRGC firing and impact the rodent SCN (Aggelopoulos & Meissl, 2000), recent data suggest that they have limited ability to support entrainment (Lall et al. 2010). Indirect evidence suggests that this latter effect reflects adaptation in cone input to the clock (Dkhissi-Benyahya et al. 2007; Dollet et al. 2010; Lall et al. 2010). This is proposed also to occur in humans, but at a much slower rate (Gooley et al. 2010). A description of the kinetics of cone-dependent responses in SCN neurons would be a very useful test of these explanations. However, this information is currently unavailable.
Here we set out to address these deficits in our knowledge of the SCN light response by using multichannel extracellular electrophysiology to record basal activity and responses to a variety of visual stimuli in chronically anaesthetised mice. Using this strategy we have sampled large numbers of neurons at the single cell level and tracked their activity over significant portions of the circadian cycle. As a result we are able to relate light responses to circadian patterns of basal firing for the first time. Because the multichannel electrodes also had recording sites outside of the SCN, we are also able to track the activity of neurones in the medial hypothalamus and ventral thalamus. Light responses in these parts of the brain have not previously been recorded to our knowledge, although the medial hypothalamus is known to be an important relay station for clock output signals, and receives sparse retinal input.
In order to determine the individual contribution of rod, cone and melanopsin photoreceptors to SCN light responses we conducted our experiments in red cone knockin mice (Opn1mwR). In these animals, a human long wavelength sensitive cone opsin coding sequence has replaced that of the native mouse medium wavelength sensitive cone opsin (Smallwood et al. 2003). Physiological and behavioural studies confirm that the visual system of these mice is intact and appears fully functional (Smallwood et al. 2003; Brown et al. 2010; Lall et al. 2010). The introduction of the human red cone opsin, however, shifts the spectral sensitivity of the mouse cone population to longer wavelengths. This renders the spectral efficiency profile of melanopsin and rod and cone photoreceptors sufficiently divergent to allow their contribution to evoked responses to be identified simply by assessing sensitivity to different wavelengths of light (Lall et al. 2010). This strategy represents an advance over alternative approaches based upon gene knockout/retinal degeneration because it explores the light response as an emergent property of the intact visual system.
We report the detection of numerous light responsive neurons in the SCN, medial hypothalamus and midline ventral thalamus. We find that these light responsive cells can be separated into four quite distinct populations on the basis of the nature of their light response and the degree to which this relies upon signals from rods, cones and melanopsin. These populations also differ in the quality of visual information that they convey, and in their circadian patterns of basal activity. A comparison with published data for behavioural light responses in Opn1mwR mice finally demonstrates that the light response of one of these cell types provides a good approximation for that of the circadian clock itself.
Methods
Animals
All animal care and experimentation received institutional ethics committee and UK Home Office approval and was in accordance with regulations laid out in the UK Animals (Scientific Procedures) Act 1986 and the policy of The Journal of Physiology (Drummond, 2009). Melatonin proficient male Opn1mwR mice (offspring of female C3H mice and hemizygous male Opn1mwR mice; Smallwood et al. 2003; Lahiri et al. 2004) were kept under a 12 h dark–light cycle at a temperature of 22°C with food and water ad libitum. Projected Zeitgeber time (pZT) 0 was designated as the time of light on in the animals’ home cage.
In vivo neurophysiology
Surgical procedures
Surgical procedures, conducted as described previously (Brown et al. 2010, 2011), were completed during the day (pZT 1–11). Adult male mice (80–160 days) were anaesthetized by i.p. injection of 30% (w/v) urethane (1.7 g kg−1; Sigma, Poole, UK) and placed in a stereotaxic apparatus (SR-15M; Narishige International Ltd, London, UK). Additional top up doses of anaesthetic (0.2 g kg−1i.p.) were applied as required. Throughout the experiment the animals’ temperature was maintained at 37°C with a homeothermic blanket (Harvard Apparatus, Edenbridge, UK). The skull surface was exposed and a small hole (∼1 mm diameter) drilled above the SCN or visual thalamus (0.4 mm posterior and 0.1 mm lateral or 2.5 mm posterior and 2.3 mm lateral to the bregma, respectively). The pupil contralateral to the craniotomy was dilated with topical application of 1% (w/v) atropine sulphate (Sigma) and the cornea kept moist with mineral oil.
For SCN recordings we used linear silicon arrays consisting of 32ch. electrodes with a spacing of 50 μm (A1X32-10mm-50-413; NeuroNexus Technologies Inc., Ann Arbor, MI, USA). These were centred on the craniotomy and lowered to a depth of 5.3 mm relative to the brain surface using a fluid filled micromanipulator (MO-10; Narishige), such that the recording sites spanned the SCN, periventricular hypothalamus and ventral midline thalamus. Unless otherwise stated, where we refer to hypothalamic recordings in the text, we include data from all cells corresponding to a particular population recorded across any of these regions. For thalamic recordings we used silicon arrays consisting of four shanks (spaced 200 μm), each with 8 recordings sites (spaced 50 μm; A4X8-5mm-50-200-413; Neuronexus). Probes were positioned centrally on the exposed skull surface, perpendicular to the midline, and lowered to a depth of 3.0 mm, such that recording sites spanned the intergeniculate leaflet (IGL) and surrounding thalamus.
Recording methodology
Once the recording probe was in position the recording chamber was covered with darkroom blackout material (Nova Darkroom, Stratford-upon-Avon, UK) and the room lights dimmed. Under these conditions background photon flux within the recording chamber was below the limit of our detectors. Mice were dark adapted for 1 h, which also allowed neuronal activity to stabilize after probe insertion/repositioning, after which we began recording. Neural signals were acquired using a Recorder64 system (Plexon, Dallas, TX, USA). Signals were amplified ×3000, highpass filtered at 300 Hz and digitized at 40 kHz. Multiunit activity (spikes with amplitudes >45 μV) was saved as time-stamped waveforms and analysed offline (see below). Light stimuli were delivered by a custom built LED based light source (Cairn Research Ltd, Faversham, UK), which consisted of independently controlled red and blue LEDs (λmax: 460 and 655 nm respectively) with appropriate bandpass filters (half peak width: ±10 nm). The light passed through a filter wheel with various neutral density filters and was focused onto a 5 mm diameter piece of opal diffusing glass (Edmund Optics Inc., York, UK) positioned 3 mm from the eye contralateral to the recording probe. LED intensity and filter wheel position were controlled by a PC running LabView 8.6 (National Instruments, Austin, TX, USA).
In most cases, irradiance response relationships for red/blue light steps spanning a 4 log unit range (starting at the lowest intensity: 60 s red/120 s darkness/60 s blue/120 s darkness) were determined every 1–2 h over the course of a 4–12 h recording session. After a set of responses were recorded, mice were allowed to dark adapt for at least 30 min before further light stimulation. Periodically we also investigated responses to a series of 20 brief bright light pulses (2 s red/18 s darkness/2 s blue/18 s darkness) to better assess the latency and reproducibility of neural responses. Mice were otherwise kept in complete darkness. Where we did not detect any clear neural responses after completing a full irradiance response measurement (4 of 23 mice), the electrode was removed and replaced to the same depth 200 μm rostral to its initial position and, after 1 h, recording commenced again. Light responsive units were recorded on average for 9.1 h (s.d.: 2.7 h, range 3.9–12.5 h).
Light measurements were performed using a calibrated spectrophotometer (Ocean Optics, Dunedin, FL, USA) and optical power meter (Irradian Ltd (Macam Photometrics), East Lothian, UK). Effective photon flux for each photoreceptor class was determined using the calculated spectra and visual pigment templates described by Govardovskii et al. (2000). Based on these calculations we matched the intensities of the blue and red LEDs to be isoluminant for Opn1mwR cones; unattenuated intensities were 8.3 × 1014 and 2.6 × 1015 photons cm−2 s−1 respectively (corresponding to an effective cone flux of 2.2 × 1014 photons cm−2 s−1). At this intensity, effective photon flux for the blue vs. red stimuli was ∼500× greater based on the spectral sensitivity of rods (6.2 × 1014vs. 1.2 × 1012 photons cm−2 s−1) and ∼5000× greater based on the melanopsin nomogram (7.5 × 1014vs. 1.5 × 1011 photons cm−2 s−1). Most mouse cones coexpress a UV-sensitive opsin (Applebury et al. 2000), which naturally, would also be more strongly activated by 460 nm stimuli. We believe this short wavelength sensitivity is unlikely to contribute significantly to any of the responses we describe here, since the reported threshold for evoking UV cone opsin-mediated responses (Nikonov et al. 2006; Yao et al. 2006) is around that of the brightest 460 nm stimuli we employed (4.5 × 1011 UV-cone stimulating photons cm−2 s−1). Consistent with this interpretation, 655 nm stimuli of a similar strength as corrected for Opn1mwR cones (2.2 × 1011 cone stimulating photons cm−2 s−1) were at the threshold for producing measurable changes in SCN cell spike rate.
Histological analysis
For each experiment the location of the probe was verified histologically. The electrode was immersed in fluorescent dye before recording (Cell Tracker CM-DiI; Invitrogen Ltd, Paisley, UK) and, at the end of the experiment, the mouse was perfused transcardially with 0.1 m phosphate buffered saline followed by 4% paraformaldehyde. The brain was removed, postfixed overnight, cryoprotected with 30% sucrose then sectioned at 100 μm on a freezing sledge microtome. For detection of DiI fluorescence, sections were mounted with Vectashield (Vector Laboratories Ltd, Peterborough, UK), coverslipped and visualised using an Olympus BX51 with appropriate filter sets. Sections were then aligned with the corresponding mouse atlas sections (Paxinos & Franklin, 2001) to estimate the anatomical location of each recording site (see online Supplemental Material, Supplemental Fig. 1).
Data analysis
Multichannel, multiunit recordings were analysed in Offline Sorter (Plexon). After removing artefacts we used principal component based sorting to discriminate single units, identifiable as a distinct cluster of spikes in principal component space with a clear refractory period in their interspike interval distribution. For hypothalamic recordings we very rarely detected more than one unit per recording site and at around half the recording sites were unable to resolve clear single units (389 units from 864 recording sites, 27 electrode placements). Our thalamic recordings provided a much higher yield with distinct units readily identifiable at most recording sites (168 units from 160 recording sites in 5 mice). Following spike sorting, data were exported to Neuroexplorer (Nex Technologies, Littleton, MA, USA) and MATLAB R2007a (The Mathworks Inc., Natick, MA, USA) for construction of peristimulus histograms and further analysis. Light responsive units were identified as those where the peristimulus average showed a clear peak (or trough) that exceeded the 99% confidence limits estimated from a Poisson distribution derived from the prestimulus spike counts.
For analysis of circadian variations in basal firing activity, for each cell we identified all epochs of recording that occurred between 1 min after the termination of a light stimulus and the start of the next stimulus and averaged these into 30 min bins across the recording session. Where applicable, curves were fitted using GraphPad prism v4 (GraphPad Software Inc., San Diego, CA, USA). For analysis of circadian variation in photic responsiveness we averaged responses of each cell to the brightest 460 nm stimuli into 2 h bins across the projected day. To determine time of day effects on sensitivity to 460 nm and 655 nm lights, we restricted our analysis to cells where we completed full irradiance response measurements during the projected day and night. Here, for each cell we averaged responses recorded between pZT5 and pZT11 (mid-late day) or pZT12 and pZT18 (early to mid-night). Significant effects of time of day, wavelength and intensity were calculated by repeated measures MANOVA in SPSS 16.0 (SPSS Inc., Chicago, IL, USA) followed by the Bonferroni post hoc test as appropriate. Where we did not separate responses according to time of day, two-way repeated measure ANOVA and Student's t test for paired data were used. Significance was set at P < 0.05.
Results
Classification of light response types
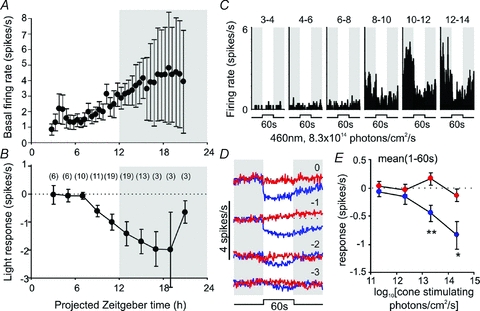
In multiunit recordings from the SCN, periventricular hypothalamus and midline ventral thalamus of 23 Opn1mwR mice, we identified 118 single units (out of 389) that showed reproducible responses to a stimulus designed to robustly activate all mouse photoreceptor classes (Supplemental Fig. 1; 60 s; 460 nm; 8.3 × 1014 photons cm−2 s−1). The response profile of each cell to repeated stimuli was consistent, but there was considerable heterogeneity between units. In fact, light responses fell into four clear categories according to the nature of their light evoked activity (Fig. 1). Two of these response types (‘transient’ and ‘sustained’) showed rapid increases in firing within 100 ms of light presentation, but differed in the persistence of light-evoked firing. In the more numerous ‘sustained’ class (n= 35), responses only partially decayed under constant illumination, being clearly above baseline throughout the last 30 s of the light step (4.2 ± 0.6 spikes s−1vs.2.4 ± 0.3 spikes s−1; paired t test, P < 0.01). By contrast, in ‘transient’ cells (n= 10), responses decayed more rapidly, with firing rates returning to basal levels by the second 30 s of light exposure (1.8 ± 0.4 vs. 1.5 ± 0.3 spikes s−1; paired t test, P > 0.05). Some ‘transient’ cells also exhibited rapid excitatory response to lights off (n= 5). The third class (‘delayed’; n= 51) lacked the initial phasic response to light on, but showed tonic increases in spike rate similar to the sustained cells (mean increase in spike rate over 60 s: 0.9 ± 0.1 vs. 2.7 ± 0.4 Hz). Light responses in ‘delayed’ cells built up over 1–2 s following light on and persisted for up to 2 min following light off. The final response type (‘suppressed’, n= 22) showed similar kinetics, but in this class light inhibited firing.
Figure 1. Diversity of hypothalamic light responses.
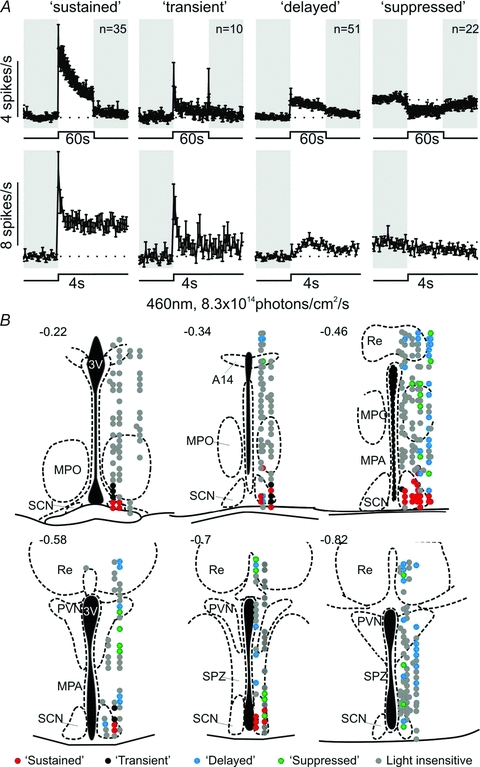
A, four different classes of light response detected in the hypothalamus and ventral midline thalamus. Top traces represent mean (±s.e.m.; bin size = 1 s) responses to 60 s, 460 nm, light steps (8.3 × 1014 photons cm−2 s−1) of all units corresponding to each class. Bottom traces represent the initial 4 s of the responses at higher temporal resolution (bin size = 100 ms). Shaded areas represent darkness. B, projected anatomical localisation of cells with different types of light response. 3V, third ventricle; SCN, suprachiasmatic nuclei; MPO, medial preoptic nucleus; MPA, medial preoptic area; Re, reuniens thalamic nucleus; PVN, paraventricular nucleus; SPZ, subparaventricular zone.
Cells exhibiting the four response types differed in their anatomical distribution. ‘Sustained’ and ‘transient’ cells were encountered exclusively within or on the borders of the SCN (Fig. 1B). By contrast, we rarely found ‘delayed’ or ‘suppressed’ cells within the SCN/peri-SCN region (n= 4 and 7, respectively). Instead these were scattered throughout the medial preoptic area, subparaventricular zone, paraventricular nuclei and the reuniens thalamic nuclei (Fig. 1B). Based on these differences in anatomical localisation, and temporal profile of light evoked activity, we were interested to determine whether these various populations also differed with respect to their daily profiles of spontaneous/light evoked activity and in the photoreceptive origin of their light responses.
Sustained cells
Daily variation in spontaneous and light evoked activity
The diurnal profile in basal firing of cells with a ‘sustained’ light response phenotype closely resembled the population activity pattern previously reported for SCN slices (Green & Gillette, 1982; Gribkoff et al. 1998; Cutler et al. 2003; Schaap et al. 2003; Brown et al. 2006). Thus, they exhibited a clear rhythm (Fig. 2A), with a peak in the mid–late projected day (mean ± 95% CI: 3.1 ± 0.3 Hz at pZT 8.4 based on fitting a sinusoidal function of periodicity 24 h) and a nadir (mean ± 95% CI: 1.2 ± 0.4 Hz) during the mid projected night.
Figure 2. Hypothalamic sustained cells exhibit day–night rhythms in basal and light-evoked activity.
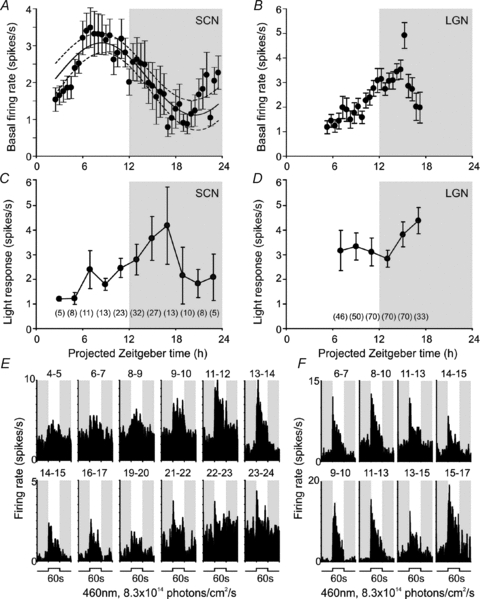
A and B, mean (±s.e.m.) basal (dark-adapted) firing rate of suprachiasmatic (SCN; A, n= 5 to 30) or lateral geniculate (LGN; B, n= 17 to 71) sustained cells at indicated time points. Curve in A represents the ±95% CI of a sinusoidal function (period = 24 h) that best fitted the data points. C and D, symbols represent mean (±s.e.m.) response of SCN (C) or LGN (D) sustained cells to 60 s light steps (change in mean spike rate during light on relative to baseline rate) at indicated time points. For C and D, numbers in brackets represent number of cells contributing to each data point. Shaded areas in A–D represent the projected night (i.e. time of light off in the animals’ home cage). E and F, examples of SCN (E) or LGN (F) sustained cells responses to 60 s light steps as a function of time. Each histogram represents the average of 2 trials between the time points indicated above the trace. Shaded areas in E and F represent epochs of darkness.
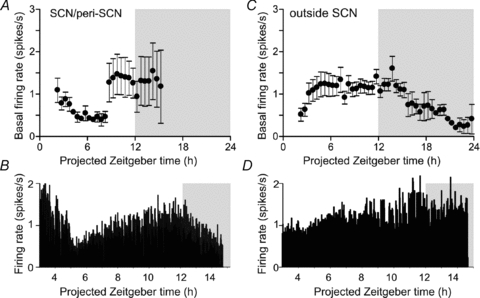
‘Sustained’ cells also showed a rhythm in light response (Fig. 2C), with light-evoked firing highest between pZT14 and 18 (an epoch when light induced circadian phase shifts are also greatest; Daan & Pittendrigh, 1976; Hughes & Piggins, 2008). This effect was apparent in the light-dependent increase in firing (Fig. 2C), but did not simply reflect changes in baseline activity as the absolute firing rate during light exposure followed a similar pattern (Fig. 2E). Thus, in 23 cells tracked across the projected day–night transition, average firing rates during light exposure were significantly greater at early to mid projected night than mid to late projected day (mean ±s.e.m.; 7.4 ± 1.3 Hz and 5.0 ± 0.7 Hz respectively; paired t test, P < 0.01). Parallel recordings in the lateral geniculate nuclei (LGN; Supplemental Fig. 2), confirmed that these circadian patterns of basal and light evoked firing were not a general feature of neural activity in our preparation (Fig. 2B, D and F). Thus, robust circadian modulation of spontaneous activity and light responsiveness is a property of the SCN and not an artifact of our experimental protocols.
Photoreceptor origins
We next turned our attention to the photoreceptive origins of the ‘sustained’ responses in our hypothalamic recordings and how these might be influenced by circadian phase. To this end, we described irradiance response relationships for 460 nm and 655 nm light steps (60 s) matched to be isoluminant for the long wavelength sensitive cones of Opn1mwR mice (Brown et al. 2010). Under these conditions, while activation of cones would be equivalent between the two stimuli, rods and melanopsin would be activated 532 and 4899 times more strongly by the shorter wavelength.
For 21 of the 23 sustained neurons tracked through the day–night transition, we completed full irradiance response measurements across both the mid to late projected day and early to mid projected night (Fig. 3A). Comparing changes in firing rate evoked by 460 nm and 655 nm stimuli revealed that, while both wavelengths initially evoked very similar excitation, response to the long wavelength light decayed much more rapidly. For this reason we analysed the influence of wavelength, intensity and circadian phase on phasic (initial peak firing within 500 ms of light on) and tonic (sustained firing over 1 to 60 s of light exposure) components separately (repeated measures MANOVA).
Figure 3. Temporal and anatomical variation in rod, cone and melanopsin input to sustained cells.
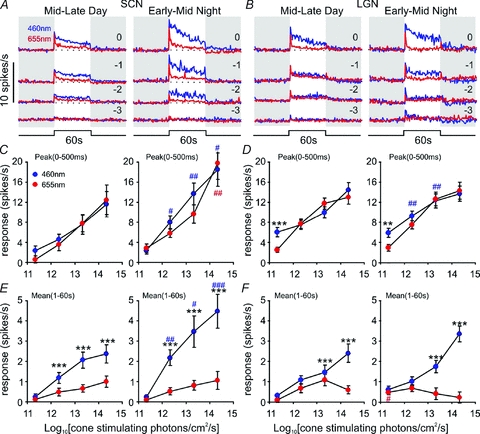
A and B, average response of SCN (A, n= 21) or LGN (B, n= 71) sustained cells to 60 s, 460 nm (blue) or 655 nm (red) light pulses of varying intensity during the mid-late projected day (left) or early-mid projected night (right). Shaded areas represent epochs of darkness, numbers above traces represent log intensity, relative to the unattenuated intensities (8.3 × 1014 and 2.6 × 1015 photons cm−2 s−1 for 460 and 655 nm respectively). C–F, quantification of data from A and B, showing peak firing rate during the first 500 ms of the response (C and D) or mean firing rate between 1 and 60 s after light on (E and F), relative to basal activity. Data in C to F were analysed by repeated measures MANOVA with intensity, wavelength and circadian time as factors, followed by the Bonferroni post hoc test. **P < 0.01 and ***P < 0.001, significant differences in responses to 460 nm and 655 nm. #P < 0.05, ##P < 0.01 and ###P < 0.001, significant difference in responses to 460 nm (blue) or 655 nm (red) between mid–late day and early–mid night.
We found that, for cone isoluminant stimuli, the phasic peak in firing rate was equivalent at both wavelengths (F1,20:1.48, P > 0.05) and was not influenced by the interaction of wavelength with either intensity or circadian phase (not shown). By contrast, the tonic component of the responses was markedly enhanced for 460 nm (F1,20: 34.36, P > 0.001), at all but the dimmest intensity employed (Fig. 3E; Bonferroni post hoc test, all P < 0.001).Thus, it seems that while the transient activation of these cells can be largely attributed to cone photoreception, a receptor maximally sensitive to shorter wavelengths makes a strong contribution to the sustained phase of the response.
In theory, the enhanced 460 nm response could arise from the activity of rods and/or melanopsin. However, the dimmest 460 nm stimuli we tested (8.3 × 1011 photons cm−2 s−1 or ∼4.2 scot cd m−2) should be sufficiently bright to evoke a maximal response from rods (Wu et al. 2004; Woodruff et al. 2008). As we observed only a very slight increase in firing rate at this lowest irradiance, it seems that rods make a modest contribution to this response type. This finding is consistent with the generally observed low sensitivity of SCN light responses in anaesthetized rodents (Groos & Mason 1978, 1980; Meijer et al. 1986, 1992; but see Aggelopoulos & Meissl, 2000). Nonetheless, behavioural assays indicate the threshold for evoking phase shifts is up to 2 log units lower than that at which changes in spike rate become detectable (Meijer et al. 1992), and we have recently shown that the spectral sensitivity of circadian phase shifts in these Opn1mwR mice matches that of rods (Lall et al. 2010). To exclude the possibility that the low amplitude of rod signals in our experiments was simply an artefact of our preparation or procedures, we therefore recorded responses in exactly the same way from a brain region where rod signals should be readily observed, the LGN (Brown et al. 2010, 2011).
The LGN contains cells whose response kinetics are similar to those of the hypothalamic ‘sustained’ population. We have previously demonstrated that melanopsin phototransduction contributes to the sustained activity of this thalamic population (Brown et al. 2010). Accordingly, we found responses of the thalamic ‘sustained’ cells to cone-isoluminant 460 and 655 nm stimuli (Fig. 3B) were generally very similar to those we observed in the hypothalamus. However, MANOVA did reveal a significant effect of wavelength on the initial transient ‘on’ response of these LGN cells (F1,69: 4.56, P < 0.05). In fact, the two wavelengths drove equivalent responses at the higher irradiances (indicating cone-dependence), but responses were significantly enhanced at the shorter wavelength for the dimmest stimuli (Fig. 3D; Bonferroni post hoc test, P < 0.01). As the enhanced 460 nm response occurred at an irradiance sub-threshold for melanopsin activation (Berson et al. 2002; Dacey et al. 2005; Tu et al. 2005; Wong et al. 2007; Mawad & van Gelder, 2008; Schmidt & Kofuji, 2009), these data represent good evidence of rod influence on LGN activity. As such they provide confidence that the limited rod input to the hypothalamus we observe is a genuine observation.
The small rod contribution to responses of the hypothalamic ‘sustained’ cells suggests that melanopsin is the major influence on the tonic phase of their response. This conclusion is consistent with published data that this aspect of the SCN light response is deficient in melanopsin knockout mice (Mure et al. 2007). To further examine this possibility, we subtracted responses to 655 nm from those elicited by the cone-isoluminant 460 nm stimuli. Assuming that (1) melanopsin makes no contribution at 655 nm, (2) any rod contribution to the firing of these cells is slight, and (3) cone responses to the two stimuli are identical, this should reveal the portion of the response that derives from melanopsin. Accordingly, the resulting response waveforms and their irradiance dependence (Fig. 4) were very similar to those previously reported for mRGCs in retinal slices (Berson et al. 2002; Dacey et al. 2005; Tu et al. 2005; Wong et al. 2007; Mawad & van Gelder, 2008; Schmidt & Kofuji, 2009).
Figure 4. Melanopsin input to sustained cells is enhanced during the projected night.
A and B, average (±s.e.m.) isolated melanopsin response of SCN (A, n= 21) or LGN (B, n= 71) sustained cells to light pulses of varying intensity during the mid–late projected day (left) or early–mid projected night (right), calculated by subtracting the response to 655 nm from the response to 460 nm (Fig 3). Shaded areas represent epochs of darkness; numbers above traces represent log intensity, relative to the unattenuated intensities (8.3 × 1014 photons cm−2 s−1 for 460 and 655 nm, respectively). C, quantification of data from A and B, showing mean firing during light on, relative to basal activity. SCN data in C were analysed by repeated measures ANOVA with intensity and circadian time as factors, followed by the Bonferroni post hoc test; LGN data were analysed by paired t test. *P < 0.05, **P < 0.01 and ***P < 0.001, significant differences in responses between mid–late day and early–mid night.
It seems then that the sustained response carries two sorts of information about the light step, and that these arise from different photoreceptor populations. The phasic ‘on’ activation is a response to the change in light intensity (visual contrast) and originates primarily with cones. The sustained phase reflects steady state illumination (irradiance or illuminance information) and is dominated by melanopsin. The influence of rods is sufficiently slight as to be hard to record, but seems to provide both sorts of information under low light levels.
Circadian changes in photoreceptor input
The time-of-day dependence of light evoked activity in the hypothalamic ‘sustained’ cells (Fig. 3) reflected differences in both phasic (cone-dependent) and tonic (melanopsin-dominated) elements of the response (peak; F1,20: 18.74, P > 0.001; tonic: F1,20: 9.21, P > 0.01). In both cases, firing was significantly enhanced during the projected night (Fig. 3C and E Bonferroni post hoc test; P < 0.05 and P < 0.001).
The rhythm in tonic aspects of the response largely reflected differences in melanopsin-evoked activity. Comparison of the 460–655 nm subtracted response waveforms (Fig. 4) revealed a ‘melanopsin-isolated’ component that was significantly enhanced during the projected night (repeated measures two-way ANOVA, F1,20: 13.56, P < 0.001; paired t tests; P < 0.05 to P < 0.001). This matches the reported rhythm in melanopsin phototransduction in the retina (Weng et al. 2009). To determine whether this was a general feature of melanopsin-driven responses, we performed the same 460–655 nm response subtraction for our recordings in the LGN. Unfortunately, the presence of substantial rod signals in the LGN population precluded us from isolating the melanopsin component of their responses across the full intensity range; however, at the highest intensity both rod and cone activation should be equivalent between the two wavelengths (since 655 and 460 nm stimuli are both predicted to be suprasaturating for rods; 655 nm =∼7.2 scot cd m−2). Performing the 460–655 nm subtraction at this intensity, to isolate the melanopsin response (Fig. 4B), revealed a profile very similar to that of the hypothalamic population. As in our hypothalamic recordings, this melanopsin-derived response was also significantly enhanced during the projected night (Fig. 4C; unpaired t test P > 0.05), indicative of a global increase in melanopsin photosensitivity at night.
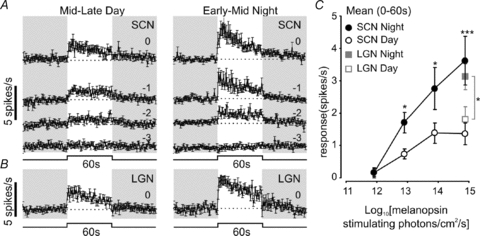
The time of day dependence of the phasic response component implies that cone-driven responses in these ‘sustained’ cells in the SCN/periSCN are also influenced by circadian phase. Our next set of experiments revealed that the daytime reduction in cone input to these cells under ‘field’ conditions may be even more marked than these responses to light steps from darkness suggest. Thus, we set out to describe cone driven responses to stimuli (modulations in light intensity over a steady background) more typically experienced by an animal under daylight conditions. To this end, we presented brief red light steps (2 s, 3.6 × 1015 photons cm−2 s−1) against a rod saturating 460 nm background (2.6 × 1014 rod-stimulating photons cm−2 s−1) light. For Opn1mwR cones this represents a high contrast stimulus (338% above background) while for rods and melanopsin the increase in effective irradiance is negligible (0.6 and 0.07% above background respectively). We found that, for six sustained neurons in the SCN/periSCN tested during both the mid to late projected day and early to mid projected night, responses were once again significantly increased during the night (Fig. 5A and C; paired t test P < 0.05). The magnitude of this effect though was surprisingly large, with cone-driven contrast responses essentially undetectable during the day phase.
Figure 5. Temporal and anatomical variation in cone-derived contrast responses of sustained cells.
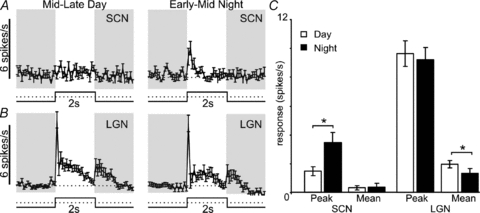
A and B, average (±s.e.m.) response of SCN (A, n= 6) or LGN (B, n= 55) sustained cells to 2 s 655 nm light pulses (2.6 × 1015 photons cm−2 s−1) presented against a bright 460 nm background light (3.4 × 1014 photons cm−2 s−1). C, quantification of data from A and B, showing peak and mean firing rate during light on, relative to basal activity. Data in C were analysed by paired t test. *Significant differences in responses between mid–late day and early–mid night (P < 0.05).
Comparison with the LGN response to this same stimulus revealed several differences from the SCN. Firstly, the response to cone-isolating contrast was much more robust at both projected day and night in the thalamus, consistent with the view that this quality of information is relatively less important for the hypothalamus. Secondly, thalamic activation was high during the day and in fact slightly reduced during the projected night (paired t test, P < 0.05), indicating that nocturnal increases in the hypothalamic cone-mediated responses (Figs 3C; 5A) could not be explained by a global upregulation of cone signalling. Finally, while LGN cells showed robust excitation at lights off, the hypothalamic cells did not (Fig. 5A and B), suggesting that the upstream retinal circuitry of ganglion cell classes communicating to these two populations is different.
Transient cells
Daily variation in spontaneous and light evoked activity
The basal firing pattern of ‘transient’ cells did not have the day time peak most commonly described for SCN neurons (Fig. 6A). Instead transient cells appeared to exhibit highest basal activity between early and mid night. We rarely encountered transient cells during our recordings across the projected day, perhaps because light-evoked spike responses of these cells were much reduced over this time epoch (Fig. 6C and E). However, the three that we did record (over the late day) had very low firing rates suggesting that these transient cells are electrically silent, and therefore largely invisible to our approaches, for much of the day. These then could correspond to SCN subpopulations recently recorded in in vitro studies that are virtually silent during the projected day (Brown & Piggins, 2009; Belle et al. 2009).
Figure 6. Temporal variation in basal and light-evoked activity of transient cells.
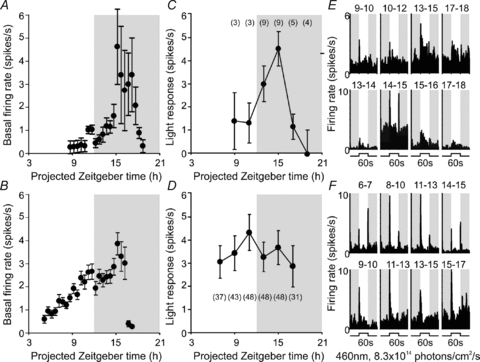
A and B, mean (±s.e.m.) basal (dark-adapted) firing rate of hypothalamic (A, n= 3 to 9) or LGN (B, n= 4 to 47) transient cells at indicated time points. C and D, symbols represent mean (±s.e.m.) response of hypothalamic (C) or LGN (D) transient cells to 60 s light steps (change in mean spike rate during first 1 s of light on relative to baseline rate) at indicated time points. For C and D, numbers in brackets represent number of cells contributing to each data point. Shaded areas in A–D represent the projected night (i.e. time of light off in the animals’ home cage). E and F, examples of SCN (E) or LGN (F) transient cells’ responses to 60 s light steps as a function of time. Each histogram represents the average of 2 trials between the time points indicated above the trace. Shaded areas in E and F represent epochs of darkness.
Photoreceptor origins
Transient cells lacked the tonic (irradiance coding) component of the sustained cells, but retained a phasic response to light increases and in some instances decreases. Due to the difficulty in detecting transient cells during the projected day, we focused on responses evoked across the early to mid projected night (Fig. 7A). We found that the phasic responses of these transient cells were equivalent for cone-isoluminant stimuli at 460 and 655 nm (two-way repeated measures ANOVA; F1,9: 0.31, P > 0.05). This suggests that their response can be explained by the action of cones, and that rods and melanopsin make at most a minor contribution to their activity.
Figure 7. Cones, but not rods, drive transient responses in the hypothalamus.
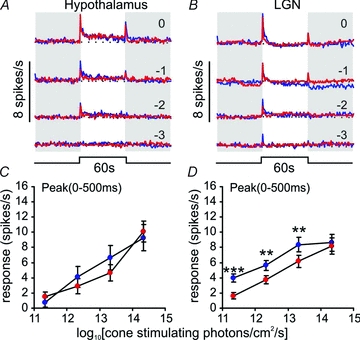
A and B, average response of SCN (A, n= 10) or LGN (B, n= 47) transient cells to 60 s, 460 nm (blue) or 655 nm (red) light pulses of varying intensity. Shaded areas represent epochs of darkness, numbers above traces represent log intensity, relative to the unattenuated intensities (8.3 × 1014 and 2.6 × 1015 photons cm−2 s−1 for 460 and 655 nm respectively). C and D, quantification of data from A and B, showing peak firing rate during the first 500 ms of the response relative to basal activity. Data in C and D were analysed by repeated measures ANOVA with intensity and wavelength as factors, followed by the Bonferroni post hoc test. **P < 0.01 and ***P < 0.001, significant differences in responses to 460 nm and 655 nm.
We next compared the behaviour of the transient cells detected in our hypothalamic recordings with thalamic neurons with similar response properties. Transient cells were much more readily detected (n= 48) in the LGN and response amplitudes were higher (Supplemental Fig. 2). Like transient cells in/around the SCN, their basal firing rates were highest during the early to mid night (Fig. 6B). However, several differences in the behaviour of ‘transient’ cells in these two retinorecipient regions were also apparent. Firstly, there was no time of day dependence to the light evoked response of transient cells in the LGN (Fig. 6D and F). Secondly, we found evidence for a substantial rod contribution to the thalamic response. Thus, when presented with cone-isoluminant 460 and 655 nm stimuli, responses of the LGN population were strongly dependent on wavelength (two-way repeated measures ANOVA; F1,47: 20.11, P < 0.001). As the response to 460 nm was greater at all but the brightest intensity (Fig. 7B and D; Bonferroni post hoc test; P < 0.001 to 0.01), we interpret this as evidence that rods contribute to the response of these thalamic transient cells. The lack of a similar rod component to transient responses in the hypothalamus recorded under identical conditions provides further support for the notion that rod influence on SCN firing is slight.
Delayed cells
Daily variation in spontaneous and light evoked activity
Because cells exhibiting a delayed response phenotype were anatomically dispersed (Fig. 1B), we further separated this population into cells located (1) in or around the SCN (n= 7); (2) in the rest of the periventricular hypothalamus (n= 17); or (3) in the midline ventral thalamic nuclei (n= 27). Basal firing rates of the periventricular hypothalamic cells and thalamic population appeared similar (Fig. 8A and B), being very low during the early projected day and increasing throughout the day, consistent with reports that electrical activity in brain regions outside the SCN is typically in phase with the animals’ rest–activity rhythm (Inouye & Kawamura, 1979; Yamazaki et al. 1998). For the thalamic cells, firing reached a peak during the early night and then declined. Although low in number, the SCN population appeared to exhibit a very different profile of spontaneous activity (Fig. 8C), with very low basal firing rates across the mid projected day to early projected night and highest activity in the morning. In one of three of these SCN cells sampled from the early morning we also observed a second peak of activity at dusk (Supplemental Fig. 3A).
Figure 8. Characterisation of basal and light evoked activity of delayed cells.
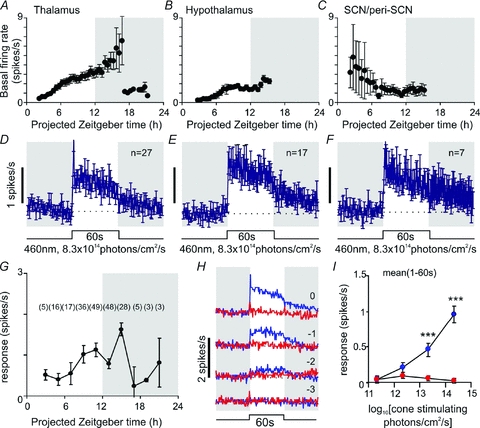
A–C, mean (±s.e.m.) basal (dark-adapted) firing rate of delayed cells recorded from the midline ventral thalamus (A, n= 5–26), perventricular hypothalamus (B, n= 3–17) or SCN region (C, n= 3–7) cells at indicated time points. D–F, mean (±s.e.m.) response of delayed cells in different anatomical subregions to bright 60 s, 460 nm, light steps. G, symbols represent mean (±s.e.m.) response of delayed cells throughout the hypothalamus and midline ventral thalamus to 60 s light steps (change in mean spike rate during light on relative to baseline rate) at indicated time points (numbers in brackets represent number of cells contributing to each data point). H, average response of all delayed cells (n= 51) to 60 s, 460 nm (blue) or 655 nm (red) light pulses of varying intensity. Shaded areas represent epochs of darkness, numbers above traces represent log intensity, relative to the unattenuated intensities (8.3 × 1014 and 2.6 × 1015 photons cm−2 s−1 for 460 and 655 nm respectively). I, quantification of data from H, showing mean firing rate 1–60 s after light on, relative to basal activity. Data in I were analysed by repeated measures ANOVA with intensity and wavelength as factors, followed by the Bonferroni post hoc test. ***P < 0.001, significant differences in responses to 460 nm and 655 nm.
In contrast to these differing profiles of basal activity, light responses were broadly similar in all anatomical locations, albeit with a suggestion that responses decayed more rapidly in the thalamic population (Fig. 8D and E). We did not detect any obvious circadian pattern to the light response in any individual region (Supplemental Fig. 3A), or indeed in the combined population (Fig. 8G).
Photoreceptor origins
Delayed cells lacked the phasic response to a change in light intensity (contrast) typical of sustained and transient cells. Indeed, these cells exhibited very little response to 655 nm stimuli at any intensity tested indicating minimal cone input. Instead, they showed only sustained (irradiance dependent) responses to 460 nm light steps. Of note, there was very little response to the lowest 460 nm stimulus tested, even though this should evoke near saturating rod responses. Rather, response amplitude built up over the melanopsin sensitivity range (Fig. 8H and I). Indeed the kinetics of delayed cell responses to 460 nm resembled our estimate of the isolated melanopsin contribution to ‘sustained’ responses (Fig. 4) and the reported profile of the melanopsin-driven component of mRGC activation (Berson et al. 2002; Dacey et al. 2005; Tu et al. 2005; Wong et al. 2007; Mawad & van Gelder, 2008; Schmidt & Kofuji, 2009). These data therefore indicate that, at least to a first approximation, delayed cells rely solely upon melanopsin phototransduction for their light response.
Suppressed cells
Daily variation in spontaneous and light evoked activity
Like the delayed cells, light suppressed cells were also encountered throughout the hypothalamus and ventral thalamus (Fig. 1B). However, due to their relative scarcity (n= 17) we were unable to reliably explore the possibility of anatomical variation in their basal or light evoked activity. As a population, suppressed cells exhibited highest spontaneous activity around the mid projected night and low firing rates during the mid projected day (Fig. 9A), again consistent with reports of circadian activity patterns in extra-SCN brain regions (Inouye & Kawamura, 1979; Yamazaki et al. 1998). In parallel with this increase in basal activity, the light-evoked change (reduction) in firing was also greater at night than mid to late projected day (Fig. 9B; mean ±s.e.m. change in firing rate; –0.6 ± 0.3 Hz and –1.1 ± 0.3 Hz respectively; paired t test; P < 0.05). However, this effect mainly seemed to reflect the nocturnal increase in spontaneous activity (Fig. 9A and C), as the absolute firing rate during light stimulation was not significantly different at night vs. day (2.0 ± 0.5 vs. 2.6 ± 0.7 Hz respectively: paired t test, P > 0.05).
Figure 9. Temporal variation in basal and light-dependent activity in suppressed cells.
A, mean (±s.e.m.) basal (dark-adapted) firing rate of suppressed cells (n= 3 to 19) at indicated time points. B, symbols represent mean (±s.e.m.) response of suppressed cells to 60 s (460 nm) light steps (change in mean spike rate during light on relative to baseline rate) at indicated time points. For B, numbers in brackets represent number of cells contributing to each data point. Shaded areas in A and B represent the projected night (i.e. time of light off in the animals’ home cage). C, example of a suppressed cells response to 60 s light steps as a function of time. Each histogram represents the average of 2 trials between the time points indicated above the trace. Shaded areas in C represent epochs of darkness. D, time-averaged response of all suppressed cells (n= 22) to 60 s, 460 nm (blue) or 655 nm (red) light pulses of varying intensity. Shaded areas represent epochs of darkness, numbers above traces represent log intensity, relative to the unattenuated intensities (8.3 × 1014 and 2.6 × 1015 photons cm−2 s−1 for 460 and 655 nm, respectively). E, quantification of data from D, showing mean firing rate 1–60 s after light on, relative to basal activity. Data in E were analysed by repeated measures ANOVA with intensity and wavelength as factors, followed by the Bonferroni post hoc test. *P < 0.05 and **P < 0.01, significant differences in responses to 460 nm and 655 nm.
Photoreceptor origins
Like the delayed cells, these light-suppressed neurons failed to show a significant response to 655 nm stimuli (Fig. 9D and E) or to the dimmest (rod-activating) 460 nm irradiance. Rather their response to the shorter wavelength stimuli built up over the predicted sensitivity range of melanopsin. It seems then that this aspect of hypothalamic photosensitivity also relies largely upon melanopsin phototransduction.
Light insensitive cells
Our experiments were designed to identify light responsive cells and on the rare occasion (4 out of 23) that we did not identify any in our first electrode placement we repositioned our electrodes. Consequently, our approach may have under-estimated the number of light insensitive cells in the hypothalamus. Nonetheless, we encountered a large number of such cells (n= 271). Of these 21 were located in or near the SCN.
The firing pattern of light-insensitive SCN cells was, at least to a first approximation, similar to that recently ascribed to SCN neurons expressing high levels of Per1 (Belle et al. 2009), which have a crepuscular profile of basal activity. Thus, at the population level these cells did not display the day-time peak in firing so widely characterized previously (Brown & Piggins, 2007), and which we found in cells exhibiting sustained light responses. Rather they appeared to display higher firing rates around dawn and dusk, with very low firing rates around midday (Fig. 10A). From six cells for which we had sufficient data, we verified that two exhibited this mid-day suppression in firing (Fig. 10B) while activity of the remaining four declined over the course of the recordings between the early projected day and early projected night. For the other 15 light-insensitive cells our recordings commenced after pZT 5, and hence we were unable to ascertain whether they also exhibited a morning peak in activity to match that observed around dusk.
Figure 10. Anatomical variation in light-insensitive cell activity.
A and C, mean (±s.e.m.) basal (dark-adapted) firing rate of light insensitive cells recorded from inside (A, n= 4 to 19) or outside the SCN region (B, n= 3 to 16) or SCN region (C, n= 3 to 168) cells at indicated time points. B and D, firing rate of two light-insensitive cells recorded similutaneously from the SCN (B) and periventricular hypothalamaus (D)
The basal firing rate of light insensitive cells outside the SCN was relatively stable throughout most of the projected day but declined to a nadir around dawn. Similar patterns were obtained when this population was split into thalamic cells and those from the periventricular hypothalamus (not shown).
Relationship between hypothalamic activity and circadian phase shifts
Among the four response types, a number of lines of evidence suggest that the sustained population are most closely associated with photoentrainment: (1) they were found exclusively and at high density in or around the SCN; (2) their light response was enhanced at night, when the circadian clock is most photosensitive; (3) of all the cell types encountered their responses were most similar to those of mRGCs, consistent with the idea that they receive direct input from this ganglion cell class, which is known to be essential for circadian phase resetting; and (4) they were the only class whose activity could be influenced by all three photoreceptor types (rods, cones and melanopsin), consistent with evidence that each of these can impact circadian entrainment (Lall et al. 2010).
For these reasons we were particularly interested to determine whether the light-driven firing of these hypothalamic sustained cells could explain the sensory characteristics of circadian phase resetting in Opn1mwR mice (Lall et al. 2010). The dimmest 460 nm intensity used in these electrophysiological experiments (8.3 × 1011 photons cm−2 s−1) elicited an, presumably rod-derived, increase in firing in the sustained population that, while low amplitude (16 ± 4 spikes/60 s), was measurable above baseline. A 15 min light pulse of equivalent irradiance produces near-saturating behavioural phase shifts in Opn1mwR mice (Lall et al. 2010). To approximate the total number of spikes in such a 15 min stimulus at this irradiance, we fitted 1 min response profiles for individual sustained cells with exponential decay functions and extrapolated these fits to 15 min. The estimated total number of light evoked spikes was low (160 ± 40). Assuming a simple relationship between spike number and phase shift suggests then that the circadian clock may be very sensitive to small increases in the activity of sustained cells.
To further explore the relationship between ‘sustained’ cell firing and behavioural responses we turned our attention to the contribution of cones. Qualitatively, the low amplitude and rapid decay of sustained cell responses to 650 nm light (Fig. 3) are consistent with previous work indicating that the ability of cones to drive phase shifts is limited (Dkhissi-Benyahya et al. 2007; Dollet et al. 2010; Lall et al. 2010). Indeed, thanks to the rapid decay of cone-dependent excitation, the total number of spikes predicted in response to a 15 min 655 nm stimulus, cone-isoluminant to the dimmest 460 nm light, was very low (Fig. 11; 35 ± 12; estimated as above). This is in agreement with our published behavioural data that Opn1mwR mice do not phase shift to such a stimulus (Lall et al. 2010). However, the same number of photons presented as fifteen 1 min light pulses (each separated by 2 min of darkness) is markedly more effective in shifting the clock (Lall et al. 2010). Consistent with this phenomenon, the estimated total number of spikes elicited by such a train of light stimuli was much higher (486 ± 170).
Figure 11. Temporal dynamics of hypothalamic light responses account for influence of rods and cones on mouse behavioural responses.
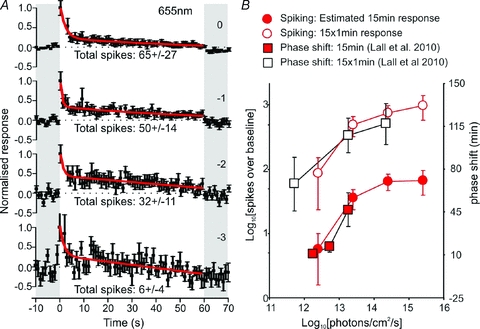
A, mean (±s.e.m.) normalized response of SCN sustained cells (n= 21) to 60 s, 655 nm, light pulses of varying intensity during the early–mid projected night. Continuous lines represent exponential decay functions fitted to the normalized response, shaded areas represent epochs of darkness, and numbers above traces represent log intensity, relative to the unattenuated intensitiy (2.6 × 1015 photons cm−2 s−1). B, filled circles represent estimates of the total number of spikes fired during fifteen 60 s, 655 nm light pulses at the indicated intensities, open circles the total number of spikes fired during one 15 min light pulse (extrapolated from the exponential decay functions fitted in A). Open and filled squares in B represent the magnitude of phase shifts in mouse behavioural activity elicted by single 15 min light pulses (filled) or fifteen 60 s light pulses (open) at circadian time 16 (replotted from Lall et al. 2010). Light intensities for the phase shifting data were corrected according to the relative stimulation of cones at 644 (Lall et al. 2010) and 655 nm (present study).
In fact when light evoked firing was estimated using this technique for a number of continuous and discontinuous, short and long wavelength stimuli over a range of irradiances, a simple relationship with the magnitude of behavioural phase shifts became apparent. This indicated that stimuli evoking maximal phase shifts elicited an average of only ∼300 spikes over baseline in each ‘sustained’ cell (equating to an increase in spike rate of 0.33 Hz for a 15 min light pulse). A prediction of this analysis is then that the brightest 460 nm stimuli we tested (8.3 × 1014 photons cm−2 s−1, evoking 272 ± 51 spikes in 60 s) would be sufficient to evoke a near-maximal phase shift if given as a 1 min pulse. Recent behavioural data support this prediction (Dollet et al. 2010).
On the basis of this simple modelling, we conclude that (1) the firing pattern of sustained cells can predict the magnitude of circadian phase shifts under all of the conditions that we have tested; (2) that the kinetics of the cone responses reported here provide a good explanation for their ability to influence circadian behaviour; and (3) small changes in sustained cell firing are associated with large phase adjustments in the SCN clock.
Discussion
By tracking the activity of large populations of cells inside and outside the SCN simultaneously, over the whole circadian cycle, we have identified four different types of light-responsive cells in the mouse hypothalamus (Fig. 12), two of which have not previously been reported. Thanks to the divergent nature of their light response these populations convey different qualities of sensory information, and rely on the three classes of retinal photoreceptor (rod, cone and melanopsin) to differing degrees. They differ also in anatomical distribution and in their circadian pattern of basal and light-evoked firing.
Figure 12. A schematic diagram of likely retinal and central influences on the four hypothalamic light-response types.
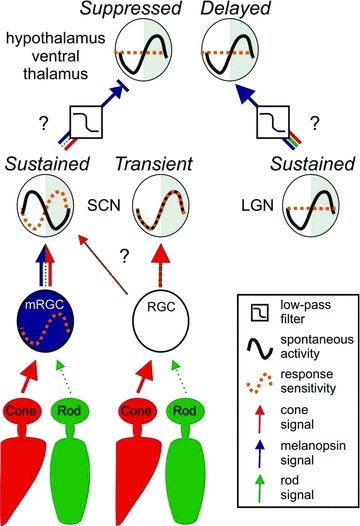
A model illustrating how photoreceptor signals, retinal/central clocks and central processing interact to produce the distinct properties of light responsive units detected in the hypothalamus. Cone: retinal cone photoreceptor; rod: retinal rod photoreceptor; mRGC: melanopsin retinal ganglion cell; RGC: conventional retinal ganglion cell; SCN: suprachiasmatic nuclei; LGN: lateral geniculate nuclei; Sustained: hypothalamic cell with sustained response type; Transient: hypothalamic cell with transient response type; Suppressed: hypothalamic cell with suppressed response type; Delayed: hypothalamic cell with delayed response type.
Sustained cells
The most commonly encountered light responses within the SCN itself were of the ‘sustained’ class. Responses of this class most closely matched those reported in previous studies of SCN light responses (Groos & Mason, 1978, 1980; Meijer et al. 1986, 1992) and here, using Opn1mwR mice, we show they were also the class whose sensory characteristics most closely matched those of the clock itself. Uniquely in our sample, the light-evoked firing pattern of sustained cells showed evidence of rod, cone and melanopsin influences. The basic response of these cells to a light step (a transient excitation decaying to a lower level of tonic firing) is similar to that reported for mRGCs, suggesting that these are the cells most likely to be directly postsynaptic to this ganglion cell class whose activity is essential for entrainment (Goz et al. 2008; Guler et al. 2008; Hatori et al. 2008).
Our experiments reveal that the sustained response profile is primarily a composite of (1) a strong, but rapidly decaying, cone excitation; and (2) a much more sustained melanopsin stimulation. By contrast, rods only drive modest changes in firing. The melanopsin-dominated sustained component can provide information about steady state levels of illumination (‘irradiance’) with obvious utility for entrainment. On the other hand, the phasic nature of the cone-driven response reveals that this photoreceptor class rather provides information about changes in light intensity (‘contrast’). This finding is consistent with behavioural data suggesting that light adaptation places a strict limit on the degree to which cone pathways can support entrainment (Dkhissi-Benyahya et al. 2007; Altimus et al. 2010; Dollet et al. 2010; Lall et al. 2010). Indeed, the phasic response is sufficiently brief that it makes only a small contribution to the cumulative change in firing of sustained cells over 60 s of light exposure. As a result, even though the higher sensitivity rod input itself changes firing very little, because its effects are more persistent, it could be more influential under extended light exposure. Indeed, our modelling suggests that the cumulated increase in firing over 15 min exposure to the dimmest 460 nm stimulus used here (evoking an average of ∼300 spikes in each sustained cell), could only be achieved from cones using multiple brief light exposures. This matches behavioural data showing that cone-stimulating long wavelength light can only reliably phase shift the Opn1mwR clock if presented as a series of pulses separated by darkness (Lall et al. 2010).
How significant might this transient cone signal be in the real world? We approached this problem by exploring the ability of cones to elicit responses to fluctuations in light intensity above a steady background, on the basis that these would provide a closer approximation of ‘natural’ patterns of exposure than do sudden steps from darkness. We found that, the cone-dependent phase of electrophysiological light responses was especially low under these conditions. Indeed, during the day the sustained cells were essentially unresponsive to this ‘cone contrast’ signal. These data further support the view that light adaptation inhibits cone input to the clock. They also could explain why, despite abundant evidence for cone signals in the retinohypothalamic tract, mice retaining cone, but not rod or melanopsin photoreception, do not reliably photoentrain (Lall et al. 2010).
We observed a nocturnal increase in the sustained light response similar to that reported by others (Meijer et al. 1996). This correlates well with the circadian phase at which the clock itself is most responsive. Until now, however, there has been little progress in determining the origin of this effect. Our data suggest that events intrinsic to mRGCs make a substantial contribution. Thus, the melanopsin-dependent tonic component of sustained cell responses is greatly enhanced at a circadian phase consistent with the recently reported peak in melanopsin phototransduction (González-Menéndez et al. 2009; Weng et al. 2009). Moreover, a similar increase in melanopsin influence is observed in the LGN, arguing for a global increase in melanopsin signals at this time of day.
Previous work in the hamster (Hamada et al. 2001; Jobst & Allen, 2002) has suggested that those SCN cells receiving retinal input may not themselves be oscillators. Thus, neurons showing light induction of period genes do not themselves show rhythms in either clock gene expression or electrical activity. At first blush our data are inconsistent with this view. Thus, the sustained cells implicated by our experiments in conveying entrainment information to the clock clearly show diurnal peaks in firing. One simple explanation for this discrepancy could be that light elicits electrophysiological and clock gene responses in different SCN populations.
A related issue is the likely origin of firing rate rhythms in the ‘sustained’ cells. While our data show that these cells clearly are rhythmic, it does not necessary follow that they are ‘oscillators’, as they could certainly attain this firing pattern thanks to inter-cellular communication. Indeed, recent data suggest that the diurnal peak in electrical activity described here for ‘sustained’ cells is in fact characteristic of SCN populations thought not to contain a molecular oscillator (Belle et al. 2009). In this regard our data are more compatible with a further interpretation of the hamster work – that SCN cells that rhythmically express period genes (‘oscillator’ cells) receive retinal input via ‘gate’ cells that are not themselves intrinsically rhythmic (Antle et al. 2003).
Transient cells
The other class of light-responsive cell that we found exclusively in the SCN region exhibited a cone-mediated phasic increase in firing at lights on (and sometimes off) but showed no evidence of melanopsin input. To our knowledge, such responses have not been observed previously in hypothalamic recordings. Nonetheless, their existence is compatible with evidence for sparse input to the SCN from conventional (non-melanopsin expressing) RGCs (Sollars et al. 2003; Guler et al. 2008).
Previous failures to record ‘transient’ responses in the SCN probably reflect the fact that, as far as we could tell, light-evoked activity of these ‘transient’ cells was largely confined to the early projected night. The origin of this strong circadian gating is unclear. It is certainly possible that the few conventional RGCs innervating the SCN provide enhanced input at night, but circadian control could also be exerted within the SCN arising from rhythmic processes intrinsic to these cells and/or input from other oscillating cells.
Since we found no evidence of melanopsin input to these cells they are unlikely to play a major role in circadian entrainment (Goz et al. 2008; Guler et al. 2008; Hatori et al. 2008). Instead, this population provides a route through which aspects of physiology could be regulated by cone-derived contrast signals. There is evidence for autonomic/reflex responses with such properties, with a report that cone signals are sufficient to stimulate cortisol secretion in humans (Figueiro & Rea, 2010).
Delayed and suppressed cells
Light-suppressed and delayed cells were found at very low frequency in or around the SCN, but had a wide distribution throughout the other regions we recorded from. We found very little evidence for rod or cone mediated responses in these cells, with the entirety of their response seeming to derive from melanopsin. This is surprising, given that all mRGCs are believed to receive input from outer retinal photoreceptors (Belenky et al. 2003; Dacey et al. 2005; Perez Leon et al. 2006; Viney et al. 2007; Wong et al. 2007).
Hence it appears that some mechanism, operating before the level of these cells’ spike output, filters out the rapid rod/cone mediated phasic excitation reliably observed in mRGCs. Such a mechanism could operate in the delayed/suppressed cells themselves. However, it could also arise from as yet unidentified afferent neurons, as it seems most likely that at least some of these cells are not directly retinorecipient. Sparse retinal/mRGC input has been observed in all the hypothalamic regions where we encountered these cells (Abrahamson & Moore, 2001; Hattar et al. 2006). However, the midline ventral thalamus (where the majority were identified) is not a known target of retinal afferents. Moreover, the slow kinetics of suppressed and delayed light responses would certainly be compatible with the inclusion of interneuron(s).
Could, then, hypothalamic cells showing one of the other light response types convey visual information to the suppressed and delayed classes? The absence of melanopsin influence in the transient response population would seem to preclude these. Inhibitory output of the ‘sustained’ cells is a plausible origin for the suppressed response, as both spontaneous and light evoked firing in these two populations are broadly antiphase. By contrast, it is harder to imagine how the delayed cell responses could arise from excitatory input from hypothalamic sustained cells since (1) the magnitude of delayed activations was not substantially increased during the projected night and (2) delayed cell spontaneous activity was not in phase with that of sustained cells in or near the SCN, as would be predicted if they were driven by this population. All of the regions in which we recorded delayed responses, including the thalamus, are known output targets not only of the SCN but also of the retinorecipient IGL/ventral LGN (Watts et al. 1987; Morin et al. 1994; Moore et al. 2000; Abrahamson & Moore, 2001). Delayed cell responses might therefore be driven by excitatory input from neurones in the IGL/vLGN. These are known to receive melanopsin input (Brown et al. 2010) and here we show they exhibit a similar night-time peak in spontaneous activity.
To our knowledge, our description of delayed and suppressed cells represents the first report of neurons whose light response relies solely on melanopsin. This is an important observation as several autonomic/reflex responses have been reported to have this characteristic (Brainard et al. 2001; Thapan et al. 2001; Mrosovsky & Hattar, 2003; Altimus et al. 2008; Lupi et al. 2008; Tsai et al. 2009). Those data have until now been hard to reconcile with the observation that all mRGCs receive rod/cone input. Our data reveal that central processing can in effect extract the melanopsin component of the visual signal to explain these findings. Such signals could be very influential, as those brain regions containing suppressed/delayed cells regulate a wide variety of systems, including cardiovascular, reproductive, attentional and memory (Van der Werf et al. 2002; Saper et al. 2005; Baskerville & Douglas, 2008; Davoodi et al. 2009).
Conclusion
In summary, our recordings provide the first comprehensive description of the photoreceptive origins of contrast and irradiance signals in the mouse hypothalamus. These rely primarily on cone and melanopsin photoreceptors, respectively, with rods exhibiting a much more subtle excitatory influence on hypothalamic firing at lower light levels. We further show that individual hypothalamic neurons sample these three information streams in different ways, giving rise to four distinct response types. While future work will need to determine the precise retinal and central circuitry that shapes these distinct visual coding properties, the existence of such cells can explain the various sensory capabilities of an array of reflex light responses. The most common response phenotype in the SCN itself is sustained activation. Cells with this behaviour respond to all three photoreceptor classes, and it is these whose light response characteristics match those of the circadian clock itself. Surprisingly, a minority of SCN neurons lack the melanopsin-derived irradiance signal and respond only to light transitions, allowing for the possibility that rod/cone contrast signals may be routed to SCN output targets without influencing neighbouring circadian oscillators. Finally, an array of cells, largely outside the SCN are excited or inhibited solely according to the activity of melanopsin. These cells then appear to reflect a filtered version of the visual signal, suitable for modulating physiology/behaviour purely according to environmental irradiance.
Acknowledgments
This work was funded by a grant from the Wellcome trust to R.J.L. and H.D.P.
Glossary
Abbreviations
- IGL
intergeniculate leaflet
- LGN
lateral geniculate nuclei
- mRGC
melanopsin expressing retinal ganglion cell
- SCN
suprachiasmatic nuclei
- pZT
projected Zeitgeber time
Author contributions
T.M.B. and R.J.L. designed the experiments. T.M.B. performed the electrophysiological studies and analysed the data. J.W. performed the histology. T.M.B. and R.J.L. wrote the manuscript with assistance from H.D.P. All authors approved the final version of the manuscript.
Supplementary material
SFig 1.
SFig 2.
SFig 3.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res. 2001;916:172–191. doi: 10.1016/s0006-8993(01)02890-6. [DOI] [PubMed] [Google Scholar]
- Aggelopoulos NC, Meissl H. Responses of neurones of the rat suprachiasmatic nucleus to retinal illumination under photopic and scotopic conditions. J Physiol. 2000;523:211–222. doi: 10.1111/j.1469-7793.2000.t01-1-00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altimus CM, Guler AD, Alam NM, Arman AC, Prusky GT, Sampath AP, Hattar S. Rod photoreceptors drive circadian photoentrainment across a wide range of light intensities. Nat Neurosci. 2010;13:1107–1112. doi: 10.1038/nn.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altimus CM, Guler AD, Villa KL, McNeill DS, Legates TA, Hattar S. Rods-cones and melanopsin detect light and dark to modulate sleep independent of image formation. Proc Natl Acad Sci U S A. 2008;105:19998–20003. doi: 10.1073/pnas.0808312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antle MC, Foley DK, Foley NC, Silver R. Gates and oscillators: a network model of the brain clock. J Biol Rhythms. 2003;18:339–350. doi: 10.1177/0748730403253840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applebury ML, Antoch MP, Baxter LC, Chun LL, Falk JD, Farhangfar F, Kage K, Krzystolik MG, Lyass LA, Robbins JT. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 2000;27:513–523. doi: 10.1016/s0896-6273(00)00062-3. [DOI] [PubMed] [Google Scholar]
- Barnard AR, Hattar S, Hankins MW, Lucas RJ. Melanopsin regulates visual processing in the mouse retina. Curr Biol. 2006;16:389–395. doi: 10.1016/j.cub.2005.12.045. [DOI] [PubMed] [Google Scholar]
- Baskerville TA, Douglas AJ. Interactions between dopamine and oxytocin in the control of sexual behaviour. Prog Brain Res. 2008;170:277–290. doi: 10.1016/S0079-6123(08)00423-8. [DOI] [PubMed] [Google Scholar]
- Belenky MA, Smeraski CA, Provencio I, Sollars PJ, Pickard GE. Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. J Comp Neurol. 2003;460:380–393. doi: 10.1002/cne.10652. [DOI] [PubMed] [Google Scholar]
- Belle MD, Diekman CO, Forger DB, Piggins HD. Daily electrical silencing in the mammalian circadian clock. Science. 2009;326:281–284. doi: 10.1126/science.1169657. [DOI] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TM, Gias C, Hatori M, Keding SR, Semo M, Coffey PJ, Gigg J, Piggins HD, Panda S, Lucas RJ. Melanopsin contributions to irradiance coding in the thalamo-cortical visual system. PLoS Biol. 2010;8:e1000558. doi: 10.1371/journal.pbio.1000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TM, Allen AE, Wynne J, Paul DL, Piggins HD, Lucas RJ. Visual responses in the lateral geniculate evoked by Cx36-independent rod pathways. Vision Res. 2011;51:280–287. doi: 10.1016/j.visres.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TM, Banks JR, Piggins HD. A novel suction electrode recording technique for monitoring circadian rhythms in single and multiunit discharge from brain slices. J Neurosci Methods. 2006;156:173–181. doi: 10.1016/j.jneumeth.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Brown TM, Piggins HD. Electrophysiology of the suprachiasmatic circadian clock. Prog Neurobiol. 2007;82:229–255. doi: 10.1016/j.pneurobio.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Brown TM, Piggins HD. Spatiotemporal heterogeneity in the electrical activity of suprachiasmatic nuclei neurons and their response to photoperiod. J Biol Rhythms. 2009;24:44–54. doi: 10.1177/0748730408327918. [DOI] [PubMed] [Google Scholar]
- Cameron MA, Barnard AR, Hut RA, Bonnefont X, Van Der Horst GT, Hankins MW, Lucas RJ. Electroretinography of wild-type and Cry mutant mice reveals circadian tuning of photopic and mesopic retinal responses. J Biol Rhythms. 2008;23:489–501. doi: 10.1177/0748730408325874. [DOI] [PubMed] [Google Scholar]
- Cameron MA, Lucas RJ. Influence of the rod photoresponse on light adaptation and circadian rhythmicity in the cone ERG. Mol Vis. 2009;15:2209–2216. [PMC free article] [PubMed] [Google Scholar]
- Challet E. Minireview: Entrainment of the suprachiasmatic clockwork in diurnal and nocturnal mammals. Endocrinology. 2007;148:5648–5655. doi: 10.1210/en.2007-0804. [DOI] [PubMed] [Google Scholar]
- Cutler DJ, Haraura M, Reed HE, Shen S, Sheward WJ, Morrison CF, Marston HM, Harmar AJ, Piggins HD. The mouse VPAC2 receptor confers suprachiasmatic nuclei cellular rhythmicity and responsiveness to vasoactive intestinal polypeptide in vitro. Eur J Neurosci. 2003;17:197–204. doi: 10.1046/j.1460-9568.2003.02425.x. [DOI] [PubMed] [Google Scholar]
- Daan S, Pittendrigh CS. A functional analysis of circadian pacemakers in nocturnal rodents II. The variability of phase response curves. J Comp Physiol A. 1976;106:253–266. [Google Scholar]
- Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- Davoodi FG, Motamedi F, Naghdi N, Akbari E. Effect of reversible inactivation of the reuniens nucleus on spatial learning and memory in rats using Morris water maze task. Behav Brain Res. 2009;198:130–135. doi: 10.1016/j.bbr.2008.10.037. [DOI] [PubMed] [Google Scholar]
- Dkhissi-Benyahya O, Gronfier C, De Vanssay W, Flamant F, Cooper HM. Modeling the role of mid-wavelength cones in circadian responses to light. Neuron. 2007;53:677–687. doi: 10.1016/j.neuron.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollet A, Albrecht U, Cooper HM, Dkhissi-Benyahya O. Cones are required for normal temporal responses to light of phase shifts and clock gene expression. Chronobiol Int. 2010;27:768–781. doi: 10.3109/07420521003695704. [DOI] [PubMed] [Google Scholar]
- Drouyer E, Rieux C, Hut RA, Cooper HM. Responses of suprachiasmatic nucleus neurons to light and dark adaptation: relative contributions of melanopsin and rod-cone inputs. J Neurosci. 2007;27:9623–9631. doi: 10.1523/JNEUROSCI.1391-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiro MG, Rea MS. The effects of red and blue lights on circadian variations in cortisol, alpha amylase, and melatonin. Int J Endocrinol. 2010;2010:829351. doi: 10.1155/2010/829351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman MS, Lucas RJ, Soni B, von Schantz M, Munoz M, David-Gray Z, Foster R. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:502–504. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Menendez I, Contreras F, Cernuda-Cernuda R, Garcia-Fernandez JM. Daily rhythm of melanopsin-expressing cells in the mouse retina. Front Cell Neurosci. 2009;3:3. doi: 10.3389/neuro.03.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooley JJ, Rajaratnam SM, Brainard GC, Kronauer RE, Czeisler CA, Lockley SW. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2010;12:31–33. doi: 10.1126/scitranslmed.3000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govardovskii VI, Fyhrquist N, Reuter T, Kuzmin DG, Donner K. In search of the visual pigment template. Vis Neurosci. 2000;17:509–528. doi: 10.1017/s0952523800174036. [DOI] [PubMed] [Google Scholar]
- Goz D, Studholme K, Lappi DA, Rollag MD, Provencio I, Morin LP. Targeted destruction of photosensitive retinal ganglion cells with a saporin conjugate alters the effects of light on mouse circadian rhythms. PLoS One. 2008;3:e3153. doi: 10.1371/journal.pone.0003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DJ, Gillette R. Circadian rhythm of firing rate recorded from single cells in the rat suprachiasmatic brain slice. Brain Res. 1982;245:198–200. doi: 10.1016/0006-8993(82)90361-4. [DOI] [PubMed] [Google Scholar]
- Gribkoff VK, Pieschl RL, Wisialowski TA, Van Den Pol AN, Yocca FD. Phase shifting of circadian rhythms and depression of neuronal activity in the rat suprachiasmatic nucleus by neuropeptide Y: mediation by different receptor subtypes. J Neurosci. 1998;18:3014–3022. doi: 10.1523/JNEUROSCI.18-08-03014.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groos G, Mason R. Maintained discharge of rat suprachiasmatic neurons at different adaptation levels. Neurosci Lett. 1978;8:59–64. doi: 10.1016/0304-3940(78)90098-8. [DOI] [PubMed] [Google Scholar]
- Groos G, Mason R. The visual properties of rat and cat suprachiasmatic neurones. J Comp Physiol A. 1980;135:349–356. [Google Scholar]
- Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, Barnard AR, Cahill H, Badea TC, Zhao H, Hankins MW, Berson DM, Lucas RJ, Yau KW, Hattar S. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada T, LeSauter J, Venuti JM, Silver R. Expression of Period genes: rhythmic and nonrhythmic compartments of the suprachiasmatic nucleus pacemaker. J Neurosci. 2001;21:7742–7750. doi: 10.1523/JNEUROSCI.21-19-07742.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori M, Le H, Vollmers C, Keding SR, Tanaka N, Buch T, Waisman A, Schmedt C, Jegla T, Panda S. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoS One. 2008;3:e2451. doi: 10.1371/journal.pone.0002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AT, Guilding C, Lennox L, Samuels RE, McMahon DG, Piggins HD. Live imaging of altered period1 expression in the suprachiasmatic nuclei of Vipr2–/– mice. J Neurochem. 2008;106:1646–1657. doi: 10.1111/j.1471-4159.2008.05520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AT, Piggins HD. Behavioral responses of Vipr2–/– mice to light. J Biol Rhythms. 2008;23:211–219. doi: 10.1177/0748730408316290. [DOI] [PubMed] [Google Scholar]
- Inouye ST, Kawamura H. Persistence of circadian rhythmicity in a mammalian hypothalamic “island” containing the suprachiasmatic nucleus. Proc Natl Acad Sci U S A. 1979;76:5962–5966. doi: 10.1073/pnas.76.11.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobst EE, Allen CN. Calbindin neurons in the hamster suprachiasmatic nucleus do not exhibit a circadian variation in spontaneous firing rate. Eur J Neurosci. 2002;16:2469–2474. doi: 10.1046/j.1460-9568.2002.02309.x. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Palm IF, La Fleur SE, Scheer FA, Perreau-Lenz S, Ruiter M, Kreier F, Cailotto C, Buijs RM. SCN outputs and the hypothalamic balance of life. J Biol Rhythms. 2006;21:458–469. doi: 10.1177/0748730406293854. [DOI] [PubMed] [Google Scholar]
- Karatsoreos IN, Yan L, LeSauter J, Silver R. Phenotype matters: identification of light-responsive cells in the mouse suprachiasmatic nucleus. J Neurosci. 2004;24:68–75. doi: 10.1523/JNEUROSCI.1666-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lall GS, Revell VL, Momiji H, Al Enezi J, Altimus CM, Guler AD, Aguilar C, Cameron MA, Allender S, Hankins MW, Lucas RJ. Distinct contributions of rod, cone, and melanopsin photoreceptors to encoding irradiance. Neuron. 2010;66:417–428. doi: 10.1016/j.neuron.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri DK, Ge YW, Sharman EH, Bondy SC. Age-related changes in serum melatonin in mice: higher levels of combined melatonin and 6-hydroxymelatonin sulfate in the cerebral cortex than serum, heart, liver and kidney tissues. J Pineal Res. 2004;36:217–223. doi: 10.1111/j.1600-079X.2004.00120.x. [DOI] [PubMed] [Google Scholar]
- Lupi D, Oster H, Thompson S, Foster RG. The acute light-induction of sleep is mediated by OPN4-based photoreception. Nat Neurosci. 2008;11:1068–1073. doi: 10.1038/nn.2179. [DOI] [PubMed] [Google Scholar]
- Mawad K, Van Gelder RN. Absence of long-wavelength photic potentiation of murine intrinsically photosensitive retinal ganglion cell firing in vitro. J Biol Rhythms. 2008;23:387–391. doi: 10.1177/0748730408323063. [DOI] [PubMed] [Google Scholar]
- Meijer JH, Groos GA, Rusak B. Luminance coding in a circadian pacemaker: the suprachiasmatic nucleus of the rat and the hamster. Brain Res. 1986;382:109–118. doi: 10.1016/0006-8993(86)90117-4. [DOI] [PubMed] [Google Scholar]
- Meijer JH, Rusak B, Ganshirt G. The relation between light-induced discharge in the suprachiasmatic nucleus and phase shifts of hamster circadian rhythms. Brain Res. 1992;598:257–263. doi: 10.1016/0006-8993(92)90191-b. [DOI] [PubMed] [Google Scholar]
- Meijer JH, Watanabe K, Detari L, Schaap J. Circadian rhythm in light response in suprachiasmatic nucleus neurons of freely moving rats. Brain Res. 1996;741:352–355. doi: 10.1016/s0006-8993(96)01091-8. [DOI] [PubMed] [Google Scholar]
- Moore RY, Weis R, Moga MM. Efferent projections of the intergeniculate leaflet and the ventral lateral geniculate nucleus in the rat. J Comp Neurol. 2000;420:398–418. doi: 10.1002/(sici)1096-9861(20000508)420:3<398::aid-cne9>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Morin LP, Goodless-Sanchez N, Smale L, Moore RY. Projections of the suprachiasmatic nuclei, subparaventricular zone and retrochiasmatic area in the golden hamster. Neuroscience. 1994;61:391–410. doi: 10.1016/0306-4522(94)90240-2. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Hattar S. Impaired masking responses to light in melanopsin-knockout mice. Chronobiol Int. 2003;20:989–999. doi: 10.1081/cbi-120026043. [DOI] [PubMed] [Google Scholar]
- Mure LS, Rieux C, Hattar S, Cooper HM. Melanopsin-dependent nonvisual responses: evidence for photopigment bistability in vivo. J Biol Rhythms. 2007;22:411–424. doi: 10.1177/0748730407306043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonov SS, Kholodenko R, Lem J, Pugh EN., Jr Physiological features of the S- and M-cone photoreceptors of wild-type mice from single-cell recordings. J Gen Physiol. 2006;127:359–374. doi: 10.1085/jgp.200609490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, Provencio I, Kay SA. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 2001. [Google Scholar]
- Perez-Leon JA, Warren EJ, Allen CN, Robinson DW, Lane Brown R. Synaptic inputs to retinal ganglion cells that set the circadian clock. Eur J Neurosci. 2006;24:1117–1123. doi: 10.1111/j.1460-9568.2006.04999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero JE, Kuhlman SJ, McMahon DG. The biological clock nucleus: a multiphasic oscillator network regulated by light. J Neurosci. 2003;23:8070–8076. doi: 10.1523/JNEUROSCI.23-22-08070.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribelayga C, Cao Y, Mangel SC. The circadian clock in the retina controls rod-cone coupling. Neuron. 2008;59:790–801. doi: 10.1016/j.neuron.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby NF, Brennan TJ, Xie X, Cao V, Franken P, Heller HC, O’Hara BF. Role of melanopsin in circadian responses to light. Science. 2002;298:2211–2213. doi: 10.1126/science.1076701. [DOI] [PubMed] [Google Scholar]
- Saeb-Parsy K, Dyball RE. Defined cell groups in the rat suprachiasmatic nucleus have different day/night rhythms of single-unit activity in vivo. J Biol Rhythms. 2003;18:26–42. doi: 10.1177/0748730402239674. [DOI] [PubMed] [Google Scholar]
- Saper CB, Lu J, Chou TC, Gooley J. The hypothalamic integrator for circadian rhythms. Trends Neurosci. 2005;28:152–157. doi: 10.1016/j.tins.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Schaap J, Albus H, VanderLeest HT, Eilers PH, Detari L, Meijer JH. Heterogeneity of rhythmic suprachiasmatic nucleus neurons: Implications for circadian waveform and photoperiodic encoding. Proc Natl Acad Sci U S A. 2003;100:15994–15999. doi: 10.1073/pnas.2436298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Kofuji P. Functional and morphological differences among intrinsically photosensitive retinal ganglion cells. J Neurosci. 2009;29:476–482. doi: 10.1523/JNEUROSCI.4117-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semo M, Peirson S, Lupi D, Lucas RJ, Jeffery G, Foster RG. Melanopsin retinal ganglion cells and the maintenance of circadian and pupillary responses to light in aged rodless/coneless (rd/rd cl) mice. Eur J Neurosci. 2003;17:1793–1801. doi: 10.1046/j.1460-9568.2003.02616.x. [DOI] [PubMed] [Google Scholar]
- Smallwood PM, Olveczky BP, Williams GL, Jacobs GH, Reese BE, Meister M, Nathans J. Genetically engineered mice with an additional class of cone photoreceptors: implications for the evolution of color vision. Proc Natl Acad Sci U S A. 2003;100:11706–11711. doi: 10.1073/pnas.1934712100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollars PJ, Smeraski CA, Kaufman JD, Ogilvie MD, Provencio I, Pickard GE. Melanopsin and non-melanopsin expressing retinal ganglion cells innervate the hypothalamic suprachiasmatic nucleus. Vis Neurosci. 2003;20:601–610. doi: 10.1017/s0952523803206027. [DOI] [PubMed] [Google Scholar]
- Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai JW, Hannibal J, Hagiwara G, Colas D, Ruppert E, Ruby NF, Heller HC, Franken P, Bourgin P. Melanopsin as a sleep modulator: circadian gating of the direct effects of light on sleep and altered sleep homeostasis in Opn4(–/–) mice. PLoS Biol. 2009;7:e1000125. doi: 10.1371/journal.pbio.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu DC, Zhang D, Demas J, Slutsky EB, Provencio I, Holy TE, Van Gelder RN. Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48:987–999. doi: 10.1016/j.neuron.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Van Der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Viney TJ, Balint K, Hillier D, Siegert S, Boldogkoi Z, Enquist LW, Meister M, Cepko CL, Roska B. Local retinal circuits of melanopsin-containing ganglion cells identified by transsynaptic viral tracing. Curr Biol. 2007;17:981–988. doi: 10.1016/j.cub.2007.04.058. [DOI] [PubMed] [Google Scholar]
- Watts AG, Swanson LW, Sanchez-Watts G. Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. J Comp Neurol. 1987;258:204–229. doi: 10.1002/cne.902580204. [DOI] [PubMed] [Google Scholar]
- Webb AB, Angelo N, Huettner JE, Herzog ED. Intrinsic, nondeterministic circadian rhythm generation in identified mammalian neurons. Proc Natl Acad Sci U S A. 2009;106:16493–16498. doi: 10.1073/pnas.0902768106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Weng S, Wong KY, Berson DM. Circadian modulation of melanopsin-driven light response in rat ganglion-cell photoreceptors. J Biol Rhythms. 2009;24:391–402. doi: 10.1177/0748730409343767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KY, Dunn FA, Graham DM, Berson DM. Synaptic influences on rat ganglion-cell photoreceptors. J Physiol. 2007;582:279–296. doi: 10.1113/jphysiol.2007.133751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff ML, Janisch KM, Peshenko IV, Dizhoor AM, Tsang SH, Fain GL. Modulation of phosphodiesterase6 turnoff during background illumination in mouse rod photoreceptors. J Neurosci. 2008;28:2064–2074. doi: 10.1523/JNEUROSCI.2973-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SM, Gao F, Pang JJ. Synaptic circuitry mediating light-evoked signals in dark-adapted mouse retina. Vision Res. 2004;44:3277–3288. doi: 10.1016/j.visres.2004.07.045. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Kerbeshian MC, Hocker CG, Block GD, Menaker M. Rhythmic properties of the hamster suprachiasmatic nucleus in vivo. J Neurosci. 1998;18:10709–10723. doi: 10.1523/JNEUROSCI.18-24-10709.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao G, Zhang K, Bellassai M, Chang B, Lei B. Ultraviolet light-induced and green light-induced transient pupillary light reflex in mice. Curr Eye Res. 2006;31:925–933. doi: 10.1080/02713680600932308. [DOI] [PubMed] [Google Scholar]
- Ying SW, Rusak B. Effects of serotonergic agonists on firing rates of photically responsive cells in the hamster suprachiasmatic nucleus. Brain Res. 1994;651:37–46. doi: 10.1016/0006-8993(94)90678-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


