Abstract
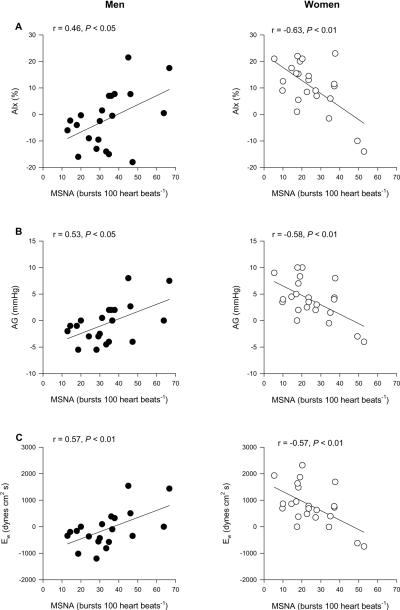
Increased arterial stiffness is associated with higher levels of aortic wave reflection and aortic blood pressure. Recent evidence suggests a link between muscle sympathetic nerve activity and indices of arterial stiffness. Therefore, the aims of this study were to examine 1) the relationship between resting muscle sympathetic nerve activity and characteristics of aortic pressure wave reflection, and 2) the influence of sex on these relationships. In forty-four subjects (23F/21M; 25 ± 1 years) we measured muscle sympathetic nerve activity via peroneal microneurography. Additionally, non-invasive aortic pressure waveforms were synthesized from radial pressure waveforms obtained from applanation tonometry. Aortic blood pressure, augmentation index, wave reflection amplitude, and wasted left ventricular energy were calculated. Resting sympathetic activity (bursts/100 heart beats) was not associated with any of the aortic pressure wave reflection characteristics for all individuals. However, there was a positive relationship between sympathetic activity and augmentation index (r = 0.46, P = 0.05) in men. Furthermore, sympathetic activity in men was related to wave reflection amplitude (r = 0.53, P < 0.05) and wasted left ventricular energy (r = 0.57, P < 0.01). In contrast to men, women demonstrated strong inverse relationships between sympathetic activity and augmentation index (r = −0.63), wave reflection amplitude (r = −0.59), and wasted left ventricular energy (r = −0.58) (P < 0.01 for all). Our results suggest another possible mechanism by which young women are protected against the development of cardiovascular disease.
Keywords: sympathetic nerve activity, aortic wave reflection, blood pressure, peripheral resistance, sex
Introduction
It has been demonstrated that sympathetic vasoconstrictor nerve traffic exhibits no relationship with resting arterial pressure among young healthy men and women.1–3 These observations are based on a lack of relationship between peroneal muscle sympathetic nerve activity (MSNA) and resting arterial blood pressure measured at the level of the brachial artery. However, there can be marked pressure differences throughout the arterial tree. Along these lines, individuals with similar peripheral arterial pressures can have drastically different aortic systolic and pulse pressures due to greater reflected pressure wave amplitude.4 Increased arterial stiffness causes an increase in pulse wave velocity (PWV) and can promote an early return of reflected waves from peripheral reflecting sites to the heart during systole. Consequently, early return and/or increased amplitude of reflected pressure waves during systole increases ascending aortic systolic and pulse pressures and elevates left ventricular afterload. In general, increases in the amplitude of the reflected pressure waves are associated with increases in cardiovascular risk.5–7
Recent evidence suggests a relationship between carotid-femoral PWV, an index of aortic stiffness, and MSNA in healthy men.8 However, whether, sympathetic vasoconstrictor nerve traffic is related to aortic pressure has not been determined. Since MSNA appears to be related to PWV and that the latter can have significant effects on pulsatile pressures in the aorta, we sought to examine the relationship between MSNA and aortic pressures and wave characteristics. We hypothesized that there would be a positive relationship between MSNA and indices of aortic wave reflection among individuals.
Based on previous findings from our group demonstrating that regulation of peripheral arterial pressure is fundamentally different between the sexes,3 a second aim of our study was to examine potential sex differences in the relationship between MSNA and aortic pressures. Therefore we also hypothesized that, because the relationship between sympathetic nerve activity and total peripheral resistance (TPR) is stronger in men,3 they would demonstrate a stronger positive relationship between resting MSNA and measures of aortic pressure wave reflection than women.
Methods
Subjects
A total of 44 young healthy subjects (23 female and 21 male) were studied. Subjects completed written informed consent and underwent a standard screening. All were healthy, non-obese, non-smokers, and were not taking any medications (except for oral contraceptives in some women). Studies were performed after an overnight fast and subjects refrained from exercise, alcohol, and caffeine for at least 24 h. Female subjects were studied during the early follicular phase of the menstrual cycle or the placebo phase of oral contraceptives.9 All study protocols were approved by the Mayo Institutional Review Board and were performed according to the Declaration of Helsinki.
Measurements
All of the studies were performed in the Clinical Research Unit laboratory at the Mayo Clinic, where ambient temperature was controlled between 22°C and 24°C. Subjects were studied at rest in the supine position. A 20 gauge, 5 cm catheter was placed in the brachial artery of the left arm under sterile conditions after local anesthesia (2% lidocaine). The catheter was connected to a pressure transducer, which was positioned at the level of the heart and interfaced with a personal computer to monitor arterial pressure. A 3-lead ECG was used for continuous recording of heart rate.
MSNA was recorded in the peroneal nerve posterior to the fibular head with a tungsten microelectrode, as described by Sundlöf and Wallin.10 The recorded signal was amplified 80,000-fold, band-pass filtered (700 to 2000 Hz), rectified and integrated (resistance-capacitance integrator circuit, time constant 0.1 s) by a nerve-traffic analyzer.
The assessment of arterial wave reflection characteristics was performed non-invasively using the SphygmoCor system (AtCor Medical, Sydney, Australia) as described previously.4 Briefly, high-fidelity radial artery pressure waveforms were recorded by applanation tonometry of the radial pulse in the right wrist using a “pencil type” micromanometer (Millar Instruments, Houston, Texas). The radial blood pressure (BP) and waveforms were calibrated from the systolic and diastolic brachial artery BP (catheter). A validated, generalized transfer function was used to generate the corresponding aortic pressure waveform (Figure S1, please see the online Data Supplement, available at http://hyper.ahajournals.org).11 The generalized transfer function has been validated using both intra-arterially11, 12 and non-invasively13 obtained radial pressure waves.
Pulse wave analysis of the aortic pressure waveform provided the following key variables of interest; aortic pressures, aortic augmentation index (AIx), AIx adjusted for a HR of 75 (AIx@75bpm), round trip travel time of the forward traveling wave from the ascending aorta to the major reflection site and back (Δtp), and wasted LV pressure energy (Ew), which is the component of extra myocardial oxygen requirement due to early systolic wave reflection.4, 14 Ew can be estimated as [(π/4)*(augmented pressure × Δtr)*1.333], where 1.333 is the conversion factor for mmHg/s to dynes·cm2·s and Δtr is the systolic duration of the reflected wave. Augmented pressure (AP) is the amplitude of the reflected wave and is defined as the difference between the first (forward wave) and second systolic shoulders of the aortic systolic blood pressure. Only high-quality recordings, defined as an in-device quality index of over 80% (derived from an algorithm including average pulse height variation, diastolic variation, and the maximum rate of rise of the peripheral waveform), were accepted for analysis. In general, 2–3 measurements were performed in order to get two measurements with an acceptable quality index.
Beat-to-beat stroke volume was calculated from the brachial arterial pulse pressure wave by model flow analysis. Model flow computes an aortic waveform based on nonlinear pressure–volume, pressure–compliance and pressure–characteristic impedance equations, incorporating age, sex, height and body mass.15 Cardiac output was calculated as the average stroke volume measured over 5 minutes multiplied by the heart rate measured over the same 5 minute rest period.
Protocol
After placement of the arterial catheter, subjects rested supine during instrumentation for microneurography. Once a satisfactory site for measurement of MSNA was located, 15 minutes of baseline data were recorded with the subject resting quietly. Subsequently, duplicate applanation tonometry measurements were made. For a more detailed description of the data analysis, please see the online Data Supplement, available at http://hyper.ahajournals.org.
Statistical Analyses
Group data are expressed as means ± SEM. Differences in MSNA, hemodynamic variables, and aortic wave reflection characteristics in men and women were evaluated using a 2-tailed independent t test. To access the relationships between MSNA and aortic wave reflection characteristics, linear regression analysis was performed and Pearson's correlation coefficients calculated. The critical α-level was set at 0.05, and data were analyzed using SigmaStat software (version 2.03, SPSS Inc).
Results
Subject demographics are presented in Table 1. All 44 subjects completed the study protocol. Arterial catheter placement was unsuccessful in four subjects (2M/2F). Therefore, TPR values were only calculated in 19 and 21 of the male and female subjects, respectively. In these subjects, an average of three automated brachial blood pressure readings was used to calibrate the radial pressure waveforms during the applanation tonometry measurements. Additionally, Ew could not be calculated in one male subject. Therefore, the relationships between MSNA and Ew are reported in 20 male subjects.
Table 1.
Demographic variables in Men (n=21) and Women (n=23)
| Demographics | Men | Women |
|---|---|---|
| Age, y | 25 ± 1 | 26 ± 1 |
| Body mass, kg | 77 ± 2 | 64 ± 2* |
| Height, cm | 178 ± 1 | 166 ± 1* |
| BMI, kg m−2 | 24.2 ± 0.5 | 23.3 ± 0.4 |
BMI, body mass index. Data are means ± SEMs.
Data are different from men (P < 0.05)
Group-Averaged Data for Neural-Hemodynamic and Aortic Wave Reflection Variables in Men and Women
Peripheral and aortic pressures were similar between sexes (Table 2). Indices of aortic wave reflection (both AIx and AG) were higher in women compared with men. Consequently, Ew was greater in women compared to their male counterparts (Table 2, P < 0.05). Women demonstrated a lower stroke volume and cardiac output, and a higher TPR compared with men.
Table 2.
Neural-Hemodynamic Variables and Aortic Wave Reflection Characteristics in Men (n=21) and Women (n=23)
| Hemodynamic/Neural Variables | Men | Women |
|---|---|---|
| PSBP, mm Hg | 126 ± 2 | 127 ± 2 |
| PDBP, mm Hg | 70 ± 2 | 71 ± 1 |
| PPP, mm Hg | 56 ± 2 | 56 ± 2 |
| ASBP, mm Hg | 102 ± 2 | 104 ± 2 |
| ADBP, mm Hg | 70 ± 1 | 70 ± 2 |
| APP, mm Hg | 32 ± 1 | 34 ± 2 |
| MAP, mm Hg | 89 ± 1 | 92 ± 2 |
| PPA | 1.74 ± 0.04 | 1.65 ± 0.06 |
| AG, mm Hg | −1 ± 1 | 4 ± 1* |
| AIx, % | −2 ± 2 | 10 ± 2* |
| AIx @ 75 beats min−1, % | −9 ± 2 | 5 ± 2* |
| Ew, dynes cm2 s | −92 ± 152 | 788 ± 161* |
| HR, beats min−1 | 60 ± 2 | 64 ± 2 |
| SV, mL | 102 ± 4 | 79 ± 3* |
| CO, L min−1 | 6.2 ± 0.3 | 5.0 ± 0.2* |
| TPR, mm Hg L min−1 | 14.8 ± 0.7 | 18.6 ± 0.7* |
| MSNA, bursts 100 heartbeats−1 | 34 ± 3 | 25 ± 3 |
| MSNA, bursts min−1 | 20 ± 2 | 16± 2 |
PSBP, peripheral systolic blood pressure; PDBP, peripheral diastolic blood pressure; PPP, peripheral pulse pressure; ASBP, aortic systolic blood pressure; ADBP, aortic diastolic blood pressure; APP, aortic pulse pressure; MAP, mean arterial pressure; PPA, pulse pressure amplification; AG, augmented aortic pressure; AIx, augmentation index; Ew, wasted left ventricular effort; HR, heart rate; SV, stroke volume; CO, cardiac output; TPR, total peripheral resistance; MSNA, muscle sympathetic nerve activity. Data are means±SEMs.
Data are different from men (P < 0.05)
Relationships Between Neural-Hemodynamic and Aortic Wave Reflection Variables
MSNA expressed both as burst incidence and frequency did not correlate with aortic systolic, diastolic, or pulse blood pressure and indices of wave reflection when all subjects were grouped together for analysis (Table 3). There was a correlation between TPR and aortic diastolic pressure (P ≤ 0.05) and an inverse relationship with pulse pressure amplification (P < 0.05) for all subjects studied.
Table 3.
Correlations between MSNA and TPR and aortic pressure and indices of wave reflection
| Hemodynamic/Neural Variables | ASBP (mmHg) | ADBP (mmHg) | APP (mmHg) | PPA | AIx (%) | AIx@75 beats min−1 (%) | AG (mmHg) | Ew (dynes cm2 s) |
|---|---|---|---|---|---|---|---|---|
| MSNA (bursts/100 heart beats) | ||||||||
| All subjects (n = 44) | −0.11 | 0.01 | −0.19 | −0.19 | −0.20 | −0.24 | −0.17 | −0.18 |
| Male (n = 21) | 0.08 | 0.05 | 0.04 | −0.40 | 0.46* (0.46*) | 0.34 | 0.53* (0.53*) | 0.57† (0.51*) |
| Female (n = 23) | −0.20 | 0.02 | −0.30 | 0.14 | −0.63† (−0.64†) | −0.57† | −0.58† (−0.59†) | −0.57† (−0.58†) |
| MSNA (bursts/min) | ||||||||
| All subjects (n = 44) | −0.13 | 0.06 | −0.26 | 0.05 | −0.16 | −0.14 | −0.16 | −0.17 |
| Male (n = 21) | 0.07 | 0.10 | −0.03 | −0.23 | 0.46* (0.45*) | 0.45* | 0.52* (0.52*) | 0.55† (0.51*) |
| Female (n = 23) | −0.21 | 0.04 | −0.39 | 0.19 | −0.63† (−0.63†) | −0.50* | −0.61† (−0.61†) | −0.60† (−0.60†) |
| TPR (mmHg/L/min) | ||||||||
| All subjects (n = 40) | 0.23 | 0.30* | −0.01 | −0.34 | 0.28 | 0.19 | 0.28 | 0.27 |
| Male (n= 19) | 0.17 | 0.39 | −0.22 | −0.42 | 0.11 | −0.18 | 0.14 | 0.13 |
| Female (n = 21) | 0.22 | 0.34 | −0.05 | −0.20 | −0.08 | −0.20 | −0.07 | −0.08 |
Values in parentheses indicate correlations where indices of wave reflection have been corrected for body height. MSNA, muscle sympathetic nerve activity; TPR, total peripheral resistance; ASBP, aortic systolic blood pressure; ADBP, aortic diastolic blood pressure; APP, aortic pulse pressure; PPA, pulse pressure amplification; AIx, augmentation index; AG, augmented pressure; Ew, wasted left ventricular effort.
P ≤ 0.05,
P < 0.01
When we split the group into men and women we observed sex specific differences in these relationships. As we have previously reported,3 MSNA was positively related to TPR in men (r = 0.55; P = 0.02), whereas no relationship was observed in women (r = 0.14, P = 0.59). MSNA was also positively related to AIx (Figure 1A; P < 0.05), AG (Figure 1B; P < 0.05), and Ew (Figure 1C; P < 0.01) in men. In contrast, in women, MSNA was inversely related to AIx (Figure 1A; P < 0.01), AG (Figure 1B; P < 0.01), and Ew (Figure 1C; P < 0.01). Similar results were observed when MSNA was expressed as burst frequency (Table 3). In men, TPR did not correlate with any measure of aortic pressure and wave reflection. Likewise, TPR did not correlate with measures of aortic pressure or wave reflection in women (Table 3). When we scaled the indices of aortic wave reflection for body height, the relationships among MSNA and wave reflection characteristics were not altered (See values in parentheses in Table 3).
Figure 1.
Linear regression analysis of the relationship between MSNA (burst incidence) and A) AIx, B) AG, and C) Ew in men and women. The correlations demonstrate that in young men when MSNA is high, indices of aortic wave reflection (AIx, AG, and Ew) are high, whereas the opposite is true in young women.
Discussion
The major new finding of the current study was that the relationships between MSNA and measures of aortic wave reflection are sex specific. Since our previous work demonstrated that sympathetic regulation of peripheral arterial pressure is fundamentally different between sexes3, we hypothesized that the relationship between MSNA and aortic wave reflection would be stronger in men than in women. Consistent with our hypothesis, men demonstrated a positive relationship between MSNA and aortic wave reflection characteristics. However, and surprisingly, these relationships were inversely related in women. Thus, greater sympathetic activity may contribute to increased aortic wave reflection and left ventricular work in men, but appears to be offset by other factors in women.
In agreement with previous studies16, 17 women demonstrated higher levels of aortic wave reflection compared to men in the current study (Table 2). These sex differences have been attributed to women being shorter with the associated shorter aorta, which in turn results in reflecting sites closer to the heart and earlier wave reflections.18 However, the differences in aortic wave reflection between the sexes remains in elderly men and women matched for body height.19 The goal of the present study was not to quantify sex differences in aortic wave reflection characteristics, but rather to examine the relationships between MSNA and aortic pressure wave reflection and whether these relationships differ between young men and women. Although a shorter body height may result in higher resting levels of wave reflection in women, it should not a priori influence the relationship between AIx and MSNA. In fact, correcting AIx, AG and Ew for body height did not alter the relationship reported in this study (see values in parentheses in Table 3). This suggests that the contrasting relationships between MSNA and aortic wave reflection characteristics in young men and women are independent of body height.
Increased arterial stiffness and early wave reflections are associated with several cardiovascular risk factors, including age, hypertension, diabetes mellitus, hypercholesterolemia, and atherosclerosis.4, 17, 20 Furthermore, increased AIx is a strong independent risk marker for premature CAD and all cause and cardiovascular mortality.7, 21 In the present study, increased AIx and AG were associated with higher levels of MSNA in men. Moreover, men also demonstrated a significant positive relationship between MSNA and Ew, an index of myocardial oxygen demand and left ventricular work. Ew is the portion of the tension-time index curve attributed to earlier reflection of the pulse pressure wave (a shortening of Δtp, Figure S1, please see online Data Supplement) that increases central aortic pressure during systole. An increase in EW can be interpreted as an excess amount of energy expended by the left ventricle without a commensurate increase in flow4 and is associated with left ventricular hypertrophy.14 Interestingly, and in contrast to men, higher levels of MSNA in women were inversely related to indices of wave reflection (AIx and AG) and Ew in the present study.
High sympathetic activity is also associated with several of the conditions in which aortic wave reflection is elevated.22, 23 Moreover, both MSNA and aortic wave reflection characteristics increase during commonly used laboratory stressors such as the cold pressor test and handgrip exercise.24, 25 26, 27 Whether sympathetic activity plays a major role in modulating the elastic properties of central arteries or the tone of peripheral muscular arteries remains unknown. Recent evidence suggests that a) resting MSNA levels are related to aortic stiffness8 in men and b) short-term sympathetic activation (via cold pressor test) decreases muscular artery compliance in normotensive young humans.28 However, Lydakis and colleagues29 demonstrated that despite similar levels of sympathetic system engagement during lower body negative pressure and isometric fatiguing handgrip exercise, only the latter resulted in an increase in blood pressure and in measures of central large artery stiffness and wave reflection. Based on these findings the authors concluded that blood pressure rather than sympathetic activity seems to play the major role in modulating the elastic properties of the central arteries. In the current study, MSNA was not related to arterial pressure (either peripheral or aortic) in men or women. Nonetheless, in our study MSNA was related to measures of aortic wave reflection. Interestingly, the direction of these relationships were dependent on sex.
Maneuvers that acutely increase sympathetic stimulation are associated with reductions in the distensibility of both muscular (radial) and elastic (carotid) type arteries.28, 30 The sympathetic nervous system may also exert a marked tonic restraint of medium-sized and large muscular arteries in men31 and may have a stiffening influence on the mechanical properties of the aorta.32 Evidence from normotensive male rats suggests that stimulation of the α-adrenergic receptors with norepinephrine increases carotid intima media thickness and reduces lumen area, which might theoretically change the mechanical properties of this artery.33 Since the nature of reflected waves are dependent upon the elastic properties of the entire arterial tree4, changes in arterial distensibility and tone of both elastic and muscular arteries can have profound effects on the aortic pressure wave. In this context, AIx is related to arterial properties via changes in PWV. Increased arterial stiffness increases PWV and causes early return of the reflected wave from the peripheral reflecting sites to the ascending aorta. Likewise, changes in smooth muscle tone of the muscular arteries, especially those in the lower body, modify the speed of travel of the pressure wave along their length and determine when the reflected wave arrives back at the heart. Along these lines, men with higher levels of MSNA demonstrate increased levels of calf vascular resistance.34
Possible Mechanisms for Discrepant Relationships in Men and Women
We were surprised and interested to note that the relationships between MSNA and aortic wave form characteristics in women were exactly opposite to that in men. These data suggest that women with high MSNA actually have a reduced aortic wave reflection and a lower index of left ventricular wasted energy compared to women with lower MSNA. In this context, previous data from our laboratory3 and others34 demonstrate that, in women, high MSNA does not necessarily translate into elevated peripheral vascular resistance as it does in men. The fact that that resting MSNA appears to have a differential effect on aortic wave form characteristics in women further adds to the body of evidence which suggests that sex influences the way that MSNA interacts with the vasculature.
The exact mechanisms underlying the inverse relationship between MSNA and aortic waveform characteristics in women are unclear, but there are several possible explanations. First, it appears that the β-adrenergic receptors are either more sensitive or up-regulated in women vs. men.35 Thus, concurrent β-mediated vasodilation via norepinephrine released from the sympathetic nerve terminal might offset α-mediated vasoconstriction in women. Consequently, high levels of MSNA in young women may decrease aortic waveform characteristics via decreased vascular tone and/or stiffness via vascular β-adrenergic receptor stimulation. Second, differences in the metabolism of norepinephrine in the vascular smooth muscle cells may exist between men and women, which could result in alterations vascular tone and/or stiffness.36 However, this can only be postulated as we have no data to support this and it is beyond the scope of the present study. Finally, circulating estrogens increase endothelial nitric oxide availability causing a decrease in resting vascular tone in women (see Miller and Duckles37 for review) and therefore have the potential to decrease indices of wave reflection.38 Consequently, the effect of estrogen on the vasculature might explain why MSNA was not positively related to augmentation index in the young women. However, if this was the case then we would expect no relationship between MSNA and augmentation index. We found an inverse relationship between MSNA and augmentation index in young women, thus other factors other than the vasodilating effects of estrogen acting alone must be important in driving this relationship.
Limitations
The characteristics of the reflected wave depend on a complex set of determinants including the speed at which the wave travels (i.e. PWV).4 Increased arterial stiffness increases PWV and causes early return of the reflected wave from peripheral reflecting sites and thus results in a greater AIx. A limitation of the present study was that we did not assess aortic PWV. Therefore, we are unable to evaluate whether the sex differences in the relationships between MSNA and wave reflection characteristics were related to differences in aortic stiffness. We also note in this context that AIx can vary considerably among large groups of young men and women despite similar aortic PWV values.17
Perspectives
This is the first study to examine the relationships between MSNA and aortic wave reflection characteristics in humans. Our current results demonstrate that young men with high MSNA have elevated levels of aortic wave reflection, whereas, the relationship between these two variables is inversely related in young women. Although these results were unexpected, they are consistent with our recent work regarding sex differences in relationships between MSNA and peripheral vascular resistance in young healthy humans.3 Taken together, the present and previous studies provide important initial insight into sympathetic-hemodynamic interactions which appear to have profound implications for development of cardiovascular risk. As such, our present findings add to our previous work by identifying additional mechanisms by which cardiovascular risk may be minimized in young women compared to young men. In men, our data suggest that increases in MSNA negatively affect aortic hemodynamics (variables shown to be related to cardiovascular risk) to a greater extent than in women. Importantly, cardiovascular risk profiles change dramatically at the menopause, where potential protective influences of estrogen in women are lost and cardiovascular risk profiles can approach or even exceed levels seen in men. To this point, future studies should address the impact of aging, specifically in women, on the relationships between sympathetic neural activity and aortic wave reflection variables.
Supplementary Material
Acknowledgments
The authors are grateful to the study volunteers for their participation. We also thank Shelly Roberts, Jean Knutson, Karen Krucker, Christopher Johnson, Nancy Meyer, Pam Engrav, and Jessica Sawyer for their technical assistance.
Sources of Funding This study was supported by National Institutes of Health grants HL083947 (MJJ, NC), HL46493 (MJJ), AR55819 (DPC), DK082424 (TBC), and CTSA RR-024150 (Mayo Clinic), and by Mayo Clinic Research Subcommittee Early Career Development Supplement (TBC) and American Heart Association grant 2170087 (ECH).
Footnotes
Disclosures None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Charkoudian N, Joyner MJ, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation. The Journal of physiology. 2005;568(Pt 1):315–321. doi: 10.1113/jphysiol.2005.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sundlof G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. The Journal of physiology. 1978;274:621–637. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance: implications for human blood pressure regulation. Hypertension. 2009;53:571–576. doi: 10.1161/HYPERTENSIONAHA.108.126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nichols WW, Singh BM. Augmentation index as a measure of peripheral vascular disease state. Current opinion in cardiology. 2002;17:543–551. doi: 10.1097/00001573-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Manisty C, Mayet J, Tapp RJ, Parker KH, Sever P, Poulter NH, Thom SA, Hughes AD. Wave reflection predicts cardiovascular events in hypertensive individuals independent of blood pressure and other cardiovascular risk factors: an ASCOT (Anglo-Scandinavian Cardiac Outcome Trial) substudy. Journal of the American College of Cardiology. 2010;56:24–30. doi: 10.1016/j.jacc.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 6.Vlachopoulos C, Aznaouridis K, O'Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. European heart journal. 2010;31:1865–1871. doi: 10.1093/eurheartj/ehq024. [DOI] [PubMed] [Google Scholar]
- 7.Weber T, Auer J, O'Rourke MF, Kvas E, Lassnig E, Berent R, Eber B. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation. 2004;109:184–189. doi: 10.1161/01.CIR.0000105767.94169.E3. [DOI] [PubMed] [Google Scholar]
- 8.Swierblewska E, Hering D, Kara T, Kunicka K, Kruszewski P, Bieniaszewski L, Boutouyrie P, Somers VK, Narkiewicz K. An independent relationship between muscle sympathetic nerve activity and pulse wave velocity in normal humans. Journal of hypertension. 2010;28:979–984. doi: 10.1097/hjh.0b013e328336ed9a. [DOI] [PubMed] [Google Scholar]
- 9.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000;101:862–868. doi: 10.1161/01.cir.101.8.862. [DOI] [PubMed] [Google Scholar]
- 10.Sundlof G, Wallin BG. The variability of muscle nerve sympathetic activity in resting recumbent man. J Physiol. 1977;272:383–397. doi: 10.1113/jphysiol.1977.sp012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pauca AL, O'Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38:932–937. doi: 10.1161/hy1001.096106. [DOI] [PubMed] [Google Scholar]
- 12.Chen CH, Nevo E, Fetics B, Pak PH, Yin FC, Maughan WL, Kass DA. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation. 1997;95:1827–1836. doi: 10.1161/01.cir.95.7.1827. [DOI] [PubMed] [Google Scholar]
- 13.Gallagher D, Adji A, O'Rourke MF. Validation of the transfer function technique for generating central from peripheral upper limb pressure waveform. American journal of hypertension. 2004;17(Pt 1):1059–1067. doi: 10.1016/j.amjhyper.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto J, Nichols WW, O'Rourke MF, Imai Y. Association between wasted pressure effort and left ventricular hypertrophy in hypertension: influence of arterial wave reflection. American journal of hypertension. 2008;21:329–333. doi: 10.1038/ajh.2007.49. [DOI] [PubMed] [Google Scholar]
- 15.Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol. 1993;74:2566–2573. doi: 10.1152/jappl.1993.74.5.2566. [DOI] [PubMed] [Google Scholar]
- 16.Lieber A, Millasseau S, Bourhis L, Blacher J, Protogerou A, Levy BI, Safar ME. Aortic wave reflection in women and men. Am J Physiol Heart Circ Physiol. 2010;299:H236–242. doi: 10.1152/ajpheart.00985.2009. [DOI] [PubMed] [Google Scholar]
- 17.McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT) Journal of the American College of Cardiology. 2005;46:1753–1760. doi: 10.1016/j.jacc.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 18.Smulyan H, Marchais SJ, Pannier B, Guerin AP, Safar ME, London GM. Influence of body height on pulsatile arterial hemodynamic data. Journal of the American College of Cardiology. 1998;31:1103–1109. doi: 10.1016/s0735-1097(98)00056-4. [DOI] [PubMed] [Google Scholar]
- 19.Gatzka CD, Kingwell BA, Cameron JD, Berry KL, Liang YL, Dewar EM, Reid CM, Jennings GL, Dart AM. Gender differences in the timing of arterial wave reflection beyond differences in body height. Journal of hypertension. 2001;19:2197–2203. doi: 10.1097/00004872-200112000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Nigam A, Mitchell GF, Lambert J, Tardif JC. Relation between conduit vessel stiffness (assessed by tonometry) and endothelial function (assessed by flow-mediated dilatation) in patients with and without coronary heart disease. The American journal of cardiology. 2003;92:395–399. doi: 10.1016/s0002-9149(03)00656-8. [DOI] [PubMed] [Google Scholar]
- 21.London GM, Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME. Arterial wave reflections and survival in end-stage renal failure. Hypertension. 2001;38:434–438. doi: 10.1161/01.hyp.38.3.434. [DOI] [PubMed] [Google Scholar]
- 22.Palatini P, Julius S. The role of cardiac autonomic function in hypertension and cardiovascular disease. Current hypertension reports. 2009;11:199–205. doi: 10.1007/s11906-009-0035-4. [DOI] [PubMed] [Google Scholar]
- 23.Tentolouris N, Argyrakopoulou G, Katsilambros N. Perturbed autonomic nervous system function in metabolic syndrome. Neuromolecular medicine. 2008;10:169–178. doi: 10.1007/s12017-008-8022-5. [DOI] [PubMed] [Google Scholar]
- 24.Fu Q, Levine BD, Pawelczyk JA, Ertl AC, Diedrich A, Cox JF, Zuckerman JH, Ray CA, Smith ML, Iwase S, Saito M, Sugiyama Y, Mano T, Zhang R, Iwasaki K, Lane LD, Buckey JC, Jr., Cooke WH, Robertson RM, Baisch FJ, Blomqvist CG, Eckberg DL, Robertson D, Biaggioni I. Cardiovascular and sympathetic neural responses to handgrip and cold pressor stimuli in humans before, during and after spaceflight. The Journal of physiology. 2002;544(Pt 2):653–664. doi: 10.1113/jphysiol.2002.025098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Padilla J, Young CN, Simmons GH, Deo SH, Newcomer SC, Sullivan JP, Laughlin MH, Fadel PJ. Increased muscle sympathetic nerve activity acutely alters conduit artery shear rate patterns. Am J Physiol Heart Circ Physiol. 2010;298:H1128–1135. doi: 10.1152/ajpheart.01133.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casey DP, Braith RW, Pierce GL. Changes in central artery blood pressure and wave reflection during a cold pressor test in young adults. European journal of applied physiology. 2008;103:539–543. doi: 10.1007/s00421-008-0746-2. [DOI] [PubMed] [Google Scholar]
- 27.Edwards DG, Mastin CR, Kenefick RW. Wave reflection and central aortic pressure are increased in response to static and dynamic muscle contraction at comparable workloads. J Appl Physiol. 2008;104:439–445. doi: 10.1152/japplphysiol.00541.2007. [DOI] [PubMed] [Google Scholar]
- 28.Boutouyrie P, Lacolley P, Girerd X, Beck L, Safar M, Laurent S. Sympathetic activation decreases medium-sized arterial compliance in humans. The American journal of physiology. 1994;267(Pt 2):H1368–1376. doi: 10.1152/ajpheart.1994.267.4.H1368. [DOI] [PubMed] [Google Scholar]
- 29.Lydakis C, Momen A, Blaha C, Herr M, Leuenberger UA, Sinoway LI. Changes of elastic properties of central arteries during acute static exercise and lower body negative pressure. European journal of applied physiology. 2008;102:633–641. doi: 10.1007/s00421-007-0637-y. [DOI] [PubMed] [Google Scholar]
- 30.Failla M, Grappiolo A, Carugo S, Calchera I, Giannattasio C, Mancia G. Effects of cigarette smoking on carotid and radial artery distensibility. Journal of hypertension. 1997;15(Pt 2):1659–1664. doi: 10.1097/00004872-199715120-00069. [DOI] [PubMed] [Google Scholar]
- 31.Failla M, Grappiolo A, Emanuelli G, Vitale G, Fraschini N, Bigoni M, Grieco N, Denti M, Giannattasio C, Mancia G. Sympathetic tone restrains arterial distensibility of healthy and atherosclerotic subjects. Journal of hypertension. 1999;17:1117–1123. doi: 10.1097/00004872-199917080-00011. [DOI] [PubMed] [Google Scholar]
- 32.Sonesson B, Vernersson E, Hansen F, Lanne T. Influence of sympathetic stimulation on the mechanical properties of the aorta in humans. Acta physiologica Scandinavica. 1997;159:139–145. doi: 10.1046/j.1365-201X.1997.581343000.x. [DOI] [PubMed] [Google Scholar]
- 33.Erami C, Zhang H, Ho JG, French DM, Faber JE. Alpha(1)-adrenoceptor stimulation directly induces growth of vascular wall in vivo. Am J Physiol Heart Circ Physiol. 2002;283:H1577–1587. doi: 10.1152/ajpheart.00218.2002. [DOI] [PubMed] [Google Scholar]
- 34.Hogarth AJ, Mackintosh AF, Mary DA. Gender-related differences in the sympathetic vasoconstrictor drive of normal subjects. Clin Sci (Lond) 2007;112:353–361. doi: 10.1042/CS20060288. [DOI] [PubMed] [Google Scholar]
- 35.Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. Journal of the American College of Cardiology. 2000;36:1233–1238. doi: 10.1016/s0735-1097(00)00849-4. [DOI] [PubMed] [Google Scholar]
- 36.Masi CM, Hawkley LC, Xu X, Veenstra TD, Cacioppo JT. Serum estrogen metabolites and systolic blood pressure among middle-aged and older women and men. American journal of hypertension. 2009;22:1148–1153. doi: 10.1038/ajh.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacological reviews. 2008;60:210–241. doi: 10.1124/pr.107.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adkisson EJ, Casey DP, Beck DT, Gurovich AN, Martin JS, Braith RW. Central, peripheral and resistance arterial reactivity: fluctuates during the phases of the menstrual cycle. Experimental biology and medicine (Maywood, N.J. 2010;235:111–118. doi: 10.1258/ebm.2009.009186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.