Pulsatile pattern of ACTH activation of the adrenal cortex is critical for corticosterone secretion and induces episodic transcription of proteins important for steroidogenesis.
Abstract
The activity of the hypothalamic-pituitary-adrenal axis is characterized by an ultradian pulsatile pattern of glucocorticoid secretion. Despite increasing evidence for the importance of pulsatility in regulating glucocorticoid-responsive gene transcription, little is known about the mechanism underlying the pulsatility of glucocorticoid synthesis and release. We tested the hypothesis that pulsatile ACTH release is critical for optimal adrenocortical function. Hypothalamic-pituitary-adrenal activity was suppressed by oral methylprednisolone, and ACTH (4 ng/h) was infused for 24h either as a constant infusion or in 5-min pulses at hourly intervals. Control methylprednisolone-treated rats had very low plasma corticosterone (CORT) levels with undetectable pulses and also had steroidogenic acute regulatory protein (StAR) and cytochrome P450 side-chain cleavage (P450scc) heteronuclear RNA levels reduced to approximately 50% of that seen in untreated animals. Pulsatile but not constant ACTH infusion restored pulsatile CORT secretion, and this was accompanied by parallel rises in StAR and P450scc heteronuclear RNA levels during the rising phase of the CORT pulse, which then fell during the falling phase. The pulsatile pattern of StAR and P450scc was paralleled by pulsatile transcription of the melanocortin 2 receptor accessory protein. Pulsatile ACTH activation of the adrenal cortex not only is critical for the secretion of CORT but also induces episodic transcription of the rate-limiting enzymes necessary for physiological steroidogenic responses. Because constant infusion of identical amounts of ACTH did not activate CORT secretion, pulsatility of ACTH provides a more effective signaling system for the activation of adrenocortical activity.
The activity of the hypothalamo-pituitary-adrenal (HPA) axis, both in man and in rodents, exhibits a circadian and an ultradian rhythm. The secretory activity underlying the circadian rhythm of the HPA axis is in fact characterized by pulsatile release of ACTH and glucocorticoids throughout the 24-h cycle (1–3). Glucocorticoids are released from the adrenal gland in hourly pulses (4) that result in rapid changes of hormone levels both in the blood and within target tissues including the brain (5).
Changes in the ultradian pattern of corticosterone secretion in the rat have been observed during different physiological states such as lactation (6) and aging (7) and after exposure to stress during both neonatal (8) and adult (9) life. Glucocorticoid pulsatility is an important factor in determining the responsivity of the HPA axis to stress. When hormone pulsatility is replaced by constant levels of glucocorticoids, a desensitization of both neuroendocrine and behavioral responses to stress is observed (10). Furthermore, the timing of the onset of a stressor in relation to the phase of an endogenous basal pulse determines the magnitude of the response to stress (3). At the tissue level, pulsatile secretion of glucocorticoids is essential for maintaining normal glucocorticoid receptor signaling and preventing desensitization of transcriptional responses of glucocorticoid target genes (11–13).
Despite our understanding of the importance of glucocorticoid pulsatility (14), little is known about the mechanisms underlying the regulation of the ultradian activity of the HPA axis. It is known that the circadian rhythm is regulated by projections from the suprachiasmatic nucleus to both the median eminence and the paraventricular nucleus of the hypothalamus (15) and disruption of normal suprachiasmatic nucleus activity in rat abolishes corticosterone circadian rhythm (16) without affecting ultradian rhythm (17). Recent studies suggest that the generation of the oscillating levels of ACTH and glucocorticoids depends on the feed-forward-feedback relationship between the pituitary corticotrophs and the adrenal cortex (18). This model proposes that independent of the pattern of CRH exposure, the peripheral interaction of ACTH on the adrenal cortex and glucocorticoids on the pituitary will result in oscillating levels of ACTH and corticosterone.
To achieve this ultradian rhythm, it is, of course, critical for the adrenal cortex to be able to release pulses of glucocorticoid hormone. Because these hormones are lipophilic, they cannot be simply packaged in readily releasable vesicles but need to be newly synthesized in response to ACTH. This therefore suggests that there must be a remarkably responsive steroid synthetic mechanism that is able to respond rapidly after ACTH receptor activation.
We hypothesized that the adrenal cortex must be adapted to respond to episodic exposure to ACTH to maintain normal ultradian rhythmicity. Therefore, in the current studies, we tested whether exposure to pulsatile ACTH was necessary for optimal release of corticosterone and how this relates to the regulation of genes involved in glucocorticoid steroidogenesis.
We developed an in vivo paradigm in which endogenous HPA activity was suppressed by the addition of methylprednisolone (MP) to the drinking water (1 g/liter) for 3 d before the experiment. Rats were then infused iv with identical doses of ACTH either as a constant infusion or in a pulsatile (one pulse/h) pattern for 24 h. The profile of corticosterone secretion was then analyzed from the blood samples collected with our automated sampling system. The adrenal glands from these animals were processed for measurement of gene transcription of steroidogenic acute regulatory protein (StAR) and cytochrome P450 side chain cleavage (P450scc) as well as the melanocortin 2 receptor (MC2R) and the MC2R accessory protein (MRAP) (19).
Materials and Methods
Animals
All experiments were conducted on adult male Sprague Dawley rats (Harlan, Oxon, UK) weighing 250–300 g at the time of surgery. Animals were group housed four to a cage and allowed to acclimatize to the housing facility for a minimum of 1 wk before the start of experiments. Rats were maintained under standard environmental conditions (21 ± 1 C) under a 14-h light, 10-h dark schedule (lights on at 0515 h), and food and water were provided ad libitum throughout the experiment. All animal procedures were approved by the University of Bristol Ethical Review Group and were conducted in accordance with Home Office guidelines and the United Kingdom Animals (Scientific Procedures) Act, 1986.
Surgery
Animals were anesthetized with a combination of Hypnorm (0.32 mg/kg fentanyl citrate and 10 mg/kg fluanisone, im; Janssen Pharmaceuticals, Oxford, UK) and diazepam (2.6 mg/kg ip; Phoenix Pharmaceuticals, Gloucester, UK). Intravenous cannulation of the jugular vein was performed as previously described (20) and modified as follows. The right jugular vein was exposed, and two silastic-tipped (Merck, Whitehouse, NJ) polythene cannulae (Portex, Hythe, UK) were inserted into the vessel to allow simultaneous blood sampling and substance infusion. Both cannulae were prefilled with pyrogen-free heparinized (10 IU/ml) isotonic saline. The free ends of both cannulae were exteriorized through a scalp incision and then tunneled through a protective spring that was anchored to the parietal bones using two stainless steel screws and self-curing dental acrylic. After recovery, animals were housed in individual cages in the automated blood sampling room. The end of the protective spring was attached to a mechanical swivel that rotated through 360° in a horizontal plane and 180° through a vertical plane, allowing the rats to maximize freedom of movement. The cannulae were flushed daily with the heparinized saline to maintain patency.
Experimental design: automated ACTH infusion and blood sampling
Starting on d 3 after surgery, animals were given the synthetic glucocorticoid MP (MP sodium succinate, Solu-Medrone; Pharmacia Limited, Sandwich, UK) dissolved in their drinking water at the minimum dose found to suppress basal (21) and restraint stress-induced plasma corticosterone and ACTH levels (1 g/liter; unpublished observations). MP was chosen for its ability to permeate the blood-brain barrier (22), and its high degree of water solubility makes this steroid an excellent choice for administration in drinking water. On d 4 after surgery, one of the implanted cannulae was connected to the automated blood sampling system as previously described (6, 23). Blood samples were collected every 10 min for a period of up to 27 h. Each blood sample (approximately 40 μl) was collected in 160 μl of heparinized saline.
Sampling began on d 5 at 0700 h. After 3 h of blood collection for assessment of basal corticosterone levels, an infusion of either synthetic ACTH [Synacthen, ACTH- (1–24) fragment; Alliance Pharmaceutical, Chippenham, UK] or 0.9% saline was started through a second cannula using the automated infusion system. Sampling was continued for up to 24 h during the infusion. We designed the study so that rats received the same dose of ACTH (or saline) either as hourly pulses of 5 min duration (4 ng/pulse; 5 min infusion followed by 55 min of pause) or as constant infusion (4 ng/h). The choice of the ACTH dose used in this study was based on a pilot study where different doses of ACTH were tested for their ability to reproduce the amplitude and duration of physiological corticosterone pulses (data not shown). Control groups of rats had no MP in drinking water and were infused with either pulsatile or constant saline. The infusion rate of ACTH and saline was set as 2 ml/h for pulsatile infusion (during the 5-min pulse) and 0.167 ml/h for constant infusion. All ACTH infusions were performed in MP-suppressed rats.
At the end of the sampling and infusion period, rats were overdosed with 0.5 ml of sodium pentobarbital (Euthatal, 200 mg/ml; Merial, Harlow, UK). Rats under pulsatile infusion were overdosed either 20 or 40 min after the start of the last ACTH pulse (between 0920 and 1020 h). Rats under constant ACTH infusion overdosed after approximately 24 h after the start of the infusion (between 0930 and 1030 h). Adrenal gland were collected and processed as described below. Blood samples collected with the automated blood sampling system were centrifuged and plasma stored at −20 C until assayed for corticosterone.
Corticosterone measurement
Plasma levels of total corticosterone were measured by RIA as previously described (20) using a citrate buffer (pH 3.0) to denature the binding globulin. Antisera was kindly supplied by Prof. G. Makara (Institute of Experimental Medicine, Budapest, Hungary), and [125I]corticosterone was purchased from Izotop (Budapest, Hungary). The intra- and interassay coefficients of variation of the corticosterone assay were 16.7 and 13.3%, respectively.
Plasma ACTH measurement
A separate cohort of animals was used to measure plasma levels of ACTH in the different experimental groups. Animals underwent surgery and MP suppression as described above. MP-suppressed rats received either pulsatile (4 ng/pulse) or constant ACTH infusions (4 ng/h), starting at 1000 h for 24 h. For rats receiving pulsatile infusion, sample collection started at 1600 h, at time 0, 5, 15, and 30 min after the onset of the last pulse. In rats receiving constant ACTH infusion, blood samples were collected before starting the infusion (time zero) and 9 and 22 h after the onset of the infusion. Control samples were taken at the same time points (0, 9, and 22 h) from an additional group of non-MP-suppressed rats receiving an infusion of saline. At the end of the experiment, all rats were overdosed with 0.5 ml sodium pentobarbital.
Blood samples of 0.2 ml were collected manually in ice-cold tubes containing 10 μl EDTA (0.5 m; pH 7.4) and 10 μl aprotinin (500,000 KIU/ml, Trasylol; Bayer, Newbury, UK). Plasma was separated by centrifugation and stored at −80 C until processed for ACTH measurement.
ACTH in plasma was measured using 100 μl plasma and RIA kit reagents (DiaSorin, Stillwater, MN) in accordance with the manufacturer's protocol. This assay was chosen for its ability to equally recognize ACTH 1–24 and 1–39. The intra- and interassay coefficients of variation of the ACTH assay were 2.2 and 7.8%, respectively.
Analysis of adrenal glands: RNA isolation and real-time quantitative PCR (qRT-PCR)
qRT-PCR assays were used to quantify relative levels of StAR and P450scc and MRAP heteronuclear RNA (hnRNA) and mRNA and MC2R mRNA in the adrenal glands. Quickly after collection, adrenal glands were dissected free of fat and decapsulated to separate into the capsule containing the zona glomerulosa and the inner zones comprising the zona fasciculata and zona reticularis of the cortex and the medulla. Individual inner zones were immediately frozen in dry ice until processing for preparation of RNA. Because it has been shown that there is no functional difference between the right and left adrenals in the rat, only right adrenals were used for RNA preparation for exonic (to measure mRNA) and intronic (to measure primary transcript or hnRNA) qRT-PCR.
RNA isolation and qRT-PCR
Total RNA was extracted from the inner zone of individual adrenals using TRIzol reagent (Invitrogen, Hopkinton, MA), followed by purification using RNeasy mini kit reagents and column deoxyribonuclease digestion (QIAGEN, Valencia, CA) to remove genomic DNA contamination. cDNA was reverse transcribed from 0.7–1 μg total RNA as previously described (24). Primary transcript and mRNA accumulation of StAR, P450scc, and MRAP genes were evaluated using primer sequences designed to amplify an intronic fragment of nascent RNA (for hnRNA) and an exonic fragment for mRNA, respectively. The sequences of the primers for hnRNA and mRNA measurements for StAR, P450scc, and GAPDH have previously been reported (25). The primers for MC2R and MRAP qRT-PCR are forward ATCTGCAGTTTGGCCATTTC and reverse GCAATCACAGACAGGCTGAA for MC2R mRNA, forward ACCTCATTCCTGTGGACGAG and reverse ACCCGCCATATTATCACTGC for MRAP hnRNA, and forward CCTCCCGGTGTGTGGCCTCT and reverse GGGGACTATGCCTTACCTGTGGGG for MRAP mRNA. The PCR procedure detects the amino terminus and transmembrane domain of the rat MRAP. Two splice variants with identical amino terminus and transmembrane domain but different carboxy terminus have been described for human MRAP. The protein structure is well conserved between rat and human, and because the primers span the conserved region in human, it is likely that if variants exist in the rat, the PCR procedure would detect them both. The rat MC2R does not contain an intron, and therefore, only mRNA could be measured. Power SYBR green PCR mix (Applied Biosystems, Foster City, CA) was used for the amplification mixture with each primer at a final concentration of 200 nm and 1.5 μl cDNA for a total reaction volume of 12.5 μl. PCR were performed on spectrofluorometric thermal cycler (7900 HT Fast Real-Time PCR System; Applied Biosystems) as previously described (24). Samples were amplified by an initial denaturation at 50 C for 2 min and 95 C for 10 min and then cycled (45 times) using 95 C for 15 sec and 60 C for 1 min.
StAR, P450scc, and MRAP hnRNA and mRNA levels and MC2R mRNA levels were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA as determined in separate qRT-PCR. The absence of RNA detection when the RT step was omitted indicated the lack of genomic DNA contamination in the RNA samples.
Statistical analysis
Data are represented as mean ± sem from the values in the number of observations indicated in the legends of the figures. Area under the curve (AUC), mean corticosterone concentration, and number of pulses were analyzed in the four experimental groups for the period of the experiment (27 h) using the PULSAR algorithm (26) as previously described (3, 20). Furthermore, parameters characterizing the pulses of corticosterone were analyzed in individual profiles during the diurnal peak phase of corticosterone secretion (1500–0300 h) using PULSAR and AutoDecon (27). All statistical analyses were performed using SPSS version 11.5 for Windows (SPSS Inc., Chicago, IL). Differences between groups were analyzed by Student's t test or ANOVA followed by Fisher protected least-significant difference post hoc test when appropriate. Statistical significance was set at P < 0.05.
Results
Effect of ACTH infusion on ultradian rhythm of corticosterone
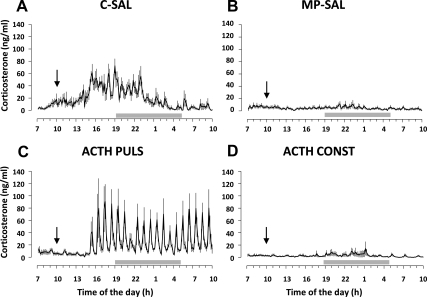
Corticosterone levels were measured in plasma obtained from blood samples collected every 10 min over a period of 27 h. Mean ± sem plasma corticosterone for each experimental group is shown in Fig. 1. Because no significant difference in AUC between constant (n = 4) and pulsatile (n = 4) infusion of saline was found in control rats, data from those groups were merged and analyzed as a single experimental group (CONTROL-SAL, n = 8; Fig. 1A). Furthermore, because no significant difference in AUC between constant (n = 6) and pulsatile (n = 5) infusion of saline was found in MP rats, data from those groups were merged and analyzed as a single experimental group (MP-SAL, n = 11; Fig. 1B). Corticosterone levels of rats treated with MP and infused with pulsatile (ACTH PULS, n = 9) or constant (ACTH CONST, n = 8) ACTH are shown in Fig. 1, C and D, respectively.
Fig. 1.
Effect of ACTH infusion on plasma corticosterone levels over 27 h of blood sample collection. Data are expressed as mean ± sem of plasma corticosterone levels. A, Corticosterone secretion in controls rats infused with saline (C-SAL, n = 8) showed the expected ultradian rhythm characterized by hourly pulses; B, corticosterone secretion was reduced in MP-treated rats chronically infused with saline (MP-SAL, n = 11); C, MP rats infused with pulsatile ACTH (ACTH PULS; 4 ng/h, 5 min pulse/h; n = 9) showed ultradian corticosterone secretion; D, MP rats infused with constant ACTH (ACTH CONST; 4 ng/h; n = 8) showed lower corticosterone levels compared with C-SAL but not different from MP-SAL. Blood samples were collected every 10 min for 27 h using an automated blood sampling system, and corticosterone levels were measured using RIA. Gray bars indicated dark period (1900–0500 h). Black arrows indicate the onset of the infusion.
ANOVA revealed a significant effect of the treatment on corticosterone secretion as shown by analysis of AUC [F(3, 36) = 13.0190; P < 1 × 10−5], mean corticosterone concentration [F(3, 36) = 29.965; P < 1 × 10−8], and number of pulses [F(3, 36) = 11.747; P < 5 × 10−5] (Table 1). Analysis of corticosterone profile in CONTROL-SAL rats showed the expected circadian rhythm, with high levels of corticosterone released during the peak phase of the diurnal cycle and low levels during the trough phase (Fig. 1A). In agreement with previous reports (3, 20, 28), those rats also showed an ultradian pattern with pulses of corticosterone occurring with hourly frequency. MP treatment induced a decrease in corticosterone levels with a complete loss of both circadian and ultradian rhythms (MP-SAL, Fig. 1B), as shown by a decreased AUC (P < 0.0005), mean concentration (P < 0.001), and number of pulses (P < 1 × 10−6) compared with CONTROL-SAL rats. However, pulsatile infusion of ACTH in MP-suppressed rats (ACTH PULS) was able to restore pulsatile corticosterone secretion, after a lag period of 5 h (Fig. 1C). This effect was characterized by an increased AUC (P < 0.0001), mean concentration (P < 0.0005), and number of pulses (P < 1 × 10−7) compared with MP-SAL rats. In contrast, constant infusion of ACTH had no effect on the reduction of corticosterone secretion induced by MP (Fig. 1D), and no difference between ACTH CONST and MP-SAL was found in any of the parameters measured, whereas there was a significant difference between ACTH CONST and CONTROL-SAL in AUC (P < 0.0005), mean corticosterone concentration (P < 0.0005) and number of pulses (P < 5 × 10−7).
Table 1.
Corticosterone secretion
| AUC | Concentration | No. of pulses | |
|---|---|---|---|
| CONTROL-SAL | 565.8 ± 84.9 | 20.3 ± 3.7 | 13.8 ± 1.1 |
| MP-SAL | 144.8 ± 49a | 4.6 ± 1.8a | 2.0 ± 1.0a |
| ACTH PULS | 607.2 ± 109.7b | 22.2 ± 4.4b | 13.6 ± 1.7b |
| ACTH CONST | 94.6 ± 12.7a | 1.6 ± 1.0a | 1.7 ± 1.0a |
Shown are mean ± sem of AUC, mean concentration, and number of pulses analyzed using PULSAR algorithm from corticosterone profiles of control rats infused with saline (CONTROL-SAL) and rats treated with MP and infused with saline (MP-SAL), pulsatile ACTH (4 ng/h, 5 min pulse, ACTH PULS), and constant ACTH (4 ng/h, ACTH CONST) for 24 h. PULSAR analysis was performed on corticosterone release data from samples collected for 27 h (0700h on d 1 to 1000 h on d 2). AUC, mean corticosterone levels, and number of pulses were lower in MP-SAL and ACTH CONST, compared with CONTROL-SAL, whereas they were increased in ACTH PULS compared with MP-SAL.
P < 0.001 vs. CONTROL-SAL.
P < 0.0005 vs. MP-SAL. (See Results for detailed P values.)
To further elucidate the effect of pulsatile ACTH on the ultradian rhythm of corticosterone, the PULSAR and AutoDecon algorithms were used to analyze the parameters characterizing the pulses of corticosterone in CONTROL-SAL and ACTH PULS rats during the diurnal peak phase of corticosterone secretion (1500–0300 h). There was no difference in mean pulse number, height, length, area, interpulse interval, and frequency between the two experimental groups when data were analyzed using PULSAR (Table 2). Furthermore, there was no difference in mean pulse number, height, mass, interpulse interval, and frequency when data were analyzed using AutoDecon (Table 3).
Table 2.
PULSAR analysis of pulse characteristics
| Treatment | Pulse number | Pulse height (ng/ml) | Pulse length (h) | Pulse area (ng/ml) | Interpulse interval (h) | Pulse frequency (no. pulses/h) |
|---|---|---|---|---|---|---|
| CONTROL-SAL | 9.2 ± 0.6 | 70.1 ± 5.7 | 0.62 ± 0.03 | 19.1 ± 1.4 | 1.23 ± 0.07 | 0.78 ± 0.06 |
| ACTH -PULS | 7.7 ± 0.9 | 83.1 ± 13.5 | 0.53 ± 0.06 | 20.6 ± 4.8 | 1.46 ± 0.21 | 0.65 ± 0.07 |
Shown are mean ± sem of pulse height, interpulse interval, pulse frequency, pulse length, and pulse area analyzed using the PULSAR algorithm calculated from 12-h corticosterone profiles during the peak phase of corticosterone secretion (1500–0300 h) of control rats infused with saline (CONTROL-SAL) and rats treated with MP for 5 d and infused with pulsatile ACTH (4 ng/h, 5 min pulse, ACTH PULS) for 24 h.
Table 3.
AutoDecon analysis of pulse characteristics
| Treatment | Pulse number | Pulse height (ng/ml·min) | Pulse mass (ng/ml) | Interpulse interval (h) | Pulse frequency (no. of pulses/h) |
|---|---|---|---|---|---|
| CONTROL-SAL | 7.9 ± 0.4 | 10.7 ± 1.4 | 121.3 ± 14.1 | 1.25 ± 0.06 | 0.65 ± 0.03 |
| ACTH PULS | 9.2 ± 0.6 | 10.7 ± 1.9 | 113.7 ± 24.8 | 1.12 ± 0.07 | 0.77 ± 0.05 |
Shown are mean ± sem of pulse number, pulse height, pulse mass, and interpulse interval and pulse frequency analyzed using the AutoDecon algorithm calculated from 12-h corticosterone profiles during the diurnal peak phase of corticosterone secretion (1500–0300 h) of control rats infused with saline (CONTROL-SAL) and rats treated with MP for 5 d and infused with pulsatile ACTH (4 ng/h, 5 min pulse, ACTH PULS) for 24 h.
Effect of ACTH infusion on plasma ACTH levels
Pulsatile ACTH
Plasma ACTH levels, measured 55 min after the previous pulse, were low (52 ± 5 pg/ml). Levels increased significantly at 5 min after the onset of the pulse (102 ± 8 pg/ml, P < 0.00005) and rapidly declined having returned to basal values by 15 min (52 ± 7 pg/ml).
Constant ACTH
Before infusion, plasma ACTH levels were significantly lower in MP-suppressed rats compared with nonsuppressed controls rats (MP-ACTH, 38.1 ± 8, vs. CONTROL-SAL, 69.8 ± 8 pg/ml; P = 0.029). Plasma ACTH levels tended to increase after ACTH infusion becoming not different from saline-infused non-MP-suppressed controls 9 h (CONTROL-SAL, 53.8 ± 5 pg/ml; MP-ACTH, 50.7 ± 14 pg/ml) or 22 h (CONTROL-SAL, 50.03 ± 2 pg/ml; MP-ACTH, 56.3 ± 8 pg/ml) after starting the infusion.
Effect of ACTH infusion on adrenal glucocorticoid steroidogenesis and transcription of StAR and P450scc genes
To investigate the effect of different patterns of ACTH infusion on steroidogenesis in the adrenal zona fasciculata, the levels of primary (hnRNA) and mature (mRNA) transcript of the genes encoding StAR and P450scc were investigated. Furthermore, to address whether ultradian secretion of corticosterone in rats infused with pulsatile ACTH is dependent on pulsatile transcription of StAR and P450scc, adrenals from rats infused with pulsatile ACTH were collected either 20 or 40 min after the onset of the ACTH pulse. These time points correspond to the rising (ACTH RISING) or the falling (ACTH FALLING) phase of the corticosterone pulse, respectively.
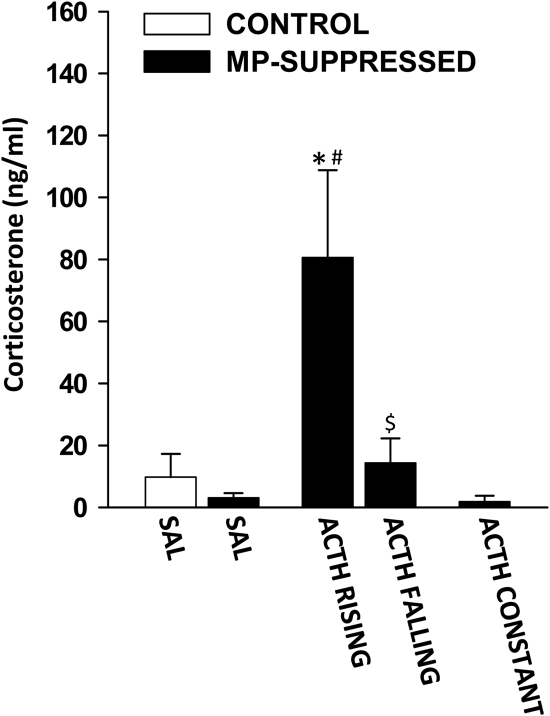
Corticosterone
Plasma corticosterone levels measured in blood samples collected immediately before collection of the adrenals are shown in Fig. 2. ANOVA revealed a significant effect of the treatment [F(4,35) = 9.448; P < 5 × 10−5]. As expected, in accordance with the diurnal trough of hormone release, corticosterone levels were low in CONTROL-SAL rats. Furthermore, corticosterone levels were low in MP-SAL rats. As expected, compared with both CONTROL-SAL and MP-SAL, plasma corticosterone levels were significantly high in ACTH RISING rats (P < 5 × 10−5 and P < 1 × 10−5, respectively) but not in ACTH FALLING rats. There was also a significant difference between ACTH RISING and ACTH FALLING (P < 0.001). In contrast, corticosterone levels were low in ACTH CONST rats.
Fig. 2.
Effect of ACTH on plasma corticosterone levels before adrenal collection. Corticosterone levels were low in both controls (white bars) and MP-suppressed rats chronically infused with saline (CONTROL-SAL, n = 8; MP-SAL, n = 11) and in MP-suppressed rats infused with constant ACTH (ACTH CONST, n = 8). In MP-suppressed rats infused with pulsatile ACTH (4 ng/h, 5 min pulse/h) corticosterone levels were high during the rising phase (ACTH RISING, n = 5) and low during the falling phase (ACTH FALLING, n = 4) of the corticosterone pulse. Blood samples were collected immediately before adrenal gland collection using an automated blood sampling system and corticosterone levels were measured using RIA. *, P < 5 × 10−5 vs. CONTROL-SAL; #, P < 1 × 10−5 vs. MP-SAL; $, P < 0.001 vs. ACTH RISING.
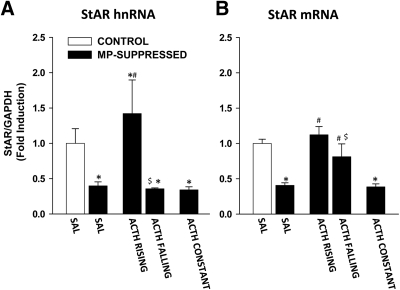
StAR protein
The effect of ACTH infusion on StAR hnRNA and mRNA levels is shown in Fig. 3. There was a significant effect of the treatment on StAR hnRNA levels [F(4,35) = 6.031; P = 0.001; Fig. 3A]. Specifically, compared with CONTROL-SAL, MP-SAL rats showed a decreased level of StAR hnRNA (P = 0.012). There was no difference in StAR hnRNA between CONTROL-SAL and ACTH RISING, whereas there was a significant decrease in ACTH FALLING (P = 0.039). Furthermore, StAR hnRNA was decreased in ACTH CONST (P = 0.011). Compared with MP-SAL, there was a significant increase in StAR hnRNA in ACTH RISING (P = 0.001), whereas no difference between MP-SAL and ACTH FALLING was found. Furthermore, there was a significant difference in StAR hnRNA between ACTH RISING and ACTH FALLING (P = 0.003).
Fig. 3.
Effect of ACTH on StAR hnRNA and mRNA levels in the adrenal cortex. StAR hnRNA (A) and mRNA (B) were measured using reverse transcript and qRT-PCR. Data are normalized to GAPDH mRNA levels (not shown) and are expressed as fold induction compared with nonsuppressed controls infused with saline (CONTROL-SAL, white bars). A, Star hnRNA. MP-suppressed rats infused with saline (MP-SAL, n = 9) showed decreased StAR hnRNA, compared with CONTROL-SAL (n = 8). StAR hnRNA was increased in ACTH RISING (n = 5) but not in ACTH FALLING (n = 4). StAR hnRNA levels were lower in rats infused with constant ACTH (ACTH CONST, n = 8). There was a difference between ACTH RISING and ACTH FALLING. B, StAR mRNA. MP-suppressed rats infused with saline (MP-SAL, n = 9) showed decreased StAR mRNA, compared with CONTROL-SAL (n = 8). StAR mRNA was increased in both ACTH RISING and ACTH FALLING. StAR mRNA levels were lower in rats infused with constant ACTH (ACTH CONST, n = 8). There was a difference between ACTH RISING and ACTH FALLING. *, P < 0.05 vs. CONTROL-SAL; #, P < 0.001 vs. MP-SAL; $, P < 0.05 vs. ACTH RISING. (See Results for detailed P values.)
There was also a significant effect of the treatment on StAR mRNA levels [F(4,35) = 23.172; P < 0.0001; Fig. 3B]. Compared with CONTROL-SAL rats, there was a decrease in StAR mRNA in MP-SAL rats (P < 0.0001), consistent with the hnRNA, whereas there was no difference between CONTROL-SAL and both ACTH RISING and ACTH FALLING. StAR mRNA levels were also decreased in ACTH CONST rats compared with CONTROL-SAL (P < 0.0001). Compared with MP-SAL, StAR mRNA levels were increased in both ACTH RISING (P < 0.001) and ACTH FALLING (P = 0.001) but not in ACTH CONST. Furthermore, there was a significant difference in StAR mRNA between ACTH RISING and ACTH FALLING (P = 0.021).
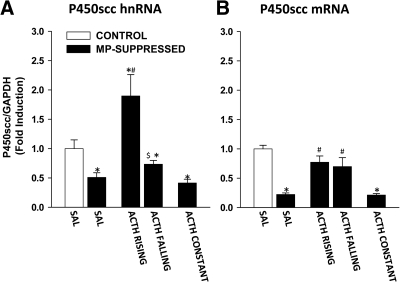
P450scc enzyme
The effect of ACTH infusion on P450scc hnRNA and mRNA levels is shown in Fig. 4. There was a significant effect of the treatment on P450scc hnRNA levels [F(4,35) = 13.5; P < 0.0001; Fig. 4A]. Specifically, compared with CONTROL-SAL, MP-SAL rats showed a decreased level of P450scc hnRNA (P = 0.012). There was also a significant increase of P450scc hnRNA in ACTH RISING (P = 0.00039), whereas there was no effect on ACTH FALLING. Furthermore, P450scc hnRNA levels were decreased in ACTH CONST (P = 0.006). Compared with MP-SAL, there was a significant increase in StAR hnRNA in ACTH RISING, whereas no difference between MP-SAL and ACTH FALLING was found. There was also a significant difference between ACTH RISING and ACTH FALLING (P < 0.0005).
Fig. 4.
Effect of ACTH on P450scc hnRNA and mRNA levels in the adrenal cortex. P450 hnRNA (A) and mRNA (B) were measured using reverse transcript and qRT-PCR. Data are normalized to GAPDH mRNA levels (not shown) and are expressed as fold induction over nonsuppressed controls infused with saline (CONTROL-SAL). A, P450scc hnRNA. MP-suppressed rats infused with saline (MP-SAL, n = 9) showed decreased P450scc hnRNA, compared with CONTROL-SAL (n = 8). P450scc hnRNA were increased in ACTH RISING (n = 5) but not in ACTH FALLING (n = 4). P450scc hnRNA levels were lower in rats infused with constant ACTH (ACTH CONST, n = 8). There was a difference between ACTH RISING and ACTH FALLING. B, P450scc mRNA. MP-suppressed rats infused with saline (MP-SAL, n = 9) showed decreased P450scc mRNA compared with CONTROL-SAL (n = 8). P450scc mRNA was increased in both ACTH RISING and ACTH FALLING. P450scc mRNA levels were lower in rats infused with constant ACTH (ACTH CONST, n = 8). *, P < 0.05 vs. CONTROL-SAL; #, P < 1 × 10−5 vs. MP-SAL; $, P < 0.0005 vs. ACTH RISING. (See Results for detailed P values.)
There was also a significant effect of the treatment on P450scc mRNA levels [F(4,35) = 37.7; P < 0.0001; Fig. 4B]. Compared with CONTROL-SAL, there was a decrease in P450scc mRNA in MP-SAL (P < 0.0001). P450scc mRNA levels were also significantly increased in both ACTH RISING (P = 0.02) and ACTH FALLING (P = 0.005). P450scc mRNA levels were decreased in ACTH CONST rats (P < 0.0001). Compared with MP-SAL, P450scc mRNA levels were increased in both ACTH RISING and ACTH FALLING (both P < 0.0001) but not in ACTH CONST. However, in contrast with P450scc hnRNA, there was no difference in P450scc mRNA between ACTH RISING and ACTH FALLING.
Effect of ACTH infusion on MC2R and MRAP transcription
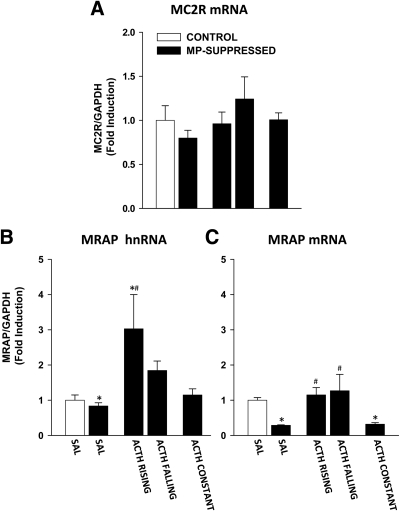
To address whether pulsatile transcription of StAR and P450scc depends on pulsatile expression of genes related to ACTH signaling in the adrenal, we investigated the effect of ACTH infusion on the transcription of genes coding for MC2R and MRAP. The effect of ACTH infusion on MC2R mRNA and on MRAP hnRNA and mRNA levels is shown in Fig. 5.
Fig. 5.
Effect of ACTH on MC2R mRNA and MRAP hnRNA and mRNA levels in the adrenal cortex. MC2R mRNA and MRAP hnRNA and mRNA were measured using reverse transcript and qRT-PCR. Rats were either nonsuppressed controls rats (white bar) or MP-suppressed rats (black bars). Data are normalized to GAPDH mRNA levels (not shown) and are expressed as fold induction over nonsuppressed controls infused with saline (CONTROL-SAL). A, MC2R mRNA. There was no significant effect of the treatment on MC2R mRNA in any of the experimental group. B, MRAP hnRNA. There was no difference between control rats (CONTROL-SAL, n = 8), MP-suppressed rats (MP-SAL, n = 9), and constant ACTH infusion (ACTH CONST, n = 8). There was an increase in MRAP hnRNA in ACTH RISING (n = 5) but not in ACTH FALLING (n = 4). C, MRAP mRNA. MRAP mRNA were decreased in MP-suppressed rats infused with saline (MP-SAL, n = 9) and constant ACTH (ACTH CONST, n = 8) compared with control rats infused with saline (CONTROL-SAL, n = 8). MRAP mRNA was increased in both ACTH RISING (n = 5) and ACTH FALLING (n = 4). *, P < 0.0005 vs. CONTROL-SAL; #, P < 0.0001 vs. MP-SAL. (See Results for detailed P values.)
No effect of the treatment was found on MC2R mRNA levels [F(4,35) = 1.273; P = 1.273; Fig. 5A].
With respect to MRAP, there was a significant effect of the treatment on the hnRNA levels [F(4,35) = 6.306; P < 0.0001; Fig. 5B]. Although no differences between CONTROL-SAL and MP-SAL were observed, MRAP hnRNA levels were increased in ACTH RISING (P = 0.0003) but not in ACTH FALLING. No differences between CONTROL-SAL and ACTH CONST were found. Compared with MP-SAL, MRAP hnRNA levels were elevated in ACTH RISING (P < 0.0001) and, although not statistically significant (P = 0.057), tended to be elevated in ACTH FALLING. Furthermore, there was a trend toward significance between ACTH RISING and ACTH FALLING (P = 0.052). No difference between MP-SAL and ACTH CONST was found.
There was also an effect of the treatment on MRAP mRNA [F(4,35) = 12.106; P < 0.0001; Fig. 5C]. In contrast to MRAP hnRNA, MRAP mRNA was a significantly reduced in MP-SAL (P < 0.0005) compared with CONTROL-SAL. There was no difference between CONTROL-SAL and both ACTH RISING and ACTH FALLING, whereas MRAP mRNA was decreased in ACTH CONST rats (P = 0.00054). Compared with MP-SAL, MRAP mRNA levels were increased in both ACTH RISING and ACTH FALLING (both P < 0.0001), but not in ACTH CONST. In addition, there was no difference in MRAP mRNA between ACTH RISING and ACTH FALLING.
Discussion
This study provides the first evidence that pulsatile release of ACTH is critical for optimal activation of adrenal glucocorticoid secretion. Our data clearly show that in animals with suppressed endogenous ACTH secretion and infused with identical amounts of ACTH, only those animals receiving ACTH in a pulsatile pattern showed normal glucocorticoid secretion, whereas those receiving a constant infusion showed no activation of glucocorticoid release. Furthermore, the resultant pulsatile secretion of corticosterone was associated with pulsatile transcription both of genes involved in steroidogenesis in the adrenal cortex and of the MC2R accessory protein MRAP.
To assess the effects of different patterns of ACTH secretion, it was necessary to inhibit endogenous pituitary ACTH secretion. This was achieved by addition of the synthetic glucocorticoid MP to the drinking water of the rats to activate negative feedback. The dose of MP used was based on pilot studies that confirmed that this was the minimum dose that resulted in a complete loss of both circadian and ultradian corticosterone secretory activity. This was confirmed in the present study (Fig. 1B). Pulsatile infusion of ACTH was able to reinstate pulsatile corticosterone secretion with normal pulse characteristics (Fig. 1C) and was comparable to that found in nonsuppressed control rats infused with saline, as analyzed using both PULSAR and AutoDecon algorithms, which, in turn, are consistent with previous reports (20, 28). On the other hand, infusion of an identical amount of ACTH by constant infusion had no effect on corticosterone secretion (Fig. 1D). Interestingly, there was a lag period of 5 h from the onset of the infusion to the emergence of pulsatile corticosterone secretion, suggesting desensitization of the adrenal to ACTH signaling. Although this delay in adrenal response to ACTH could be due to a reduced sensitivity to ACTH during the nadir of corticosterone circadian rhythm, we did not see any changes in adrenal sensitivity to ACTH during the diurnal nadir on d 2 of the experiment. Furthermore, when rats were infused with pulsatile ACTH starting during the diurnal peak, a variable delay in the adrenal response to ACTH was also observed (data not shown). It was also noteworthy that MP-treated rats infused with pulsatile ACTH did not show the physiological diurnal rhythm of corticosterone secretion normally characterized by changes in pulse amplitude during the peak and trough of normal circadian variation. Although the rats predominately drank the MP during their nocturnal active phase, it is still possible that the diurnal variation in adrenal sensitivity to ACTH (29–31) may have been abolished by the MP.
The complete ineffectiveness of the constant infusion of ACTH was unexpected. This could be due to the short half-life of ACTH (<10 min) preventing plasma levels of ACTH from reaching sufficient concentrations to activate MC2R on the adrenal cortex. However, plasma ACTH 1–24 levels, although lower than peak levels during pulsatile exogenous ACTH infusion, were comparable to those found in nonsuppressed control rats. Furthermore, rats infused with a constant very high dose of ACTH (48 ng/h) did not show any consistent pulsatile secretion of corticosterone (data not shown). Alternatively, the rapid increase in ACTH during each pulse could be more effective for signaling through the MC2R. Regardless of the explanation, it is clear that pulsatile exposure to ACTH is crucial for normal physiological activation of the adrenal cortex.
Given the key role of StAR and P450scc genes in adrenal glucocorticoid steroidogenesis in regulating the intramitochondrial transport of cholesterol (32, 33) and its enzymatic processing to pregnenolone (34), respectively, we investigated the transcriptional regulation of these proteins by ACTH. Consistent with the corticosterone data, we found that reduction of endogenous ACTH induced by MP treatment led to decreased levels of both StAR and P450scc primary transcript (hnRNA) and mature mRNA. This is in accordance with a previous report where dexamethasone was found to reduce StAR mRNA and protein levels (35), and it may contribute to the lag period before the adrenal start responding to the pulsatile ACTH infusion in MP-suppressed rats. However, when ACTH was infused with a pulsatile pattern for a longer time, we found a pulsatile pattern of StAR and P450scc gene transcription and a pulsatile pattern in the accumulation of StAR mRNA, which paralleled the pulsatile secretion of corticosterone. The sustained increase in P450scc mRNA suggests that rapid regulation of the enzyme activity depends on translational and posttranslational (e.g. phosphorylation) processes rather than on new RNA synthesis. Notwithstanding the lack of pulsatility of P450scc mRNA levels, the transient increases in transcription in response to the ACTH pulse indicates rapid regulation of the enzyme synthesis corresponding to the steroidogenic event. Our data therefore suggest that pulsatile secretion of corticosterone depends on pulsatile steroidogenesis and support the hypothesis that adrenal steroidogenesis is a dynamic and transient process. The lack of pulsatile pattern in P450scc mRNA levels is likely the result of rapid mRNA turnover due to activation of translation. Consistent with the lack of effect of constant ACTH on corticosterone secretion, we did not find an effect of this pattern of infusion on either StAR or P450scc hnRNA or mRNA.
Based on our data showing pulsatile synthesis of corticosterone, we investigated whether the pulsatile pattern of StAR and P450scc transcription was associated with changes in the mechanism for ACTH signaling. We studied the effect of pulsatile and constant ACTH on mRNA accumulation of the ACTH-specific receptor MC2R. This receptor has the unique characteristic to be up-regulated by its own ligand (36), and this positive feedback mechanism is thought to contribute to amplify ACTH signals leading to an enhancement of the adrenal response (37). We therefore hypothesized that ACTH reduction induced by MP will lead to decreased synthesis of MC2R, with a reduction of MC2R-mediated ACTH signaling leading to the observed decrease in StAR and P450scc transcription in those rats. However, we did not find any effect of MP on MC2R mRNA or any effect of ACTH infusion, either pulsatile or constant, on MC2R mRNA. In contrast, prolonged exposure (48 h) of primary cultures of human adrenocortical cells to relatively higher ACTH concentrations (10 nm compared with about 0.1 nm plasma concentrations in the present study) have been shown to increase MC2R mRNA levels (38). Thus, it is possible that the lack of change in MC2R mRNA observed here is due to rapid mRNA translation and turnover, which would mask small increases in newly transcribed RNA induced by exogenous ACTH pulses mimicking the physiological ultradian pattern.
In addition to MC2R synthesis, adrenal responsiveness to ACTH depends on the availability of functional receptor in the cell membrane. Indeed, it is known that availability and functionality of the MC2R depend on the presence of MRAP (39, 40). We found that rats treated with MP had lower levels of MRAP mRNA, although there were no changes in hnRNA. This suggests that reductions in MRAP protein may contribute to decreased cell surface MC2R in this group. This, in conjunction with the decreases in StAR protein and P450scc expression observed in MP-suppressed rats, could be responsible for the lag period seen between the initiation of the pulsatile ACTH infusion and the emergence of corticosterone pulsatility. Furthermore, we found that pulsatile ACTH increased the levels of both MRAP hnRNA and mRNA. Although MRAP transcription decreased during the falling phase of corticosterone pulses, no significant difference in MRAP mRNA was observed between the rising and the falling phase of the corticosterone pulse. These data are consistent with a previous microarray study showing up-regulation of MRAP after 48 h incubation of human adrenal cells with ACTH (38). The rapid induction of MRAP transcription by ACTH observed in this study supports the hypothesis that ACTH-mediated stimulation of MRAP expression serves a feed-forward mechanism leading to amplification of adrenal responsiveness. Interestingly, our data show a pulsatile pattern of MRAP transcription, suggesting that pulsatile transcription of StAR and P450scc could be regulated at the level of ACTH signaling through cyclic availability of functional MC2R at the cell surface.
In conclusion, our data clearly show that pulsatile ACTH exposure is necessary for optimal adrenal activity, characterized by ultradian secretion of glucocorticoid. Our use of intronic qRT-PCR to determine changes in primary transcript made it possible for us to estimate pulsatile transcriptional activity of genes involved in steroidogenesis (StAR and P450scc) and in MC2R signaling (MRAP), suggesting a transient and dynamic mechanism regulating adrenal signaling leading to steroidogenesis.
Acknowledgments
This work was supported by the Biotechnology and Biological Sciences Research Council (Grant BB/H015779/1), The Wellcome Trust (Program Grant 074112/Z/04/Z), the Neuroendocrinology Charitable Trust, and the Intramural Research program of the National Institutes of Health (NIH)/National Institute of Child Health and Human Development. The collaborative visit of F.S. to NIH was supported by the British Society for Neuroendocrinology.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- Area under the curve
- hnRNA
- heteronuclear RNA
- HPA
- hypothalamo-pituitary-adrenal
- MC2R
- melanocortin 2 receptor
- MP
- methylprednisolone
- MRAP
- MC2R accessory protein
- P450scc
- cytochrome P450 side-chain cleavage
- qRT-PCR
- real-time quantitative PCR
- StAR
- steroidogenic acute regulatory protein.
References
- 1. Carnes M, Kalin NH, Lent SJ, Barksdale CM, Brownfield MS. 1988. Pulsatile ACTH secretion: variation with time of day and relationship to cortisol. Peptides 9:325–331 [DOI] [PubMed] [Google Scholar]
- 2. Carnes M, Lent S, Feyzi J, Hazel D. 1989. Plasma adrenocorticotropic hormone in the rat demonstrates three different rhythms within 24 h. Neuroendocrinology 50:17–25 [DOI] [PubMed] [Google Scholar]
- 3. Windle RJ, Wood SA, Shanks N, Lightman SL, Ingram CD. 1998. Ultradian rhythm of basal corticosterone release in the female rat: dynamic interaction with the response to acute stress. Endocrinology 139:443–450 [DOI] [PubMed] [Google Scholar]
- 4. Jasper MS, Engeland WC. 1991. Synchronous ultradian rhythms in adrenocortical secretion detected by microdialysis in awake rats. Am J Physiol 261:R1257–R1268 [DOI] [PubMed] [Google Scholar]
- 5. Droste SK, de Groote L, Atkinson HC, Lightman SL, Reul JM, Linthorst AC. 2008. Corticosterone levels in the brain show a distinct ultradian rhythm but a delayed response to forced swim stress. Endocrinology 149:3244–3253 [DOI] [PubMed] [Google Scholar]
- 6. Windle RJ, Wood S, Shanks N, Perks P, Conde GL, da Costa AP, Ingram CD, Lightman SL. 1997. Endocrine and behavioural responses to noise stress: comparison of virgin and lactating female rats during non-disrupted maternal activity. J Neuroendocrinol 9:407–414 [DOI] [PubMed] [Google Scholar]
- 7. Lightman SL, Windle RJ, Julian MD, Harbuz MS, Shanks N, Wood SA, Kershaw YM, Ingram CD. 2000. Significance of pulsatility in the HPA axis. Novartis Found Symp 227:244–257; discussion 257–260 [DOI] [PubMed] [Google Scholar]
- 8. Shanks N, Windle RJ, Perks PA, Harbuz MS, Jessop DS, Ingram CD, Lightman SL. 2000. Early-life exposure to endotoxin alters hypothalamic-pituitary-adrenal function and predisposition to inflammation. Proc Natl Acad Sci USA 97:5645–5650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Windle RJ, Wood SA, Kershaw YM, Lightman SL, Ingram CD, Harbuz MS. 2001. Increased corticosterone pulse frequency during adjuvant-induced arthritis and its relationship to alterations in stress responsiveness. J Neuroendocrinol 13:905–911 [DOI] [PubMed] [Google Scholar]
- 10. Sarabdjitsingh RA, Conway-Campbell BL, Leggett JD, Waite EJ, Meijer OC, de Kloet ER, Lightman SL. 2010. Stress responsiveness varies over the ultradian glucocorticoid cycle in a brain-region-specific manner. Endocrinology 151:5369–5379 [DOI] [PubMed] [Google Scholar]
- 11. Conway-Campbell BL, McKenna MA, Wiles CC, Atkinson HC, de Kloet ER, Lightman SL. 2007. Proteasome-dependent down-regulation of activated nuclear hippocampal glucocorticoid receptors determines dynamic responses to corticosterone. Endocrinology 148:5470–5477 [DOI] [PubMed] [Google Scholar]
- 12. Conway-Campbell BL, Sarabdjitsingh RA, McKenna MA, Pooley JR, Kershaw YM, Meijer OC, De Kloet ER, Lightman SL. 2010. Glucocorticoid ultradian rhythmicity directs cyclical gene pulsing of the clock gene period 1 in rat hippocampus. J Neuroendocrinol 22:1093–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stavreva DA, Wiench M, John S, Conway-Campbell BL, McKenna MA, Pooley JR, Johnson TA, Voss TC, Lightman SL, Hager GL. 2009. Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat Cell Biol 11:1093–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lightman SL, Conway-Campbell BL. 2010. The crucial role of pulsatile activity of the HPA axis for continuous dynamic equilibration. Nat Rev Neurosci 11:710–718 [DOI] [PubMed] [Google Scholar]
- 15. Engeland WC, Arnhold MM. 2005. Neural circuitry in the regulation of adrenal corticosterone rhythmicity. Endocrine 28:325–332 [DOI] [PubMed] [Google Scholar]
- 16. Sage D, Maurel D, Bosler O. 2001. Involvement of the suprachiasmatic nucleus in diurnal ACTH and corticosterone responsiveness to stress. Am J Physiol Endocrinol Metab 280:E260–E269 [DOI] [PubMed] [Google Scholar]
- 17. Waite EJ, McKenna MA, Kershaw YM, Lightman SL. Ultradian corticosterone secretion is maintained in the absence of circadian cues. Program of the 92nd Annual Meeting of The Endocrine Society, San Diego, CA, 2010, p 44 (Abstract P1-630) [DOI] [PubMed] [Google Scholar]
- 18. Walker JJ, Terry JR, Lightman SL. 2010. Origin of ultradian pulsatility in the hypothalamic-pituitary-adrenal axis. Proc Biol Sci 277:1627–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Metherell LA, Chapple JP, Cooray S, David A, Becker C, Rüschendorf F, Naville D, Begeot M, Khoo B, Nürnberg P, Huebner A, Cheetham ME, Clark AJ. 2005. Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nat Genet 37:166–170 [DOI] [PubMed] [Google Scholar]
- 20. Spiga F, Harrison LR, Wood SA, Atkinson HC, MacSweeney CP, Thomson F, Craighead M, Grassie M, Lightman SL. 2007. Effect of the glucocorticoid receptor antagonist Org 34850 on basal and stress-induced corticosterone secretion. J Neuroendocrinol 19:891–900 [DOI] [PubMed] [Google Scholar]
- 21. Waite E, Kershaw Y, Spiga F, Lightman SL. 2009. A glucocorticoid sensitive biphasic rhythm of testosterone secretion. J Neuroendocrinol 21:737–741 [DOI] [PubMed] [Google Scholar]
- 22. Chen TC, Mackic JB, McComb JG, Giannotta SL, Weiss MH, Zlokovic BV. 1996. Cellular uptake and transport of methylprednisolone at the blood-brain barrier. Neurosurgery 38:348–354 [DOI] [PubMed] [Google Scholar]
- 23. Clark RG, Chambers G, Lewin J, Robinson IC. 1986. Automated repetitive microsampling of blood: growth hormone profiles in conscious male rats. J Endocrinol 111:27–35 [DOI] [PubMed] [Google Scholar]
- 24. Liu Y, Kamitakahara A, Kim AJ, Aguilera G. 2008. Cyclic adenosine 3′,5′-monophosphate responsive element binding protein phosphorylation is required but not sufficient for activation of corticotropin-releasing hormone transcription. Endocrinology 149:3512–3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spiga F, Liu Y, Aguilera G, Lightman SL. 2011. Temporal effect of ACTH on adrenal glucocorticoid steroidogenesis: Involvement of the Transducer of Regulated CREB activity. J Neuroendocrinol 23:136–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Merriam GR, Wachter KW. 1982. Algorithms for the study of episodic hormone secretion. Am J Physiol 243:E310–E318 [DOI] [PubMed] [Google Scholar]
- 27. Veldhuis JD, Johnson ML. 1992. Deconvolution analysis of hormone data. Methods Enzymol 210:539–575 [DOI] [PubMed] [Google Scholar]
- 28. Spiga F, Harrison LR, Wood SA, MacSweeney CP, Thomson FJ, Craighead M, Grassie M, Lightman SL. 2008. Effect of the glucocorticoid receptor antagonist Org 34850 on fast and delayed feedback of corticosterone release. J Endocrinol 196:323–330 [DOI] [PubMed] [Google Scholar]
- 29. Dallman MF, Engeland WC, Rose JC, Wilkinson CW, Shinsako J, Siedenburg F. 1978. Nycthemeral rhythm in adrenal responsiveness to ACTH. Am J Physiol 235:R210–R218 [DOI] [PubMed] [Google Scholar]
- 30. Kaneko M, Kaneko K, Shinsako J, Dallman MF. 1981. Adrenal sensitivity to adrenocorticotropin varies diurnally. Endocrinology 109:70–75 [DOI] [PubMed] [Google Scholar]
- 31. Ulrich-Lai YM, Arnhold MM, Engeland WC. 2006. Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. Am J Physiol Regul Integr Comp Physiol 290:R1128–R1135 [DOI] [PubMed] [Google Scholar]
- 32. Lin D, Sugawara T, Strauss JF, 3rd, Clark BJ, Stocco DM, Saenger P, Rogol A, Miller WL. 1995. Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science 267:1828–1831 [DOI] [PubMed] [Google Scholar]
- 33. Stocco DM, Clark BJ. 1996. Role of the steroidogenic acute regulatory protein (StAR) in steroidogenesis. Biochem Pharmacol 51:197–205 [DOI] [PubMed] [Google Scholar]
- 34. Churchill PF, Kimura T. 1979. Topological studies of cytochromes P-450scc and P-45011β in bovine adrenocortical inner mitochondrial membranes. Effects of controlled tryptic digestion. J Biol Chem 254:10443–10448 [PubMed] [Google Scholar]
- 35. Lehoux JG, Fleury A, Ducharme L. 1998. The acute and chronic effects of adrenocorticotropin on the levels of messenger ribonucleic acid and protein of steroidogenic enzymes in rat adrenal in vivo. Endocrinology 139:3913–3922 [DOI] [PubMed] [Google Scholar]
- 36. Mountjoy KG, Bird IM, Rainey WE, Cone RD. 1994. ACTH induces up-regulation of ACTH receptor mRNA in mouse and human adrenocortical cell lines. Mol Cell Endocrinol 99:R17–R20 [DOI] [PubMed] [Google Scholar]
- 37. Penhoat A, Jaillard C, Saez JM. 1994. Regulation of bovine adrenal cell corticotropin receptor mRNA levels by corticotropin (ACTH) and angiotensin-II (A-II). Mol Cell Endocrinol 103:R7–R10 [DOI] [PubMed] [Google Scholar]
- 38. Xing Y, Parker CR, Edwards M, Rainey WE. 2010. ACTH is a potent regulator of gene expression in human adrenal cells. J Mol Endocrinol 45:59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cooray SN, Almiro Do Vale I, Leung KY, Webb TR, Chapple JP, Egertová M, Cheetham ME, Elphick MR, Clark AJ. 2008. The melanocortin 2 receptor accessory protein exists as a homodimer and is essential for the function of the melanocortin 2 receptor in the mouse y1 cell line. Endocrinology 149:1935–1941 [DOI] [PubMed] [Google Scholar]
- 40. Webb TR, Chan L, Cooray SN, Cheetham ME, Chapple JP, Clark AJ. 2009. Distinct melanocortin 2 receptor accessory protein domains are required for melanocortin 2 receptor interaction and promotion of receptor trafficking. Endocrinology 150:720–726 [DOI] [PMC free article] [PubMed] [Google Scholar]