We detail the in-depth characterization of insulin-regulated raptor phosphorylation and report the novel finding that raptor acts as an insulin-stimulated substrate for MAPK.
Abstract
The adaptor protein raptor is the functional identifier for mammalian target of rapamycin (mTOR) complex 1 (mTORC1), acting to target mTOR to specific substrates for phosphorylation and regulation. Using HPLC-electrospray ionization tandem mass spectrometry, we confirmed the phosphorylation of raptor at Ser696, Thr706, Ser721, Ser722, Ser855, Ser859, Ser863, Thr865, Ser877, Ser881, Ser883, and Ser884 and identified Tyr692, Ser699, Thr700, Ser704, Ser854, Ser857, Ser882, Ser886, Ser887, and Thr889 as new, previously unidentified raptor phosphorylation sites. Treatment of cells with insulin increased the phosphorylation of raptor at Ser696, Ser855, Ser863, and Thr865 and suppressed the phosphorylation of Ser722. Ser696 phosphorylation was insensitive to mTOR inhibition with rapamycin, whereas treatment of cells with the MAPK inhibitor PD98059 inhibited the insulin-stimulated phosphorylation of raptor at Ser696. In vitro incubation of raptor with p42 MAPK significantly increased raptor phosphorylation (P < 0.01), whereas phosphorylation of a Ser696Ala mutant was decreased (P < 0.05), suggesting MAPK is capable of directly phosphorylating raptor at Ser696. Mutation of Ser696 to alanine interfered with insulin-stimulated phosphorylation of the mTOR downstream substrate p70S6 kinase. Incubation of cells with the MAPK inhibitor PD98059 and the phosphatidylinositol 3-kinase inhibitor wortmannin decreased the insulin stimulated phosphorylation of raptor, suggesting that the MAPK and phosphatidylinositol 3-kinase pathways may merge at mTORC1.
Mammalian target of rapamycin (mTOR) is central to regulating metabolism and growth in response to insulin and nutrient availability, in particular the branched-chain amino acids. mTOR localizes to two functionally distinct complexes, known as mTOR complex 1 (mTORC1) and mTORC2 (1). The identity of each complex is defined by the presence of the protein raptor for mTORC1 (2, 3) or the protein rictor for mTORC2 (4, 5). mTORC1 activity is sensitive to rapamycin inhibition and is responsible for the insulin-stimulated phosphorylation of p70S6 kinase (p70S6K) and 4E-binding protein 1 (4E-BP1) (2–5), whereas mTORC2 phosphorylates the serine/threonine protein kinase (AKT) at Ser473 (6) in response to insulin.
Upon energy deprivation, AMP-activated protein kinase (AMPK) catalyzes the phosphorylation and activation of tuberous sclerosis complex 1 (TSC1)/TSC2, functioning to inactivate the Rheb GTPase and ultimately mTORC1 (7–9). In addition, two AMPK-mediated phosphorylation sites on raptor have been identified: Ser722 and Ser792. Raptor phosphorylation at Ser722 and Ser792 was found to create targeting motifs for 14-3-3 binding, and although 14-3-3 binding to raptor did not affect mTOR/raptor association, serine to alanine mutation of these sites did increase mTOR-catalyzed phosphorylation of p70S6K (10).
To study mechanisms regulating raptor function, Carrière et al. (11) identified Ser719 and Ser722 (and possibly Ser721) as phorbol myristate acetate (PMA)-stimulated phosphorylation sites in raptor, whereas phosphorylation of raptor at Ser877 was unaltered upon PMA stimulation. Using pharmacological inhibitors and RNA interference, p90 ribosomal S6 kinases (RSK) 1 and 2 were found to be required for raptor phosphorylation at Ser719/721/722 in cells, and RSK was shown to directly phosphorylate raptor in vitro. Overexpression of RSK increased the phosphorylation of S6K and 4E-BP1, suggesting that RSK positively regulates mTORC1 (11).
Recently, Wang et al. (12) identified Ser859/Ser863 of raptor as phosphorylation sites catalyzed by mTOR. Raptor phosphorylation at Ser859 was found to be dependent upon Ser863 phosphorylation, whereas Ser863 phosphorylation was shown to be insulin stimulated and appears to regulate raptor function through the small GTP-binding protein Rheb (12). Foster et al. (13) confirmed the dependence of Ser859 phosphorylation on the phosphorylation of Ser863 and, in addition, found a similar dependence for the newly discovered Ser855 phosphorylation site. Raptor phosphorylation at Ser863 was found to be stimulated by amino acids, epidermal growth factor/MAPK signaling, and cellular energy. Rheb overexpression increased phosphorylation of raptor at Ser696, Thr706, Ser855, Ser859, Ser863, and Ser877, whereas mTORC1 containing phosphorylation site-defective raptor exhibited reduced in vitro kinase activity toward the substrate 4E-BP1 (13).
The large majority of raptor phosphorylation sites remain uncharacterized with respect to their potential role in insulin action. Because insulin stimulates the association of Rheb with mTOR, and Rheb overexpression increases the phosphorylation of raptor at Ser696, Thr706, Ser855, Ser859, Ser863, and Ser877 (13), we hypothesized that insulin stimulates the phosphorylation of raptor at multiple sites, through activation of kinases within the phosphatidylinositol 3-kinase (PI 3-K) and MAPK pathways. In this report, we detail the in-depth characterization of insulin-regulated raptor phosphorylation and report the finding that raptor acts as an insulin-stimulated substrate for MAPK and that MAPK phosphorylates raptor at Ser696.
Materials and Methods
Materials
The following supplies were used: sequencing-grade trypsin from Sigma Chemical Co. (St. Louis, MO), C18 ZipTip from Millipore (Billerica, MA), and anti-HA.11 antibody from Covance (Berkeley, CA). Chinese hamster ovary cells overexpressing the insulin receptor (CHO/IR) were a gift from Dr. Feng Liu (University of Texas Health Science Center at San Antonio, TX). The cDNA encoding full-length wild-type, myc-tagged human raptor cloned into the pRK-5 vector was purchased from Addgene (Cambridge, MA; plasmid 1859, pRK5-myc-raptor-WTge, MA), which was supplied by Dr. D. M. Sabatini (Whitehead Institute for Biomedical Research, Cambridge, MA). The cDNA encoding full-length wild-type rat, hemagglutinin (HA)-tagged p70S6K cloned into the pRK-7 vector was purchased from Addgene (plasmid 8984, pRK7-HA-S6K1-WT), which was supplied to Addgene by Dr. John Blenis (Harvard Medical School, Boston, MA). Anti-pSer473 (catalog item 9271) for AKT, anti-AKT (catalog item 9272), anti-p-Thr378 (catalog item 9234) for p70S6K, anti-p70S6K (catalog item 9202), and anti-p44/42 MAPK (catalog item 9102) antibodies were purchased from Cell Signaling Technologies (Beverly, MA). Anti-myc (catalog item sc-40) and anti-p-ERK (catalog item sc-7383) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell culture, transfection, immunoprecipitation, and Western blot analysis
CHO/IR cells were grown in HAM'S F-12 medium (Invitrogen, Carlsbad, CA), supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. Transfections were performed with Lipofectamine reagent (Invitrogen), according to the manufacturer's protocol. For cell treatment experiments, cells were transfected, and 24 h after transfection, cells were serum starved for 5 h, treated with or without 100 nm insulin for the time designated, and lysed on ice in 300 μl lysis buffer [50 mm HEPES (pH 7.6), 150 mm NaCl, 1% Triton X-100, 10 mm NaF, 20 mm sodium pyrophosphate, 20 mm β-glycerol phosphate, 1 mm sodium orthovanadate, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 1 mm phenylmethylsulfonyl fluoride]. Cell lysates were centrifuged (10,000 × g at 4 C for 10 min), and the clarified supernatants were used for immunoprecipitation. For immunoprecipitation, cell lysates were incubated with specific antibodies conjugated to protein G-agarose beads overnight at 4 C with gentle rotation. Immunoprecipitates were washed extensively with ice-cold PBS, and the proteins bound to beads were eluted by heating at 95 C for 4 min in sodium dodecyl sulfate (SDS) sample loading buffer. Eluted proteins were separated by 10% SDS-PAGE and were either stained with Coomassie blue or transferred to a nitrocellulose membrane for subsequent Western blotting.
In-gel digestion
The gel portions containing raptor were excised, placed in a 0.6-ml polypropylene tube, destained twice with 300 μl 50% acetonitrile (ACN) in 40 mm NH4HCO3 and dehydrated with 100% ACN for 15 min. After removal of ACN by aspiration, the gel pieces were dried in a vacuum centrifuge at 60 C for 30 min. Trypsin (250 ng; Sigma) in 20 μl 40 mm NH4HCO3 was added, and the samples were maintained at 4 C for 15 min before the addition of 50 μl 40 mm NH4HCO3 containing three 10 fmol/μl peptides to serve as internal standards (bradykinin fragment 2–9, B1901, β-sheet breaker peptide, S7563, and anaphylatoxin C3a fragment, A8651; Sigma). The digestion was allowed to proceed at 37 C overnight and was terminated by addition of 10 μl 5% formic acid (FA). After additional incubation at 37 C for 30 min and centrifugation for 1 min, each supernatant was transferred to a clean polypropylene tube. The extraction procedure was repeated using 40 μl 0.5% FA, and the two extracts were combined. The resulting peptide mixtures were purified by solid-phase extraction (C18 ZipTip; Millipore) after sample loading in 0.05% heptafluorobutyric acid/5% FA (vol/vol) and elution with 4 μl 50% ACN/1% FA (vol/vol) and 4 μl 80% ACN/1% FA (vol/vol), respectively. The eluates were combined and dried by vacuum centrifugation and 10 μl of 0.025% heptafluorobutyric acid/0.1% FA/2% ACN (vol/vol) were added.
Mass spectrometry
HPLC-electrospray ionization (ESI)-tandem mass spectrometry (MS/MS) was performed on a Thermo Finnigan (San Jose, CA) linear ion trap-Fourier transform ion cyclotron resonance-FTICR fitted with a PicoView nanospray source (New Objective, Woburn, MA). Online HPLC was performed using a Michrom BioResources Paradigm MS4 micro two-dimensional HPLC (Alburn, CA) with a PicoFrit column (New Objective; 75 μm inner diameter, packed with ProteoPep II C18 material, 300 Å), with a mobile phase consisting of a linear gradient of 2–27% ACN in 0.1% FA in 65 min, a hold of 5 min at 27% ACN, followed by a step to 50% ACN, a hold of 5 min, and then a step to 80% and a flow rate of 400 nl/min.
A top-10 data-dependent MS/MS analysis was performed [acquisition of a full scan spectrum followed by collision-induced dissociation (CID) mass spectra of the 10 most abundant ions in the survey scan] to identify raptor peptides and to obtain their HPLC retention times, as described before (14). For quantification, the following multisegment strategy was employed: one survey scan followed by one parent-list CID scan and six targeted CID scans. Included in the parent list were the 2+ or 3+ charge states of seven representative raptor peptides selected from the prominent ions reproducibly observed in the top-10 data-dependent MS/MS analysis. These peptides were used as internal standards for raptor protein content (see below). They were selected according to the following criteria: 1) detected by HPLC-ESI-MS with high intensity among raptor peptides, 2) no missed cleavage observed, and 3) no methionine in the sequence to avoid variability due to methionine oxidization and no N-terminal Gln residues. The 2+ or 3+ ions of the phosphopeptides of interest were placed on the target list. To assess the relative quantities of a large number of phosphopeptides in each experiment and yet still maintain acceptable mass analysis cycle times, the targeted mass to charge ratio values were grouped into segments based on their expected HPLC retention times with a minimum 10-min retention time window for each peptide. Tandem mass spectra were extracted from Xcalibur RAW files, and charge states were assigned using the Extract_MSN script that is a component of Xcalibur version 2.0 SR2 (Thermo Fisher, San Jose, CA). The fragment mass spectra were then searched against the human SwissProt version 52.2 database (16,135 entries) using Mascot (Matrix Science, London, UK; version 2.1). The search variables that were used were 10 ppm mass tolerance for precursor ion masses and 0.5 Da for product ion masses, digestion with trypsin, a maximum of two missed tryptic cleavages, and variable modifications of oxidation of methionine and phosphorylation of serine, threonine, and tyrosine. Cross-correlation of Mascot search results with X! Tandem was accomplished with Scaffold (version Scaffold-01-06-19; Proteome Software, Portland, OR). Probability assessment of peptide assignments and protein identifications were made through the use of Scaffold. Only peptides with at least 95% probability were considered. Assignments of the phosphopeptides were confirmed by manual comparison of the MS/MS spectra with the predicted fragmentation generated in silico by the MS-Product component of Protein Prospector (http://prospector.ucsf.edu). Peak areas for each peptide were obtained by integration of the appropriate reconstructed ion chromatograms with 10 ppm error tolerance for precursor ion masses acquired using the Fourier transform ion cyclotron resonance and 0.5 Da for the fragment ions acquired using the linear ion trap mass analyzer. The reconstructed ion chromatograms allow one to express the intensity (abundance) of ions relative to HPLC retention times in chromatogram form, analogous to analysis of UV absorbance during an HPLC run. The peak areas for the phosphopeptides were then normalized against the average peak area (using precursor ions) for the seven representative raptor peptides (endogenous standards). Relative quantification of each phosphopeptide was obtained by comparing normalized peak-area ratios for control and insulin/inhibitor-treated samples.
ERK2 in vitro kinase assay
Myc-tagged raptor and Ser696Ala raptor were transiently transfected into CHO/IR cells. The cells were lysed in a low-stringency lysis buffer containing CHAPS detergent as a substitute for Triton X-100 [40 mm HEPES (pH 7.6), 120 mm NaCl, 0.3% CHAPS, 2 mm sodium orthovanadate, 10 mm NaF, 10 mm β-glycerol phosphate, 1 mm EDTA, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 1 mm phenylmethylsulfonyl fluoride] to preserve protein-protein interactions potentially important for raptor phosphorylation, and either myc-tagged wild-type or Ser696Ala raptor was successfully immunopurified. The immunoprecipitates were washed three times in lysis buffer and once in ERK kinase buffer, followed by incubation at 30 C for 30 min in 30 μl ERK kinase buffer [20 mm HEPES (pH 7.5), 100 mm NaCl, 10 mm MgCl2, and 15 μm cold ATP] that included recombinant active ERK2 (active ERK2, catalog item 14-173; Millipore/Upstate, Billerica, MA) and 5 μCi [γ-32P]ATP. The reactions were stopped by addition of SDS loading buffer and boiled at 95 C for 4 min. The proteins were resolved on a 10% SDS-polyacrylamide gel and transferred electrophoretically from the gel to a nitrocellulose membrane. The phosphorylation of raptor was examined by autoradiography, and the membrane was subsequently probed with anti-myc antibody to visualize the presence of raptor.
Results
Identification of raptor phosphorylation sites
Raptor phosphorylation at Ser722 and Ser792, catalyzed by AMPK, was shown to play an important role in negatively regulating mTORC1 activity (10). We therefore hypothesized that additional phosphorylation sites on raptor may exist. To test this hypothesis, myc-tagged raptor was transiently transfected into CHO/IR cells, followed by immunoprecipitation and subsequent analysis by HPLC-ESI-MS/MS, as previously described (14–20). In all, 22 raptor phosphorylation sites were detected throughout our studies, 10 of which were previously unidentified. Phosphorylation of raptor at Ser863, Thr865, and Ser877, originally observed by Gwinn et al. (10) and published as supplemental data, was confirmed in CHO/IR cells. We also confirmed the phosphorylation of raptor at Ser696, Ser722, Ser855, Ser859, Ser881, Ser882, and Ser844. MS analysis was unable to detect phosphorylation of raptor at Ser190, Ser738, Ser771, and Ser795 (10), most likely due to a lack of sequence coverage of the tryptic digest (which was typically ∼40%). Phosphorylation of Ser859 was detectable in a doubly phosphorylated manner, in tandem with phosphorylation at Ser863, whereas phosphorylation of Ser863 was detectable as a monophosphorylated peptide. These data are in agreement with the previous reports that raptor phosphorylation at Ser859 appears to be dependent upon Ser863 phosphorylation (12, 13). All phosphorylation sites of raptor were detected on four phosphopeptides (Table 1). For the amino acids (aa)691-709 phosphopeptide, phosphorylation of raptor at the additional sites Tyr692, Thr699, Thr700, Thr704, and Thr706 was also detected, although these ambiguous sites were not reproducibly identified by Scaffold analysis, whereas phosphorylation of Ser696 was consistently the predominant site detected. Similar findings were recorded for the phosphorylation of aa850–867 at Ser854, because this ambiguous site was not reproducibly identified by Scaffold analysis, whereas pSer863 was consistently present and abundant, with phosphorylation at Thr857 and Thr865 being less abundant, although consistently observed. Ser881, Ser882, Thr883, Ser884, Ser886, Ser887, and Thr889 were all detected as phosphorylation sites for the aa868–894 phosphopeptide, although phosphorylation at Ser877 was predominant. The aa868–894 peptide contains a cluster of nine Ser/Thr residues, rendering phosphorylation analysis by MS difficult for this region. Of the four phosphopeptides analyzed, aa719–727 was the phosphopeptide detected almost exclusively with one phosphorylation site (Ser722), although phosphorylation of Ser721 was detected, albeit to a much lesser degree, whereas phosphorylation of this peptide at the previously observed Ser719 site (11) was undetected.
Table 1.
Raptor phosphopeptides
| Start | Stop | Peptide sequence | Phosphorylation site |
|---|---|---|---|
| 691 | 709 | NYALPSPATTEGGSLTPVR | Tyr692, Ser696, Thr699, Thr700, Ser704, Thr706 |
| 719 | 727 | SVSSYGNIR | Ser721, Ser722 |
| 850 | 867 | VLDTSSLTQSAPASPTNK | Ser854, Ser855, Thr857, Ser859/863, Ser863, Thr865 |
| 868 | 894 | GVHIHQAGGSPPASSTSSSSLTNDVAK | Ser877, Ser881, Ser882, Thr883, Ser884, Ser886, Ser887, Thr889 |
The effect of insulin and rapamycin on raptor phosphorylation
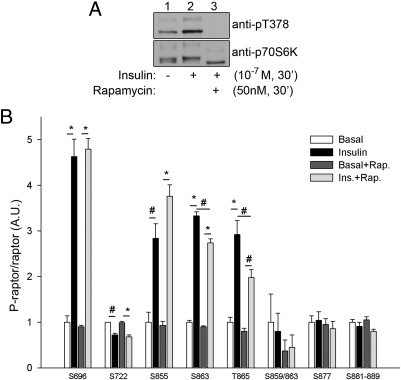
Sequence analysis of the raptor phosphorylation sites identified reveals that Ser696, Thr706, Ser863, and Ser877 conform to the consensus phosphorylation sequence recognized by mTOR (S/T-Pro) (21). Because mTORC1 activity is sensitive to rapamycin inhibition and insulin stimulation (22), we tested whether rapamycin inhibition or insulin treatment affected raptor phosphorylation in cells. Cells overexpressing myc-tagged raptor were serum starved and either left untreated or treated with insulin alone, rapamycin alone, or rapamycin pretreatment followed by insulin stimulation. Whole-cell lysates were probed for the phosphorylation of the mTOR substrate p70S6K to verify insulin action and rapamycin inhibition of mTOR activity (Fig. 1A). Raptor was immunopurified and resolved by 7.5% SDS-PAGE and stained with Coomassie blue for protein visualization, and the gel area corresponding to the position of raptor was excised and trypsinized. The resulting tryptic peptides were then prepared for subsequent phospho-quantification by HPLC-ESI-MS/MS, in a manner similar to our published studies that analyzed insulin-stimulated phosphorylation of insulin receptor substrate-1 both in vitro and in vivo (16, 18–20). Seven nonphosphorylated raptor peptides that were detected in all MS analyses were chosen to serve as endogenous internal standards as a measure of total raptor present per sample (Table 2). Analysis of raptor expressed in CHO/IR cells revealed that only one phospho-raptor peptide is predominantly monophosphorylated (aa719–727, pSer722), whereas the remainder of the raptor tryptic phosphopeptides contain multiple phosphorylation sites (Table 1). These different monophosphorylated versions of the same peptide possess the same mass/charge value, rendering them indistinguishable from each other during peak area analysis of precursor ions because they coelute. As a result, we chose to target the mass/charge value of the parent ions of the particular phosphopeptides for MS2/MS3 fragmentation and use the resulting peak areas of the fragment b and y ions specific to the sites of interest for quantification of phosphorylation. A list of the fragment b and y ions that were found to be suitable for analysis for each phospho-site within each phosphopeptide of interest is shown in Table 3. It is noted that for some phosphopeptides, fragment ions from MS2 spectra gave high-quality quantification results, whereas for other phosphopeptides, fragment ions from MS3 were superior, whereas fragmentation for analysis of pSer722 was not required. Quantification of pSer696, pSer722, pSer855, and pSer877 phosphorylation was straightforward, facilitated by either the placement of the phosphorylation site relative to the other phosphorylation sites on the peptide (Ser696, Ser855, and Ser877) or the predominance of the phosphorylation site (Ser722). Due to the tight clustering of Ser881, Ser882, Thr883, Ser884, Ser886, Ser887, and Thr889 phosphorylation, we grouped quantification of these phosphorylation sites together as 1-fold change over the basal value. Unfortunately, we were unable to quantify Thr699, Thr700, Ser704, and Ser706 phosphorylation with any of the strategies used, perhaps due to suboptimal fragmentation capacity. For the quantification of Ser863 and Thr865, we chose to target the MS2-generated y7 (+1) phosphorylated fragment ion (m794.34) for further fragmentation by MS3. Significant insulin stimulation was observed for the phosphorylation of raptor at Ser696, Ser855, Ser863, and Thr865 (Fig. 1B). Surprisingly, Ser722 phosphorylation was significantly suppressed by insulin, which has previously been unreported, revealing a novel mechanism of insulin-regulated raptor phosphorylation. Phosphorylation of the Ser859/863 doubly phosphorylated peptide, Ser877, and the aa881–889 cassette was unaffected by insulin treatment (Fig. 1B). Although phosphorylation of raptor at Ser696, Ser722, Ser855, Ser877, and the Ser881–889 cluster was unaffected by rapamycin treatment, detection of Ser863 and Thr865 phosphorylation, and the doubly phosphorylated Ser859/Ser863 peptide, was reduced after rapamycin inhibition, implicating a role for mTORC1, and possibly mTOR directly in the phosphorylation of raptor in cells at Ser859, Ser863, and Thr865.
Fig. 1.
The effect of insulin (Ins.) and rapamycin (Rap.) on raptor phosphorylation. A, Cells overexpressing myc-tagged raptor were serum starved and left untreated, treated with insulin alone, or pretreated with rapamycin followed by stimulation with insulin. Whole-cell lysates were probed for either the phosphorylation of p70S6K1 (upper panel) or p70S6K1 protein (lower panel). B, Immunoprecipitated raptor was resolved by 7.5% SDS-PAGE and stained with Coomassie blue for protein visualization, and the gel area corresponding to the position of raptor was excised and processed for MS analysis as described in Materials and Methods. The peak area for each phosphopeptide was normalized against the average peak area of the seven representative raptor peptides per sample (endogenous standards). Each raptor phosphorylation site was normalized by the average value of the respective basal sample and then expressed as a fold change over basal ± sem (n = 3). #, P < 0.01; *, P < 0.0001 (ANOVA). A.U., Arbitrary units.
Table 2.
Raptor internal standard peptides
| Start | Stop | Peptide sequence | Molecular mass (Da) | Quantified m/z (+2) |
|---|---|---|---|---|
| 98 | 108 | ALETIGANLQK | 1157.65 | 579.33 |
| 339 | 348 | QDLLVASLFR | 1161.66 | 581.34 |
| 604 | 616 | LYSLLSDPIPEVR | 1501.83 | 751.42 |
| 833 | 840 | VLNSIAYK | 907.52 | 454.27 |
| 1013 | 1021 | LDDQIFLNR | 1133.59 | 567.3 |
| 1022 | 1030 | NPGVPSVVK | 896.52 | 448.76 |
| 1179 | 1191 | SLIVAGLGDGSIR | 1257.72 | 629.36 |
m/z, Mass to charge ratio.
Table 3.
Raptor fragment ions for quantification
| Start | Stop | Peptide sequence | Phosphorylation site | Parent ion, MS2, or MS3? | Product ion (charge state) | Mass (Da) |
|---|---|---|---|---|---|---|
| 691 | 709 | NYALPpSPATTEGGSLTPVR | Ser696 | MS2 | y13 (+1) | 1285.67 |
| 719 | 727 | SVSpSYGNIR | Ser722 | FT | Parent ion (+2) | 531.73 |
| 850 | 867 | VLDTSpSLTQSAPASPTNK | Ser855 | MS3 | b10 (+1) | 1014.51 |
| b11 (+1) | 1085.55 | |||||
| VLDTSSLTQSAPApSPTNK | Ser863 | MS3 | y4 (+1) | 459.26 | ||
| VLDTSSLTQSAPASPpTNK | Thr865 | MS3 | y4 −H3PO4 | 441.25 | ||
| 850 | 867 | VLDTSSLTQpSAPApSPTNK | Ser859/863 | MS3 | Parent ion (+2) | 988.93–>935.95 |
| 868 | 894 | GVHIHQAGGpSPPASSTSSSSLTNDVAK | Ser877 | MS2 | y14 (+1) | 1383.66 |
| GVHIHQAGGSPPA//pSSTSSSSLTNDVAK | Ser881/882/Thr883/884/886/887/Thr889 | MS2 | y15 (+1) | 1534.66 | ||
| y14 −H3PO4 | 1365.65 | |||||
| b13 (+1) | 1209.61 |
PI 3-K and MAPK pathways are involved in insulin-stimulated raptor phosphorylation
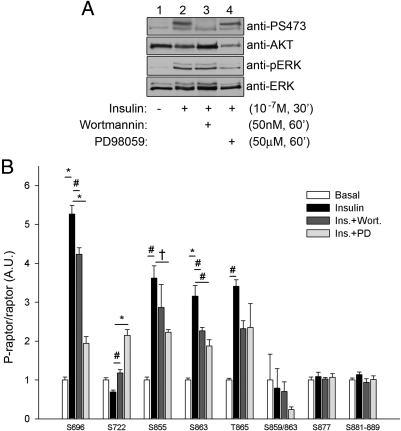
To identify the signal transduction pathway(s) responsible for insulin-stimulated phosphorylation of raptor, myc-tagged raptor was transiently transfected into CHO/IR cells. Twenty-four hours after transfection, the cells were serum starved and either left untreated or pretreated with 50 nm wortmannin or 50 μm PD98059 for 1 h to inhibit the insulin-stimulated activation of the PI 3-K and MAPK pathways, respectively. The cells were then either left untreated for basal analysis or treated with 10−7 m insulin for 30 min. The cells were subsequently lysed, and the whole-cell lysates were probed for the phosphorylation of either AKT or MAPK to ensure efficient insulin stimulation and either wortmannin or PD98059 inhibition of the PI 3-K and MAPK pathways, respectively (Fig. 2A). Raptor was immunoprecipitated with an anti-myc antibody, and the proteins were resolved by SDS-PAGE. The area on the gel corresponding to the molecular mass of raptor was then excised, and the proteins within the gel slice were subjected to in-gel trypsin digestion. The resulting tryptic peptides were then prepared for subsequent phospho-quantification by HPLC-ESI-MS/MS (Fig. 2B) as described above. As observed before, phosphorylation of both Ser877 and the Ser881–889 cassette was unaffected by insulin treatment, nor was this phosphorylation affected by PI 3-K inhibition or MAPK inhibition. Raptor phosphorylation at Ser696, Ser855, Ser863, and Thr865 all underwent significant insulin-stimulated phosphorylation, whereas Ser722 phosphorylation was suppressed upon insulin treatment (results that repeat the findings presented in Fig. 1B). The insulin-stimulated phosphorylation of raptor at Ser696 underwent a highly significant decrease upon inhibition of the MAPK pathway and to a lesser extent by inhibition of the PI 3-K pathway (a trend observed for the phosphorylation of Ser855 as well). The insulin-induced suppression of raptor phosphorylation at Ser722 was significantly reversed by treatment with wortmannin, whereas inhibition of the MAPK pathway not only reversed the insulin-induced suppression of Ser722 phosphorylation, but rather, inhibition of MAPK actually increased phosphorylation of this site approximately 2-fold over basal levels. Insulin-stimulated phosphorylation of raptor at Ser863 and Thr865 were both inhibited to a similar extent by treatment with either wortmannin or PD98059, whereas detection of the tandem Ser859/Ser863 phosphopeptide was not significantly affected by any treatment, although an inhibitory trend was observed in the presence of either inhibitor.
Fig. 2.
PI 3-K and MAPK pathways are involved in insulin-stimulated raptor phosphorylation. A, Cells overexpressing myc-tagged raptor were serum starved and left untreated, treated with insulin alone, or pretreated with 50 nm wortmannin or 50 μm PD98059 for 1 h and then either left untreated or treated with 10−7 m insulin for 30 min. Whole-cell lysates were probed for the phosphorylation of either AKT (top panel) or MAPK (third panel), or the expression of AKT (second panel) or MAPK (fourth panel). B, Immunoprecipitated raptor was resolved by 7.5% SDS-PAGE and stained with Coomassie blue for protein visualization, and the gel area corresponding to the position of raptor was excised and processed for MS analysis as described in Materials and Methods. The peak area for each phosphopeptide was normalized against the average peak area of the seven representative raptor peptides per sample (endogenous standards). Each raptor phosphorylation site was normalized by the average value of the respective basal sample and then expressed as a fold change over basal ± sem (n = 3). †, P < 0.05; #, P < 0.01; *, P < 0.0001 (ANOVA). A.U., Arbitrary units; Ins., insulin; PD, PD98059; Wort., wortmannin.
ERK2 phosphorylates raptor in vitro at Ser696
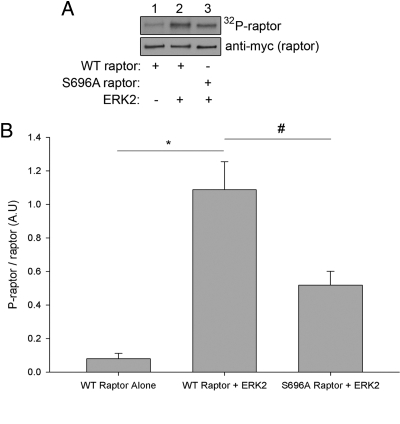
Because insulin-stimulated raptor phosphorylation at Ser696 was strongly decreased in response to inhibition of the MAPK pathway, and Ser696 lies within the consensus phosphorylation sequence targeted by p44/42 MAPK (pS/T-Pro) (23), we next tested whether MAPK acts as a kinase capable of phosphorylating raptor. Myc-tagged raptor was transiently transfected into CHO/IR cells. The cells were lysed in a low-stringency lysis buffer containing CHAPS detergent as a substitute for Triton X-100 to preserve protein-protein interactions potentially important for raptor phosphorylation, and either myc-tagged wild-type or Ser696Ala raptor was successfully immunopurified and incubated in vitro with recombinant active ERK2 (p42 MAPK). Figure 3A shows typical results for the raptor/ERK2 in vitro kinase assays, whereas Fig. 3B shows the quantification of raptor phosphorylation by ERK2 for all experiments (n = 3). The addition of ERK2 significantly increased raptor phosphorylation (P < 0.01; Student's t test), whereas phosphorylation of the Ser696Ala mutant was significantly decreased compared with wild-type raptor (P < 0.05; Student's t test), suggesting that ERK acts as a kinase that can directly phosphorylate raptor at Ser696.
Fig. 3.
ERK2 phosphorylates raptor in vitro at Ser696. A, Either myc-tagged wild-type (WT) or Ser696Ala raptor was transiently transfected and immunoprecipitated from CHO/IR cells. The immunoprecipitates were washed three times in lysis buffer and once in kinase buffer, followed by incubation at 30 C for 30 min in kinase buffer that included recombinant active ERK2 and 5 μCi [γ-32P]ATP. The reactions were stopped by addition of SDS loading buffer and boiled at 95 C for 4 min. The proteins were resolved on a 10% SDS-polyacrylamide gel and transferred electrophoretically from the gel to a nitrocellulose membrane. The phosphorylation of raptor was examined by autoradiography (upper panel), and the membrane was subsequently probed with anti-myc antibody to visualize the presence of raptor (lower panel). B, Quantification of raptor phosphorylation by ERK2. Raptor phosphorylation was normalized to the amount of raptor and expressed as the fold change over raptor incubated alone (n = 3). #, P < 0.05; *, P < 0.01 (Student's t test). A.U., Arbitrary units.
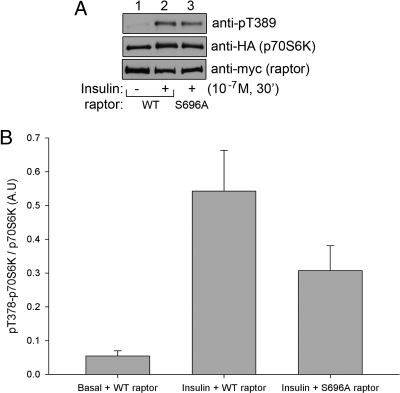
Phosphorylation of raptor at Ser696 is important for insulin-stimulated phosphorylation of p70S6K
Raptor phosphorylation at Ser696 undergoes robust insulin-stimulated phosphorylation, which hypothetically could be required for proper insulin-stimulated mTORC1 downstream action. To test this hypothesis, CHO/IR cells were transfected with HA-tagged p70S6K together with either wild-type or S696A raptor. Twenty-four hours after transfection, the cells were serum starved and either left untreated or treated with insulin. Analysis of p70S6K phosphorylation (Fig. 4, A and B) reveals that mutation of Ser696 to alanine interferes with the insulin-stimulated phosphorylation of p70S6K, supporting the hypothesis that phosphorylation of raptor at Ser696 plays a role in insulin-stimulated mTOR substrate phosphorylation.
Fig. 4.
Phosphorylation of raptor at Ser696 is important for insulin-stimulated phosphorylation of p70S6K. A, CHO/IR cells were transfected with HA-tagged p70S6K together with either wild-type (WT) or S696A raptor. Twenty-four hours after transfection, the cells were serum starved and either left untreated or treated with insulin. The cells were then lysed, and the whole-cell lysates were immunoprecipitated with anti-HA for p70S6K. The immunoprecipitates were resolved by SDS-PAGE and transferred to nitrocellulose membranes. The membranes containing the immunoprecipitated p70S6K were subjected to Western blot for p70S6K phosphorylation at Thr389 (upper panel) or with anti-HA antibodies for p70S6K protein (middle panel). The whole-cell lysates were also resolved by SDS-PAGE and transferred to nitrocellulose membranes and probed with anti-myc for raptor expression (lower panel). B, Quantification of p70S6K phosphorylation. P70S6K phosphorylation was normalized to the amount of p70S6K and expressed as the fold change over basal (n = 3). P value = 0.09 (Student's t test).
Discussion
The phosphorylation of raptor is emerging as an important step in the insulin-stimulated regulation of mTORC1. To date, raptor has been reported to be phosphorylated at 23 sites: Ser8, Ser190, Ser696, Thr706, Ser719, Ser721, Ser722, Tyr723, Ser738, Ser771, Ser792, Ser795, Ser853, Ser855, Ser857, Ser859, Ser863, Thr865, Ser877, Ser881, Ser882, Thr883, and Ser884 (10, 11, 13, 24–35). This study now adds Tyr692, Ser699, Thr700, Ser704, Ser854, Ser886, Ser887, and Thr889, bringing the total to 31. Four kinases have been identified to phosphorylate eight of these sites: Cdc2 for Ser696 and Thr706 (34), AMPK for Ser792 (and most likely Ser722) (10), RSK for Ser719 and Ser722 (and possibly Ser721) (11), and mTOR for Ser859 and Ser863 (12, 13). Intestinal cell kinase was also found to phosphorylate raptor, although no specific sites were identified (36). We report here that p44/42 MAPK acts as a kinase capable of phosphorylating raptor, and we identify Ser696 as a MAPK phosphorylation site, although other sites might be phosphorylated by MAPK as well. MS analysis of raptor phosphorylation in this report resulted in a focus on phosphorylation cassettes for raptor, in a manner similar to that reported by Foster et al. (13)with the difference being that the previous report focused on two clusters of raptor phosphorylation, whereas our findings now broaden that number to four regions of phosphorylation, which is based on the sensitivity of each raptor phosphorylation cassette to insulin treatment. Cassette 1 revolves around the phosphorylation of Ser696, a site that was consistently observed to be heavily phosphorylated and, importantly, underwent a strong insulin-stimulated increase. Cassette 1 includes the secondary phosphorylation sites of Tyr692, Ser699, Thr700, Ser704, and Thr706, which were not repeatedly observed and, when identified, were less abundant compared with the phosphorylation of Ser696 (resulting in the designation of these ancillary sites as ambiguous raptor phosphorylation sites). Cassette 2, which is suppressed upon insulin treatment, is centralized around Ser722, the dominant phosphorylation site, and includes the phosphorylation of Ser721 and possibly Ser719 (11, 27) and Tyr723 (27). Cassette 3 is defined by the insulin-stimulated phosphorylation of Ser863 and includes the strong secondary sites of Ser855 and Thr865, both of which were also found to undergo insulin-stimulated increases. Cassette 3 might also involve the phosphorylation of Ser853 and Ser854, although we were not able to detect pSer853, and pSer854 was not repeatedly observed and, when detected, was much less abundant compared with the phosphorylation of Ser863. Although the Scaffold program repeatedly assigned Thr857 as a raptor phosphorylation site, we were unable to quantify phosphorylation of this site by MS due to the complexity of the phosphopeptide. The fourth and final cassette, which was found to be unresponsive to insulin treatment, is divided between Ser877, a site consistently observed to be highly phosphorylated, and the cluster of eight Ser/Thr residues at aa881-889, which, with the exception of Ser885, have all been found to be phosphorylated, albeit to a much lesser extent than that of Ser877. Of the four phosphopeptides studied, only aa850-867 was detected as a doubly phosphorylated peptide, predominantly at Ser859/863. From all the MS analysis conducted for this study, four predominant raptor phosphorylation sites were consistently observed: Ser696, Ser722, Ser863, and Ser877.
During the preparation of this manuscript, Foster et al. (13) reported the phosphorylation of raptor at Ser696, a site found to be stimulated by overexpression of the upstream mTORC1 activator Rheb. We present data confirming the phosphorylation of raptor at Ser696 and that Ser696 acts as one of the major insulin-stimulated raptor phosphorylation sites. We also show that insulin-stimulated Ser696 phosphorylation of raptor is strongly decreased through inhibition of the p44/42 MAPK pathway and, to a lesser extent, the PI 3-K pathway. We introduce the findings that raptor acts a substrate for p44/42 MAPK phosphorylation and that MAPK phosphorylates raptor at Ser696. This identifies both mTOR (12, 13) and MAPK as kinases involved in the insulin-stimulated phosphorylation of raptor, whereas RSK- and AMPK-mediated phosphorylation of raptor acts independently of insulin action (10, 11). Raptor mutation of Ser696 to alanine reduced the insulin-stimulated phosphorylation of the mTORC1 downstream substrate p70S6K in a manner similar to that for the previously observed mutation of Ser863 (12), suggesting that the MAPK and mTOR pathways may synergize at the level of raptor phosphorylation for the insulin-stimulated activation of mTORC1. During the review of this manuscript, Carriere et al. (35) presented evidence confirming our findings that ERK phosphorylates raptor at Ser696.
We present the novel finding that Ser722 phosphorylation is reduced upon insulin treatment and that inhibition of the MAPK pathway and, to a lesser extent, the PI 3-K pathway reversed the suppressive effect of insulin on Ser722 phosphorylation. mTOR activity is optimal in a nutrient-rich, energy-favorable, anabolic environment, as evidenced by the fact that amino acid availability directs mTOR signaling. AMPK is the protein sensor activated by low levels of AMP during periods of energy deprivation (37), and upon energy deprivation, AMPK catalyzes the phosphorylation and activation of TSC1/TSC2, functioning to inactivate the Rheb GTPase and mTORC1 (7–9). AMPK also suppresses mTORC1 activity by phosphorylating raptor at Ser792 and most likely Ser722, which creates targeting motifs for 14-3-3 binding, and although 14-3-3 binding to raptor did not affect mTOR/raptor association, serine to alanine mutation of these sites did increase mTOR-catalyzed phosphorylation of S6K (10). Therefore, although AMPK increases the phosphorylation of raptor at Ser722 in the catabolic state, insulin decreases raptor phosphorylation in the opposing anabolic state, solidifying a role for the phosphorylation of raptor at Ser722 as a pivotal control point for regulating the activity of mTORC1. Future experiments will focus on elucidating the mechanism of insulin-suppressed phosphorylation of raptor at Ser722 mediated by the MAPK pathway.
We found that phosphorylation of raptor at Ser863 and Thr865 and dual phosphorylation at Ser859/Ser863 is reduced by treatment of cells with the mTOR inhibitor rapamycin. Although the finding that insulin stimulated and rapamycin inhibited phosphorylation of raptor at Thr865 is novel, the Ser859 and Ser863 findings are confirmatory to the previously published data by Wang et al. (12) and Foster et al. (13). Our data are in agreement that phosphorylation of Ser859 appears to be dependent upon Ser863 phosphorylation. Foster et al. (13) reported a similar dependence on the phosphorylation of raptor at Ser855 on Ser863 phosphorylation, although we were unable to analyze this phenomenon, possibly due to the low abundance of the Ser855/863 doubly phosphorylated peptide. It is important to note that insulin increased Ser863 phosphorylation of raptor, although insulin did not increase the dual phosphorylation of Ser859/Ser863. This discovery suggests that raptor may exist in functionally distinct, alternatively phosphorylated forms, where singly phosphorylated Ser863 raptor is insulin regulated, whereas dual, Ser859/Ser863-phosphorylated raptor is regulated in a non-insulin-dependent manner.
To date, of the four major raptor phosphorylation sites, Ser877 stands as the least studied. Our data found no effect of insulin, rapamycin, PD98059, or wortmannin on Ser877 phosphorylation, whereas Carrière et al. (11) reported no effect of the Ras/MAPK pathway agonist PMA on Ser877 phosphorylation. In the studies conducted by Foster et al. (13) aimed at characterizing the phosphorylation of raptor, overexpression of the mTORC1 upstream activator Rheb stimulated an increase in Ser877 phosphorylation, suggesting a potential positive role for this phosphorylation site in an as-of-yet uncharacterized pathway.
One of the major findings in this report is that insulin-stimulated phosphorylation of Ser696, Ser855, Ser863, and Thr865 is sensitive to inhibition of MAPK and PI 3-K, suggesting the involvement of both pathways in the phosphorylation of each of these sites. It has been shown that both MAPK and AKT are responsible for the inactivation of TSC1/TSC2, which allows for Rheb binding to mTOR and activation of mTORC1. Foster et al. (13) reported that Rheb overexpression increased phosphorylation of raptor at Ser696, Thr706, Ser855, Ser859, Ser863, and Ser877. Because inhibition of either the MAPK (38) or PI 3-K (39) pathway theoretically blocks the insulin-induced inactivation of TSC1/TSC2 and subsequent Rheb binding to mTORC1, and Rheb increases the phosphorylation of raptor, the decrease observed in raptor phosphorylation upon inhibition of MAPK and PI 3-K may be due to the loss of Rheb association with mTOR. This suggests that Rheb may be vital for the insulin-stimulated phosphorylation of raptor. Future studies will be aimed at understanding the potential requirement of Rheb in the phosphorylation of raptor.
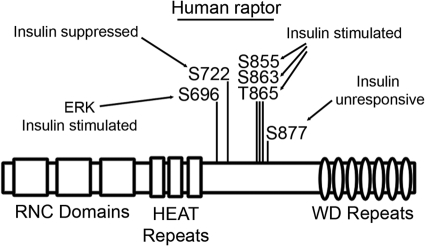
The results of these experiments are summarized in Fig. 5. Scaffold analysis of raptor phosphorylation consistently assigned the majority of spectra from each of the four phosphopeptides to one major phosphorylation site per peptide: Ser696, Ser722, Ser863, and Ser877. Each major phosphorylation site was found to be accompanied by ancillary phosphorylation sites that were detected with much less frequency: Ser696 is complimented by phosphorylation of Tyr692, Ser699, Thr700, Ser704, and Thr706; Ser722 is accompanied by Ser721 and possibly Ser719 and Tyr723 phosphorylation; Ser863 phosphorylation is proximal to the phosphorylation of Ser853, Ser854, Ser855, Thr857, Ser859, and Thr865; and phosphorylation of Ser877 is situated near the long stretch of Ser881, Ser882, Thr883, Ser884, Ser886, Ser887, and Thr889 phosphorylation (Fig. 5). Interestingly, the phosphorylation sites within each cassette respond uniformly to insulin or inhibitor treatments, establishing the specificity of each cluster and imparting a function of raptor for stimuli-specific direction of mTORC1 function. Understanding the function of these key raptor phosphorylation sites in insulin signaling will be crucial for determining how insulin regulates mTORC1 action.
Fig. 5.
Schematic of the effect of insulin on raptor phosphorylation. Ser696, Ser855, Ser863, and T865 underwent insulin-stimulated phosphorylation, whereas S722 phosphorylation was suppressed upon insulin treatment, and S877 phosphorylation remained unchanged upon insulin treatment. RNC, Raptor N-terminal conserved; HEAT, Huntingtin elongation factor 3 (EF3), protein phosphatase.
Acknowledgments
This work was supported by National Institutes of Health Grants R01DK47936 and R01DK66483 (to L.J.M.) and 5F32DK078460-02 (to P.L.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACN
- Acetonitrile
- aa
- amino acids
- AKT
- serine/threonine protein kinase
- AMPK
- AMP-activated protein kinase
- CHO/IR
- Chinese hamster ovary cells overexpressing the insulin receptor
- CID
- collision-induced dissociation
- 4E-BP1
- 4E-binding protein 1
- ESI
- electrospray ionization
- FA
- formic acid
- HA
- hemagglutinin
- MS/MS
- tandem mass spectrometry
- mTOR
- mammalian target of rapamycin
- mTORC1
- mTOR complex 1
- PI 3-K
- phosphatidylinositol 3-kinase
- PMA
- phorbol myristate acetate
- p70S6K
- p70S6 kinase
- RSK
- p90 ribosomal S6 kinase
- SDS
- sodium dodecyl sulfate
- TSC1
- tuberous sclerosis complex.
References
- 1. Laplante M, Sabatini DM. 2009. mTOR signaling at a glance. J Cell Sci 122:3589–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K. 2002. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110:177–189 [DOI] [PubMed] [Google Scholar]
- 3. Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. 2002. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110:163–175 [DOI] [PubMed] [Google Scholar]
- 4. Jacinto E, Loewith R, Schmidt A, Lin S, Rüegg MA, Hall A, Hall MN. 2004. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol 6:1122–1128 [DOI] [PubMed] [Google Scholar]
- 5. Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. 2004. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol 14:1296–1302 [DOI] [PubMed] [Google Scholar]
- 6. Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. 2005. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307:1098–1101 [DOI] [PubMed] [Google Scholar]
- 7. Shaw RJ, Bardeesy N, Manning BD, Lopez L, Kosmatka M, DePinho RA, Cantley LC. 2004. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell 6:91–99 [DOI] [PubMed] [Google Scholar]
- 8. Corradetti MN, Inoki K, Bardeesy N, DePinho RA, Guan KL. 2004. Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes Dev 18:1533–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Inoki K, Zhu T, Guan KL. 2003. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115:577–590 [DOI] [PubMed] [Google Scholar]
- 10. Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. 2008. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30:214–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carrière A, Cargnello M, Julien LA, Gao H, Bonneil E, Thibault P, Roux PP. 2008. Oncogenic MAPK signaling stimulates mTORC1 activity by promoting RSK-mediated raptor phosphorylation. Curr Biol 18:1269–1277 [DOI] [PubMed] [Google Scholar]
- 12. Wang L, Lawrence JC, Jr, Sturgill TW, Harris TE. 2009. Mammalian target of rapamycin complex 1 (mTORC1) activity is associated with phosphorylation of raptor by mTOR. J Biol Chem 284:14693–14697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Foster KG, Acosta-Jaquez HA, Romeo Y, Ekim B, Soliman GA, Carriere A, Roux PP, Ballif BA, Fingar DC. 2010. Regulation of mTOR complex 1 (mTORC1) by raptor Ser863 and multisite phosphorylation. J Biol Chem 285:80–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yi Z, Langlais P, De Filippis EA, Luo M, Flynn CR, Schroeder S, Weintraub ST, Mapes R, Mandarino LJ. 2007. Global assessment of regulation of phosphorylation of insulin receptor substrate-1 by insulin in vivo in human muscle. Diabetes 56:1508–1516 [DOI] [PubMed] [Google Scholar]
- 15. Højlund K, Yi Z, Lefort N, Langlais P, Bowen B, Levin K, Beck-Nielsen H, Mandarino LJ. 2010. Human ATP synthase beta is phosphorylated at multiple sites and shows abnormal phosphorylation at specific sites in insulin-resistant muscle. Diabetologia 53:541–551 [DOI] [PubMed] [Google Scholar]
- 16. Luo M, Langlais P, Yi Z, Lefort N, De Filippis EA, Hwang H, Christ-Roberts CY, Mandarino LJ. 2007. Phosphorylation of human insulin receptor substrate-1 at serine 629 plays a positive role in insulin signaling. Endocrinology 148:4895–4905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Luo M, Reyna S, Wang L, Yi Z, Carroll C, Dong LQ, Langlais P, Weintraub ST, Mandarino LJ. 2005. Identification of insulin receptor substrate 1 serine/threonine phosphorylation sites using mass spectrometry analysis: regulatory role of serine 1223. Endocrinology 146:4410–4416 [DOI] [PubMed] [Google Scholar]
- 18. Yi Z, Luo M, Carroll CA, Weintraub ST, Mandarino LJ. 2005. Identification of phosphorylation sites in insulin receptor substrate-1 by hypothesis-driven high-performance liquid chromatography-electrospray ionization tandem mass spectrometry. Anal Chem 77:5693–5699 [DOI] [PubMed] [Google Scholar]
- 19. Yi Z, Luo M, Mandarino LJ, Reyna SM, Carroll CA, Weintraub ST. 2006. Quantification of phosphorylation of insulin receptor substrate-1 by HPLC-ESI-MS/MS. J Am Soc Mass Spectrom 17:562–567 [DOI] [PubMed] [Google Scholar]
- 20. Langlais P, Mandarino LJ, Yi Z. 2010. Label-free relative quantification of co-eluting isobaric phosphopeptides of insulin receptor substrate-1 by HPLC-ESI-MS/MS. J Am Soc Mass Spectrom 21:1490–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brunn GJ, Fadden P, Haystead TA, Lawrence JC., Jr 1997. The mammalian target of rapamycin phosphorylates sites having a (Ser/Thr)-Pro motif and is activated by antibodies to a region near its COOH terminus. J Biol Chem 272:32547–32550 [DOI] [PubMed] [Google Scholar]
- 22. Wullschleger S, Loewith R, Hall MN. 2006. TOR signaling in growth and metabolism. Cell 124:471–484 [DOI] [PubMed] [Google Scholar]
- 23. English JM, Cobb MH. 2002. Pharmacological inhibitors of MAPK pathways. Trends Pharmacol Sci 23:40–45 [DOI] [PubMed] [Google Scholar]
- 24. Ballif BA, Villén J, Beausoleil SA, Schwartz D, Gygi SP. 2004. Phosphoproteomic analysis of the developing mouse brain. Mol Cell Proteomics 3:1093–1101 [DOI] [PubMed] [Google Scholar]
- 25. Beausoleil SA, Jedrychowski M, Schwartz D, Elias JE, Villén J, Li J, Cohn MA, Cantley LC, Gygi SP. 2004. Large-scale characterization of HeLa cell nuclear phosphoproteins. Proc Natl Acad Sci USA 101:12130–12135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Daub H, Olsen JV, Bairlein M, Gnad F, Oppermann FS, Körner R, Greff Z, Kéri G, Stemmann O, Mann M. 2008. Kinase-selective enrichment enables quantitative phosphoproteomics of the kinome across the cell cycle. Mol Cell 31:438–448 [DOI] [PubMed] [Google Scholar]
- 27. Dephoure N, Zhou C, Villén J, Beausoleil SA, Bakalarski CE, Elledge SJ, Gygi SP. 2008. A quantitative atlas of mitotic phosphorylation. Proc Natl Acad Sci USA 105:10762–10767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Imami K, Sugiyama N, Kyono Y, Tomita M, Ishihama Y. 2008. Automated phosphoproteome analysis for cultured cancer cells by two-dimensional nanoLC-MS using a calcined titania/C18 biphasic column. Anal Sci 24:161–166 [DOI] [PubMed] [Google Scholar]
- 29. Moser K, White FM. 2006. Phosphoproteomic analysis of rat liver by high capacity IMAC and LC-MS/MS. J Proteome Res 5:98–104 [DOI] [PubMed] [Google Scholar]
- 30. Munton RP, Tweedie-Cullen R, Livingstone-Zatchej M, Weinandy F, Waidelich M, Longo D, Gehrig P, Potthast F, Rutishauser D, Gerrits B, Panse C, Schlapbach R, Mansuy IM. 2007. Qualitative and quantitative analyses of protein phosphorylation in naive and stimulated mouse synaptosomal preparations. Mol Cell Proteomics 6:283–293 [DOI] [PubMed] [Google Scholar]
- 31. Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M. 2006. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 127:635–648 [DOI] [PubMed] [Google Scholar]
- 32. Sui S, Wang J, Yang B, Song L, Zhang J, Chen M, Liu J, Lu Z, Cai Y, Chen S, Bi W, Zhu Y, He F, Qian X. 2008. Phosphoproteome analysis of the human Chang liver cells using SCX and a complementary mass spectrometric strategy. Proteomics 8:2024–2034 [DOI] [PubMed] [Google Scholar]
- 33. Villén J, Beausoleil SA, Gerber SA, Gygi SP. 2007. Large-scale phosphorylation analysis of mouse liver. Proc Natl Acad Sci USA 104:1488–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gwinn DM, Asara JM, Shaw RJ. 2010. Raptor is phosphorylated by cdc2 during mitosis. PLoS One 5:e9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carriere A, Romeo Y, Acosta-Jaquez HA, Moreau J, Bonneil E, Thibault P, Fingar DC, Roux PP. 2011. ERK1/2 phosphorylate raptor to promote Ras-dependent activation of mTOR complex 1 (mTORC1). J Biol Chem 286:566–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fu Z, Kim J, Vidrich A, Sturgill TW, Cohn SM. 2009. Intestinal cell kinase, a MAP kinase-related kinase, regulates proliferation and G1 cell cycle progression of intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 294:G632–G640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Towler MC, Hardie DG. 2007. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res 100:328–341 [DOI] [PubMed] [Google Scholar]
- 38. Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. 2005. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell 121:179–193 [DOI] [PubMed] [Google Scholar]
- 39. Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J. 2005. Rheb binds and regulates the mTOR kinase. Curr Biol 15:702–713 [DOI] [PubMed] [Google Scholar]