Abstract
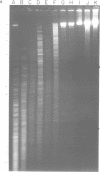
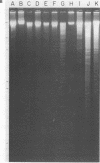
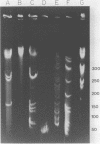
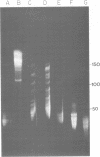
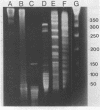
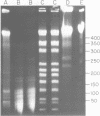
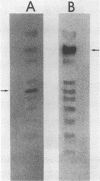
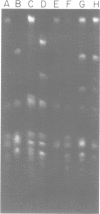
Fundamental to many bacterial genome mapping strategies currently under development is the need to cleave the genome into a few large DNA fragments that can be resolved by pulsed field gel electrophoresis. Identification of endonucleases that infrequently cut a genome is of key importance in this process. We show that the tetranucleotide CTAG is extremely rare in most bacterial genomes with G+C contents above 45%. As a consequence, most of the sixteen bacterial genomes we have tested are cleaved less than once every 100,000 base pairs by one or more endonucleases that have CTAG in their recognition sequences: Xba I (TCTAGA), Spe I (ACTAGT), Avr II (CCTAGG) and Nhe I (GCTAGC). Similarly, CCG and CGG are the rarest trinucleotides in many genomes with G+C content of less than 45%. Thus, Sma I (CCCGGG), Rsr II (CGGWCCG), Nae I (GCCGGC) and Sac II (CCGCGG) are often suitable endonucleases for producing fragments that average over 100,000 base pairs from such genomes. Pulsed field gel electrophoresis of the fragments that result from cleavage with endonucleases that cleave only a few times per genome should assist in the physical mapping of many prokaryotic genomes.
Full text
PDF




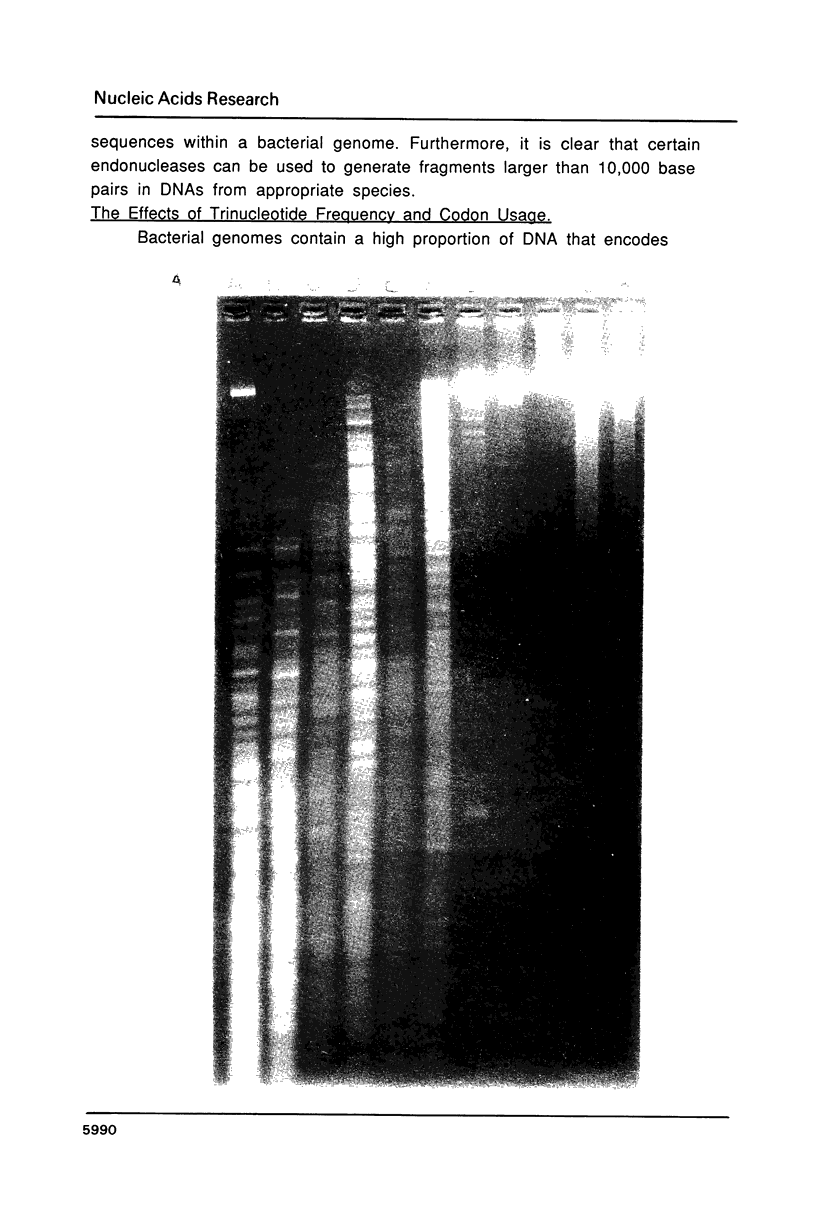
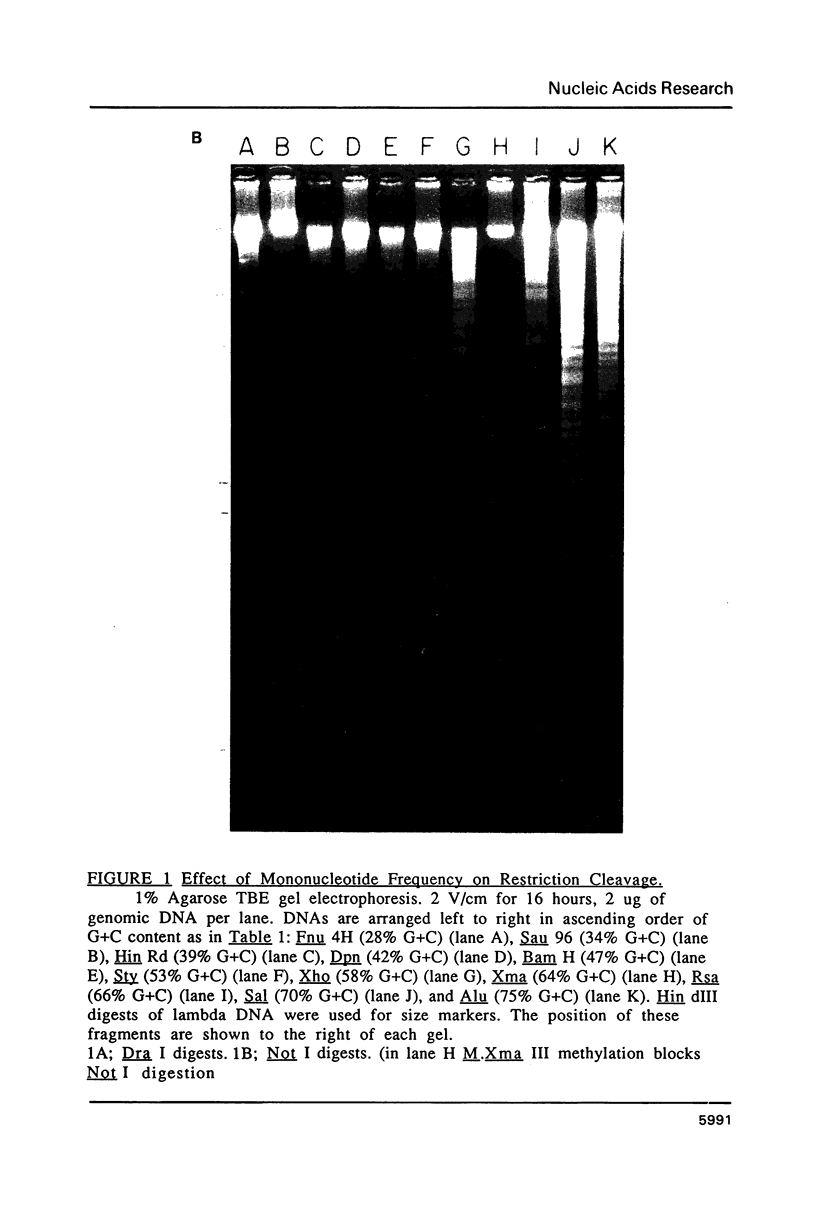
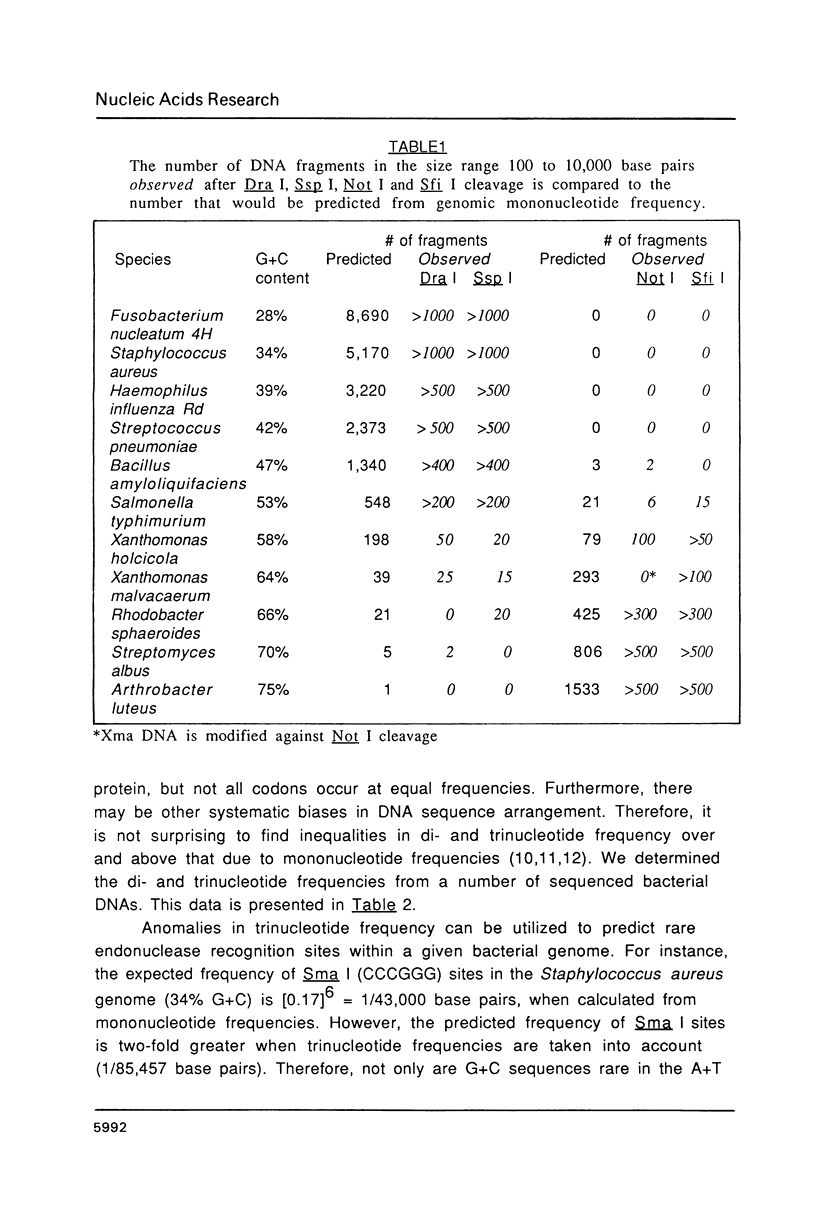
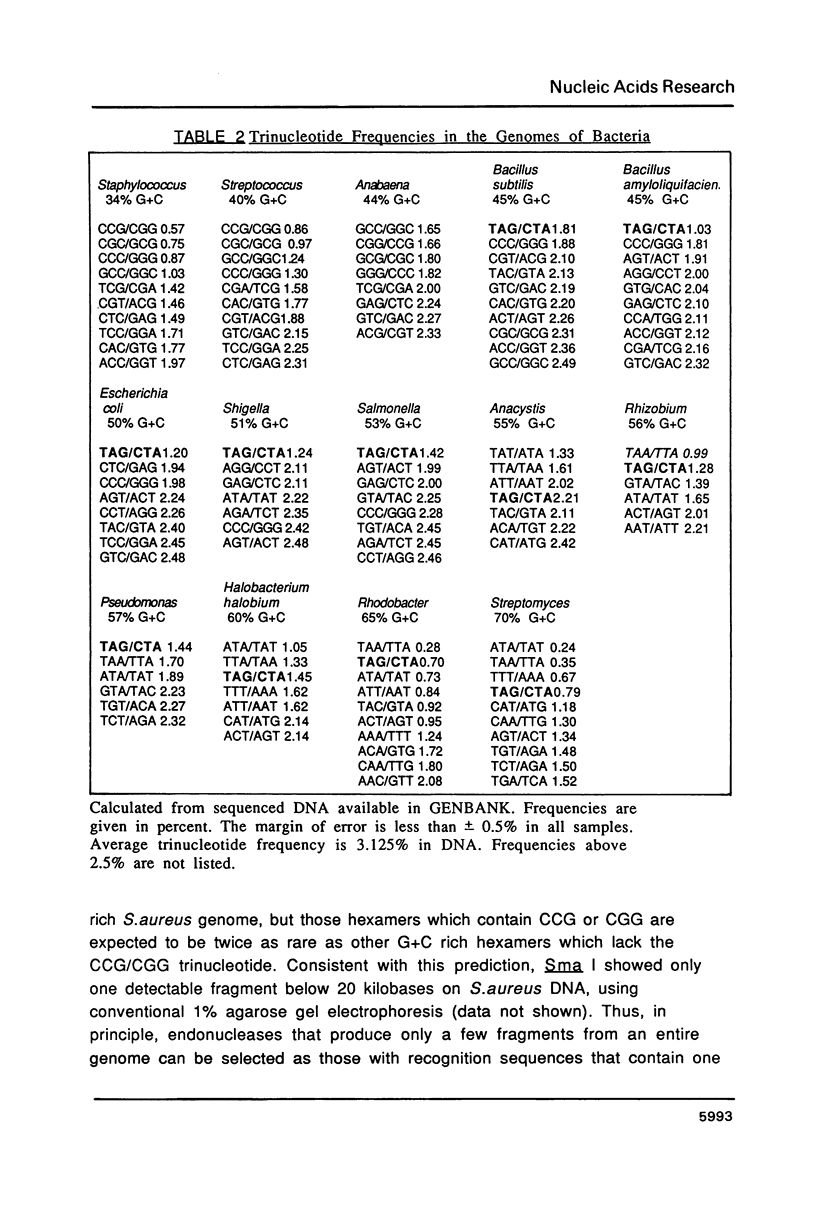

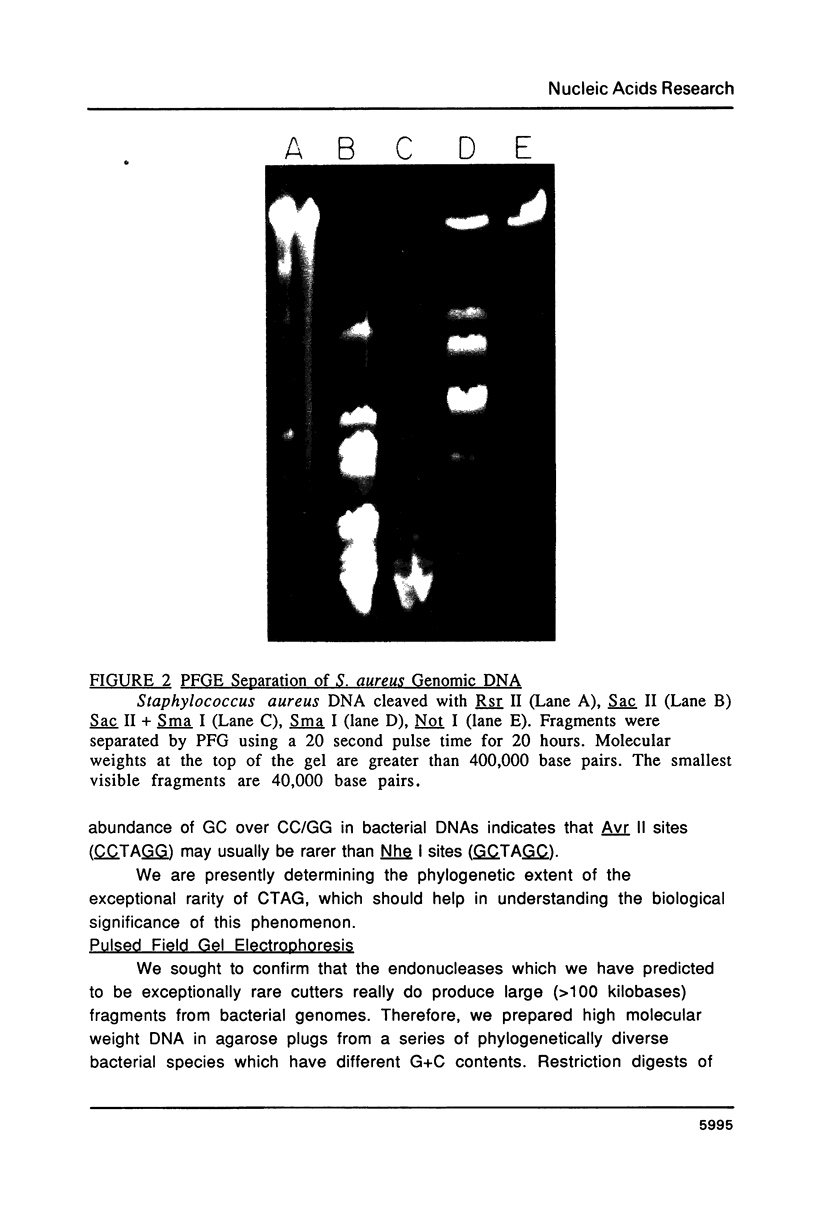

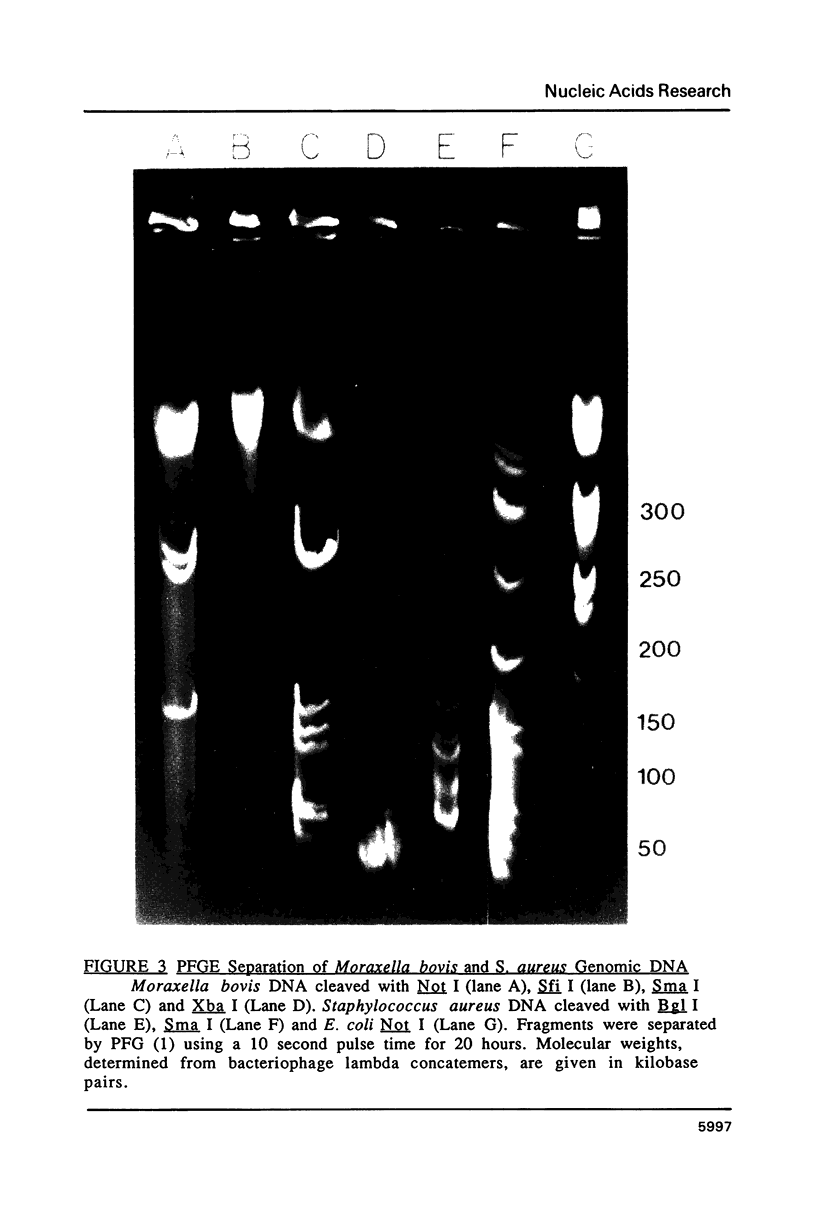
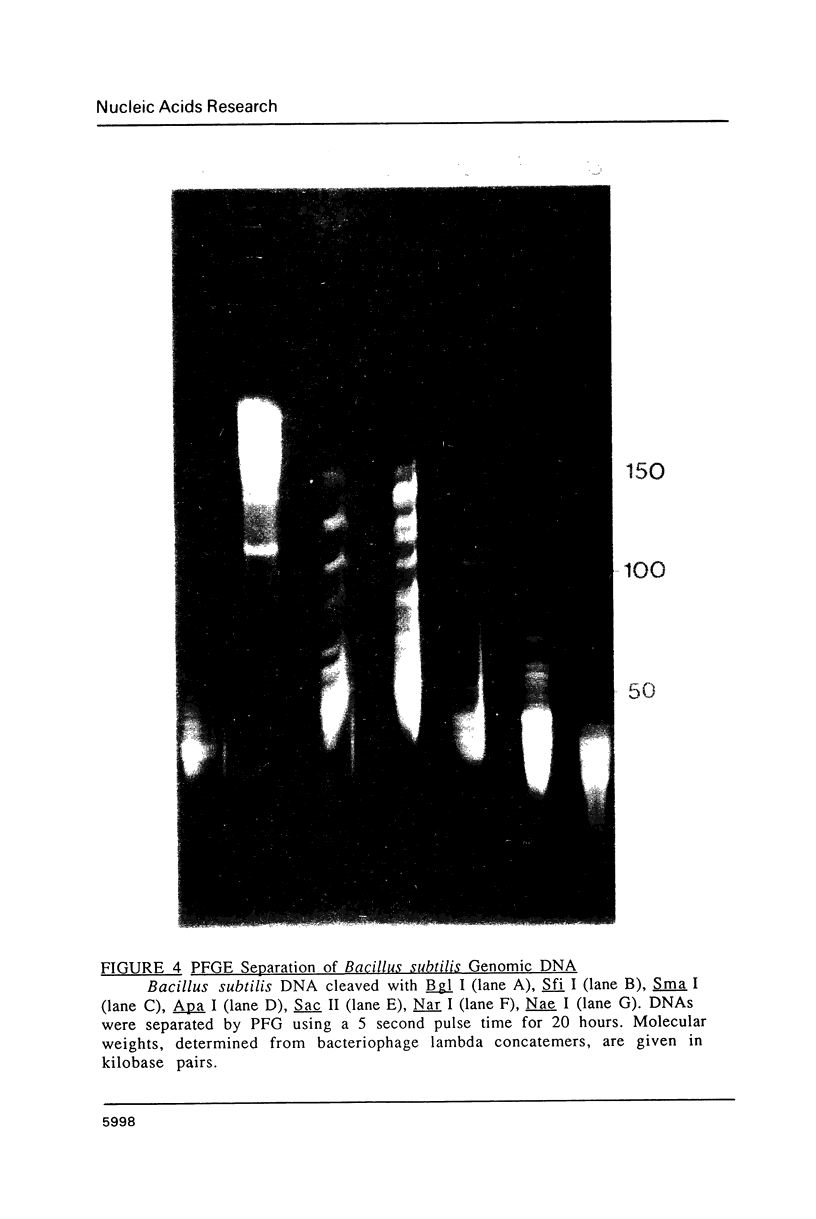
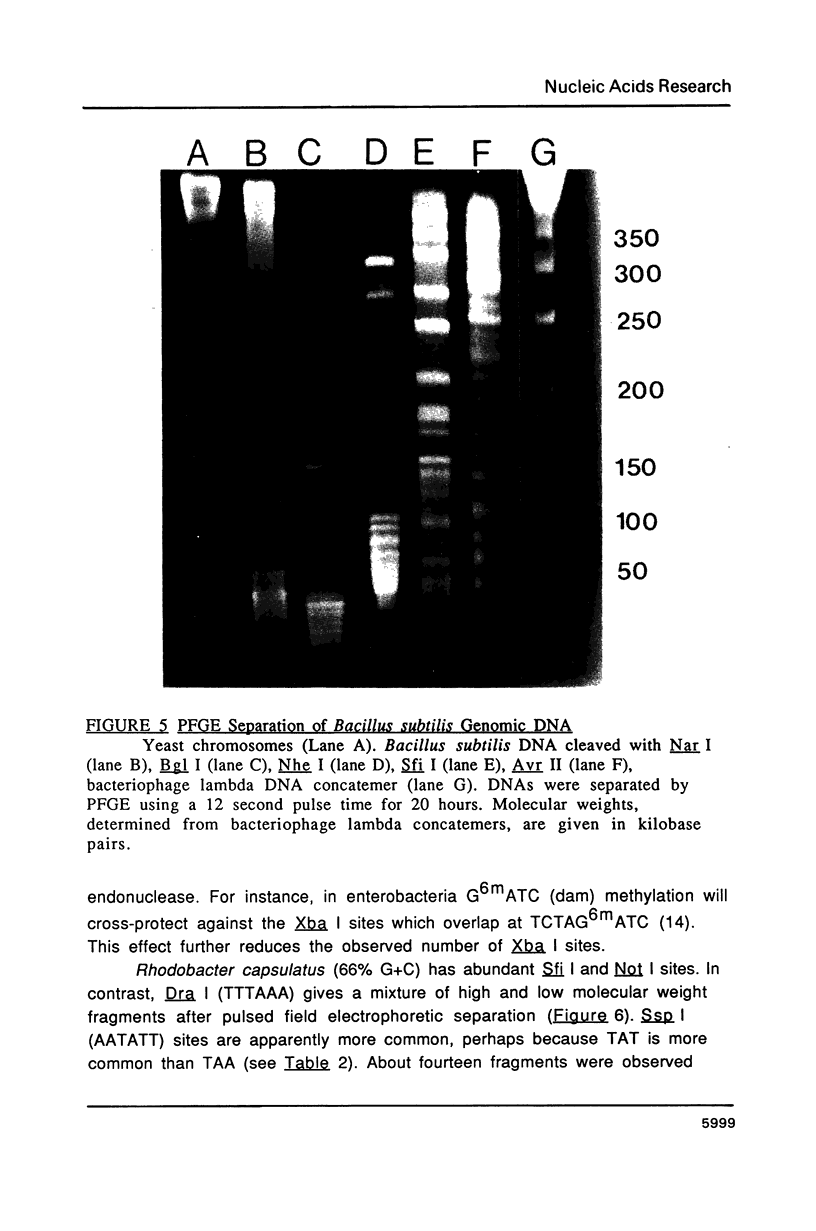
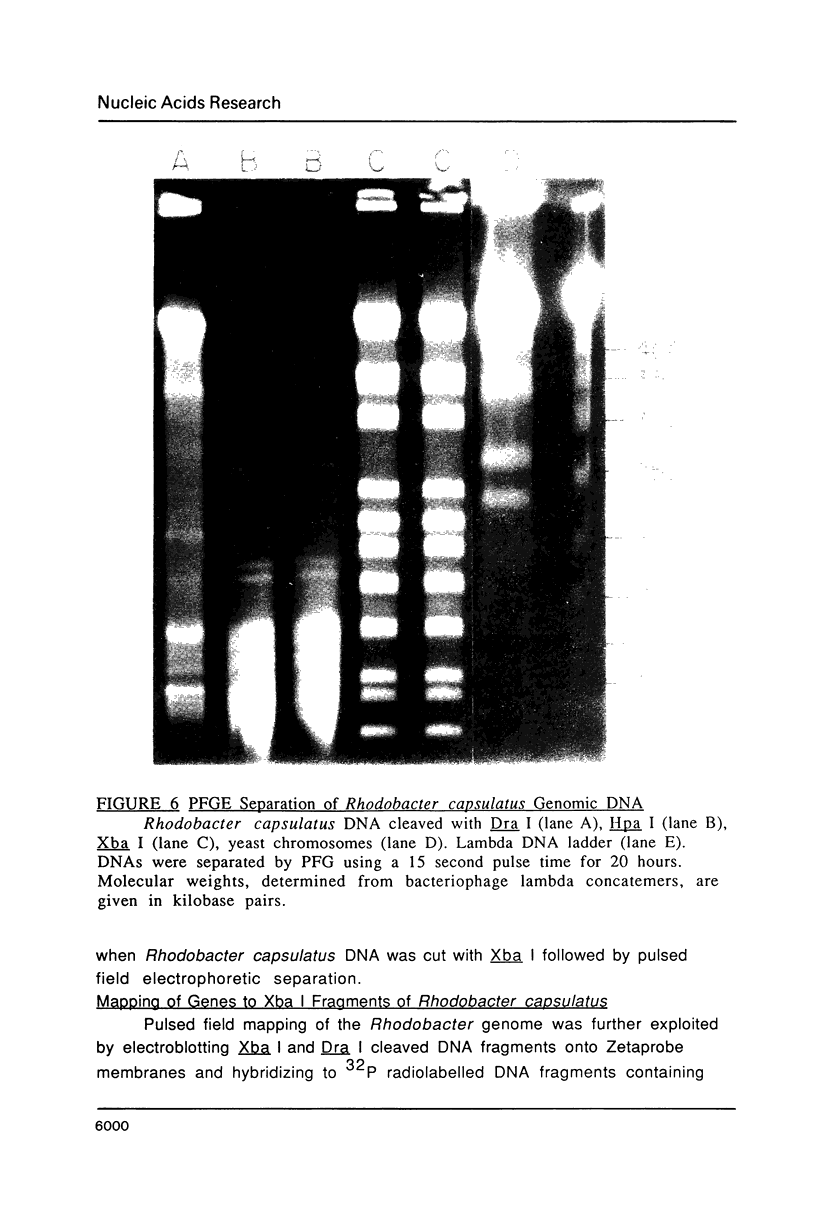
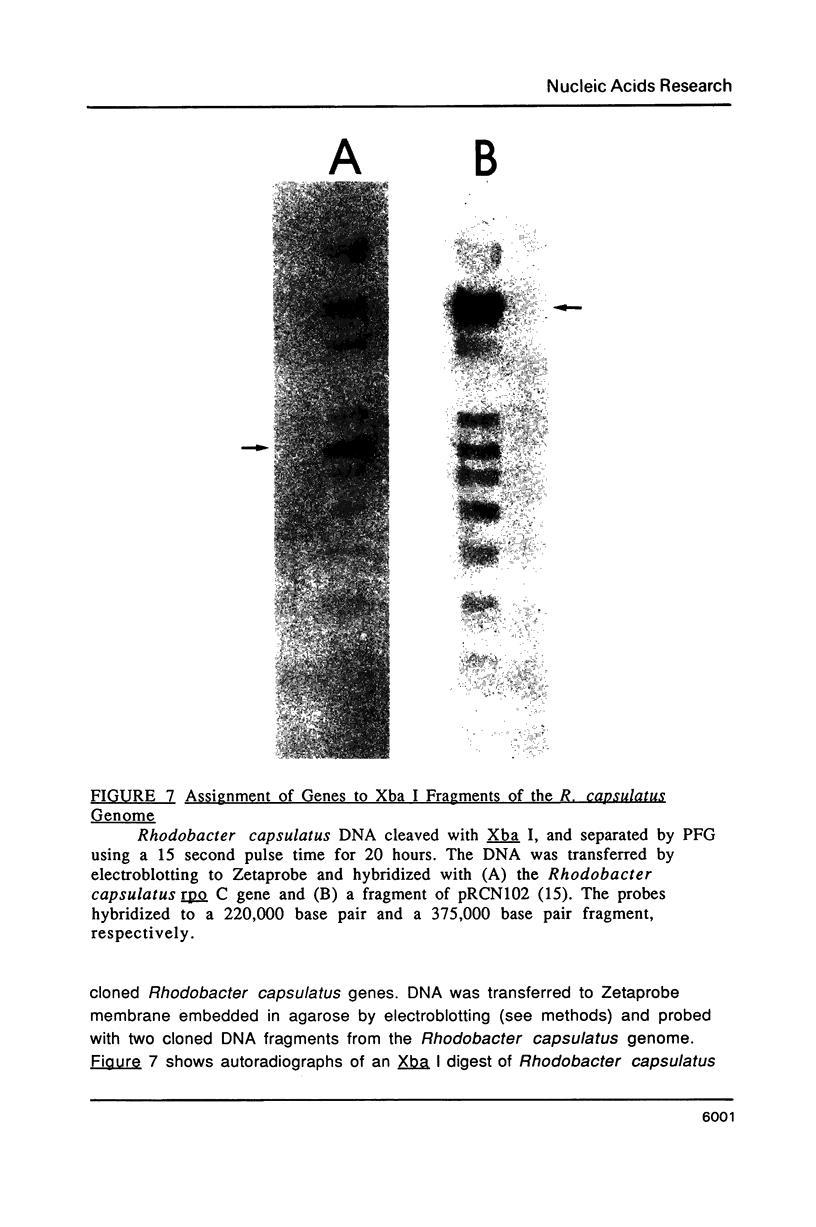
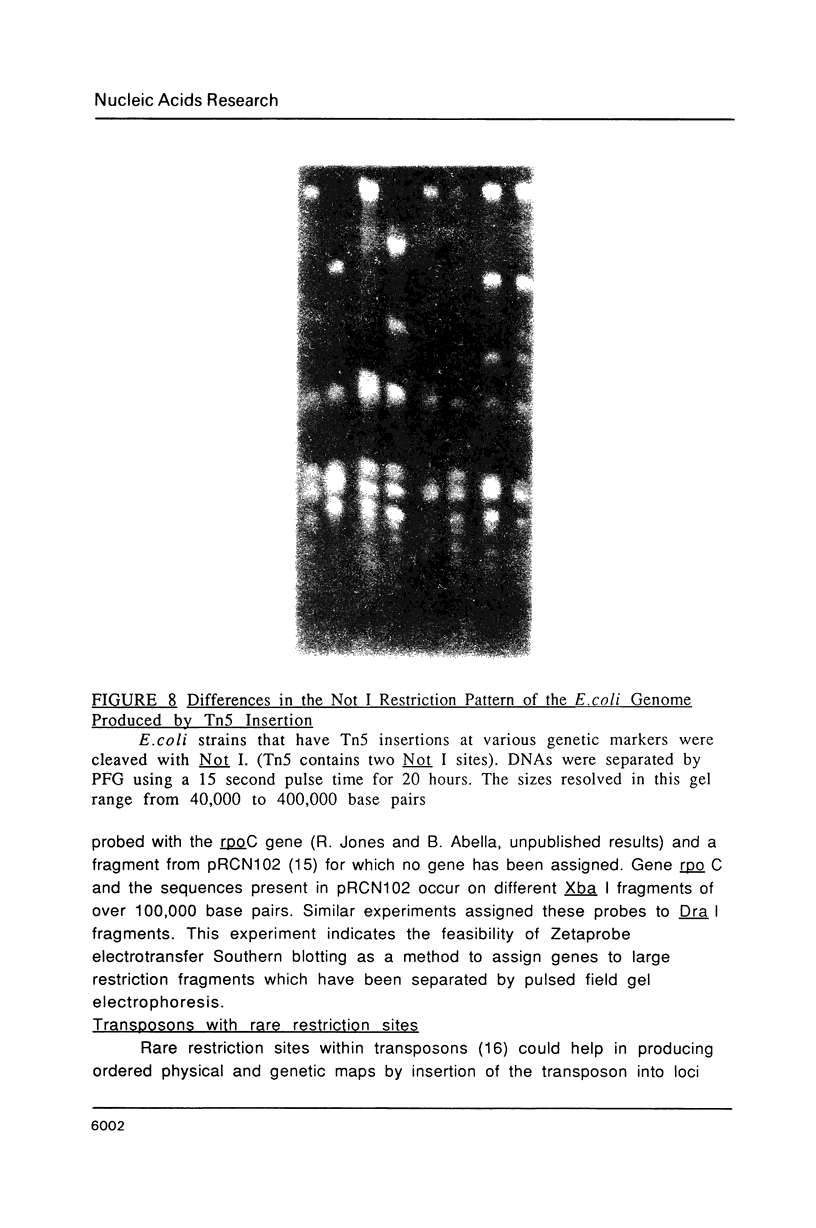



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ehrlich M., Gama-Sosa M. A., Carreira L. H., Ljungdahl L. G., Kuo K. C., Gehrke C. W. DNA methylation in thermophilic bacteria: N4-methylcytosine, 5-methylcytosine, and N6-methyladenine. Nucleic Acids Res. 1985 Feb 25;13(4):1399–1412. doi: 10.1093/nar/13.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner K., Laas W., Patterson D. Fractionation of large mammalian DNA restriction fragments using vertical pulsed-field gradient gel electrophoresis. Somat Cell Mol Genet. 1986 Mar;12(2):185–195. doi: 10.1007/BF01560665. [DOI] [PubMed] [Google Scholar]
- Gillis M., De Ley J., De Cleene M. The determination of molecular weight of bacterial genome DNA from renaturation rates. Eur J Biochem. 1970 Jan;12(1):143–153. doi: 10.1111/j.1432-1033.1970.tb00831.x. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. T. Estimate of the genome size of various microorganisms. J Bacteriol. 1969 Jun;98(3):1400–1401. doi: 10.1128/jb.98.3.1400-1401.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N. Transposable elements in prokaryotes. Annu Rev Genet. 1981;15:341–404. doi: 10.1146/annurev.ge.15.120181.002013. [DOI] [PubMed] [Google Scholar]
- Kranz R. G., Haselkorn R. Characterization of nif regulatory genes in Rhodopseudomonas capsulata using lac gene fusions. Gene. 1985;40(2-3):203–215. doi: 10.1016/0378-1119(85)90043-5. [DOI] [PubMed] [Google Scholar]
- Luchansky J. B., Pattee P. A. Isolation of transposon Tn551 insertions near chromosomal markers of interest in Staphylococcus aureus. J Bacteriol. 1984 Sep;159(3):894–899. doi: 10.1128/jb.159.3.894-899.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland M. Selection against dam methylation sites in the genomes of DNA of enterobacteriophages. J Mol Evol. 1984;21(4):317–322. doi: 10.1007/BF02115649. [DOI] [PubMed] [Google Scholar]
- Phillips G. J., Arnold J., Ivarie R. The effect of codon usage on the oligonucleotide composition of the E. coli genome and identification of over- and underrepresented sequences by Markov chain analysis. Nucleic Acids Res. 1987 Mar 25;15(6):2627–2638. doi: 10.1093/nar/15.6.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Shepherd J. C. Method to determine the reading frame of a protein from the purine/pyrimidine genome sequence and its possible evolutionary justification. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1596–1600. doi: 10.1073/pnas.78.3.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace D. C., Morowitz H. J. Genome size and evolution. Chromosoma. 1973;40(2):121–126. doi: 10.1007/BF00321457. [DOI] [PubMed] [Google Scholar]
- Waterbury P. G., Lane M. J. Generation of lambda phage concatemers for use as pulsed field electrophoresis size markers. Nucleic Acids Res. 1987 May 11;15(9):3930–3930. doi: 10.1093/nar/15.9.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]