Testosterone during postnatal development organizes the adult display of anxiety-related behaviors, activity, and sensorimotor gating in rats independent of androgen receptors.
Abstract
Perinatal exposure to testosterone (T), which can act upon both the androgen receptor (AR) and, via aromatization of T into estrogens, upon estrogen receptors, organizes many adult behaviors in rodents. We compared behaviors in wild-type (WT) male rats and AR-deficient rats with the testicular feminization mutation (Tfm), which on the day of birth were either gonadectomized (Neo-Gdx) or sham operated. In adulthood, all rats were either gonadectomized or sham operated and implanted with T capsules to equilibrate circulating androgens. In each of four tests of behavior related to anxiety (open field, novel object exposure, light/dark box, and elevated plus maze), Neo-Gdx rats showed decreased indices of anxiety and increased activity compared with rats sham operated on the day of birth, with no differences between WT or Tfm males within treatment groups. These results indicate that testicular hormones act in development to increase adult indices of anxiety and decrease activity in males and that functional ARs are not required for this effect. Acoustic startle response was also reduced by Neo-Gdx, suggesting that postnatal testicular secretions potentiate this behavior as well. Adult corticosterone levels and sensorimotor gating, as measured by prepulse inhibition of the acoustic startle response, were increased by neonatal castration in both WT and Tfm rats. These findings indicate a role of T before adulthood in the organization of anxiety-related behaviors, activity, the hypothalamic-pituitary-adrenal axis, and sensorimotor gating in rats, all of which appears to be AR independent.
In rodents, exposure to hormones around the time of birth can “organize” adult sex differences in both the central nervous system and behavior (1–4). Traditionally, sex differences in behavior and brain morphology are believed to result from testicular production of testosterone (T) in males in early development. According to the aromatization hypothesis, T is converted locally in the brain by the enzyme aromatase to estrogens, which then activate estrogen receptors (ERs) to masculinize the rodent brain (5). However, emerging evidence suggests that activation of the androgen receptor (AR) also contributes to the masculinization of brain and behavior in rodents (6–9), so the issue of the relative contribution of ER and AR to masculinization of rodent behavior remains open.
The most apparent sex differences in rodent behavior are related to sexual behavior. Prenatal administration of T to guinea pigs masculinizes sexual behaviors in both females and males gonadectomized before adulthood (1), indicating a role of T in the organization of this sex difference. Androgen's effects on sexual behavior have commonly been attributed to effects of estrogens acting upon ERs to mediate these effects (10, 11), although recent studies using nervous system-specific AR deletion have demonstrated that ARs also normally play a role in the full masculinization of sexual behavior in mice (12, 13). Early hormone exposure also contributes to sex differences in rodent spatial memory performance in the Morris water maze (males perform better than females). Neonatal castration of males or administration of T to newborn females eliminates such sex differences (14), an effect that appears to be mediated through both ERs and ARs (15–17).
In rats there are also sex differences in tests of anxiety, with females typically showing decreased indices of anxiety and greater activity compared with males (18–23). These differences appear to depend on both organizational and activational effects of hormones. Neonatal gonadectomy of male rats eliminates sex differences in anxiety-related behaviors in the elevated plus maze (EPM) (22). Furthermore, female rats receiving either neonatal treatment with an ER antagonist (tamoxifen) or prepubertal ovariectomy show greater indices of anxiety in the EPM compared with control females (23). In adulthood, administration of either estrogens or androgens generally results in decreased anxiety-related behavior in rodents (24–30) apparently by acting on both ERs and ARs (6, 26, 30–32). Sex differences in anxiety-related behaviors are also associated with sex differences in hypothalamic-pituitary-adrenal (HPA) axis activation, in which gonadally intact female rats show a greater release of stress hormones from the pituitary (ACTH) and adrenal glands (corticosterone) in both basal and anxiety-provoking situations (33, 34). Androgens and estrogens acting in development and adulthood appear to contribute to these differences (33, 35–37).
Sex differences in sensorimotor gating have also been reported in humans and some strains of mice and rats (38–41) as assessed by an experimental model of sensorimotor gating, prepulse inhibition (PPI) of the acoustic startle response (ASR). In adult rodents, circulating levels of both androgens and estrogens can influence aspects of PPI (42–44), and these effects appear to be largely mediated through activation of ERs rather than ARs (6, 43).
Previous studies have indicated that androgens in adulthood modulate anxiety-related behaviors (24, 27), sensorimotor gating (44), and HPA axis activity in rodents (33–35). However, less is known about the effects of androgens during postnatal development and whether ARs participate in the regulation of these functions. To investigate these effects, we compared behavioral and hormonal responses in neonatally gonadectomized (Neo-Gdx) or sham-operated (Neo-Sham) wild-type (WT) and AR-deficient male rats with the testicular feminization mutation (Tfm). Tfm rats represent a genetic model for exploring the role of the AR in brain and behavior (45). In rats, this mutation consists of a single base pair replacement in the AR gene resulting in the expression of a normal sized but dysfunctional AR protein; consequently, sensitivity to androgens through ARs is greatly reduced (46, 47). In the present report, newborn WT and Tfm male rats were either gonadectomized (Neo-Gdx) or sham operated (Neo-Sham), then in adulthood provided with equivalent T before assessing anxiety, ASR, PPI, and adrenal responses. We confirm reports that testicular hormones during postnatal development lead to greater indices of anxiety in adulthood, and because we find almost all effects of neonatal castration are equivalent in WT and Tfm males, conclude that these developmental effects of T increasing adult anxiety are independent of ARs. This contrasts with our previous findings in Tfm mice, which indicate that in adulthood, the anxiolytic effects of circulating T are dependent on functional ARs (6). Thus, T can activate ARs in adulthood to reduce anxiety-related behaviors, but it acts through some other mechanism in development to increase those behaviors. We also report a role for developmental androgens in the regulation of PPI and stress hormone levels that are independent of ARs.
Materials and Methods
Animals
Our colony of Long Evans rats carrying the Tfm mutation was maintained at Michigan State University and group housed in a vivarium with a 12-h light, 12-h dark cycle, lights on at 0600, with food and water ad libitum. On the day of birth, Tfm and WT male pups were either gonadectomized (Neo-Gdx Tfms, n = 11; Neo-Gdx males, n = 9) or sham operated (Neo-Sham Tfms, n = 10; Neo-Sham males, n = 9; procedure described in detail below). All rats were weaned at 21 d old, at which point ear punches were taken, and rats were genotyped using a modified PCR described elsewhere (48). Products of this reaction differentiated between the Tfm and WT alleles for AR, as well as male vs. female, based on the presence or absence of the Sry gene found only on the Y chromosome. In adulthood, animals were either gonadectomized (for those that were sham operated at birth) or sham operated and implanted with T capsules to equilibrate adult circulating T (procedure described in detail below). All procedures were reviewed and approved by the Michigan State University Institutional Animal Care and Use Committee.
Neonatal castration
Within 12 h after birth, pups were removed from their home cage and anesthetized by hypothermia. After pups were deeply anesthetized, incisions were made on either side of the lower abdominal cavity, and the gonads were visualized and removed or left intact (sham operated). Incisions were closed with surgical glue, and pups were placed under a lamp until they were warm and mobile before returning to their home cage.
Adult castration and hormone replacement
At 120 d, rats were anesthetized with isoflurane and either gonadectomized (for those that were sham operated at birth) or sham operated (for those that were gonadectomized at birth). In rats receiving gonadectomy, testes were externalized via bilateral incisions made in the scrotum (WT males) or in the lower abdominal area (Tfm males), and testes were tied off with silk suture and removed. In sham-operated rats, bilateral incisions were also made into either the scrotum (WT males) or lower abdominal area (Tfm males). Incisions in the abdominal muscles were closed with silk suture, and the overlying skin was closed with wound clips, whereas scrotal incisions were closed with wound clips. All rats also received two sc SILASTIC (Dow Corning Corp., Midland, MI) capsules (1.57 mm inner diameter, 3.18 mm outer diameter; 20 mm effective release length) containing free T via a 2-cm incision dorsally at the nape of the neck. We recently demonstrated that these SILASTIC capsules deliver normal male physiological levels of T to adult rats (49). The analgesic buprenorphine (0.05 mg/kg) was injected sc postoperatively.
Behavior testing
Beginning 2 wk after adult gonadectomy or sham operation, animals were tested for anxiety-related behavior, sensorimotor gating, and blood corticosterone levels. Testing took place in an order we judged was least to most anxiety provoking: open field/novel object test, light/dark box (LD box), EPM, PPI, and blood corticosterone collection at killing (all described in detail below), with a minimum of 72 h between tests. To control for circadian variations in behavior, all anxiety-related tests were administered between 1000 and 1400 h. PPI was conducted during the dark cycle beginning at 1900 h.
Open field/novel object test
Open field/novel object testing took place in a 48 × 48 in. white plastic box illuminated from directly above by a 60-W light. A grid was drawn in the box to demarcate entries into the center area and activity (grid crossings). Rats were first placed into a corner of the empty open field, and behavior was recorded via an overhead video camera for 5 min. After 5 min, rats were removed, the box was cleaned with 70% ethanol, and a novel object (a 4 in. diameter × 8 in. high cylindrical metal oxygen tank cap) was placed in the center of the chamber. Three minutes after removal from the open field, the rat was replaced into the chamber now containing the novel object, and behavior was recorded for another 5 min. The number of entries into the center area, time spent in the center area, visits to the novel object, and time spent visiting the novel object were assessed as indices of anxiety-related behavior. The number of grid crossings and rearings was assessed as measures of activity, and all behaviors were coded at a later time by a blind observer. The box was again cleaned with 70% ethanol before testing the next subject.
Light/dark box
The LD box (Phenome Technologies, Inc., Lincolnshire, IL) consisted of a rectangular box that was divided into two regions, one light (40 cm length × 38 cm width × 29 cm height) and one dark (31 cm length × 28 cm width × 29 cm height). The dark region was constructed of black plastic and was covered by a black lid, whereas the light region was constructed of transparent Plexiglas and was illuminated by a 60-W light that was 3 feet directly above it. The two chambers were connected by a small opening (7.5 cm height × 7.5 cm width) that allowed the animals to freely enter either area. Animals were placed in the light side of the chamber facing the opening to the dark chamber and were allowed to move freely between the two compartments for 10 min. Behavior was recorded via an overhead video camera for coding at a later time by a blind observer. The number of entries into and time spent in the two compartments, as well as the number of rearings in the light compartment, was assessed as indices of anxiety and activity. The box was cleaned with 70% ethanol between subjects.
Elevated plus maze
The EPM consisted of two open and two closed arms (50 × 10 cm) that extended from a center platform and was elevated 50 cm above the floor. Testing took place in a dimly lit room, in which rats were placed in the center area of the EPM facing an open arm and allowed to move freely between the arms for 10 min. Behavior was recorded using an overhead video camera and was coded at a later time by a blind observer. The number of entries into and the amount of time spent on the open arms were assessed as indices of anxiety-related behavior. Total arm entries (open and closed) were assessed as a measure of activity. The maze was cleaned with 70% ethanol between each test.
PPI and ASR
Rats were tested for PPI 1 h after lights off in a room illuminated by dim red light. PPI was measured in ASR chambers (SR Lab startle response system; San Diego Instruments, San Diego, CA). Animals were placed into the chamber for 18 min, the first 5 min of which is an acclimation period. For the remaining 13 min, the fast muscle twitch startle responses of animals to a 100 decibel tone alone (ASR), or preceded by 100 msec by tones of 3, 8, 10, or 15 decibels were recorded via SR Lab software (San Diego Instruments). The prepulse should permit the subject to anticipate the loud pulse and consequently startle less severely. Each of these trials was repeated six times at pseudorandom intervals. After the test, animals were removed, and the chamber was cleaned with 70% ethanol.
Plasma collection and corticosterone assay
One week after PPI testing, blood was collected from rats at baseline between 0900 and 1100 h. Rats were removed from their home cage, deeply anesthetized with isoflurane, and decapitated, with trunk blood collected within 2 min of cage disturbance. All blood was collected in 1.5 ml tubes containing 250 μl of heparin and held on crushed blue ice until centrifugation. After centrifugation, plasma was collected and frozen at −20 C until the assay was performed. Plasma was assayed for corticosterone at the Diagnostic Center for Population and Animal Health at Michigan State University using a Coat-A-Count Corticosterone kit (Diagnostics Products Corp., Los Angeles, CA). All plasma samples were run in duplicate, and results were averaged. Intra- and interassay coefficients of variation were less than 5 and less than 7%, respectively. Brains were removed and weighed immediately after killing.
Statistical analysis
For PPI, a mixed design repeated measures ANOVA was used to analyze data with genotype and neonatal treatment as a between groups factors and prepulse intensity as within (or repeated) groups factor. Anxiety-related behaviors, ASR, and corticosterone levels were each analyzed separately using two-way ANOVA (neonatal treatment as one factor, genotype as another). All significant main effects or interactions were further analyzed using Tukey's multiple comparisons tests. Differences were considered significant when P < 0.05, and all data are reported as means ± sem with N the number of animals in each group.
Results
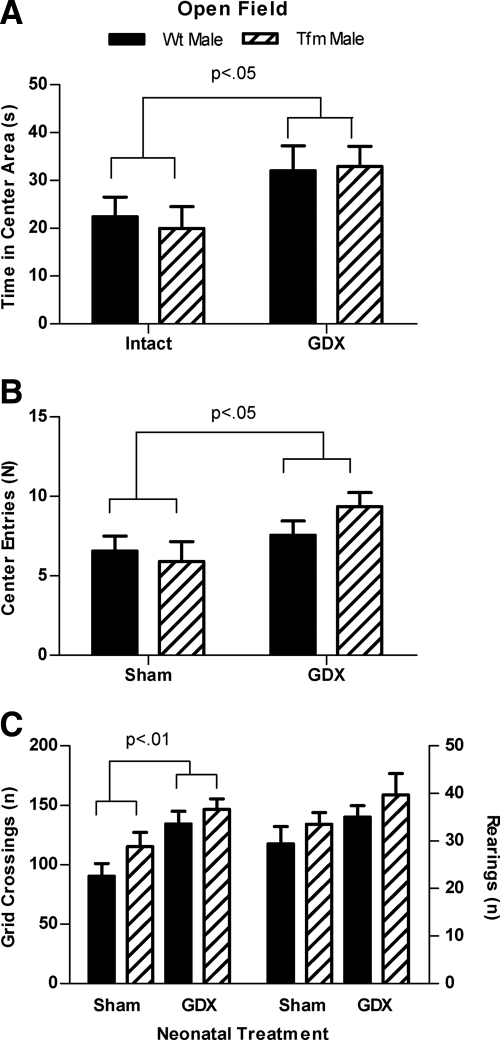
Neonatal castration of male rats reduced adult indices of anxiety in four different tests, whether the males carried a WT or Tfm allele for AR. In the open field test, a 2 × 2 ANOVA revealed a significant main effect of neonatal treatment, as Neo-Gdx rats spent more time in the center area, and visited the center area more often, than did Neo-Sham rats [F(1,35) = 6.191, P <.05; Fig. 1A] [F(1,35) = 4.763, P <.05; Fig. 1B], with no main effects of genotype nor any interactions. Neonatal castration also affected the number of grid crossings in the open field test [significant main effect of treatment F(1,35) = 12.79, P < 0.01], indicating a greater number of crossings in Neo-Gdx compared with Neo-Sham males (Fig. 1C, left), but there were no significant effects on number of rearings (Fig. 1C, right). Post hoc comparisons revealed that Neo-Sham males had significantly fewer grid crossings than did either Neo-Gdx male or Neo-Gdx Tfm groups (P < 0.05 and P < 0.01, respectively). No significant main effects of genotype or interactions were found for any measures in the open field test.
Fig. 1.
Testicular secretions during development increase indices of anxiety in the open field. Neonatal castration increased the amount of time spent in (A) and the number of visits to (B) the center area of the open field in both Tfm and WT male rats (P < 0.05). C, Neo-Gdx rats also showed a greater number of grid crossings (P < 0.01) than did Neo-Sham rats (left), but the number of rearings did not differ between groups (right). These data indicate testicular secretions during postnatal development increase anxiety-related behavior and open field activity in adulthood and can do so in the absence of functional AR. Mean ± sem.
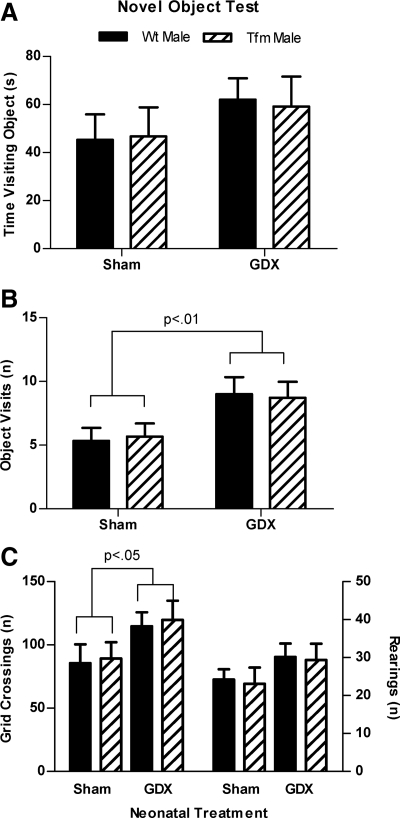
In the novel object test, neonatal gonadectomy elevated the number of novel object visits [F(1,35) = 7.872, P < 0.01; Fig. 2B] and the number of grid crossings [F(1,35) = 4.660, P < 0.05; Fig. 2C, left] with no main effects of genotype or interactions. Post hoc comparisons did not reveal any other significant group differences, indicating only an overall effect of neonatal treatment in these measures. No significant effects of treatment or genotype were found for time spent visiting the novel object (Fig. 2A) or the number of rearings (Fig. 2C, right).
Fig. 2.
Neonatal castration increases visits to a novel object in the open field. The amount of time spent with (A) and number of visits to (B) a novel object in the open field arena in Neo-Gdx or Neo-Sham Tfm and WT males. C, The number of grid crossing (left) and rearings (right) in the novel object test. Neo-Gdx rats visited the novel object more frequently than Neo-Sham rats (P < 0.05) and showed a greater number of grid crossings than did Neo-Sham rats (P < 0.05). Time spent visiting the object and the number of rearings did not significantly differ between groups. These data indicate that neonatal castration of male rats decreases indices of anxiety and increases activity in the novel object test and that an intact AR is not required for these effects. Mean ± sem.
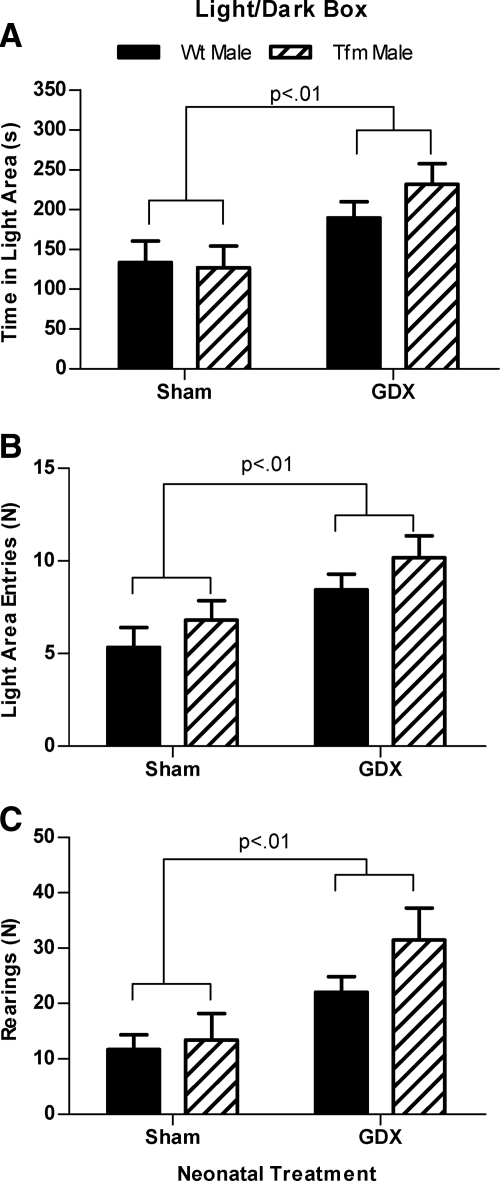
In the LD box, Neo-Gdx rats showed an elevation in time spent in the light area [F(1,34) = 9.663, P < 0.01], the number of entries into the light area [F(1,34) = 9.663, P < 0.01], and the number of rearings in the light area [F(1,34) = 9.894, P < 0.01], with no significant main effects of genotype or interaction (Fig. 3, A–C).
Fig. 3.
Developmental testicular secretions increase indices of anxiety in the LD box. A, The amount of time spent in the light area; B, number of visits to the light area; and C, rearings in the light area of the LD box. As a group, Neo-Gdx rats spent more time in (P < 0.01), showed more visits to (P < 0.01), and reared more frequently in the light area (P < 0.01) than did Neo-Sham rats. These data indicate decreased anxiety-related behavior and greater activity in the LD box after neonatal castration in rats. Mean ± sem.
Neo-Gdx rats also had more open arm entries in the EPM than Neo-Sham rats [F(1,34) = 9.894, P < 0.01; Fig. 4B]. Neonatal treatment did not significantly affect time spent on the open arms [F(1,34) = 3.682, P = 0.063; Fig. 4A] or total number of arm entries [F(1,34) = 3.707, P = 0.062; Fig. 4C], although there was a trend for neonatally gonadectomized rats to show elevations in both measures. No significant main effects of genotype or interactions were found for any measures in the EPM.
Fig. 4.
Neonatal castration increases visits to the open arms of an EPM. The amount of time spent on (A) and number of entries into (B) the open arms of the EPM in Neo-Gdx or Neo-Sham Tfm and WT males. C, The number of total (open and closed) arm entries. Neo-Gdx rats had greater number of open arm visits than Neo-Sham rats (P < 0.01). They also tended to spend more time on the open arms (P = 0.063) and show a greater number of total arm entries (P = 0.062) than Neo-Sham males. These data indicate that neonatal castration decreases several indices of anxiety and may increase activity in adult rats in the EPM. Mean ± sem.
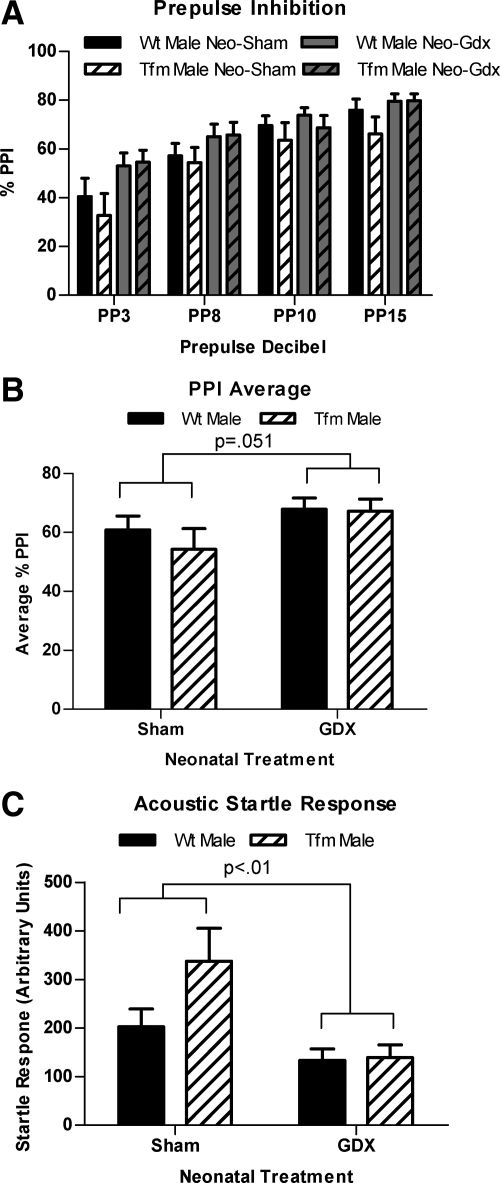
A mixed design ANOVA using genotype and neonatal treatment as between groups factors and prepulse intensity as a within groups factor revealed the expected effect of prepulse intensity [F(3,140) = 22.86, P < 0.001] in which PPI increased as intensity of the prepulse increased. There was also a significant main effect of neonatal treatment [F(1,35) = 9.970, P < 0.01) as Neo-Gdx rats showed increased PPI compared with Neo-Sham rats (Fig. 5A). There was no significant effect of genotype and no interaction. A two-way ANOVA of averaged overall PPI revealed a marginally significant main effect of neonatal treatment (P = 0.051), which again reflected increased PPI in Neo-Gdx compared with Neo-Sham rats (Fig. 5B).
Fig. 5.
Neonatal castration increases PPI. PPI across prepulses (A), averaged overall PPI (B), and ASRs to pulse alone (C) in Neo-Gdx and Neo-Sham WT and Tfm male rats. PPI was increased in Neo-Gdx compared with Neo-Sham rats across prepulses (P <0.01), an effect that was equivalent in WT and Tfm males. Similarly, averaged overall PPI was also marginally increased in Neo-Gdx compared with Neo-Sham rats (P = 0.051). ASR was decreased in Neo-Gdx compared with Neo-Sham rats (P < 0.01), a difference largely accounted for by an increased ASR in Neo-Sham Tfm males. Because developmental testicular secretions increase adult ASR only in Tfm males, there may be both AR- and ER-mediated developmental influences on this behavior. Mean ± sem.
The only effect of a dysfunctional AR on behavior was seen in ASR to a pulse alone, without any prepulse. Neonatal castration decreased ASR [F(1,35) = 9.760, P < 0.01], because Neo-Gdx rats showed a reduced ASR compared with Neo-Sham rats (Fig. 5C), which is consistent with the above findings that Neo-Gdx reduces measures of adult anxiety. Post hoc tests indicated that Neo-Sham Tfms displayed an increased ASR compared with Neo-Gdx Tfm and Neo-Gdx male groups (P < 0.05 and P < 0.01, respectively). No significant main effect of genotype or interaction was found for average ASR.
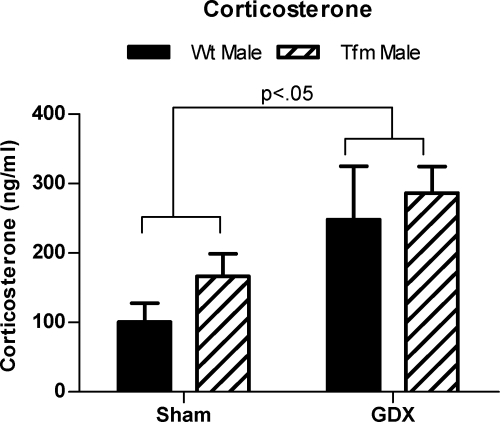
A two-way ANOVA (neonatal treatment x genotype) was performed to compare basal corticosterone levels. This analysis revealed that Neo-Gdx rats had higher basal corticosterone levels than Neo-Sham rats [F(1,34) = 8.546, P < 0.05], with no main effect of genotype or interaction (Fig. 6A). Post hoc comparisons did not reveal any further significant group differences, indicating only an overall effect of neonatal treatment in this measure.
Fig. 6.
Neonatal castration elevates corticosterone levels. Adult basal corticosterone levels were elevated by neonatal castration in both WT and Tfm male rats (P < 0.05). Mean ± sem.
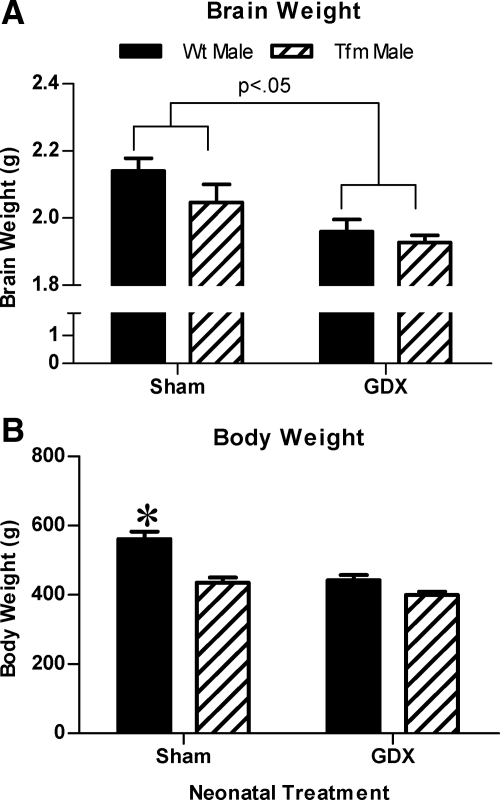
A two-way ANOVA for brain weight also revealed a significant main effect of neonatal treatment [F(1,34) = 8.546, P < 0.05], with no main effect of genotype or interaction. Post hoc comparisons revealed reduced brain weights in Neo-Gdx Tfm and Neo-Gdx male groups compared with Neo-Sham males (P < 0.01; Fig. 7A). There was a significant main effect of genotype [F(1,35) = 30.53, P < 0.001], neonatal treatment [F(1,35) = 25.66, P < 0.001], and an interaction between the two [F(1,35) = 7.60, P < 0.01) on body weight. Specifically, body weight was greater in Neo-Sham males than all other groups (all P < 0.001, Fig. 7B).
Fig. 7.
Neonatal castration affects weight of the adult body and brain. Brain (A) and body (B) weight in Neo-Gdx and Neo-Sham WT and Tfm male rats. Brain weight was greater in Neo-Sham compared with Neo-Gdx rats (P < 0.05) and was equivalent in WT and Tfm males. Thus developmental stimulation of ER may be responsible for the larger brain weight in male vs. female rats. In contrast, body weight was greater in Neo-Sham WT males compared with all other groups, suggesting that AR stimulation may normally contribute to masculinization of adult body weight. *, P < 0.001 compared with all other groups. Mean ± sem.
Discussion
Neonatal castration of male rats reduced adult indices of anxiety-related behavior in all the tests we administered. Specifically, Neo-Gdx males displayed a greater number of visits to the center area of the open field, to a novel object in the open field, to the light area of the LD box, and to the open arms of the EPM, as well as a decreased ASR compared with Neo-Sham males. Neo-Gdx rats also spent more time in the center area of the open field and light area of the LD box and tended to spend more time in the open arms of the EPM. These findings are in accord with a previous report of decreased anxiety-related behavior in the EPM in male rats that were gonadectomized at birth compared with sham-operated rats (22). Unlike that previous study, in our study, T levels were equilibrated in adulthood, strengthening the idea that T exposure during development increases adult expression of anxiety. Thus, these findings also indicate that sex differences in anxiety behaviors that have been reported in rats (females less than males, see Refs. 18–23, 50) may, at least partially, be related to organizational effects of T, which could not have been concluded from studies that failed to equilibrate adult androgen levels. However, because we did not directly compare females in this study, this possibility is only speculative. Because we see these same effects of neonatal castration in Tfm rats carrying a dysfunctional AR, these organizational effects of T to increase adult anxiety-related behavior do not appear to be regulated through AR. That conclusion is in contrast with our previous findings that the “activational” effects of adult T treatment, which reduces anxiety-related behavior in WT males, has no such effect on Tfm mice (6). Thus, although neonatal and postnatal T increases adult anxiety-related behaviors in an AR-independent manner, adult T exerts an opposing effect by acting through AR.
Several activity measures (grid crossings in the open field and novel object tests, transitions between compartments and rearings in the light area of the LD box, and total arm entries in the EPM) were elevated in Neo-Gdx compared with Neo-Sham rats. These data are also in accord with previous reports that Neo-Gdx rats show increased ambulation in the open field and more total arm entries in the EPM (51, 22). Therefore, data from this study and others indicate that the presence of T during development contributes to sex differences in activity in adult rodents (females generally more active than males). Because there were no differences in activity measures between Neo-Sham Tfm and Neo-Sham WT males, our data also indicate that T does not act through the AR to have these effects on activity. Thus T's organizational influence on activity may be ER mediated.
There was only a single exception to the above pattern that AR is irrelevant to developmental T effects on adult anxiety-related behaviors, and that was in the ASR, which has also been suggested to reflect anxiety in mice and rats (52–55). Again, Neo-Sham rats showed a greater ASR than Neo-Gdx rats, but this difference was largely due to effects in Tfm rats. Neo-Sham Tfm males showed a moderately increased startle response compared with Neo-Sham males and a significantly increased startle response compared with Neo-Gdx males of both genotypes (see Fig. 5C). Thus, removing developmental testicular secretions from Tfm rats, but not WT rats, decreases ASR. This result indicates a complex relationship between AR and possibly ER in the display of this behavior.
PPI was increased in Neo-Gdx rats compared with Neo-Sham rats, indicating an organizational effect of T in sensorimotor gating. Gonadal hormone administration in adulthood was previously shown to influence PPI in rats (43, 44), but to our knowledge, this is the first evidence of an organizational effect of hormones on PPI in rodents. A recent report in nonhuman primates indicated that gonadectomy before puberty also resulted in increased PPI (56). Thus, differences in PPI in the present study may also be regulated by pubertal rather than neonatal hormones, as further studies would be needed to define the sensitive period. Again, this effect on PPI appears to be independent of AR activation, because there were no differences between Neo-Sham Tfm and Neo-Sham WT males. Because deficits in sensorimotor gating are associated with disorders, including schizophrenia, autism, and Tourette's syndrome (57–59), our findings suggest that exposure to androgens during development predisposes males to these disorders.
Our finding of elevated basal corticosterone levels in Neo-Gdx rats agrees with another report of increased adult basal corticosterone in neonatally gonadectomized rats (37). Prenatal administration of flutamide (an AR antagonist) or 1,4,6-androstatriene-3,17-dione (an aromatase inhibitor) also results in increased basal corticosterone levels in rats, indicating that androgens act through both ARs and ERs around the time of birth to organize basal corticosterone levels (36). However, we found no significant differences in basal corticosterone levels between Tfm and WT males among Neo-Sham groups, which suggest that AR deficiency during ontogeny does not affect basal adrenal hormone levels in rats. In contrast, stress induced levels of corticosterone do appear to be AR dependent. Our previous studies found that exposure to an open field with a novel object triggered a larger corticosterone response in Tfm males than WT males, in both mice (6) and rats (Zuloaga, D. Z., unpublished observation).
In the present study, increased basal corticosterone levels did not correlate positively with anxiety-related behaviors as in other studies (6, 26), because neonatal castration increased levels of the hormone while decreasing anxiety. However, occasional disconnects between these two measured have been shown, particularly in the context of hormone effects. For instance, treatment of adult rats with estradiol results in decreases in anxiety-related behaviors (25) but increases in corticosterone levels (60). Similarly, female rats commonly show the same pattern of decreased anxiety and increased stress hormone levels compared with male rats (23, 34). Together, these findings indicate that anxiety and stress hormone levels do not necessarily correlate and underlines the point that these functions are mechanistically distinct and may therefore be differentially affected by developmental androgens.
Brain weight analysis also revealed an effect of neonatal treatment on brain weight with Neo-Sham rats having heavier brains than Neo-Gdx rats. This difference was largely accounted for by heavier brain weights in Neo-Sham WT males compared with both Neo-Gdx groups, although Neo-Sham Tfm males also tended to have heavier brains than did Neo-Gdx rats. Decreased brain weight in Neo-Gdx rats compared with Neo-Sham WT males resembles generalized sex differences in brain weight (males more than females, see Refs. 45, 61) and indicate that adult brain weight is increased by testicular secretions during development in male rats. Confirming our previous findings in gonadally intact rats (45), brain weight did not significantly differ between Tfm and WT males, indicating that T increases brain weight via an AR-independent mechanism. Because the Tfm mutation decreases body weight, but has no effect on brain weight, an effect seen in both rats and mice (45), masculinization of these two parameters are at least partially independent in rodents.
AR signaling is not completely eliminated in Tfm males but is estimated to be reduced by 85–90% (46). This reduced sensitivity is enough to result in a completely feminine exterior, in terms of feminized anogenital distance, the presence of nipples, and an external vagina (45). Alternatively, postnatal androgens may act through aromatization and stimulation of ERs to affect behaviors measured in this study. Supporting this possibility are reports that ovarian hormones, acting both in development and adulthood, also appear to contribute to these sex differences (23, 25). Studies in Tfm rats indicate that estrogen binding in the brain is similar to WT males and aromatization also normally occurs in the adult Tfm brain (developmental levels are unknown), although it is reduced within several brain regions (62). Testicular secretions during the neonatal period in Tfm rats have also been shown to permanently masculinize sexual behavior (63). These findings (along with the evidence that androgens act through ERs during this period to masculinize sexual behavior) (10–11) indicate that ER activation is near normal in developing Tfm rats. However, because ER activation was not a focus of the current study, we cannot conclude that the effects reported here result from such activation.
Presumably, all of these behavioral and endocrine effects of developmental androgens occur as a consequence of alterations in specific brain regions. Androgens may act on several stress/androgen responsive brain regions to affect anxiety-related behaviors and the HPA axis, including the amygdala, hippocampus, paraventricular nucleus of the hypothalamus, and bed nucleus of the stria terminalis (31, 55, 64, 65). Sensorimotor gating (specifically PPI) is also mediated by a complex network of brain regions, including the prefrontal cortex, basolateral amygdala, nucleus accumbens, and ventral tegmental area among many others (66). During postnatal development, androgens may interact with these brain regions to alter the adult display of behaviors.
In conclusion, Neo-Gdx rats showed reduced indices of anxiety compared with sham-operated rats in several different tests thought to reflect anxiety (open field, novel object test, LD box, EPM, and ASR), indicating that testicular secretions during postnatal development normally result in increased anxiety in adult male rats. Virtually all of these effects of neonatal testicular secretions are equivalent in WT and Tfm males, indicating that AR does not mediate these developmental effects, and stands in contrast to our previous findings in Tfm mice that indicate AR in adulthood have anxiolytic effects (6). Compared with Neo-Sham rats, Neo-Gdx rats also showed increased PPI and corticosterone levels as well as decreased brain weights, and these effects are also independent of AR. Only in ASR, which was decreased by neonatal gonadectomy only in Tfm males, did we find evidence that developmental AR stimulation may affect adult anxiety.
Together, these findings indicate a role of T before adulthood in the organization of anxiety-related behaviors, locomotor activity, the HPA axis, sensorimotor gating, and brain weight and indicate that a functional AR is not required for these masculinizing effects of T during postnatal development.
Acknowledgments
We thank Diane Redenius, Heather Malinowski, Sandra Troxell, and Susan Beyerlein for their expert technical assistance.
This work was supported by National Institutes of Health (NIH) Grant R01 NS028421 and NIH Predoctoral Fellowship F31 MH78273.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AR
- Androgen receptor
- ASR
- acoustic startle response
- EPM
- elevated plus maze
- ER
- estrogen receptor
- HPA
- hypothalamic-pituitary-adrenal
- LD box
- light/dark box
- Neo-Gdx
- neonatally gonadectomized
- Neo-Sham
- neonatally sham operated
- PPI
- prepulse inhibition
- T
- testosterone
- Tfm
- testicular feminization mutation
- WT
- wild type.
References
- 1. Phoenix CH, Goy RW, Gerall AA, Young WC. 1959. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65:369–382 [DOI] [PubMed] [Google Scholar]
- 2. Jacobson CD, Csernus VJ, Shryne JE, Gorski RA. 1981. The influence of gonadectomy, androgen exposure, or a gonadal graft in the neonatal rat on the volume of the sexually dimorphic nucleus of the preoptic area. J Neurosci 1:1142–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Breedlove SM, Jacobson CD, Gorski RA, Arnold AP. 1982. Masculinization of the female rat spinal cord following a single neonatal injection of testosterone propionate but not estradiol benzoate. Brain Res 237:173–181 [DOI] [PubMed] [Google Scholar]
- 4. Arnold AP, Breedlove SM. 1985. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav 19:469–498 [DOI] [PubMed] [Google Scholar]
- 5. MacLusky NJ, Naftolin F. 1981. Sexual differentiation of the central nervous system. Science 211:1294–1302 [DOI] [PubMed] [Google Scholar]
- 6. Zuloaga DG, Morris JA, Jordan CL, Breedlove SM. 2008. Mice with the testicular feminization mutation demonstrate a role for androgen receptors in the regulation of anxiety-related behaviors and the hypothalamic-pituitary-adrenal axis. Horm Behav 54:758–766 [DOI] [PubMed] [Google Scholar]
- 7. Dugger BN, Morris JA, Jordan CL, Breedlove SM. 2007. Androgen receptors are required for full masculinization of the ventromedial hypothalamus (VMH) in rats. Horm Behav 51:195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Durazzo A, Morris JA, Breedlove SM, Jordan CL. 2007. Effects of the testicular feminization mutation (Tfm) of the androgen receptor gene on BSTMPM volume and morphology in rats. Neurosci Lett 419:168–171 [DOI] [PubMed] [Google Scholar]
- 9. Morris JA, Jordan CL, Dugger BN, Breedlove SM. 2005. Partial demasculinization of several brain regions in adult male (XY) rats with a dysfunctional androgen receptor gene. J Comp Neurol 487:217–226 [DOI] [PubMed] [Google Scholar]
- 10. Ogawa S, Chester AE, Hewitt SC, Walker VR, Gustafsson JA, Smithies O, Korach KS, Pfaff DW. 2000. Abolition of male sexual behaviors in mice lacking estrogen receptors α and β (α β ERKO). Proc Natl Acad Sci USA 97:14737–14741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Södersten P. 1978. Effects of anti-oestrogen treatment of neonatal male rats on lordosis behaviour and mounting behaviour in the adult. J Endocrinol 76:241–249 [DOI] [PubMed] [Google Scholar]
- 12. Juntti SA, Tollkuhn J, Wu MV, Fraser EJ, Soderborg T, Tan S, Honda S, Harada N, Shah NM. 2010. The androgen receptor governs the execution, but not programming, of male sexual and territorial behaviors. Neuron 66:260–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raskin K, de Gendt K, Duittoz A, Liere P, Verhoeven G, Tronche F, Mhaouty-Kodja S. 2009. Conditional inactivation of androgen receptor gene in the nervous system: effects on male behavioral and neuroendocrine responses. J Neurosci 29:4461–4470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Isgor C, Sengelaub DR. 2003. Effects of neonatal gonadal steroids on adult CA3 pyramidal neuron dendritic morphology and spatial memory in rats. J Neurobiol 55:179–190 [DOI] [PubMed] [Google Scholar]
- 15. Williams CL, Barnett AM, Meck WH. 1990. Organizational effects of early gonadal secretions on sexual differentiation in spatial memory. Behav Neurosci 104:84–97 [DOI] [PubMed] [Google Scholar]
- 16. Jones BA, Watson NV. 2005. Spatial memory performance in androgen insensitive male rats. Physiol Behav 85:135–141 [DOI] [PubMed] [Google Scholar]
- 17. Isgor C, Sengelaub DR. 1998. Prenatal gonadal steroids affect adult spatial behavior, CA1 and CA3 pyramidal cell morphology in rats. Horm Behav 34:183–198 [DOI] [PubMed] [Google Scholar]
- 18. Archer J. 1975. Rodent sex differences in emotional and related behavior. Behav Biol 14:451–479 [DOI] [PubMed] [Google Scholar]
- 19. Masur J, Schutz MT, Boerngen R. 1980. Gender differences in open-field behavior as a function of age. Dev Psychobiol 13:107–110 [DOI] [PubMed] [Google Scholar]
- 20. Slob AK, Bogers H, van Stolk MA. 1981. Effects of gonadectomy and exogenous gonadal steroids on sex differences in open field behaviour of adult rats. Behav Brain Res 2:347–362 [DOI] [PubMed] [Google Scholar]
- 21. Seliger DL. 1977. Effects of age, sex, and brightness of field on open-field behaviors of rats. Percept Mot Skills 45(3 Pt 2):1059–67 [DOI] [PubMed] [Google Scholar]
- 22. Lucion AB, Charchat H, Pereira GA, Rasia-Filho AA. 1996. Influence of early postnatal gonadal hormones on anxiety in adult male rats. Physiol Behav 60:1419–1423 [DOI] [PubMed] [Google Scholar]
- 23. Zimmerberg B, Farley MJ. 1993. Sex differences in anxiety behavior in rats: role of gonadal hormones. Physiol Behav 54:1119–1124 [DOI] [PubMed] [Google Scholar]
- 24. Frye CA, Lacey EH. 2001. Posttraining androgens' enhancement of cognitive performance is temporally distinct from androgens' increases in affective behavior. Cogn Affect Behav Neurosci 1:172–182 [DOI] [PubMed] [Google Scholar]
- 25. Walf AA, Frye CA. 2005. Antianxiety and antidepressive behavior produced by physiological estradiol regimen may be modulated by hypothalamic-pituitary-adrenal axis activity. Neuropsychopharmacology 30:1288–1301 [DOI] [PubMed] [Google Scholar]
- 26. Lund TD, Rovis T, Chung WC, Handa RJ. 2005. Novel actions of estrogen receptor-β on anxiety-related behaviors. Endocrinology 146:797–807 [DOI] [PubMed] [Google Scholar]
- 27. Frye CA, Edinger K, Sumida K. 2008. Androgen administration to aged male mice increases anti-anxiety behavior and enhances cognitive performance. Neuropsychopharmacology 33:1049–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bing O, Heilig M, Kakoulidis P, Sundblad C, Wiklund L, Eriksson E. 1998. High doses of testosterone increase anticonflict behaviour in rat. European Neuropsychopharmacology 8:321–323 [DOI] [PubMed] [Google Scholar]
- 29. Bitran D, Kellogg CK, Hilvers RJ. 1993. Treatment with an anabolic-androgenic steroid affects anxiety-related behavior and alters the sensitivity of cortical GABAA receptors in the rat. Horm Behav 27:568–583 [DOI] [PubMed] [Google Scholar]
- 30. Imwalle DB, Gustafsson JA, Rissman EF. 2005. Lack of functional estrogen receptor β influences anxiety behavior and serotonin content in female mice. Physiol Behav 84:157–163 [DOI] [PubMed] [Google Scholar]
- 31. Edinger KL, Frye CA. 2006. Intrahippocampal administration of an androgen receptor antagonist, flutamide, can increase anxiety-like behavior in intact and DHT-replaced male rats. Horm Behav 50:216–222 [DOI] [PubMed] [Google Scholar]
- 32. Fernández-Guasti A, Martínez-Mota L. 2005. Anxiolytic-like actions of testosterone in the burying behavior test: role of androgen and GABA-benzodiazepine receptors. Psychoneuroendocrinology 30:762–770 [DOI] [PubMed] [Google Scholar]
- 33. Seale JV, Wood SA, Atkinson HC, Harbuz MS, Lightman SL. 2004. Gonadal steroid replacement reverses gonadectomy-induced changes in the corticosterone pulse profile and stress-induced hypothalamic-pituitary-adrenal axis activity of male and female rats. J Neuroendocrinol 16:989–998 [DOI] [PubMed] [Google Scholar]
- 34. Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. 1994. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav 28:464–476 [DOI] [PubMed] [Google Scholar]
- 35. Handa RJ, Nunley KM, Lorens SA, Louie JP, McGivern RF, Bollnow MR. 1994. Androgen regulation of adrenocorticotropin and corticosterone secretion in the male rat following novelty and foot shock stressors. Physiol Behav 55:117–124 [DOI] [PubMed] [Google Scholar]
- 36. Seale JV, Wood SA, Atkinson HC, Lightman SL, Harbuz MS. 2005. Organizational role for testosterone and estrogen on adult hypothalamic-pituitary-adrenal axis activity in the male rat. Endocrinology 146:1973–1982 [DOI] [PubMed] [Google Scholar]
- 37. Bingham B, Viau V. 2008. Neonatal gonadectomy and adult testosterone replacement suggest an involvement of limbic arginine vasopressin and androgen receptors in the organization of the hypothalamic-pituitary-adrenal axis. Endocrinology 149:3581–3591 [DOI] [PubMed] [Google Scholar]
- 38. Swerdlow NR, Auerbach P, Monroe SM, Hartston H, Geyer MA, Braff DL. 1993. Men are more inhibited than women by weak prepulses. Biol Psychiat 34:253–260 [DOI] [PubMed] [Google Scholar]
- 39. Lehmann J, Pryce CR, Feldon J. 1999. Sex differences in the acoustic startle response and prepulse inhibition in Wistar rats. Behav Brain Res 104:113–117 [DOI] [PubMed] [Google Scholar]
- 40. Ralph RJ, Paulus MP, Fumagalli F, Caron MG, Geyer MA. 2001. Prepulse inhibition deficits and perseverative motor patterns in dopamine transporter knock-out mice: differential effects of D1 and D2 receptor antagonists. J Neurosci 21:305–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ison JR, Allen PD. 2007. Pre- but not post-menopausal female CBA/CaJ mice show less prepulse inhibition than male mice of the same age. Behav Brain Res 185:76–81 [DOI] [PubMed] [Google Scholar]
- 42. Jovanovic T, Szilagyi S, Chakravorty S, Fiallos AM, Lewison BJ, Parwani A, Schwartz MP, Gonzenbach S, Rotrosen JP, Duncan EJ. 2004. Menstrual cycle phase effects on prepulse inhibition of acoustic startle. Psychophysiology 41:401–406 [DOI] [PubMed] [Google Scholar]
- 43. Van den Buuse M, Eikelis N. 2001. Estrogen increases prepulse inhibition of acoustic startle in rats. Eur J Pharmacol 425:33–41 [DOI] [PubMed] [Google Scholar]
- 44. Gogos A, van den Buuse M. 2003. Castration reduces the effect of serotonin-1A receptor stimulation on prepulse inhibition in rats. Behav Neurosci 117:1407–1415 [DOI] [PubMed] [Google Scholar]
- 45. Zuloaga DG, Puts DA, Jordan CL, Breedlove SM. 2008. The role of androgen receptors in the masculinization of brain and behavior: what we've learned from the testicular feminization mutation. Horm Behav 53:613–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yarbrough WG, Quarmby VE, Simental JA, Joseph DR, Sar M, Lubahn DB, Olsen KL, French FS, Wilson EM. 1990. A single base mutation in the androgen receptor gene causes androgen insensitivity in the testicular feminized rat. J Biol Chem 265:8893–8900 [PubMed] [Google Scholar]
- 47. Naess O, Haug E, Attramadal A, Aakvaag A, Hansson V, French F. 1976. Androgen receptors in the anterior pituitary and central nervous system of the androgen “insensitive” (Tfm) rat: correlation between receptor binding and effects of androgens on gonadotropin secretion. Endocrinology 99:1295–1303 [DOI] [PubMed] [Google Scholar]
- 48. Fernandez R, Collado P, Garcia Doval S, Garcia-Falgueras A, Guillamon A, Pasaro E. 2003. A molecular method for classifying the genotypes obtained in a breeding colony from testicular feminized (Tfm) rats. Horm Metab Res 35:197–200 [DOI] [PubMed] [Google Scholar]
- 49. Morris JA, Jordan CL, King ZA, Northcutt KV, Breedlove SM. 2008. Sexual dimorphism and steroid responsiveness of the posterodorsal medial amygdala in adult mice. Brain Res 1190:115–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Imhof JT, Coelho ZM, Schmitt ML, Morato GS, Carobrez AP. 1993. Influence of gender and age on performance of rats in the elevated plus maze apparatus. Behav Brain Res 56:177–180 [DOI] [PubMed] [Google Scholar]
- 51. Slob AK, Huizer T, Van der Werff ten Bosch JJ. 1986. Ontogeny of sex differences in open-field ambulation in the rat. Physiol Behav 37:313–315 [DOI] [PubMed] [Google Scholar]
- 52. Walker DL, Davis M. 1997. Anxiogenic effects of high illumination levels assessed with the acoustic startle response in rats. Biol Psychiat 42:461–471 [DOI] [PubMed] [Google Scholar]
- 53. Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. 1997. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacol Berl 132:107–124 [DOI] [PubMed] [Google Scholar]
- 54. Belzung C, Le Guisquet AM, Crestani F. 2000. Flumazenil induces benzodiazepine partial agonist-like effects in BALB/c but not C57BL/6 mice. Psychopharmacol 148:24–32 [DOI] [PubMed] [Google Scholar]
- 55. Hode Y, Ratomponirina C, Gobaille S, Maitre M, Kopp C, Misslin R. 2000. Hypoexpression of benzodiazepine receptors in the amygdala of neophobic BALB/c mice compared to C57BL/6 mice. Pharmacol Biochem Behav 65:35–38 [DOI] [PubMed] [Google Scholar]
- 56. Morris RW, Fung SJ, Rothmond DA, Richards B, Ward S, Noble PL, Woodward RA, Weickert CS, Winslow JT. 2010. The effect of gonadectomy on prepulse inhibition and fear-potentiated startle in adolescent rhesus macaques. Psychoneuroendocrinology 35:896–905 [DOI] [PubMed] [Google Scholar]
- 57. Kumari V, Aasen I, Sharma T. 2004. Sex differences in prepulse inhibition deficits in chronic schizophrenia, Schizophr Res 69:219–235 [DOI] [PubMed] [Google Scholar]
- 58. Perry W, Minassian A, Lopez B, Maron L, Lincoln A. 2007. Sensorimotor gating deficits in adults with autism, Biol Psychiat 61:482–486 [DOI] [PubMed] [Google Scholar]
- 59. Castellanos FX, Fine EJ, Kaysen D, Marsh WL, Rapoport JL, Hallett M. 1996. Sensorimotor gating in boys with Tourette's syndrome and ADHD: preliminary results. Biol Psychiat 39:33–41 [DOI] [PubMed] [Google Scholar]
- 60. Weiser MJ, Handa RJ. 2009. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor α within the hypothalamus. Neuroscience 159:883–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Peiffer AM, Fitch RH, Thomas JJ, Yurkovic AN, Rosen GD. 2003. Brain weight differences associated with induced focal microgyria. BMC Neurosci 24:4–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Roselli CE, Salisbury RL, Resko JA. 1987. Genetic evidence for androgen-dependent and independent control of aromatase activity in the rat brain. Endocrinology 121:2205–2210 [DOI] [PubMed] [Google Scholar]
- 63. Olsen KL. 1979. Androgen-insensitive rats are defeminised by their testes. Nature 279:238–239 [DOI] [PubMed] [Google Scholar]
- 64. Gerrits M, Grootkarijn A, Bekkering BF, Bruinsma M, Den Boer JA, Ter Horst GJ. 2005. Cyclic estradiol replacement attenuates stress-induced c-Fos expression in the PVN of ovariectomized rats. Brain Res Bull 67:147–155 [DOI] [PubMed] [Google Scholar]
- 65. Toufexis D, Davis C, Hammond A, Davis M. 2005. Sex differences in hormonal modulation of anxiety measured with light-enhanced startle: possible role for arginine vasopressin in the male. J Neurosci 25:9010–9016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Koch M. 1999. The neurobiology of startle. Prog Neurobiol 59:107–128 [DOI] [PubMed] [Google Scholar]