Nearby construction can cause subtle, but important activation of the hypothalamic-pituitary-adrenal axis in laboratory rats.
Abstract
Although loud noise and intense vibration are known to alter the behavior and phenotype of laboratory animals, little is known about the effects of nearby construction. We studied the effect of a nearby construction project on the classic stress hormones ACTH, corticosterone, renin, and aldosterone in rats residing in a barrier animal facility before, for the first 3 months of a construction project, and at 1 month after all construction was completed. During some of the construction, noise and vibrations were not obvious to investigators inside the animal rooms. Body weight matched for age was not altered by nearby construction. During nearby construction, plasma ACTH, corticosterone, and aldosterone were approximately doubled compared with those of pre- and postconstruction levels. Expression of CRH mRNA in the paraventricular nucleus of the hypothalamus, CRH receptor and POMC mRNA in the anterior pituitary, and most mRNAs for steroidogenic genes in the adrenal gland were not significantly changed during construction. We conclude that nearby construction can cause a stress response without long-term effects on hypothalamic-pituitary-adrenal axis gene expression and body weight.
It is generally accepted that the hypothalamic-pituitary-adrenal (HPA) axis is the archetypal stress-responsive system (1). Although stress is difficult to precisely define, it is known that noise and vibration are stressful stimuli that can activate the HPA axis (2–4).
Laboratory animals are usually housed under highly controlled environmental conditions with preset light-dark cycles and temperature and humidity ranges. Obviously, construction projects are occasionally necessary in or adjacent to animal facilities, and most veterinarians understand that minimizing the disruption to resident animals should be of paramount importance.
It is therefore surprising that there are only a few published reports suggesting that nearby construction projects are stressful to laboratory animals (5–9). This is more than a mere annoyance because it has been demonstrated that nearby construction can actually alter changes in phenotype associated with changes in background genotype (5). A build-out, remodeling, and outside landscaping project in and around our animal facility gave us a unique opportunity to evaluate the effects of construction, noise, and vibration on a colony of laboratory rats residing in our barrier facility. We measured plasma ACTH, corticosterone, renin activity, and aldosterone in groups of rats before, during, and then after construction (when construction had ceased for at least a month). We also evaluated hypothalamic, pituitary, and adrenal gene expression in tissue samples of these rats.
Materials and Methods
WAG/Rij/MCW male rats (n = 40; aged 8–18 wk at the time of experimentation), were maintained on sterilized rat chow in a moderate-security barrier facility at the Biomedical Resource Center of the Medical College of Wisconsin (Milwaukee, WI). All rats were bred and born in the barrier facility in a room just adjacent to where they were housed for experimentation. Rats were moved to the adjacent housing room at 5–8 wk of age and were housed two per cage on a 12-h light, 12h dark cycle (lights on at 0600 h) at an ambient temperature of 21 C. Rats were handled daily on the 3 d before experimentation as described previously (10) to minimize the acute stress responses due to handling on the experimental day. Rats were killed by rapid decapitation between 0800 and 0900 h. This occurred on 4 separate days for 2 months before construction started (four rats per day; 16 total), on 4 separate days separated by 2 wk for the first 3 months of construction (four rats per day; 16 total), and on one occasion at least 1 month after all construction had stopped (eight rats). This last time point was about 14 months after the construction had started. Only the preconstruction rats were two different ages at the time of study (two groups of 8 wk old rats and two groups of 18 wk old rats). The rats studied during construction were all 18 wk old when decapitated, had been in the facility since birth, and had been housed during construction for the last 6 wk (one group of four rats), 10 wk (one group of four rats), or all 18 wk (two groups of four rats each) before the day of decapitation. The rats studied 1 month after the end of construction were 18 wk old on the day of decapitation and had been housed during construction for the first 12 wk of life. Animal care was in accordance with National Institutes of Health guidelines. The Animal Care and Use Committee at the Medical College of Wisconsin approved all procedures.
Construction started at 0600 h each day. Construction inside and outside the animal facility started with low noise levels involving exterior work, building a construction fence, and, most importantly, use of earth-moving equipment and their associated noises. For most of the during-construction period, the noise and vibration levels were high as reported by the animal facility personnel and the construction company, although the noise was not always audible to workers inside the rat room. Construction involved wall demolition, jack-hammering, underground sanitation installation, carpentry, wall excavation, trench digging, and landscaping directly outside the facility. The proximity of the construction to the animal rooms was no less than 100 ft. Subjective observations of several investigators revealed a lack of correlation between the construction company's daily assessment of noise/vibration levels and the impression of investigators inside the animal facility. Therefore, for the purposes of this report, during construction was assumed to be any construction occurring in or near the facility.
Trunk blood was obtained from all rats. Anterior pituitary glands and adrenal glands (four per day) were frozen in liquid nitrogen. Brains were frozen in methylbutane on dry ice and then kept frozen on dry ice. All frozen tissue was subsequently stored in a −70 C freezer until further analysis.
Plasma ACTH, corticosterone, and aldosterone were measured by RIA as described previously (11). Plasma renin activity was measured as angiotensin I generation in vitro as described previously (12). Pituitary CRH receptor (Crhr1) and proopiomelanocortin (Pomc) mRNA expression was analyzed by quantitative PCR as described previously (10). Essential components of the adrenal steroidogenic pathway were also evaluated by quantitative PCR (13). Each sample was assayed in triplicate. Gene expression was quantified using the number of cycles to reach a predetermined threshold value in the intensity of the PCR signal (the cycle threshold value). An index of fold change in mRNA (2-ΔΔCt) was calculated as described previously (14). In situ hybridization histochemistry using four brains from before and four from during construction was performed to assess hypothalamic paraventricular nucleus (PVN) Crh mRNA expression as described previously (15–17).
Data were analyzed by t test or one-factor ANOVA followed by Duncan's multiple range test. The ACTH results were not normally distributed and were analyzed by Kruskal-Wallis one-way ANOVA on ranks followed by the Dunn's all pairwise comparison procedure. All analyses were performed using Sigmastat 3.0 (Systat Software, San Jose, CA), and P < 0.05 was considered statistically significant. Data are reported as mean ± se.
Results
Plasma ACTH, corticosterone, and aldosterone were significantly increased, being approximately doubled during construction, and returned to normal after construction had completely ceased for at least 1 month (Fig. 1). Plasma renin activity was not significantly increased during construction (5.4 ± 0.5 ng/ml · h; n = 16) compared with before (5.0 ± 0.5 ng/ml · h; n = 16) or after (3.8 ± 0.3 ng/ml · h; n = 8) construction (P = 0.19). Body weight matched for age was not different before (302 ± 12 g), during (307 ± 6 g), or after (298 ± 11 g) construction (P = 0.745). There were no statistical differences in these variables between the four groups of rats studied during construction, regardless of how many weeks during their life they had been exposed to construction.
Fig. 1.
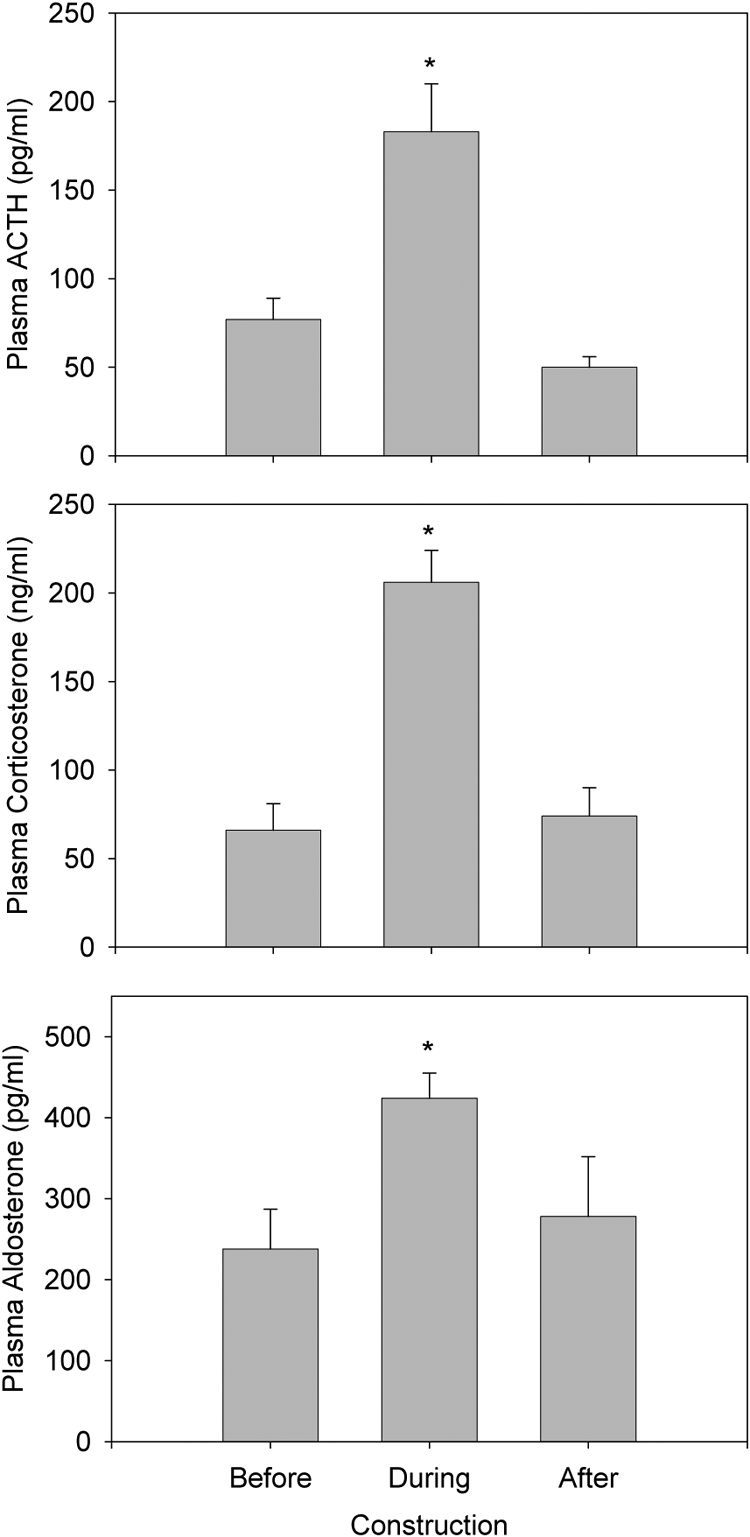
Plasma ACTH, corticosterone, and aldosterone over the 2 months before construction (four rats each day; n = 16), during the first 3 months of construction (four rats each day; n = 16), and on one occasion 1 month after the construction had stopped (n = 8). *, Different from before and after construction (P < 0.015).
There were no significant effects of construction on pituitary or adrenocortical gene expression except for a trivial (4%) increase in Star mRNA (Table 1). There was no significant difference in the PVN Crh mRNA expression before (67.7 ± 3.3, corrected gray area) compared with during construction (59.5 ± 2.4, corrected gray area) as assessed by in situ hybridization histochemistry (P = 0.08).
Table 1.
Pituitary and adrenal mRNA by quatitative PCR
| Gene symbol | Cycles to threshold (Ct) |
P | 2−ΔΔCt | |
|---|---|---|---|---|
| Before construction | Construction | |||
| Pituitary | ||||
| Pomc | 18.16 ± 0.16 | 18.22 ± 0.08 | 0.794 | |
| Crhr1 | 28.48 ± 0.06 | 28.38 ± 0.06 | 0.265 | |
| Adrenal | ||||
| Mc2r | 27.73 ± 0.39 | 28.17 ± 0.09 | 0.312 | |
| Ldlr | 29.80 ± 0.30 | 28.83 ± 0.52 | 0.156 | |
| Star | 20.78 ± 0.03 | 20.63 ± 0.03 | 0.009 | 1.04 |
| Cyp11a1 | 22.02 ± 0.06 | 22.22 ± 0.06 | 0.057 | |
| Cyp21a1 | 21.78 ± 0.10 | 21.87 ± 0.07 | 0.462 | |
| Cyp11b1 | 25.07 ± 0.07 | 25.36 ± 0.16 | 0.150 | |
| Cyp11b2 | 28.02 ± 0.24 | 28.34 ± 0.17 | 0.321 | |
| Actb | 20.76 ± 0.12 | 20.68 ± 0.09 | 0.624 | |
The full name of each gene listed above is as follows: proopiomelanocortin (Pomc); CRH receptor-1 (Crhr1); steroidogenic acute regulatory protein (Star); low-density lipoprotein receptor (Ldlr); P450scc (Cyp11a1); P45021a1 (Cyp21a1); melanocortin 2/ACTH receptor (Mc2r); P45011b1 (Cyp11b1); P45011b2 (Cyp11b2); β-actin (Actb) (n = 4 tissue pools per experimental group). Note that a decrease in Ct reflects an increase in mRNA.
Discussion
This study demonstrated a doubling of basal plasma ACTH, corticosterone, and aldosterone concentrations in laboratory rats during nearby construction. The magnitude of the increases in plasma ACTH and corticosterone are similar to those obtained with stimuli acknowledged to be stressors in laboratory rodents (6). Interestingly, plasma renin activity, body weight, and HPA axis gene expression were not significantly altered by nearby construction, except for an extremely minor increase in adrenocortical Star mRNA, unlikely to have an important physiological effect (13). The increase in plasma aldosterone was likely due to the effect of an acute increase in plasma ACTH (18), rather than due to renin-induced angiotensin II.
The results demonstrate an acute stress-induced increase in HPA axis activity, particularly because body weight, renin, and a comprehensive list of mRNA expression of relevant genes were not affected. This suggests that the early morning construction noise resulted in an acute stress response but that food, salt, and water intake were probably normal. Consistent with this assessment, the observed increases in plasma ACTH and corticosterone are comparable with levels seen at the peak of the circadian rhythm and after mild acute stressors such as open-field or elevated plus maze (19–21). In fact, noise alone and/or construction seems to cause a subtle increase in the activity of the HPA axis (6, 22). As little as 85 dB (equivalent to city noise while inside an automobile with the windows closed) can result in a significant increase in plasma ACTH and corticosterone in laboratory rats (2). Given this construction-induced elevation in ACTH and corticosterone secretion, it is somewhat surprising that these changes were not accompanied by elevated CRH mRNA, as is the case after a chronic variable stress regimen that also produces more modest increases in basal ACTH and corticosterone than in the present study (15). The lack of elevated CRH mRNA seen in this study most likely reflects the net effect of opposing factors: increased excitatory neural drive to the PVN and greater activation of glucocorticoid feedback mechanisms. Previous studies have also demonstrated effects of construction and building work on glucose transporter-2-mediated glucose absorption (7), immune function (8), and addictive behavior (9) in rats and food intake in laboratory mice and rats (5).
Although it is difficult to quantify the magnitude of the disruption caused by construction, our finding is an important warning to investigators that even normal body weight and plasma renin activity does not exclude the potential for a significant effect of nearby construction and noise on physiological systems sensitive to stress. What was remarkable was that the disruption caused by construction was often not audible to workers inside the animal rooms. Every attempt should be made to isolate laboratory animals from levels of construction rated as low by facility managers and construction companies. It is also important for investigators to be fully informed of any construction work in or nearby animal facilities.
Acknowledgments
The authors acknowledge the technical assistance of Barbara Jankowski, Marylou Mader, and Ashley Shock.
This work was supported by National Institutes of Health/National Institute of Allergy and Infectious Diseases Pilot Grant U19AI067734 (principal investigator, John Moulder).
Disclosure Summary: The authors have nothing to disclose.
For editorial see page 1197
- HPA
- Hypothalamic-pituitary-adrenal
- PVN
- paraventricular nucleus.
References
- 1. Dallman MF, Akana SF, Scribner KA, Bradbury MJ, Walker DC, Strack AM, Cascio CS. 1992. Stress, feedback and facilitation in the hypothalamo-pituitary-adrenal axis. J Neuroendocrinol 4:517–526 [DOI] [PubMed] [Google Scholar]
- 2. Burow A, Day HE, Campeau S. 2005. A detailed characterization of loud noise stress: intensity analysis of hypothalamo-pituitary-adrenocortical axis and brain activation. Brain Res 1062:63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eisenberg RM. 1993. Sound vibration, a non-invasive stress: antagonism by diazepam. Psychopharmacology 110:467–470 [DOI] [PubMed] [Google Scholar]
- 4. Coppola CL, Enns RM, Grandin T. 2006. Noise in the animal shelter environment: building design and the effects of daily noise exposure. J Appl Anim Welf Sci 9:1–7 [DOI] [PubMed] [Google Scholar]
- 5. Dallman MF, Akana SF, Bell ME, Bhatnagar S, Choi S, Chu A, Gomez F, Laugero K, Soriano L, Viau V. 1999. Warning! Nearby construction can profoundly affect your experiments. Endocrine 11:111–113 [DOI] [PubMed] [Google Scholar]
- 6. Akana SF, Dallman MF, Bradbury MJ, Scribner KA, Strack AM, Walker CD. 1992. Feedback and facilitation in the adrenocortical system: unmasking facilitation by partial inhibition of the glucocorticoid response to prior stress. Endocrinology 131:57–68 [DOI] [PubMed] [Google Scholar]
- 7. Shepherd EJ, Helliwell PA, Mace OJ, Morgan EL, Patel N, Kellet GL. 2004. Stress and glucocorticoid inhibit apical GLUT2-trafficking and intestinal glucose absorption in rat small intestine. J Physiol 560:281–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Folch H, Waksman BH. 1974. The splenic suppressor cell. I. Activity of thymus-dependent adherent cells: changes with age and stress. J Immunol 113:127–139 [PubMed] [Google Scholar]
- 9. Lê AD, Quan B, Juzytch W, Fletcher PJ, Joharchi N, Shaham Y. 1998. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology 135:169–174 [DOI] [PubMed] [Google Scholar]
- 10. Cohen EP, Bruder ED, Cullinan WE, Ziegler D, Raff H. 2011. The effect of high dose total body irradiation on ACTH, corticosterone, and catecholamines in the rat. Transl Res 157:38–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Raff H, Lee JJ, Widmaier EP, Oaks MK, Engeland WC. 2004. Basal and adrenocorticotropin-stimulated corticosterone in the neonatal rat exposed to hypoxia from birth: modulation by chemical sympathectomy. Endocrinology 145:79–86 [DOI] [PubMed] [Google Scholar]
- 12. Rieder MJ, Roman RJ, Greene AS. 1997. Reversal of microvascular rarefaction and reduced renal mass hypertension. Hypertension 30:120–127 [DOI] [PubMed] [Google Scholar]
- 13. Bruder ED, Taylor JK, Kamer KJ, Raff H. 2008. Development of the ACTH and corticosterone response to acute hypoxia in the neonatal rat. Am J Physiol Regul Integr Comp Physiol 295:R1195–R1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-ΔΔC(T)] method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 15. Herman JP, Adams D, Prewitt C. 1995. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology 61:180–190 [DOI] [PubMed] [Google Scholar]
- 16. Raff H, Jacobson L, Cullinan WE. 2007. Augmented hypothalamic corticotrophin-releasing hormone mRNA and corticosterone responses to stress in adult rats exposed to perinatal hypoxia. J Neuroendocrinol 19:907–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ziegler DR, Cullinan WE, Herman JP. 2005. Organization and regulation of paraventricular nucleus glutamate signaling systems: N-methyl-d-aspartate receptors. J Comp Neurol 484:43–56 [DOI] [PubMed] [Google Scholar]
- 18. Raff H, Chadwick CJ. 1986. Aldosterone responses to ACTH during hypoxia in conscious rats. Clin Exp Pharmacol Physiol 13:827–830 [DOI] [PubMed] [Google Scholar]
- 19. Mueller NK, Dolgas CM, Herman JP. 2004. Stressor-selective role of the ventral subiculum in regulation of neuroendocrine stress responses. Endocrinology 145:3763–3768 [DOI] [PubMed] [Google Scholar]
- 20. Herman JP, Dolgas CM, Carlson SL. 1998. Ventral subiculum regulates hypothalamo-pituitary-adrenocortical and behavioural responses to cognitive stressors. Neuroscience 86:449–459 [DOI] [PubMed] [Google Scholar]
- 21. Watts AG, Tanimura S, Sanchez-Watts G. 2004. Corticotropin-releasing hormone and arginine vasopressin gene transcription in the hypothalamic paraventricular nucleus of unstressed rats: daily rhythms and their interactions with corticosterone. Endocrinology 145:529–540 [DOI] [PubMed] [Google Scholar]
- 22. Michaud DS, Miller S, Ferrarotto C, Keith S, Bowers W, Kumarathsan P, Marro L, Trivedi A. 2005. Exposure to chronic noise and fractionated X-ray radiation elicits biochemical changes and disrupts body weight gain in rat. Int J Radiat Biol 81:299–307 [DOI] [PubMed] [Google Scholar]


