17β-Estradiol does not restore the cardiac heat shock protein response in myocytes from aged menopausal rats but does protect against inflammatory stress and restore cardiac function.
Abstract
Heat shock proteins (HSPs) are a cardioprotective class of proteins induced by stress and regulated by the transcription factor, heat shock factor (HSF)-1. 17β-estradiol (E2) indirectly regulates HSP expression through rapid activation of nuclear factor-κB (NF-κB) and HSF-1 and protects against hypoxia. As males experience a loss of protective cellular responses in aging, we hypothesized that aged menopausal (old ovariectomized) rats would have an impaired HSP response, which could be prevented by immediate in vivo E2 replacement. After measuring cardiac function in vivo, cardiac myocytes were isolated from ovariectomized adult and old rats with and without 9 weeks of E2 replacement. Myocytes were treated with E2 in vitro and analyzed for activation of NF-κB, HSF-1, and HSP expression. In addition, we measured inflammatory cytokine expression and susceptibility to hypoxia/reoxygenation injury. Cardiac contractility was reduced in old ovariectomized rats and could prevented by immediate E2 replacement in vivo. Subsequent investigations in isolated cardiac myocytes found that in vitro E2 activated NF-κB, HSF-1, and increased HSP 72 expression in adult but not old rats. In response to hypoxia/reoxygenation, myocytes from adult, but not old, rats had increased HSP 72 expression. In addition, expression of the inflammatory cytokines TNF-α and IL-1β, as well as oxidative stress, were increased in myocytes from old ovariectomized rats; only the change in cytokine expression could be attenuated by in vivo E2 replacement. This study demonstrates that while aging in female rats led to a loss of the cardioprotective HSP response, E2 retains its protective cellular properties.
Heat shock proteins (HSPs) are molecular chaperones that fold macromolecules and stabilize protein structure during stress to reduce injury and preserve cellular function. In addition, HSPs are protective through antiapoptotic and antiinflammatory mechanisms (1–3). A hallmark of cardiac aging is a reduced cellular capacity to respond to stress and protect function during cardiac injury, which translates into an increased susceptibility to apoptosis and necrosis (4). As HSPs protect against inflammation and apoptosis, a loss of the ability to increase the expression of the cardioprotective HSPs could explain in part why aging is associated with diminished recovery from cardiac injury. Several studies have shown in male animal models that aging leads to a loss in the ability to induce HSPs in response to a variety of stressors (5, 6). However, the cardiac HSP response in aging females and the role of estrogens has not been investigated.
We have previously reported that 17β-estradiol (E2), the most active estrogen metabolite, indirectly regulates cardiac HSP 72 expression (7). In addition to its role in protein folding, HSP 72 has cardioprotective effects in transgenic models and reduces apoptosis by stabilizing the mitochondrial membrane and preventing apoptosome formation (8–11). In isolated adult Sprague Dawley female cardiomyocytes, E2 treatment increased the expression of HSP 72 through sequential activation of the transcription factors nuclear factor-κB (NF-κB) and heat shock factor-1 (HSF-1) (7). E2 pretreatment also protected against hypoxia/reoxygenation injury (7, 12). Although we have reported that E2 can induce cardiac HSP 72 expression in adults, little is known about how the loss of estrogens in aging female rats affects the cardiac HSP response and the adaptive response to stress.
The purpose of our study was to assess the role of E2 on cardiac function and the regulation of the HSPs in a model of menopausal aging in rats. To accomplish this we ovariectomized adult (6-month-old) and old (22-month-old) Norway Brown rats and supplemented half of the animals with E2 immediately upon ovariectomy. As female rats display wide variations in levels of estrogens during their cycles and enter a state of constant estrus as they age, they cannot be readily compared with intact adult females. Thus, the optimal sham control in this setting is quite debatable, and therefore the focus of this article is on the effects of E2 replacement, compared with ovariectomy alone, and how aging factors into the response.
Materials and Methods
Animals
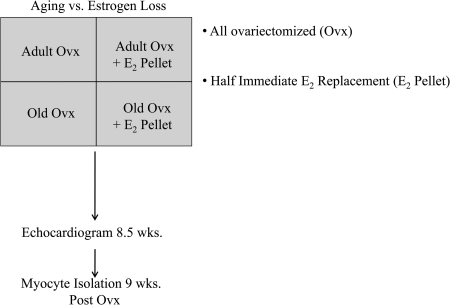
The animal protocol was approved by the University of California, Davis Animal Research Committee in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Norwegian brown rats were obtained from the National Institute of Aging and housed under standard conditions in female rat only housing. Adult (4-month-old) and old (20-month-old) Norway Brown female rats were ovariectomized (ovx) under sterile conditions using standard methods. In one-half of the ovx rats, immediate hormone replacement was done with 0.36-mg E2 90-day slow-release pellets (Innovative Research, Sarasota, FL) implanted subcutaneously as previously described (13), forming four groups: adult ovx, adult ovx + E2 pellet, old ovx, and old ovx + E2 pellet (Fig. 1). Nine weeks after ovx (i.e., at 6 and 22 months), the rats were euthanized by exsanguination under anesthesia. Plasma estrogen levels were measured by a core lab at the Veterinary School.
Fig. 1.
Timeline: Adult (6-month-old) and old (22-month-old) rats were ovariectomized. Half of the animals received immediate E2 replacement in vivo (ovx + E2 pellet), while the other half did not. After a recovery period of 9 weeks, myocytes were isolated from the respective animals and analyzed.
Two-dimensional cardiac echocardiogram
Nine-week postoperative rats were anesthetized with 50 mg/kg ketamine and 5 mg/kg xylazine, their chests were shaved, and an echocardiogram was done (Acuson, Sequoia model C512, 15-MHz probe). Two-dimensional imaging was used to identify the short-axis position. Three consecutive M-mode images were collected in the short-axis view and saved for analysis of chamber size [left ventricular (LV) diastolic dimension] and fractional shortening, as previously described (14).
Isolation of adult cardiac myocytes
Cardiac myocytes were isolated from 6- or 22-month-old ovx female Norway Brown rats with and without E2 replacement and cultured as previously described (15, 16). Butadione monoxide (2.5 mm; Sigma Chemicals, St. Louis, MO) was added to the perfusion buffer for all isolations to aid in the isolation of aged cardiac myocytes.
Treatment protocol
When myocytes became adherent, the medium was exchanged for fresh supplemented phenol red-free M199 containing E2 in doses ranging from 5 nm to 100 nm, based on previous reports (7, 12). Control cells were treated with vehicle only (equal volume of ethanol). Experiments were performed with three or four replicates in each of the three separate experiments. For Western analyses, cells were collected 12 h after E2 treatment, for NF-κB cells were collected 15 min after treatment, and for HSF-1 cells were collected 3 h after treatment.
NF-κB and HSF-1 activation assay
Cells were trypsinized and processed for nuclear isolation for the NF-κB activation assay (Pierce Biotechnology, Rockford, IL). NF-κB was assayed as previously described (7). This assay, which uses a microtiter plate coated with the binding domain for NF-κB and measures binding of NF-κB with an antibody for p50, allows for precise and reproducible measurement of NF-κB activation. The results correlate well with EMSA, as we have previously shown (7). HSF-1 activation was assayed as previously described (12). Briefly, 0.5 μg of nuclear protein was processed and incubated with a double stranded heat shock element sequence which was biotinylated at the 3′ ends using a commercially available kit (Pierce Biotechnologies, Rockford, IL).
Western blotting
Western blotting was performed as previously described (13, 16). After transfer, all membranes were stained with Ponceau S to verify quality of transfer and equal loading. Blots were blocked with Blotto (Bio-Rad, Hercules, CA) and developed with the following primary antibodies: 1:250 ER-α, 1:250 ER-β, 1:1000 HSP 25, 1:1000 Phospho HSF-1 (Ser303/307), 1:5000 HSF-1 (Novus Biologicals, Littleton, CO), 1:1000 β-myosin heavy chain (MHC) (Sigma, St. Louis, MO), 1:1000 Cu/Zn-SOD, 1:1000 HSP 32, 1:250 HSP 72, 1:5000 Mn-SOD (Assay Designs, Ann Arbor, MI), 1:1000 HSP 90 (BD Biosciences, San Jose, CA), 1:1000 Catalase, 1:1000 Acetyl Lysine (Abcam), and 1:20,000 GAPDH (RDI, Concord, MA). Antimouse or antirabbit IgG-horseradish peroxidase (Amersham, Piscataway, NJ) was used at 1:1,000. Blots were developed with a chemiluminescent agent (Pierce, Rockford, IL) and exposed to x-ray film. Densitometric analysis was done as previously described (16). For immunoprecipitation experiments, 100 μg of untreated cardiomyocyte lysate was pulled down using a total HSF-1 antibody as previously described (17).
Hypoxia-reoxygenation (H/R)
H/R was done using a hypoxia workstation (Forma), which produces near-zero oxygen. The medium was changed to DMEM base (no glucose, glutamine, or phenol red to prevent switching to glycolysis), and the cells were subjected to hypoxia for 12 h in an anaerobic workstation (model 1025, Forma Scientific, Marietta, OH; 4.8% CO2, 10.3% H2, and 84.9% N2) as previously described (18). After hypoxia, cells were returned to phenol red–free supplemented Medium 199 for an additional 12 h. The supernatant was assayed for lactase dehydrogenase (LDH) release and cell lysates were collected for Western analysis.
Real-time PCR
Total RNA was extracted from isolated cardiomyocytes using the modified guanidine isothiocyanate method (19). First-strand complementary DNA was generated using 2 μg of total RNA with a high-capacity cDNA RT system (Applied Biosystems, Foster City, CA). qPCR Primers were purchased from Superarray technologies (Frederick, MD). PCR reactions were set up in 20-μl volumes, consisting of 1 μl of cDNA, 1 μl of 10 μm primers, 10 μl of SYBR Green PCR Master Mix (Applied Biosystems), and 8 μl Rnase-Dnase free water. For all primer sets, a denaturing step at 94 C for 10 min was followed by 40 cycles of denaturing at 94 C for 30 s, annealing at 60 C for 45 s, and 72 C extension for 30 s. Real-time PCR was performed using an AbiPrism 7900HT Sequence Detector, and data were analyzed using SDS 2.1 Software (Applied Biosystems). The relative concentration of the corresponding mRNA was measured using the standard curve method and normalized against that of an internal control gene (GAPDH).
Reactive oxygen species (ROS) assay
ROS were assayed using the methods of Wang and Joseph (20). In brief, cells were plated in triplicate in a 96-well plate and incubated with 10 μm carboxy-dichlorodihydrofluorescin diacetate (Carboxy-H2DCFDA, Invitrogen, Carlsbad, CA) in M199 for 25 min at 37 C. Carboxy-H2DCFDA is a cell-permeant indicator for ROS that is nonfluorescent until the acetate groups are removed by intracellular esterases and oxidation occurs within the cell. After loading with Carboxy-H2DCFDA, cells were washed two times with PBS and replaced with fresh M199 with and without 100 μm H2O2. Fluorescence was recorded using a fluorometer (excitation wavelength 485 nm, emission wavelength 530 nm, Packard Instrument Co., Meriden, CT) at 0 and 30 min, and ROS formation was calculated by subtracting the baseline from the 30 min reading.
Statistical analyses
The results are presented as the mean ± sem of at least three separate experiments. Data were analyzed by a one-way ANOVA or ANOVA on Ranks, where appropriate, followed by the Student-Newman-Keuls or Dunn's test (Sigma Stat). A P < 0.05 was considered significant.
Results
Ovariectomized rats had decreased plasma E2 levels (27.3 ± 6.2 pg/ml) and uterine atrophy, which were restored by E2 replacement to that seen in intact cycling animals (59.48 ± 9.6 pg/ml) as previously reported (13). Body weight did not differ significantly among adult ovx, adult ovx + E2 pellet, old ovx, and old ovx + E2 pellet (225.6 ± 15.6, 196.2 ± 12.6, 263.8 ± 6.4, 233.0 ± 9.6 g). In addition there was no difference in myocyte viability or yield between adult (2.5 × 106 ± 2.6 × 105 myocytes, 51.6 ± 2.1% viability as determined by number of rod-shaped/total myocytes) and old (2.6 × 106 ± 4.0 × 105 myocytes, 49.2 ± 2.6% viability) rats, regardless of whether the rats received in vivo E2 replacement.
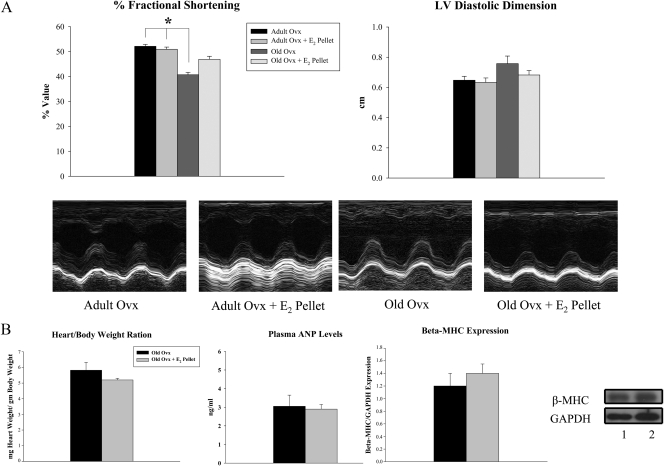
Aging and ovariectomy leads to reductions in fractional shortening
Ventricular function was assessed by cardiac ultrasound 3–4 d before the isolation of cardiac myocytes. Old ovx animals had a significant decrease in fractional shortening in vivo compared with both adult groups, while no differences were detected in LV diastolic dimension among the groups (Fig. 2A). In addition, there was no difference in heart to body weight ratio, an index of cardiac hypertrophy among aged animals (Fig. 2B). We measured plasma levels of atrial natriuretic peptide and expression of β-MHC in myocytes isolated from aged animals to determine whether the decreased fractional shortening in the old ovx was associated with hypertrophy or heart failure, respectively. As shown in Figure 2B, no changes were seen in these parameters in old ovx vs. old ovx + E2 pellet.
Fig. 2.
Aging and estrogen withdrawal impairs fractional shortening. A, Graphs summarize fractional shortening and LV diastolic dimension 9 weeks postovariectomy. Lower panels are representative m-mode echo images showing wall motion in adult and old animals with and without E2 replacement. B, Graphs summarize heart to body weight ratios, plasma atrial natriuretic peptide, and β-MHC expression in isolated cardiomyocytes from old ovx and old ovx + E2 pellet rats (parameters increased in congestive heart failure and/or LV hypertrophy). β-MHC was normalized to GAPDH expression. Representative Western blots are shown on the right. Lanes: 1, old ovx; 2, old ovx + E2 pellet. n = 4–7 per group. *, P < 0.05 vs. adult ovx and adult ovx + E2 pellet.
NF-κB transcription factor activation
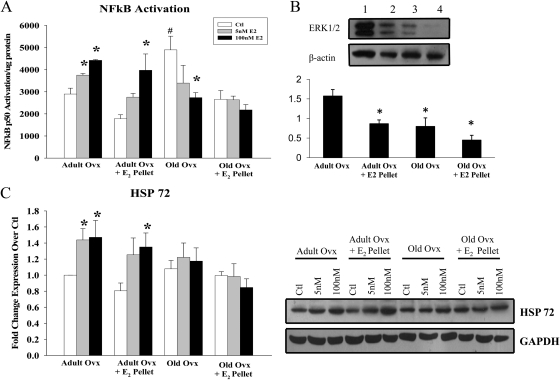
NF-κB activation in isolated cardiomyocytes was measured after 15 min of treatment with 5 and 100 nm E2 in vitro. In myocytes isolated from adult animals, 5 nm (adult ovx) and 100 nm E2 (adult ovx and ovx + E2 pellet) activated NF-κB at 15 min (Fig. 3A). Neither 5 nor 100 nm E2 treatment activated NF-κB in myocytes from old animals. However, myocytes from old ovx had significantly greater basal activation of NF-κB compared with all other controls, and this activation was reduced by 100 nm E2 treatment. ERK 1/2, a mediator of NF-κB activation, was decreased in myocytes from aged rats and adult ovx + E2 pellet rats (Fig. 3B, P < 0.05 vs. adult ovx).
Fig. 3.
NF-κB activation and HSP 72 expression in myocytes from adult and old rats treated with E2 in vitro. A, Graph summarizes the results of NF-κB activation in adult and old rats treated with 5 and 100 nm E2 for 15 min in vitro. B, Graph summarizes ERK 1/2 expression, normalized to β-actin, in the isolated cardiomyocytes. Upper panel, A representative Western blot for ERK 1/2 and β-actin. Lanes: 1, adult ovx; 2, adult ovx + E2 pellet; 3, old ovx; 4, old ovx + E2 pellet. *, P < 0.05 vs. adult ovx. C, Graph summarizes the results of HSP 72 expression in adult and old rats treated with 5 and 100 nm E2 for 12 h in vitro. HSP 72 was normalized to GAPDH expression and to adult ovx. A representative Western blot is shown to the right. n = 5–6 per group and 7–12 plates per group. *, P < 0.05 vs. control (ctl); #, P < 0.05 vs. all other ctls.
Changes in HSP 72 expression with E2 and aging
We measured expression of HSP 72 in isolated cardiomyocytes both basally and after 12 h of E2 treatment in vitro. As shown in Figure 3C, 5 nm E2 increased HSP 72 in myocytes from adult ovx, but 100 nm was needed to increase HSP 72 in myocytes from adult ovx + E2 pellet, a similar result to NF-κB activation. Neither 5 nor 100 nm E2 increased expression of HSP 72 in myocytes from old ovx or old ovx + E2 pellet rats. Basal expression of HSP 72 did not differ among any of the groups.
Estrogen receptor expression in cardiomyocytes
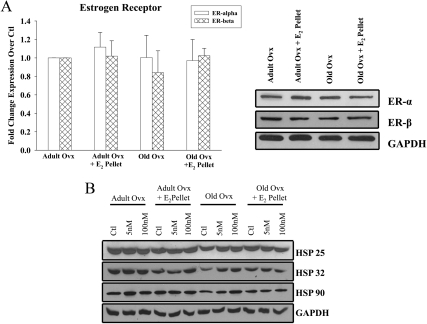
Expression of ER-α and ER-β in isolated cardiomyocytes was measured. Neither aging nor E2 replacement affected the expression of either ER-α or ER-β in isolated myocytes from any of the groups (Fig. 4A).
Fig. 4.
ER, HSP 25, 32, and 90 expression in adult and old myocytes. A, Graph summarizes the basal ER-α and β expression in cardiac myocytes from adult and old rats with or without in vivo E2 replacement. ER expression was normalized to GAPDH expression and to adult ovx. Right panel, Representative western blots. n = 3 animals and 3–5 plates per group. Ctl, control. B, Representative Western blots of HSP 25, 32, 90, and GAPDH expression in adult and old rats treated with 5 and 100 nm E2 for 12 h in vitro. n = 6 animals and 6–11 plates per group. P = n.s., data not shown.
Changes in HSP 25, 32, and 90 expression with E2 and aging
The expression of other cardioprotective HSPs were similarly measured in isolated cardiomyocytes both basally and with E2 treatment in vitro. We examined HSP 25, which has an estrogen response element, HSP 32 (heme oxygenase-1 or HO-1), and HSP 90, which is important in estrogen signaling. As shown in Fig. 4A, neither 5 nm nor 100 nm of E2 treatment increased the expression of any of these HSPs in the isolated myocytes (Fig. 4B, P = n.s., data not shown).
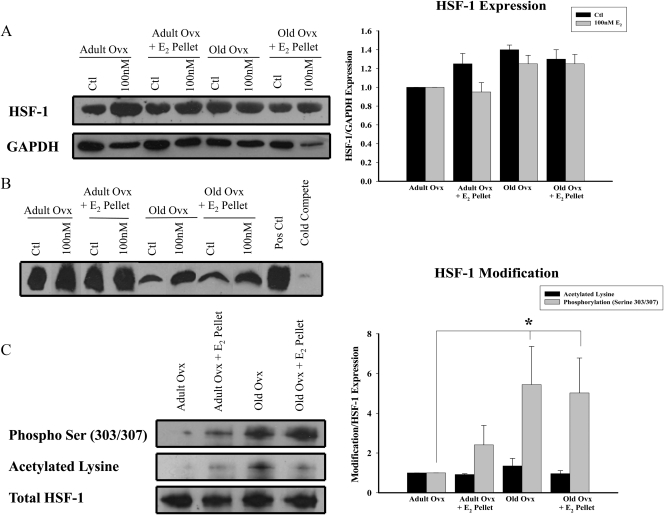
HSF-1 expression and activation
HSF-1 is the main transcription factor regulating HSP expression. No differences were found in HSF-1 expression levels in control or E2-treated cardiomyocytes among the groups (Fig. 5A). As shown in Figure 5B, myocytes from both adult and old rats had increased binding in response to 100 nm E2, albeit the response was smaller in myocytes from old rats. We have previously shown this band is HSF-1 by supershift assay (7). Total HSF-1 was immunoprecipitated and analyzed for acetylation and phosphorylation of serine303/307. These modifications inhibit HSF-1 binding and transcriptional activity, respectively. As shown in Fig. 5C, decreased activation of HSF-1 was associated with increased phosphorylation of the serine303/307 residues for both aged groups (P < 0.05 vs. adult ovx) but not with increased acetylation.
Fig. 5.
Differences in HSF-1 activation and expression with age. A, A representative blot for HSF-1 and GAPDH expression in cardiomyocytes in adult and ovx rats with and without E2 replacement untreated or treated with 100 nm E2 in vitro. Graph on right summarizes HSF-1 expression normalized to GAPDH and to adult ovx ctl. B, EMSA showing HSF-1 binding in myocytes from adult and old rats with and without E2 replacement untreated or treated with 100 nm E2 in vitro. The image is representative of five independent experiments. C, Graph summarizes the results of immunoprecipitated HSF-1 blotted for acetylation and phospho Ser303/307 and normalized to total HSF-1. The immunoprecipitated samples were untreated cardiac myocytes from adult and old ovx rats with and without E2 replacement. Representative Western blots are shown on left. n = 7–10 animals and plates per group. *, P < 0.05 vs. adult ovx.
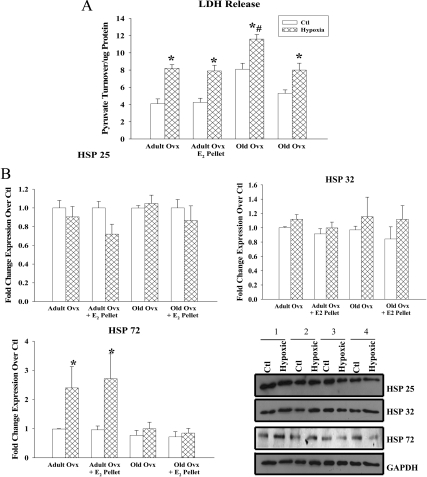
H/R injury
H/R was used to evaluate the effect of aging vs. E2 replacement on the cardiac myocytes stress response. While myocytes from all groups had increased LDH release upon H/R injury, myocytes from old ovx rats had increased LDH release upon H/R injury compared with all other groups (Fig. 6A). Expression of HSP 25, HSP32, and HSP 72 were also analyzed after H/R injury. Adult rats responded to H/R injury with increased expression of HSP 72, but not HSP 25 or HSP 32. No changes were detected in myocytes from old rats with H/R injury for any of the HSPs (Fig. 6B).
Fig. 6.
Hypoxia-reoxygenation induced release of lactate dehydrogenase (LDH), a marker of cell necrosis. A, LDH release after myocyte hypoxia (12 h) followed by reoxygenation (12 h). B, Graphs summarize HSP 25, 32, and 72 expression in adult and old myocytes with and without E2 replacement under normoxic and hypoxic conditions. All HSP expression was normalized to GAPDH and to adult ovx. Representative Western blots for HSP 25, 32, 72, and GAPDH are shown on the right. Lanes: 1, adult ovx; 2, adult ovx + E2 pellet; 3, old ovx; 4, old ovx + E2 pellet. n = 6 animals and 6–11 plates per group. *, P < 0.05 vs. ctl; #, P < 0.05 vs. all other groups.
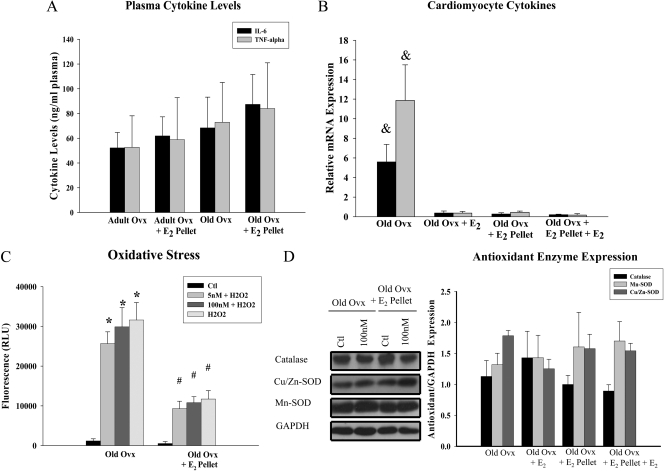
Aging, E2, and inflammation
The inflammatory cytokines IL-6 and TNF-α were measured in plasma samples collected at the time of removal of the heart, and subsequent work assessed IL-6 and TNF-α expression in the isolated cardiac myocytes. A subset of myocytes was pretreated with 100 nm in vitro E2 before measuring cytokine expression, based on our findings that 100 nm E2 inhibited NF-κB activation in myocytes from old ovx rats (Fig. 3A). No differences were detected in circulating plasma levels of IL-6 or TNF-α among old ovx and old ovx + E2 pellet (Fig 7A) or between adult and old animals (data not shown, P = n.s.). At the cellular level, old ovx myocytes had increased expression of IL-6 and TNF-α compared with old ovx + E2 pellet. Pretreatment with 100 nm E2 for 6 h in old ovx decreased the gene expression of IL-6 and TNF-α (Fig. 7B).
Fig. 7.
Inflammatory and oxidative stress is increased in old ovx cardiac myocytes. A, Graph summarizes IL-6 and TNF-α levels in the plasma of old rats with and without E2 replacement. n = 6–11 animals per group. Subsequent studies focused on the isolated cardiac myocytes. B, Graph summarizes the relative mRNA expression of IL-6 and TNF-α in isolated myocytes from old ovx and old ovx + E2 pellet rats treated with or without 100 nm E2 pretreatment in vitro. Cytokine expression was normalized to GAPDH. n = 5 animals and plates per group. &, P < 0.05 vs. all other groups. C, Graph summarizes the increase in oxidative stress after treating myocytes from old ovx and old ovx + E2 pellet rats with 100 μm H2O2. A subset of cardiac myocytes were pretreated with 5 and 100 nm E2 for 6 h before insult with H2O2. n = 3 animals and 9 plates per group. *, P < 0.05 vs. ctl; #, P < 0.05 vs. ctl and identical group in old ovx. D, Representative Westerns and a graph summarizing the expression of catalase, Cu/Zn-SOD, and Mn-SOD, basally and with 100 nm E2 treatment for 24 h. Blots were normalized to GAPDH and to old ovx. Graph summarizing the results are shown to the right. n = 4–5 animals and plates per group.
Aging, E2, and oxidative stress
Aging increases the formation of ROS, which are reduced by E2 (21, 22). Therefore, studies were done to determine whether old ovx myocytes had increased ROS formation compared with old ovx + E2 pellet. At baseline there was no difference in ROS. However, as shown in Fig. 7C, cardiac myocytes from old ovx had increased ROS formation after challenge with H2O2 compared with myocytes from old ovx + E2 pellet rats as shown by oxidized DCF fluorescence (20). ROS formation was also measured in samples pretreated with E2 for 6 h as this was shown to inhibit cytokine expression in isolated myocytes. Pretreatment with either 5 or 100 nm E2 for 6 h before H2O2 treatment did not attenuate ROS formation. No changes were found in the expression of the three major antioxidant enzymes catalase, Cu/Zn, or Mn-SOD between old ovx and old ovx + E2 pellet basally or upon stimulation with E2 in vitro (Fig. 7D).
Discussion
Rapid induction of the HSP response attenuates cellular injury in response to injury and oxidative stress. The ability for HSPs to be induced diminishes with age in male rats (5, 6), and the present study extends these observations to show that a similar reduction occurs in female animals independent of estrogen replacement. While aging led to a loss of HSP induction, myocytes from old ovx rats displayed increased basal activation of NF-κB, increased cytokine expression, and decreased ability to handle ROS, all of which could be prevented by immediate E2 replacement (old ovx + E2 pellet). These inflammatory changes in aged ovx cardiac myocytes, which could be expected to lead to myocyte loss and fibrosis, correlated with impaired ventricular function, which was prevented by E2 replacement at the time of ovariectomy. These studies demonstrate that while E2 replacement does not affect the HSP response in aged animals, it prevents inflammation, preserves the response to oxidative stress, and can protect cardiac function.
Cardiovascular studies using menopausal animal models have focused on the effect of ovariectomy on recovery from ischemia or the response to pressure overload (23). Our findings that ovariectomy in combination with aging impairs fractional shortening in combination with significant cellular abnormalities adds to our understanding of the cardiovascular effects of menopause. Using this same model we have previously shown that reduced soluble guanylyl cyclase expression causes impaired vascular relaxation during aging and estrogen loss (13), and impaired vascular relaxation is a characteristic of menopausal women (24). Although there are likely multiple events leading to the impaired fractional shortening, it is interesting that isolated myocytes from the old ovx animals displayed increased inflammation and basal NF-κB activation, both of which were not present in myocytes from old ovx + E2 pellet rats. Inhibition of NF-κB has previously been shown to restore impaired fractional shortening in transgenic mice with cardiac specific overexpression of TNF-α (25). The results of the current study suggest that abnormal NF-κB activation may underlie some of the detrimental changes observed in the cardiovascular system of surgically menopausal rodents and has implications for the cardiovascular dysfunction seen with aging in humans (4, 26).
HSPs reduce injury during cell stress by decreasing protein denaturation and aggregation and by promoting proper refolding (1). In males, aging leads to depressed HSP induction after various stressors (27–30). An important finding in this study is that in female rats, aging similarly led to a loss of the cardiac HSP response, independent of E2 replacement upon ovariectomy. The reduced induction of HSPs in myocytes from old animals upon E2 treatment was not attributable to decreased ER expression, rather to the inhibition of HSF-1, the main transcription factor controlling the expression of HSPs. Although controversial, aging is thought to lead to a loss in the ability of HSF-1 to bind to DNA and induce transcription and is not necessarily accompanied by decreased HSF-1 expression (31, 32). Decreased activity of HSF-1 in aging could be potentially attributable to posttranslational changes. Acetylation of HSF-1 at lysine80 reduces the ability of HSF-1 to bind to the heat shock element (33), while phosphorylation of Serine303/307 does not inhibit HSF-1 binding but prevents transcription (34, 35). In our studies, we found that HSF-1 expression did not differ, but HSF-1 DNA binding was decreased in myocytes from old ovx rats compared with those from adults both basally and upon E2 treatment. Total HSF-1 from old myocytes had increased phosphorylation at Ser303/307 but no change in acetylated lysine, which could explain the loss of increased HSP 72 expression with E2 and H/R injury, as the phosphorylation at Ser 303/307 would inhibit activation of HSP 72 gene transcription by HSF-1.
In the current study, myocytes from adult but not old animals had increased NF-κB activation in response to E2 in vitro. The in vitro E2 concentrations used in the current study, and in much of the cell culture literature, are higher than the levels measured in vivo. It may be that a plasma protein(s), such as albumin, enhances E2 delivery to the cells in vivo, or in some other way alter its distribution. Nonetheless, cardiac myocytes from both aged groups were quite insensitive to E2 and required high concentrations for effect. There was no difference in expression of either ER-α or ER-β, suggesting that changes in downstream signaling are involved. Recent work by our lab has shown that E2 activates NF-κB ultimately through phosphorylation of ERK 1/2 (Stice and Knowlton, unpublished data). In this study, we similarly show that ERK 1/2 expression is markedly reduced in myocytes from aged and adult + E2 pellet rats compared with myocytes from adult ovx rats, thus providing a possible mechanism for the diminished ability to activate NF-κB in the aged. The decrease of ERK 1/2 expression in adult + E2 pellet provides a mechanism for the decreased responsiveness of these cells to E2 in vitro. While rapid activation of NF-κB induces HSP 72 and protects cells from hypoxic injury (7, 12), as well as reduces oxidative stress (36), chronic activation leads to inflammation, as seen with the aged ovx myocytes, and to the development of pathologic conditions (37). Furthermore, E2 and the estrogen receptor have been shown to inhibit NF-κB binding and cytokine induction (38).
Myocytes from the old ovx rats had increased basal NF-κB activation and increased expression of the inflammatory cytokines IL-6 and TNF-α and were more susceptible to oxidative stress compared with myocytes from old ovx + E2 pellet rats. The H2O2 challenge tested the ability of the myocytes to respond to an increase in ROS. Clearly, the aging ovx cardiac myocytes had an inadequate response to ROS consistent with impaired antioxidant enzyme function, despite the fact that the major antioxidant enzyme levels were not decreased by Western. Others have found little difference in cardiac levels of the antioxidant enzymes with aging but found decreased enzyme activity (39). In fact, overexpression of catalase targeted to the mitochondria has been found to mitigate cardiac aging in a mouse model (40). The increased cytokine expression could be reduced by pretreatment with high dose E2, which similarly inhibited the higher basal NF-κB activation; however, pretreatment with E2 had no effect on the impaired response to the H2O2 challenge. These results suggest that aging and E2 withdrawal leads to activation of NF-κB and chronic inflammation and that these effects can be partially mitigated by E2 in the aged.
The exact role of estrogens and hormone replacement therapy (HRT) in the aging female population remains controversial. Results from several large randomized clinical trials showed no cardiovascular benefit in postmenopausal women taking conjugated equine estrogens (41, 42). Currently, it is thought that the time between menopause and the initiation of HRT may be a crucial factor in determining the benefit of HRT (24, 43). An important finding of this study is that myocytes from adult but not old animals can activate the HSP response regardless of E2 replacement at time of ovariectomy. However, E2 replacement in the aged prevented multiple dysfunctions associated with aging, supporting the idea that HRT can confer beneficial effects if given immediately. Further work is needed to understand how aging alters the cardioprotective properties of E2 and how this impacts the progression of heart disease in the aging female population.
Acknowledgments
This work was supported by grants from the Treadwell Foundation (to A.A.K.), National Institutes of Health (AG19327; to A.A.K.), and the American Heart Association Western States Affiliate (to J.P.S.).
Disclosure Summary: The authors have nothing to declare.
Footnotes
- E2
- 17β-Estradiol
- H/R
- hypoxia-reoxygenation
- HRT
- hormone replacement therapy
- HSF
- heat shock factor
- HSP
- heat shock protein
- LDH
- lactase dehydrogenase
- LV
- left ventricular
- MHC
- myosin heavy chain
- NF-κB
- nuclear factor-κB
- ovx
- ovariectomized
- ROS
- reactive oxygen species.
References
- 1. Christians ESP, Yan L-JP, Benjamin IJMD. 2002. Heat shock factor 1 and heat shock proteins: Critical partners in protection against acute cell injury. [Review]. Crit Care Med 30:S43–S50 [PubMed] [Google Scholar]
- 2. Knowlton AA. 1995. The role of heat shock proteins in the heart. J Mol Cell Cardiol 27:121–131 [DOI] [PubMed] [Google Scholar]
- 3. Mosser DD, Morimoto RI. 2000. Molecular chaperones and the stress of oncogenesis. Oncogene 23:2907–2918 [DOI] [PubMed] [Google Scholar]
- 4. Juhaszova M, Rabuel C, Zorov DB, Lakatta EG, Sollott SJ. 2005. Protection in the aged heart: preventing the heart-break of old age? Cardiovasc Res 66:233–244 [DOI] [PubMed] [Google Scholar]
- 5. Chung HY, Sung B, Jung KJ, Zou Y, Yu BP. 2006. The molecular inflammatory process in aging. Antioxid Redox Signal 8:572–581 [DOI] [PubMed] [Google Scholar]
- 6. Roth DA, White CD, Podolin DA, Mazzeo RS. 1998. Alterations in myocardial signal transduction due to aging and chronic dynamic exercise. J Appl Physiol 84:177–184 [DOI] [PubMed] [Google Scholar]
- 7. Hamilton KL, Gupta S, Knowlton AA. 2004. Estrogen and regulation of heat shock protein expression in female cardiomyocytes: cross-talk with NF-kB signaling. J Mol Cell Cardiol 36:577–584 [DOI] [PubMed] [Google Scholar]
- 8. Marber MS, Mestril R, Chi SH, Sayen MR, Yellon DM, Dillmann WH. 1995. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Invest 95:1446–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Trost SU, Omens JH, Karlon WJ, Meyer M, Mestril R, Covell JW, Dillmann WH. 1998. Protection against myocardial dysfunction after a brief ischemic period in transgenic mice expressing inducible heat shock protein 70. J Clin Invest 101:855–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suzuki K, Murtuza B, Sammut IA, Latif N, Jayakumar J, Smolenski RT, Kaneda Y, Sawa Y, Matsuda H, Yacoub MH. 2002. Heat shock protein 72 enhances manganese superoxide dismutase activity during myocardial ischemia-reperfusion injury, associated with mitochondrial protection and apoptosis reduction. Circulation 106:I-270–276 [PubMed] [Google Scholar]
- 11. Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR. 2000. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol 2:469–475 [DOI] [PubMed] [Google Scholar]
- 12. Hamilton KL, Mbai FN, Gupta S, Knowlton AA. 2004. Estrogen, Heat Shock Proteins, and NF{kappa}B in Human Vascular Endothelium. Arterioscler Thromb Vasc Biol 24:1628–1633 [DOI] [PubMed] [Google Scholar]
- 13. Stice JP, Eiserich JP, Knowlton AA. 2009. Role of aging versus the loss of estrogens in the reduction in vascular function in female rats. Endocrinology 150:212–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin L, Kim SC, Wang Y, Gupta S, Davis B, Simon SI, Torre-Amione G, Knowlton AA. 2007. HSP60 in heart failure: abnormal distribution and role in cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol 293:H2238–H2247 [DOI] [PubMed] [Google Scholar]
- 15. Gupta S, Knowlton AA. 2007. HSP60 trafficking in adult cardiac myocytes: role of the exosomal pathway. Am J Physiol Heart Circ Physiol 292:H3052–H3056 [DOI] [PubMed] [Google Scholar]
- 16. Kirchhoff SR, Gupta S, Knowlton AA. 2002. Cytosolic heat shock protein 60, apoptosis, and myocardial injury. Circulation 105:2899–2904 [DOI] [PubMed] [Google Scholar]
- 17. Knowlton AA, Sun L. 2001. Heat-shock factor-1, steroid hormones, and regulation of heat-shock protein expression in the heart. Am J Physiol Heart Circ Physiol 280:H455–H464 [DOI] [PubMed] [Google Scholar]
- 18. Gupta S, Knowlton AA. 2002. Cytosolic heat shock protein 60, hypoxia, and apoptosis. Circulation 106:2727–2733 [DOI] [PubMed] [Google Scholar]
- 19. Chomczynski P, Sacchi N. 2006. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on. Nat Protocols 1:581–585 [DOI] [PubMed] [Google Scholar]
- 20. Wang H, Joseph JA. 1999. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med 27:612–616 [DOI] [PubMed] [Google Scholar]
- 21. Kim JK, Pedram A, Razandi M, Levin ER. 2006. Estrogen prevents cardiomyocyte apoptosis through inhibition of reactive oxygen species and differential regulation of p38 kinase isoforms. J Biol Chem 281:6760–6767 [DOI] [PubMed] [Google Scholar]
- 22. Strehlow K, Rotter S, Wassmann S, Adam O, Grohe C, Laufs K, Bohm M, Nickenig G. 2003. Modulation of antioxidant enzyme expression and function by estrogen. Circ Res 93:170–177 [DOI] [PubMed] [Google Scholar]
- 23. Xu Y, Armstrong SJ, Arenas IA, Pehowich DJ, Davidge ST. 2004. Cardioprotection by chronic estrogen or superoxide dismutase mimetic treatment in the aged female rat. Am J Physiol Heart Circ Physiol 287:H165–H171 [DOI] [PubMed] [Google Scholar]
- 24. Mendelsohn ME, Karas RH. 2007. HRT and the young at heart. N Engl J Med 356:2639–2641 [DOI] [PubMed] [Google Scholar]
- 25. Kawamura N, Kubota T, Kawano S, Monden Y, Feldman AM, Tsutsui H, Takeshita A, Sunagawa K. 2005. Blockade of NF-κB improves cardiac function and survival without affecting inflammation in TNF-α-induced cardiomyopathy. Cardiovasc Res 66:520–529 [DOI] [PubMed] [Google Scholar]
- 26. Dai DF, Rabinovitch PS. 2009. Cardiac aging in mice and humans: the role of mitochondrial oxidative stress. Trends Cardiovasc Med 19:213–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Demirel HA, Hamilton KL, Shanely RA, Tumer N, Koroly MJ, Powers SK. 2003. Age and attenuation of exercise-induced myocardial HSP 72 accumulation. Am J Physiol Heart Circ Physiol 285:H1609–H1615 [DOI] [PubMed] [Google Scholar]
- 28. Lee CE, McArdle A, Griffiths RD. 2007. The role of hormones, cytokines and heat shock proteins during age-related muscle loss. Clin Nutr 26:524–534 [DOI] [PubMed] [Google Scholar]
- 29. Wu B, Gu MJ, Heydari AR, Richardson A. 1993. The Effect of Age on the Synthesis of Two Heat Shock Proteins in the HSP70 Family. J Gerontol 48:B50–B56 [DOI] [PubMed] [Google Scholar]
- 30. Isoyama S, Nitta-Komatsubara Y. 2002. Acute and chronic adaptation to hemodynamic overload and ischemia in the aged heart. Heart Failure Rev 7:63–69 [DOI] [PubMed] [Google Scholar]
- 31. Heydari AR, You S, Takahashi R, Gutsmann-Conrad A, Sarge KD, Richardson A. 2000. Age-related alterations in the activation of heat shock transcription factor 1 in rat hepatocytes. Exper Cell Res 256:83–93 [DOI] [PubMed] [Google Scholar]
- 32. Lee YK, Liu DJ, Lu J, Chen KY, Liu AY-C. 2009. Aberrant regulation and modification of heat shock factor 1 in senescent human diploid fibroblasts. J Cell Biochem 106:267–278 [DOI] [PubMed] [Google Scholar]
- 33. Saunders LR, Verdin E. 2009. Cell biology: stress response and aging. Science 323:1021–1022 [DOI] [PubMed] [Google Scholar]
- 34. Pirkkala L, Nykanen P, Sistonen L. 2001. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J 15:1118–1131 [DOI] [PubMed] [Google Scholar]
- 35. Chu B, Soncin F, Price BD, Stevenson MA, Calderwood SK. 1996. Sequential phosphorylation by mitogen-activated protein kinase and glycogen synthase kinase 3 represses transcriptional activation by heat shock factor-1. J Biol Chem 271:30847–30857 [DOI] [PubMed] [Google Scholar]
- 36. Borrás C, Gambini J, Gómez-Cabrera MC, Sastre J, Pallardó FV, Mann GE, Viña J. 2005. 17B-oestradiol up-regulates longevity-related, antioxidant enzyme expression via the ERK1 and ERK2[MAPK]/NFkB cascade. Aging Cell 4:113–118 [DOI] [PubMed] [Google Scholar]
- 37. Hall G, Hasday JD, Rogers TB. 2006. Regulating the regulator: NF-[kappa]B signaling in heart. Journal of Molecular and Cellular Cardiology 41:580–591 [DOI] [PubMed] [Google Scholar]
- 38. Xing D, Nozell S, Chen Y-F, Hage F, Oparil S. 2009. Estrogen and mechanisms of vascular protection. Arterioscler Thromb Vasc Biol 29:289–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Meng Q, Wong YT, Chen J, Ruan R. 2007. Age-related changes in mitochondrial function and antioxidative enzyme activity in Fischer 344 rats. Mech Ageing Dev 128:286–292 [DOI] [PubMed] [Google Scholar]
- 40. Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K, Martin GM, Loeb LA, Ladiges WC, Rabinovitch PS. 2009. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation 119:2789–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, Rodabough RJ, Gilligan MA, Cyr MG, Thomson CA, Khandekar J, Petrovitch H, McTiernan A. 2003. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women's Health Initiative Randomized Trial. JAMA 289:3243–3253 [DOI] [PubMed] [Google Scholar]
- 42. Kuhl H. 2004. Effects of estrogen-only treatment in postmenopausal women. JAMA 292:683; author reply 685–686 [DOI] [PubMed] [Google Scholar]
- 43. Stice JP, Lee JS, Pechenino AS, Knowlton AA. 2009. Estrogen, aging and the cardiovascular system. Future Cardiol 5:93–103 [DOI] [PMC free article] [PubMed] [Google Scholar]