Neonatal testosterone programs a masculinization of POMC neurons in female mice that may participate in the gender dimorphism in energy intake.
Abstract
In mammals, males consume more food, which is considered a masculinized behavior, but the underlying mechanism of this sex-specific feeding behavior is unknown. In mice, neonatal testosterone (NT) is critical to masculinize the developing brain, leading to sex differences in reproductive physiology. The proopiomelanocortin (POMC) neurons of the hypothalamic arcuate nucleus (ARC) are critical to suppress energy intake and POMC innervation of hypothalamic feeding circuits develops to a large extent neonatally. We hypothesized that NT programs the masculinization of energy intake by programming POMC neurons. We tested this hypothesis by comparing control females and control males (CMs) with female mice neonatally androgenized with testosterone (NTFs). We show that increased food intake in CMs is associated with reduced POMC expression and decreased intensity of neuronal projections from POMC neurons within the ARC compared with control females. We found that NTFs display a masculinized energy intake and ARC POMC expression and innervation as observed in CMs, which can be mimicked by neonatal exposure to the androgen receptor agonist dihydrotestosterone (DHT). NTFs also exhibit hyperleptinemia and a decreased ability of leptin to up-regulate POMC, suppress food intake, and prevent adipose tissue accumulation, independent of signal transducer and activator of transcription 3. However, this leptin resistance is specific to NTFs, is not a consequence of masculinization, and is reproduced by neonatal exposure to estrogen but not DHT. Thus, NT programs a sexual differentiation of POMC neurons in female mice via DHT but also predisposes to leptin resistance and obesity in an estrogen-dependent manner.
There are fundamental aspects of energy metabolism, including energy intake, that are regulated differently in males and females (1). In mammals, males consume more food than females, which is considered a masculinized behavior (2). Although it is assumed that the increased energy intake of males supports their higher muscle mass, the underlying mechanism of this feeding-related sex dimorphism is unknown. In many mammals including humans, the testis produces two perinatal testosterone surges that are critical to masculinize the organism (3). Early in development, the brain, like the reproductive system, is inherently feminine, disregarding the animal's genetic sex. If the brain is exposed to testosterone during a sensitive developmental window (either from its own testes in males or from an exogenous source in females), it will be masculinized (4–9). To study brain sexual differentiation, investigators have extensively used the model of neonatal testosterone (NT) exposure in female rodents (4–9). In females, NT defeminizes and masculinizes the structure and function of the hypothalamus. There is extensive evidence of sexual dimorphisms in copulatory behavior and physiology that are secondary to brain programming by NT (4–9). For example, Kiss1 encodes for kisspeptins that are instrumental in generating the preovulatory gonadotropin surge in females. Males express less Kiss1 in the hypothalamus, and in females, NT exposure suppresses Kiss1 expression, thus preventing the preovulatory surge of gonadotropin (10).
The arcuate nucleus (ARC) is a key hypothalamic area mediating the inhibition of energy intake by the main appetite-suppressing hormone, leptin. Leptin acts via anorexigenic proopiomelanocortin (POMC) neurons, which express the long form of the leptin receptor (11). In POMC neurons, leptin up-regulates the expression of POMC, which suppresses food intake via its endoproteolitic product, α-MSH, the main ligand of melanocortin 4 receptors (MC4Rs) (12). In rodents, neural projections from the ARC develop to a large extent during the first 2 wk of postnatal life to connect with hypothalamic feeding circuits (13). Thus, during this sensitive period, the perinatal environment plays a crucial role in programming energy-balance set points in adults. For example, in mice, neonatal leptin acts as a neurotrophic factor, promoting the development of projections from the ARC into other hypothalamic areas (13). This neutrophic action of leptin is restricted to a critical neonatal period that precedes leptin's anorexigenic action in adults (13). Similarly, NT mediates many aspects of sexual differentiation of the male rodent brain during a restricted developmental neonatal period ending on d 10 (4–5). Thus, NT may also program hypothalamic ARC neurons involved in energy intake. Still the effect of NT in organizing the ARC POMC neurons is unknown. Here we hypothesized that NT programs the organization of ARC POMC neurons during the critical neonatal period of ARC development, thus masculinizing energy intake and sex differences in feeding behavior. We tested this hypothesis by comparing control females (CFs) with control males (CMs) and female mice neonatally androgenized with testosterone (NTFs). We studied the central regulation of energy intake and POMC neuron architecture and function in adulthood.
Materials and Methods
Animals
Neonatally androgenized and estrogenized mice were produced by injecting C57BL/6 pups with 100 μg testosterone, 100 μg dihydrotestosterone, or 50 μg 17β-estradiol (Steraloids, Inc., Newport, RI) sc in sesame oil (Sigma-Aldrich, St. Louis, MO) at neonatal d 1 and 2. Control pups of the same age were injected with vehicle in sesame oil. Mice were studied on a standard rodent chow. All animal experiments were approved by Northwestern University Animal Care and Use Committee in accordance with the National Institutes of Health Guide for the Care and Use of Animals.
Food intake measurement
Animals were housed individually for 1 wk to accommodate to the new environment. Food intake was measured daily for 1 wk after accommodation. For measurement of food intake after prolonged fasting, mice were fasted for 24 h and food intake was measured at the indicated time points.
Serum hormone concentrations measurement
Serum leptin concentrations were measured by ELISA (Linco Research, Inc., St. Louis, MO). Serum testosterone (Siemens Medical Solutions Diagnostics, Los Angeles, CA), estradiol (Beckman Coulter, Inc., Fullerton, CA) and progesterone (Siemens Medical Solutions Diagnostics) were measured by RIA at the core of the University of Virginia Center for Research in Reproduction.
Gene expression analysis by real-time quantitative PCR (qRT-PCR)
Gene expression was quantified in tissues by real-time quantitative PCR (qRT-PCR) (iCycler; Bio-Rad Laboratories, Hercules, CA) and normalized to β-actin expression. Briefly, total RNA was extracted in TRIzol Reagent (Invitrogen, Carlsbad, CA). One microgram of RNA was reversed transcribed using iScript cDNA synthesis kit (Bio-Rad Laboratories) with random hexamers. Primer sequences are available upon request.
Western blotting
Frozen hypothalami were homogenized in lysis buffer [50 mm HEPES (pH 7.6), 1% Triton X-100, 150 mm NaCl, 100 mm NaF, 50 mm sodium pyrophosphate, 10 mm EDTA (pH 8.0), 10 μg/ml aprotinin, 10 μg/ml leupeptin, 2 mm phenylmethylsulfonyl fluoride, 10 mm Na3VO4, and 2 mm benzamidine], centrifuged at 12,000 × g, and supernatant removed. POMC was detected with rabbit polyclonal antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Detection was performed by an enhanced chemiluminescence kit (Pierce, Rockford, IL).
In vivo leptin stimulation
Mice were separated into individual cages for 1 wk to accommodate to their surroundings. Food intake was measured daily for 1 wk to obtain basal values. Leptin [(25 μg/20 g ip), National Hormone and Peptide Program, Torrance, CA] was injected daily for 4 d during which food intake and body weight were measured daily. For hypothalamic neuropeptide gene expression studies, PBS or leptin (3 μg/g) were injected ip after a 24-h fast. Six hours later, mice were killed followed by isolation of hypothalami.
Free-floating brain immunohistochemistry (IHC)
Mice were fasted for 24 h and exposed to ip PBS or leptin (5 μg/g body weight) for 30 min. Mice were killed after cardiac perfusion with 10% formalin, and brains were postfixed in 10% formalin overnight at 4 C and transferred to 30% sucrose solution. Tissues were frozen on dry ice, cut into 30-μm coronal sections on a sliding microtome, collected, and stored in antifreeze solution (50% PBS, 15% ethyl glycol, and 35% glycerol) at −20 C. Brain sections were used for free-floating IHC. Sections were washed and pretreated in methanol containing 1% H2O2, 0.3% glycine, and 0.03% sodium dodecyl sulfate. Sections were blocked in 3% normal donkey serum, followed by incubation with rabbit antiphospho-(Y705)-signal transducer and activator of transcription 3 (STAT3) (1:1000; Cell Signaling, Beverly, MA) for 3 d at 4 C. After extensive washing, the sections were incubated with a secondary biotinylated donkey antirabbit antibody (1:1000; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) and finally detected with an avidin-biotin-complex and developed in a metal-enhanced diaminobenzidine solution (Thermo Scientific, Rockford, IL) in a peroxide buffer (Fisher Scientific). For double IHC and the identification of phosphorylated (p) STAT3 colocalization with POMC cell bodies, sections were further subjected to incubation with rabbit anti-ACTH [1:100; from the National Hormone and Peptide Program, Torrance, CA] overnight at room temperature and visualized with a secondary donkey antirabbit-Alexa488 (Invitrogen).
Neuronal cell counts
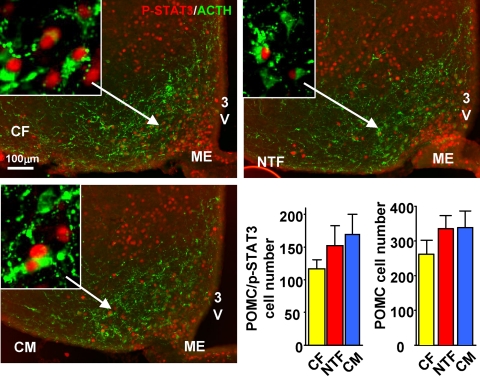
To analyze for changes in leptin-induced pSTAT3 in POMC neurons, we quantified the number of colocalized pSTAT3/ACTH neurons in three sections of the rostral ARC that were anatomically matched between animals (n = 3–4 per groups). Images were obtained with a digital camera (DP30W; Olympus, Tokyo, Japan) under a bright-field or fluorescent microscope (BX51; Olympus) using a ×20 objective or with a confocal microscope for analysis of the ACTH fibers. For better visualization images, brightness and contrast were adjusted using exact parameters for all samples. Scale bars indicate 200 μm. Identical images were captured for pSTAT3 (using bright field) and ACTH (using fluorescent light and appropriate filters), and images were merged using Adobe Photoshop software (Adobe Systems, San Jose, CA). All adjustments for brightness and contrast were kept identical to ensure comparability. Data were analyzed for the total number of POMC neurons as well as the total number of pSTAT3/POMC neurons.
Quantification of ACTH fibers
To quantify ACTH fiber density in mouse brains, we took confocal images (Leica Microsystems, Heidelberg, Germany) from anatomically closely matched sites of the paraventricular nucleus and ARC. In each focused image, the gain and offset was kept constant, and a stack of 75 optical sections was scanned through a volume of 10.1 μm covering the florescent signal. Further image analysis was performed with Image J (National Institutes of Health, Bethesda, MD), and three rectangles (identical in size) were chosen per stack to measure average fluorescence intensity throughout the individual optical sections. Background signals were subtracted after ensuring that no significant difference in background signal was found between groups.
Statistical analysis
Results are presented as mean ± sem unless otherwise stated. Data were analyzed using one-way ANOVA followed by post hoc analysis using Dunnett's multiple comparison test or two-way ANOVA followed by post hoc analysis using Bonferroni test as appropriate. A value of P < 0.05 was considered statistically significant.
Results
To address the hypothesis that neonatal androgenization programs the masculinization of energy intake, we compared littermate CF, NTF, and CM mice. NTF mice showed effective neonatal androgenization as demonstrated by decreased serum progesterone concentrations and the constitution of an anovulatory state (Table 1) (14). Other than that, adult NTFs showed no alteration in serum estradiol or testosterone levels (Table 1).
Table 1.
Serum sex steroid hormone concentrations
| CF | NTF | CM | |
|---|---|---|---|
| Testosterone (ng/dl) | 12.83 ± 1.13 | 15.49 ± 1.81 | 113.97 ± 27.27a |
| 17β-Estradiol (pg/μl) | 42.68 ± 3.99 | 48.17 ± 6.74 | 14.48 ± 2.23a |
| Progesterone (ng/ml) | 4.73 ± 0.45 | 1.56 ± 0.22b | N.D. |
Values represent mean ± se. N.D., Not determined.
P < 0.01, NTF, CM vs. CF [n = 3–11 (12 wk old)].
P < 0.001, NTF, CM vs. CF [n = 3–11 (12 wk old)].
NT programs energy intake
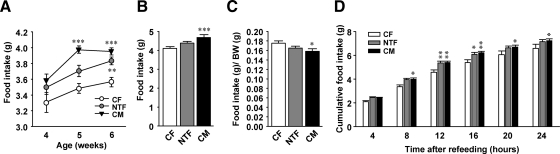
To determine whether NT programs energy intake, we monitored food intake in young mice after weaning. Young NTF mice showed an increase in food intake that reached significance at 6 wk of age compared with CFs, as observed in CMs, consistent with a masculinization of food intake (Fig. 1A). This increase in food intake persisted in adult CMs and as a trend in adult NTFs (Fig. 1B). When food intake was normalized to body weight, and consistent with a linear increase in body weight in NTFs and CMs (Supplemental Fig. 1A, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org), the trend toward masculinization was reversed in the three groups of mice (Fig. 1C).The male response to food deprivation is to gain fat by increasing energy intake, whereas, conversely, that of females is to gain fat by decreasing energy expenditure (15). Thus, to explore masculinization of feeding behavior after food deprivation, we studied food intake after a 24-h fast. During ad libitum refeeding, NTFs further increased their food intake to a level equal to that of CMs, with a maximum between 12 and 16 h (Fig. 1D), supporting a masculinized starvation behavior in NTF mice. Furthermore, consistent with brain masculinization, locomotor activity and energy expenditure were both significantly lower in NTFs and CMs compared with CFs (Supplemental Fig. 1, B and C).
Fig. 1.
NT masculinizes energy intake. A, Food intake was measured in young mice at the indicated age (n = 7–17). B, Daily food intake in 12-wk-old mice (n = 10–19). C, Ratio food intake to body weight in mice from B (n = 10–19). D, Cumulative food intake was measured after refeeding after 24 h fasting in 12-wk-old mice (n = 8–14). Results represent mean ± se. *, P < 0.05; **, P < 0.01; ***, P < 0.001; NTF, CM vs. CF.
NT programs hypothalamic POMC neurons
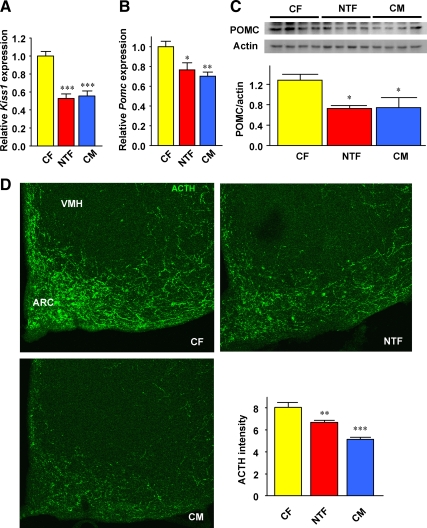
We explored the possibility that NT masculinizes energy intake by programming ARC POMC neurons. The ARC is a key hypothalamic area that mediates leptin's anorexigenic action. It contains first-order, leptin-responsive, anorexigenic POMC/cocaine and amphetamine-regulated transcript neurons and orexigenic neuropeptide Y/agouti-related peptide neurons (11). In these neurons, leptin up-regulates POMC/cocaine and amphetamine-regulated transcript anorexigenic and down-regulates neuropeptide Y/agouti-related peptide orexigenic gene expression, thereby suppressing energy intake (11). We quantified ARC neuropeptide gene expression after refeeding, when leptin levels are at the highest. We used Kiss1 as a genetic marker of appropriate masculinization of the hypothalamus. Indeed, NT masculinization of the ARC decreases Kiss1 in female mice (10). Accordingly, we observed that Kiss1 was suppressed in NTF mice to levels observed in CMs (Fig. 2A). Furthermore, POMC gene and protein expression was decreased in the hypothalamus from NTFs to an extent similar to that of CM mice (Fig. 2, B and C). Conversely, the expression of Cart, Agrp, and Npy were not altered between CFs and NTFs (Supplemental Fig. 2A). We next looked at the possibility that ARC POMC neuron number and/or architecture had been altered by NT exposure. Consistent with the decreased POMC gene and protein expression, we observed a decrease or masculinization of the intensity of POMC neuronal projections in the ARC of NTFs and CMs compared with CFs (Fig. 2D). Together, these data suggest that first, ARC POMC neurons function and projections are sexually dimorphic, and second, that they are masculinized in NTFs as observed in CMs.
Fig. 2.
NT masculinizes POMC neurons. A and B, Kiss1 and Pomc expression was measured by qRT-PCR in hypothalami from 20-wk-old mice after refeeding (n = 10–26). C, POMC protein levels were measured by Western blotting in whole hypothalami in fed condition (n = 4). D, Hypothalamic POMC neuron projections were detected by IHC after ACTH staining followed by quantification as described in Materials and Methods (n = 3). All studies were performed in 12-wk-old mice. Results represent the mean ± se. *, P < 0.05; **, P < 0.01; ***, P < 0.001; NTF, CM vs. CF; VMH, ventromedial hypothalamus.
NT impairs leptin's ability to stimulate Pomc and suppress energy intake
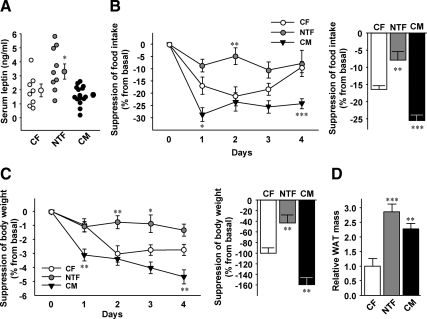
To explore the mechanism underlying NT programming of hyperphagia and POMC neurons, we measured serum concentrations of the main appetite-suppressing hormone, leptin. Adult NTF mice showed hyperleptinemia compared with CFs and CMs (Fig. 3A), suggesting that they are leptin resistant. To test that possibility, we studied leptin anorexigenic action after peripheral leptin injection. Leptin significantly suppressed food intake in CF and CM mice (Fig. 3B). Conversely, and inconsistent with a process of masculinization, leptin's ability to suppress food intake was dramatically reduced in NTFs compared with CFs and CMs (Fig. 3B). Whereas CMs showed an increased reduction of body weight in response to leptin compared with CFs, conversely, leptin-induced body weight loss was blunted in NTFs compared with CFs and CMs (Fig. 3C). This leptin resistance was further reflected in an increased adiposity in NTFs compared with CFs and CMs (Fig. 3D).
Fig. 3.
NT impairs leptin's ability to suppress energy intake. A, Fed serum leptin concentrations were measured in 12-wk-old mice (n = 8–9). Suppression of food intake (B) and reduction of body weight (C) were assessed daily for 4 d after ip leptin injection (25 μg per 20 g/d) in 12-wk-old mice. Daily values and 4 d averages are shown (n = 7–10). D, Total fat pad weights (percent of body weight) were measured in 32-wk-old mice (n = 10–23). Results represent mean ± se. *, P < 0.05; **, P < 0.01; ***, P < 0.001; NTF, CM vs. CF.
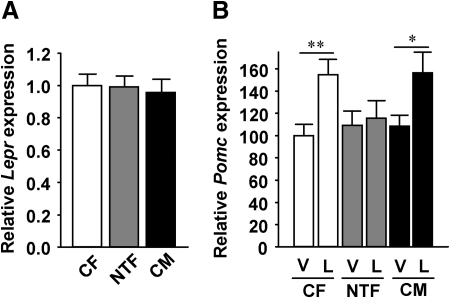
In the hypothalamus, leptin decreases energy intake via the long leptin receptor isoform. However, Lepr expression was similar in all three groups (Fig. 4A). We further studied whether the known effect of leptin to stimulate Pomc expression was altered in NTF mice. Consistent with leptin anorexigenic action, peripheral leptin significantly increased Pomc expression in CFs and CMs (Fig. 4B). Conversely, but consistent with lack of leptin anorexigenic action, leptin failed to stimulate Pomc expression in NTFs (Fig. 4B). Note that this experiment used fasted animals for vehicle and leptin treatment in contrast to Fig. 3, and thus, in the fasted state, differences in Pomc expression were no longer present. No significant difference was observed in leptin suppression of orexigenic neuropeptides between groups (Supplemental Fig. 2B).
Fig. 4.
NT impairs leptin's ability to stimulate Pomc. A, Lepr expression in 12-wk-old mouse hypothalami after refeeding (n = 10–26). B, Pomc expression in 20-wk-old mouse hypothalami after 24 h fasting followed by 6 h of 3 μg/g ip leptin (L) or vehicle (V) treatment (n = 11–13). Results represent mean ± se. *, P < 0.05; **, P < 0.01; NTF, CM vs. CF.
Thus, NT masculinizes feeding behavior and POMC expression in females. Conversely, the impaired leptin action is specific for NTFs and is therefore inconsistent with masculinization. This latter observation supports the hypothesis that testosterone induces leptin resistance selectively in females, as further indicated below.
NT programs a novel form of leptin resistance
The best-defined signaling pathway for leptin to stimulate Pomc expression is the Janus kinase 2-STAT3 pathway (16). To define the mechanism of the observed leptin resistance in NTFs, we quantified leptin-induced pSTAT3 in hypothalamic sections. Surprisingly, despite a marked decrease in leptin sensitivity in NTFs, we were not able to observe any differences in leptin-induced pSTAT3 in the ARC or other hypothalamic sites between NTFs, CFs, or CMs (Supplemental Fig. 3). We thus sought to determine whether leptin signaling via STAT3 was selectively altered in POMC neurons in the rostral part of the ARC, in which most leptin-responsive POMC neurons are located (17). In leptin-stimulated mice, we observed a similar trend in NTFs and CMs toward an increase in total number of POMC neurons and number of POMC neurons coexpressing pSTAT3, suggestive of masculinization in NTFs (Fig. 5). Still leptin-induced pSTAT3 was not decreased in POMC neurons of NTFs.
Fig. 5.
NT does not decrease leptin-stimulated pSTAT3 in POMC neurons. IHC and quantification of pSTAT3/POMC cell number and total POMC neurons in hypothalami from 12-wk-old CF, NTF, and CM mice treated with leptin for 60 min (n = 3). 3V, Third ventricle; ME, median eminence.
Thus, NTFs exhibit a specific form of leptin resistance to suppress energy intake via a STAT3-independent mechanism that is not observed in CMs and is not a mere masculinization.
NT programs MC4R sensitivity
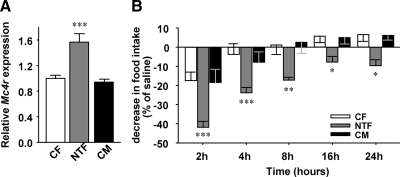
In diet-induced obese and leptin-resistant mice, the melanocortin system is overresponsive due to increased MC4R expression (18). Similarly, we observed a significant increase in hypothalamic Mc4r expression in NTFs compared with CFs and CMs, suggesting a compensatory increase in sensitivity to residual α-MSH (Fig. 6A). Indeed, after ip injection with the MC4R agonist, melanotan II, we observed an increased suppression of food intake in NTF compared with CF and CM mice, confirming increased MC4R sensitivity (Fig. 6B). Thus, NTF mice fail to increase POMC expression in response to leptin but conversely increase MC4R expression and sensitivity to respond to melanocortins.
Fig. 6.
NT increases MC4R sensitivity. A, Mc4r expression was measured by qRT-PCR in hypothalami from 20-wk-old mice after refeeding (n = 10–36). B, Suppression of food intake was measured after ip saline or melanotan II (1 μg/g) injection in 20-wk-old mice (n = 5–10). Results represent the mean ± se. *, P < 0.05; **, P < 0.01; ***, P < 0.001; NTF, CM vs. CF.
NT programs POMC neurons and leptin resistance via distinct pathways
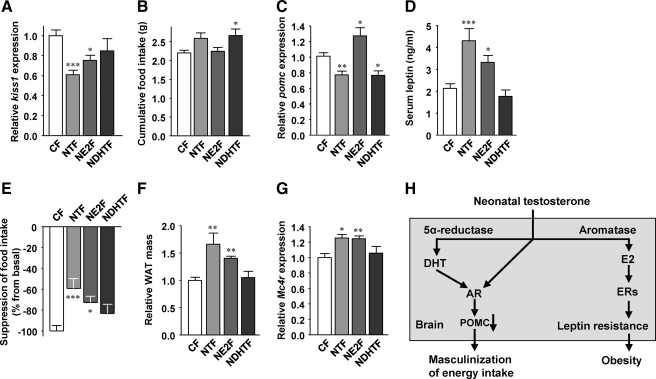
We finally focused on the receptor underlying NT programming of POMC neurons and leptin resistance. Testosterone is a prohormone that is locally converted to either the pure androgen 5α-dihydrotestosterone (DHT), the main ligand of the androgen receptor (AR), or estradiol (E2) acting on estrogen receptors (ERs) (19). Thus, we sought to determine whether NT programs POMC neurons via ER- or AR-dependent pathways. To address this issue, we compared NTF mice to female mice neonatally exposed to DHT (NDHTF) or E2 (NE2F). Again, Kiss1 expression was used as a genetic marker of hypothalamic masculinization (Fig. 3B). We observed that NE2F, but not NDHTF, mice exhibited a suppression of Kiss1 expression to an extent similar to NTF mice (Fig. 7A).We next measured food intake after a 24-h fast. Whereas NE2F mice showed no increase in food intake after refeeding (Fig. 7B), NDHTF mice were hyperphagic similar to NTF mice (Fig. 7B). Consistent with the food intake measurement, hypothalamic Pomc expression was normal in NE2F mice (Fig. 7C). However, in NDHTF mice, Pomc expression was reduced compared with CFs and as observed in NTF mice (Fig. 7C). NDHTF mice maintained normal serum leptin concentrations, in contrast to NTF mice (Fig. 7D). Conversely, NE2F mice, like NTF mice, showed hyperleptinemia, suggesting that they are leptin resistant (Fig. 7D). To test that possibility, we studied the anorexigenic effect of peripheral leptin. NDHTF mice showed normal leptin suppression of food intake (Fig. 7E), and accordingly they retained normal adiposity (Fig. 7F). Conversely, and consistent with hyperleptinemia, NE2F mice exhibited a failure of leptin to suppress food intake (Fig. 7E) and developed increased adiposity as observed in NTF mice (Fig. 7F). Finally, as expected from the leptin resistance data, NDHTF mice retained normal Mc4r expression, whereas NE2F mice, like NTF mice, developed a compensatory increased Mc4r expression (Fig. 7G). Thus, neonatal exposure to DHT programs Pomc and energy intake, whereas neonatal exposure to E2 leads to leptin resistance and obesity.
Fig. 7.
Effects of neonatal DHT and E2 on the melanocortin system. A, Kiss1 expression was measured by qRT-PCR in hypothalami from 20-wk-old mice after refeeding (n = 12–21). B, Cumulative food intake was measured 4 h after refeeding after 24 h fasting in 12-wk-old mice (n = 8–15). C, Hypothalamic Pomc expression was measured qRT-PCR in fed condition in 20-wk-old mice (n = 10–34). D, Fed serum leptin concentrations were measured in 12-wk-old mice (n = 9–26). E, Suppression of food intake was assessed daily for 4 d after ip leptin injection (25 μg per 20 g/d) in 18-wk-old mice. Four-day averages are shown (n = 11–22). F, Total fat pad weights (relative to CF) were measured in 12- to 20-wk-old mice (n = 8–31). G, Mc4r expression was measured by qRT-PCR in hypothalami from 20-wk-old mice after refeeding (n = 8–14). Results represent the mean ± se. *, P < 0.05; **, P < 0.01; ***, P < 0.001; NTF, NE2F, NDHTF vs. CF. H, Proposed pathways whereby NT programs energy intake and leptin resistance. NT is metabolized to the potent androgen DHT, and DHT masculinizes POMC neurons via the AR. NT is aromatized to E2 and programs leptin resistance via ERs.
Discussion
Exposure of female mice to testosterone during a critical neonatal developmental window programs a sexual differentiation of hypothalamic POMC neurons. Conversely, and inconsistent with a masculinization, NT programs a developmental failure of leptin to stimulate hypothalamic POMC production.
The developing brain is feminine by default, regardless of the animal's genetic sex. To achieve a male-specific brain, the differentiated testis produces two perinatal testosterone surges that program the organization of neural circuits permissive to the activation of male behavior at puberty. The sex-specific brain differentiation, however, can also be achieved by neonatal exposure to exogenous testosterone in female rodents (6–8). We used this paradigm to explore the role of NT in programming the sex-specific dimorphisms in energy intake. The evolutionary survival strategy of male mammals after food deprivation is to gain fat by increasing energy intake, whereas females reduce fat loss by decreasing energy expenditure (15). Our study suggests that the male increase in energy intake results from sex dimorphism of the melanocortin system. CM mice exhibit a decreased density of POMC neuronal fibers in the ARC compared with CF mice. This male phenotype is associated with decreased POMC expression during refeeding possibly caused by decreased POMC protein and/or an altered POMC neuron wiring of the ARC. POMC is an important suppressor of energy intake (20, 21). Thus, the male-specific differences in POMC neurons may explain the elevated energy intake characteristic of males compared with females. We find that NTF mice have developed a masculinized POMC neuron architecture and function which may cause NTF mice increased energy intake in normal feeding conditions and in response to starvation. Thus, NT has imprinted male patterns of melanocortin-dependent feeding behavior in female mice. This suggests that first, the POMC neuron architecture/function is inherently feminine in mice and can be masculinized by perinatal sex steroids. Second, this suggests that adult sex dimorphisms in POMC neurons could be determined neonatally by the testicular testosterone surge acting in the developing central nervous system of males. This latter issue has not been directly addressed in our study, however, and deserves further investigation. Furthermore, the causality between masculinization of POMC neurons and increase in food intake still remains to be established.
Testosterone is a prohormone that is converted to the more potent androgen DHT that binds the AR. Testosterone can also be aromatized to E2, which binds at least two ERs, ERα and ERβ. E2 masculinizes the neonatal brain, which accounts for most sex differences in neural structure and behavior (4, 5, 7, 9). Accordingly, we observe that NT masculinizes Kiss1 and thus prevents the preovulatory surge of gonadotropin via aromatization in E2 acting on ERs because it is recapitulated by neonatal E2 but not DHT. It appears, however, that NT masculinizes the hypothalamic POMC neurons, via AR action, because it is reproduced by neonatal DHT but not E2. This is consistent with the presence of the AR in the mouse neonatal ARC (22). Thus, the AR seems to have an organizational function in the hypothalamic melanocortin system. The mechanism through which the AR programs POMC neurons is still unknown. Gonadal steroids exert permanent organizational effects on the developing brain of rodents through alteration in cell death, neuronal connectivity, or epigenetic changes in gene promoters (6, 23, 24). Because POMC neuron number is not decreased in NTF females, the possibility that an AR-dependent pathway promotes POMC neuron apoptosis is implausible. Rather, the possibility that the AR influences POMC synaptogenesis in the ARC or programs epigenetic modification of the POMC promoter, thus decreasing its expression, deserves investigation.
NTFs exhibit a defect independent of masculinization: a failure of leptin to stimulate Pomc expression. We believe that the decreased ability of leptin to stimulate Pomc expression is instrumental in the development of obesity in NTFs. First, POMC neurons are considered to be critical in mediating leptin's anorexigenic action (11), and a decrease in leptin's ability to stimulate POMC expression is expected to favor obesity. Indeed, mice lacking leptin receptors in POMC neurons exhibit a moderate decrease in Pomc but still show hyperleptinemia and develop obesity as can be observed in NTFs (25). Second, NT-induced leptin resistance is characterized by a defect that is also observed during high-fat feeding-induced leptin resistance and obesity (18). This defect is a compensatory increase in sensitivity of the downstream melanocortin system via MC4R up-regulation. Thus, NT-induced leptin resistance may blunt the increase in POMC when serum leptin rises, thus favoring adipose accumulation. However, NT-induced leptin resistance also exhibits a specific feature. In classical forms of leptin resistance such as high-fat feeding or pregnancy, impairment of leptin's ability to suppress food intake and regulate body weight is characterized by decreased leptin signaling through STAT3 (26, 27). Precisely, the site of defective leptin-induced pSTAT3 during diet-induced obesity is the ARC (26). The paradigm here is that NTF mice display a novel form of leptin resistance that is also observed in the ARC but, conversely, is independent of STAT3 signaling. STAT3 is the major transcription factor inducing Pomc via a STAT3 response element on the Pomc promoter (16). Still, in NTF mice, leptin stimulation of pSTAT3 is normal in POMC neurons, whereas leptin induction of Pomc is altered. This suggests that NTF hypothalami exhibit a developmental abnormality of a STAT3-independent leptin signaling pathway. This abnormality in leptin action is programmed in an ER-dependent manner because the obesity phenotype associated with hyperleptinemia, leptin resistance, and compensatory increase in MC4R is reproduced by neonatal E2. Studies using isoform-specific, ER-deficient mice are ongoing to address this issue.
This study has clinical implications. In mice, the neonatal window of developmental plasticity of ARC feeding circuits (13) shares similarities with ARC synaptogenesis in humans during the second trimester of pregnancy (28). Accordingly, in humans, there is evidence of masculinization by prenatal testosterone. Females from opposite sex twin pairs exposed to prenatal testosterone from a male cotwin develop masculinized eating behaviors as adults (29), suggesting that our model recapitulates human pathology. Furthermore, evidence suggests that the polycystic ovarian syndrome, the most common endocrine disorder of premenopausal women, has a developmental origin in which maternal androgen excess during pregnancy programs both the reproductive and metabolic abnormalities (30, 31). Because the NTF mouse shows both kiss1 suppression with anovulation and metabolic abnormalities, we speculate that perinatal testosterone excess programming POMC neurons and leptin resistance in female fetus may participate in the pathogenesis of polycystic ovarian syndrome.
In summary, NT programs a sexual differentiation of energy homeostasis in female mice leading to two independent types of events: 1) DHT-dependent masculinization of hypothalamic POMC neurons associated with elevated energy intake; and 2) E2-dependent leptin resistance which favors obesity. Figure 7H summarizes the programming effect of NT on energy homeostasis.
Supplementary Material
Acknowledgments
We are grateful to Surabhi Bhatt for animal husbandry. We acknowledge the core of the University of Virginia Center for Research in Reproduction (National Institute of Child Health and Human Development Grant U54-HD28934) for measurement of gonadal hormones and the Seattle Mouse Metabolic Phenotype Core(Grant U24-DK076126) for measurement of locomotor activity and energy expenditure.
This work was supported by Grants P50 HD044405 and RO1 DK074970-01 from the National Institutes of Health, the March of Dimes Grant 6-FY07-678, and Northwestern University Institute for Women's Health Research Pioneer Award (to F.M.-J.). Immunohistochemistry experiments (Figs. 4 and 5) were supported by Grant AHA053298N and National Institutes of Health Grant P20 RR02195 (to H.M.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AR
- Androgen receptor
- ARC
- arcuate nucleus
- CF
- control female
- CM
- control male
- DHT
- 5α-dihydrotestosterone
- E2
- estradiol
- ER
- estrogen receptor
- IHC
- immunohistochemistry
- MC4R
- melanocortin 4 receptor
- NDHTF
- NTF mice to female mice neonatally exposed to DHT
- NE2F
- female mice neonatally exposed to E2
- NT
- neonatal testosterone
- NTF
- female mice neonatally androgenized with testosterone
- p
- phosphorylated
- POMC
- proopiomelanocortin
- qRT-PCR
- real-time quantitative PCR
- STAT3
- signal transducer and activator of transcription 3.
References
- 1. Shi H, Seeley RJ, Clegg DJ. 2009. Sexual differences in the control of energy homeostasis. Front Neuroendocrinol 30:396–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wade GN. 1972. Gonadal hormones and behavioral regulation of body weight. Physiol Behav 8:523–534 [DOI] [PubMed] [Google Scholar]
- 3. Corbier P, Edwards DA, Roffi J. 1992. The neonatal testosterone surge: a comparative study. Arch Int Physiol Biochim Biophys 100:127–131 [DOI] [PubMed] [Google Scholar]
- 4. Arnold AP, Gorski RA. 1984. Gonadal steroid induction of structural sex differences in the central nervous system. Annu Rev Neurosci 7:413–442 [DOI] [PubMed] [Google Scholar]
- 5. MacLusky NJ, Naftolin F. 1981. Sexual differentiation of the central nervous system. Science 211:1294–1302 [DOI] [PubMed] [Google Scholar]
- 6. Simerly RB. 2002. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci 25:507–536 [DOI] [PubMed] [Google Scholar]
- 7. Morris JA, Jordan CL, Breedlove SM. 2004. Sexual differentiation of the vertebrate nervous system. Nat Neurosci 7:1034–1039 [DOI] [PubMed] [Google Scholar]
- 8. Negri-Cesi P, Colciago A, Pravettoni A, Casati L, Conti L, Celotti F. 2008. Sexual differentiation of the rodent hypothalamus: hormonal and environmental influences. J Steroid Biochem Mol Biol 109:294–299 [DOI] [PubMed] [Google Scholar]
- 9. Wu MV, Manoli DS, Fraser EJ, Coats JK, Tollkuhn J, Honda S, Harada N, Shah NM. 2009. Estrogen masculinizes neural pathways and sex-specific behaviors. Cell 139:61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. 2007. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology 148:1774–1783 [DOI] [PubMed] [Google Scholar]
- 11. Elmquist JK, Elias CF, Saper CB. 1999. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron 22:221–232 [DOI] [PubMed] [Google Scholar]
- 12. Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. 1997. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385:165–168 [DOI] [PubMed] [Google Scholar]
- 13. Bouret SG, Draper SJ, Simerly RB. 2004. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 304:108–110 [DOI] [PubMed] [Google Scholar]
- 14. Barraclough CA. 1961. Production of anovulatory, sterile rats by single injections of testosterone propionate. Endocrinology 68:62–67 [DOI] [PubMed] [Google Scholar]
- 15. Shi H, Strader AD, Woods SC, Seeley RJ. 2007. Sexually dimorphic responses to fat loss after caloric restriction or surgical lipectomy. Am J Physiol Endocrinol Metab 293:E316–E326 [DOI] [PubMed] [Google Scholar]
- 16. Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr 2003. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 421:856–859 [DOI] [PubMed] [Google Scholar]
- 17. Münzberg H, Huo L, Nillni EA, Hollenberg AN, Bjørbaek C. 2003. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology 144:2121–2131 [DOI] [PubMed] [Google Scholar]
- 18. Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, Grove KL, Cowley MA. 2007. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab 5:181–194 [DOI] [PubMed] [Google Scholar]
- 19. Simpson ER, Misso M, Hewitt KN, Hill RA, Boon WC, Jones ME, Kovacic A, Zhou J, Clyne CD. 2005. Estrogen—the good, the bad, and the unexpected. Endocr Rev 26:322–330 [DOI] [PubMed] [Google Scholar]
- 20. Krude H, Biebermann H, Luck W, Horn R, Brabant G, Grüters A. 1998. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet 19:155–157 [DOI] [PubMed] [Google Scholar]
- 21. Challis BG, Coll AP, Yeo GS, Pinnock SB, Dickson SL, Thresher RR, Dixon J, Zahn D, Rochford JJ, White A, Oliver RL, Millington G, Aparicio SA, Colledge WH, Russ AP, Carlton MB, O'Rahilly S. 2004. Mice lacking pro-opiomelanocortin are sensitive to high-fat feeding but respond normally to the acute anorectic effects of peptide-YY(3–36). Proc Natl Acad Sci USA 101:4695–4700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Juntti SA, Tollkuhn J, Wu MV, Fraser EJ, Soderborg T, Tan S, Honda S, Harada N, Shah NM. 2010. The androgen receptor governs the execution, but not programming, of male sexual and territorial behaviors. Neuron 66:260–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCarthy MM, Auger AP, Bale TL, De Vries GJ, Dunn GA, Forger NG, Murray EK, Nugent BM, Schwarz JM, Wilson ME. 2009. The epigenetics of sex differences in the brain. J Neurosci 29:12815–12823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McCarthy MM. 2010. How it's made: organisational effects of hormones on the developing brain. J Neuroendocrinol 22:736–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, Lowell BB. 2004. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42:983–991 [DOI] [PubMed] [Google Scholar]
- 26. Münzberg H, Flier JS, Bjørbaek C. 2004. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 145:4880–4889 [DOI] [PubMed] [Google Scholar]
- 27. Ladyman SR, Grattan DR. 2004. Region-specific reduction in leptin-induced phosphorylation of signal transducer and activator of transcription-3 (STAT3) in the rat hypothalamus is associated with leptin resistance during pregnancy. Endocrinology 145:3704–3711 [DOI] [PubMed] [Google Scholar]
- 28. Koutcherov Y, Mai JK, Ashwell KW, Paxinos G. 2002. Organization of human hypothalamus in fetal development. J Comp Neurol 446:301–324 [DOI] [PubMed] [Google Scholar]
- 29. Culbert KM, Breedlove SM, Burt SA, Klump KL. 2008. Prenatal hormone exposure and risk for eating disorders: a comparison of opposite-sex and same-sex twins. Arch Gen Psychiatry 65:329–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abbott DH, Barnett DK, Bruns CM, Dumesic DA. 2005. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update 11:357–374 [DOI] [PubMed] [Google Scholar]
- 31. Xita N, Tsatsoulis A. 2006. Review: fetal programming of polycystic ovary syndrome by androgen excess: evidence from experimental, clinical, and genetic association studies. J Clin Endocrinol Metab 91:1660–1666 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.